Abstract
Background:
Merkel cell carcinoma is among the most aggressive and lethal of primary skin cancers, with a high rate of distant metastasis. Anti-PD1/PDL1 monotherapy is currently standard-of-care for unresectable recurrent or metastatic Merkel cell carcinoma. We assessed treatment with combined nivolumab plus ipilimumab (NIVO/IPI) with or without stereotactic body radiation therapy (SBRT) in patients with advanced Merkel cell carcinoma as a first-line therapy or following prior treatment with anti-PD1/PDL1 monotherapy.
Methods:
We randomly assigned adults with unresectable Merkel cell carcinoma stratified by prior immune-checkpoint inhibitor (ICI) status to receive nivolumab 240mg intravenously every 2 weeks plus ipilimumab 1mg/kg intravenously every 6 weeks (NIVO/IPI, Arm A) or NIVO/IPI plus stereotactic body radiation therapy (SBRT) to ≥1 lesion (24Gy in 3 fractions at week 2, Arm B). The trial was non-blinded and open label. Patients had to have ≥2 measurable sites of disease so one non-irradiated site could be followed for response. The primary endpoint was objective response rate (ORR). Response was assessed every 12 weeks by immune-related Response Evaluation Criteria in Solid Tumors.
Findings:
50 patients (24 ICI naïve, 26 following prior ICI) received ≥1 dose of NIVO/IPI and 24 received SBRT (median follow-up 14.6 months). ORR in ICI naïve patients was 100% [95%CI, 82%-100%], 41% with complete response. ORR in patients with prior ICI exposure was 31% [95%CI, 15%-52%], 15% with complete response. No significant differences in ORR were observed between Arms A and B (72% vs. 52%, p=0.26). Median duration of response was not reached [95%CI, 33.9 months-NE] in the ICI naïve cohort and was 10.8 months [95% CI, 4.9 months-NE] in the prior ICI cohort. The toxicity profile was similar to that previously observed for the study drug dose and schedule.
Interpretation:
First-line NIVO/IPI in patients with advanced Merkel cell carcinoma demonstrated an exceptionally high ORR with durable responses and an expected safety profile. NIVO/IPI also showed clinical benefit in patients with prior anti-PD1/PDL1 treatment. Addition of SBRT did not improve efficacy of NIVO/IPI. NIVO/IPI represents a new first-line and salvage therapeutic option for advanced Merkel cell carcinoma.
Funding:
Bristol Myers Squibb Rare Population Malignancy Program clinicaltrials.gov Identifier: NCT03071406
Introduction
Merkel cell carcinoma is among the most aggressive and lethal of primary skin cancers, with a high rate of distant metastases and poor survival outcome1,2. Merkel cell carcinoma pathogenesis is driven by Merkel cell polyomavirus (MCPyV) or ultraviolet light (UV) exposure1,3. In recent years, a major breakthrough in Merkel cell carcinoma was achieved with immune-checkpoint inhibitors (ICI) targeting programmed death ligand 1 (PDL1) and programmed death receptor 1 (PD1). Avelumab and pembrolizumab were approved for the treatment of advanced Merkel cell carcinoma by the Food and Drug Administration (FDA) in 2017 and 2018, respectively. Avelumab was associated with an objective response rate (ORR) of 39.7% as first-line therapy and 33% in patients with chemotherapy-refractory metastatic Merkel cell carcinoma, with durable responses4-6. First-line therapy with pembrolizumab was associated with an ORR of 58% in a phase II study of 50 patients with advanced Merkel cell carcinoma7,8, with the majority of responses being durable7. Pembrolizumab was effective in both MCPyV-positive and UV-induced MCPyV-negative Merkel cell carcinoma8. While these studies have established anti-PD1/PDL1 monotherapy as standard-of-care in advanced Merkel cell carcinoma4,8, the majority of patients who fail anti-PD1/PDL1 monotherapy have limited therapeutic options, and identifying strategies to overcome immunologic barriers leading to tumor resistance to anti-PD1/PDL1 monotherapy is of pivotal importance in the management of Merkel cell carcinoma.
Combining PD1/PDL1 blockade with modulation of alternative immunologic targets has been the mainstay of recent innovation in cancer immunotherapy. Notably, cytotoxic T lymphocyte-associated antigen 4 (CTLA4), expressed on activated effector and regulatory T cells, dampens antitumor immune responses by disrupting CD28 costimulation. Ipilimumab (IPI), an anti-CTLA4 antibody, was the first FDA-approved ICI for cancer. While the single agent activity of IPI has not been extensively investigated in Merkel cell carcinoma, the efficacy of the nivolumab (NIVO) and IPI combination has been demonstrated in several tumor types9-11. Cancer immunotherapy may be also combined with conventional cytotoxic therapy to harness antitumor immune responses elicited by immunogenic cell death12. Radiation therapy in combination with ICI has previously shown clinical benefit13,14. Further, Merkel cell carcinoma is profoundly radiosensitive and radiation therapy has an established role in the locoregional management of Merkel cell carcinoma15; the addition of stereotactic body radiation therapy (SBRT) to ICI is therefore worthy of further investigation. Notably, the abscopal effect of radiation therapy has been most extensively studied in combination with CTLA4 blockade. Subablative hypofractionated radiation therapy ranging 21 – 24Gy delivered over 3 fractions as well as CTLA4 blockade are thought to induce a priming event for eliciting anti-tumor immune responses, and synergy of these two modalities has been demonstrated in multiple preclinical studies16. Interestingly, PD-L1 expression has been proposed as the major mechanism of resistance to the combination of radiation therapy and ipilimumab17. In this context, there is a strong rationale to test whether ipilimumab will potentiate the efficacy of SBRT and Nivolumab in Merkel cell carcinoma. Therefore, we conducted a randomized phase II study with two experimental arms to evaluate the safety and efficacy of NIVO/IPI with or without SBRT in advanced Merkel cell carcinoma.
Methods
PATIENTS
Eligible patients were at least 18 years old with an Eastern Cooperative Oncology Group (EGOG) performance status of 0 or 1, and had histologically-proven recurrent or metastatic Merkel cell carcinoma with a minimum of two measurable tumor lesions, to ensure the presence of at least one unirradiated lesion to assess response. Prior chemotherapy or immunotherapy given in either adjuvant or unresectable/metastatic settings was allowed if new or progressive measurable sites of disease were present at enrollment. Prior radiation therapy was allowed if current measurable lesions were not previously treated with radiation therapy. Patients were stratified based on whether they had received prior immunotherapy with anti-PD1/PDL1 monotherapy. Key exclusion criteria were history of Grade 3 toxicity or use of infliximab with prior immunotherapy, active brain metastasis, autoimmune disease or other conditions requiring systemic treatment with either corticosteroids (>10mg daily prednisone or equivalent) or other immunosuppressive medications, or history of non-Merkel cell carcinoma malignancies except indolent diseases such as chronic lymphocytic leukemia that were not requiring active therapy or adequately treated cancer in complete remission.
STUDY DESIGN
This phase II randomized study, sponsored by the Moffitt Cancer Center and funded through the Bristol Myers Squibb Rare Population Malignancy Program, was developed by the authors. Patients were enrolled at the Moffitt Cancer Center and Ohio State University James Cancer Hospital and Solove Research Institute. A Bayesian pick-the-winner design18 was used with each arm using a Simon’s Mini-Max two-stage design with 10% for type I and II error rates, which required 3 or more responders in the 1st stage of 16 patients to advance to the 2nd stage for a total of 24 patients. Interim analysis was planned following the completion of the 1st stage to evaluate efficacy and unexpected drug-related adverse events. If the total number responding is 6 or less, we would conclude that the treatment was not effective. Arm B would be considered superior if the posterior probability of a higher response rate for Arm B than for Arm A was >80% based on a non-informative prior of beta distribution, beta(1,1). Each arm would have 25 patients with 24 efficacy-evaluable patients, for a total of 50 patients. Eligible patients were randomized with a ratio of 1:1, stratified based on prior ICI. Randomization of patients for this trial took place within the Moffitt SRARS program, a web delivered application that records subject registrations and provides randomization assignment. The trial was non-blinded and open label. Patients were treated with intravenous NIVO 240mg every 2 weeks and intravenous IPI 1mg/kg every 6 weeks (Fig. 1). Patients enrolled onto Arm B also received SBRT to at least one tumor site at a dose of 24Gy in 3 fractions during week 2, beginning before the second NIVO infusion. Non-skin lesions were treated with SBRT delivered on 3 consecutive days, but skin lesions were treated every other day. Simultaneous integrated boost or dose-painting was allowed to meet normal tissue dose constraints. At least one measurable lesion was not to be irradiated to evaluate response outside the radiation field. Subjects received systemic treatment with NIVO/IPI until disease progression, unacceptable toxicity, or withdrawal of consent. Patients were allowed to continue treatment post-progression as long as investigator-assessed clinical benefit was present and the subject was tolerating the study drugs.
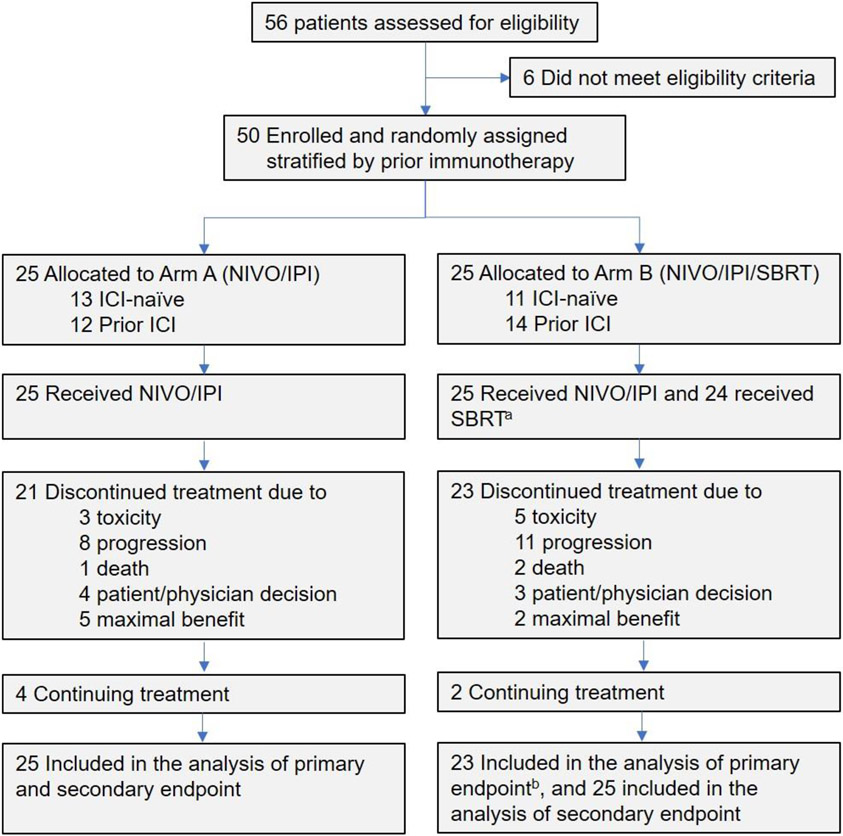
Figure 1. Flowchart of Study Population.
aOne patient failed to receive SBRT due to concern for excess toxicity from overlap with the prior radiation field.
bTwo patients received SBRT to the target lesion and were excluded from the objective response rate.
Abbreviations: NIVO: nivolumab, IPI: ipilimumab, SBRT: stereotactic body radiation therapy
The study was designed with two experimental arms to determine the clinical efficacy of NIVO/IPI and the role of SBRT in augmenting NIVO/IPI, as first-line therapy or following prior ICI failure. The primary endpoint was ORR, and key secondary endpoints included progression-free survival (PFS), overall survival (OS), local control (LC) of irradiated tumors and safety.
STUDY OVERSIGHT
The protocol was approved by the institutional review board at each participating center, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent before study entry. The principal investigators and co-investigators were responsible for the design and oversight of the study. The Moffitt Cancer Center was responsible for centralizing and maintaining the collected data. The manuscript was written and prepared by the authors, who adhered to the study protocol to report complete and accurate data.
CLINICAL ASSESSMENTS
All patients underwent computed tomographic scanning of the thorax, abdomen, and pelvis (as well as any other areas with target lesions) at the time of screening and every 12 weeks after starting therapy. Evaluations of scans according to immune-related Response Evaluation Criteria in Solid Tumors (irRECIST)19 were conducted at the institutional level. Response assessment was to be based on non-irradiated target lesions. Adverse events were recorded according to National Cancer Institute Common Terminology Criteria for Adverse Events, v4.020.
STATISTICAL ANALYSIS
Patients who received at least one dose of NIVO/IPI were included in the analysis. Data are reported as of July 17, 2022. Radiologic and physical examination assessments according to irRECIST were used to determine treatment responses. The best overall response was defined as the best response recorded between the start of the treatment until disease progression. The ORR was calculated as the percentage of patients who had a complete or partial response according to irRECIST among all the patients who received at least one dose of NIVO/IPI. Clopper–Pearson exact confidence intervals were generated for the response rates. Two-sample proportion test and the Bayesian posterior probability were used for comparison of response rate between the two arms. Subgroup analysis was performed based on the ICI naïve and prior ICI stratification. PFS was defined as the interval from the date of the first dose of NIVO/IPI to the date of disease progression or death, whichever occurred earlier. OS was defined as the interval from the date of the first dose of NIVO/IPI to the date of death. DOR was defined as the interval from date of first response to the date of disease progression or death. LC was defined as the interval from the date of SBRT to the date of progression of the irradiated lesion. PFS, OS, DOR and LC were estimated with the Kaplan-Meier method21.
Results
PATIENT CHARACTERISTICS
A total of 50 patients with unresectable stage IIIB or IV Merkel cell carcinoma were enrolled from March 2017 until December 2021. All received protocol-assigned treatment except one patient in Arm B who failed to receive SBRT due to concerns for excess toxicity from overlap with the prior radiation field (Fig 1). Median age was 74 years (IQR 66–81) for Arm A and 73 years (range 68–76) for Arm B. The majority of patients had ECOG PS of 1. 20% of patients on Arm A and 32% of patients on Arm B had recurrent stage IIIB disease; the remainder were stage IV at the time of enrollment (Table 1). Notably, 48% of patients on Arm A and 56% of patients on Arm B were previously treated with pembrolizumab or avelumab (Prior ICI cohort) (Table 1). Of the 26 patients in the Prior ICI cohort, 21 patients demonstrated disease progression while on pembrolizumab or avelumab, 2 patients progressed after discontinuation of these agents following initial response, and 3 patients received ICI as adjuvant therapy and subsequently relapsed. 8% of patients on Arm A and 12% of patients on Arm B had previously received chemotherapy, all of which also received prior ICI. Randomization yielded reasonable balance of demographic and clinical factors in both arms (p>0.05, Table 1).
Table 1.
Patient Characteristics
| Characteristic | Total Cohort (N=50) |
Arm A (NIVO/IPI) (N=25) |
ArmB (NIVO/IPI/SBRT) (N=25) |
P Value |
|---|---|---|---|---|
| Age median [IQR] | 73 [67-81] | 74 [66-81] | 73 [68-76] | 0.81 |
| Gender, No. (%) | ||||
| Female | 11 (22) | 5 (20) | 6 (24) | 0.99 |
| Male | 39 (78) | 20 (80) | 19 (76) | |
| Race: White, No. (%) | 50 (100) | 25 (100) | 25 (100) | 0.99 |
| ECOG Performance Status, No. (%) | 0.99 | |||
| 0 | 23 (46) | 11 (44) | 12 (48) | |
| 1 | 27 (54) | 14 (56) | 13 (52) | |
| Stage, No. (%) | 0.32 | |||
| IIIB | 12 (24) | 4(16) | 8 (32) | |
| IV | 38 (76) | 21 (84) | 17 (68) | |
| Primary Tumor Site, No. (%) | 0.48 | |||
| Head and Neck | 16 (32) | 11 (44) | 5 (20) | |
| Trunk | 2 (4) | 1 (4) | 1 (4) | |
| Extremities | 26 (52) | 11 (44) | 15 (60) | |
| Unknown Primary | 6 (12) | 2 (8) | 4 (16) | |
| M Stage, No. (%) | 0.26 | |||
| MO | 12 (24) | 4 (16) | 8 (32) | |
| M1a | 19 (38) | 12 (48) | 7 (28) | |
| M1b | 0 (0) | 0 (0) | 0 (0) | |
| M1c | 19 (38) | 9 (36) | 10 (40) | |
| #Distant Metastastic Sites, No. (%) | ||||
| 0 | 12 (24) | 4 (16) | 8 (32) | 0.81 |
| 1-2 | 16 (32) | 9 (36) | 7 (28) | |
| 3-4 | 14 (28) | 9 (36) | 5 (20) | |
| >5 | 7 (14) | 3 (12) | 4 (16) | |
| LDH, No. (%) | ||||
| Normal | 26 (52) | 10 (40) | 16 (64) | 0.16 |
| Elevated | 24 (48) | 15 (60) | 9 (36) | |
| Prior Immunotherapy Status, No. (%) | 0.78 | |||
| ICI Naïve | 24 (48) | 13 (52) | 11 (44) | |
| Prior ICI | 26 (52) | 12 (48) | 14 (56) | |
| Prior Chemotherapy Status, No. (%) | 0.99 | |||
| Chemotherapy-Naïve | 45 (90) | 23 (92) | 22 (88) | |
| Prior Chemotherapy | 5 (10) | 2 (8) | 3 (12) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ICI, immune-checkpoint inhibitor; IQR, interquartile range; NIVO, nivolumab; IPI, ipilimumab; SBRT, stereotactic body radiation therapy; LDH, lactate dehydrogenase.
CLINICAL ACTIVITY
Both arms completed the two stages of accrual with 25 patients each (Fig 1). In Arm B, two ICI naïve patients received SBRT to the only target lesion that was measurable by irRECIST. Target lesions in both patients responded. In one patient, both the irradiated target lesion and the non-irradiated non-target lesion completely resolved. In the second patient, the irradiated lesion achieved partial response and the non-irradiated non-target lesions remain stable and without new lesions at 9 months. Because the target lesions were irradiated, these two patients were deemed nonevaluable and excluded from analysis of ORR.
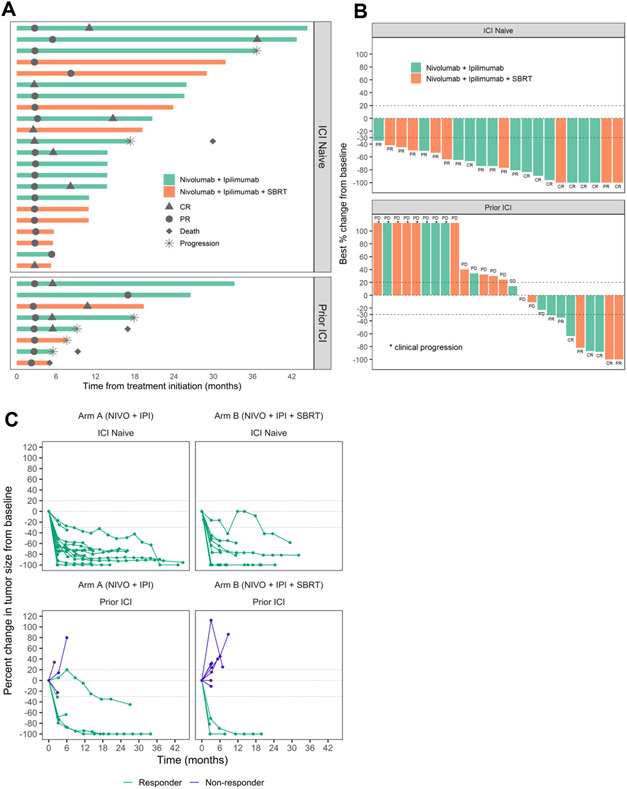
There were 18 responders on Arm A (72%) and 12 responders on Arm B (52%) with no statistical difference between the two arms (p=0.26 by two-sample proportion test; 0.2 for the Bayesian posterior probability of higher response rate in Arm B than in Arm A). Pooled analysis stratified by ICI status demonstrated that 22 of 22 evaluable patients (100%) who had not previously received anti-PD1/PDL1 monotherapy achieved an objective response, 9 (41%) with complete response (Table 2). By contrast, 8 of 26 patients (31%) who had previously received anti-PD1/PDL1 monotherapy treatment achieved radiographic response, 4 (15%) with complete response (Table 2). A representative radiographic response of a long-term survivor following response to NIVO/IPI is shown (Supplementary Fig 1). Among the 30 responders, response was confirmed on a subsequent evaluation in 28 patients. Two responding patients in the Prior ICI cohort had unconfirmed partial responses: one patient subsequently progressed, and one patient expired prior to the confirmation scan.
Table 2.
Objective Response and Durability of Response
| Outcomesa | Total Cohort | Arm A (NIVO + IPI) | Arm B (NIVO + IPI + SBRT) | |||
|---|---|---|---|---|---|---|
| ICI Naïve (N=24) |
Prior ICI (N=26) |
ICI Naïve (N=13) |
Prior ICI (N=12) |
ICI Naïve (N=11) |
Prior ICI (N=14) |
|
| ORR [95%CI] | 100 [82-100] | 31 [15-52] | 100 [72-100] | 42 [16-71] | 100 [63-100] | 21 [6-51] |
| BOR, No. (%) | ||||||
| Complete Response | 9 (41) | 4 (15) | 7 (54) | 3 (25) | 2 (22) | 1 (7) |
| Partial Response | 13 (59) | 4 (15) | 6 (46) | 2 (17) | 7 (78) | 2 (14) |
| Stable Disease | 0 (0) | 1 (4) | 0 (0) | 1 (8) | 0 (0) | 0 (0) |
| Progressive Disease | 0 (0) | 17 (65) | 0 (0) | 6 (50) | 0 (0) | 11 (79) |
| Nonevaluableb | 2 | 0 | 0 | 0 | 2 | 0 |
| Progression following initial response, No. (%) | 2 (9) | 4 (50) | 2 (15) | 3 (60) | 0 (0) | 1 (33) |
| Median DOR, mo [95%CI]c | NE [34-NE] | 11 [5-NE] | NE [34-NE] | 15 [7-NE] | NE | 5 [3-NE] |
Numbers were rounded to the nearest whole number.
Two patients deemed nonevaluable as the target lesion was irradiated.
Includes 30 responders with at least 6 months of follow-up.
Abbreviations: BOR, best overall response; 95%CI, 95% confidence interval, ICI, immune-checkpoint inhibitor; NIVO, nivolumab; IPI, ipilimumab; ORR, objective response rate; SBRT, stereotactic body radiation therapy, DOR, duration of response, NE, non-estimable.
Following initial response, 2 of 22 responders in the ICI naïve cohort have subsequently progressed, whereas 4 of 8 responders have progressed in the Prior ICI cohort (Table 2, Fig 2A). All except one patient were still receiving protocol treatment at the time of progression. Additionally, 1 responder in the Prior ICI cohort expired following initial response unrelated to the disease status or toxicity. Median duration of response was not reached [95% CI, 33.9 months-NE] in the ICI naïve cohort and was 10.8 months [95% CI, 4.9 months-NE] in the Prior ICI cohort (Table 2). The best overall response, disease progression, and death, are summarized in Fig 2A, 2B. To gain further insight into the durability of response, a spider plot of target lesion size measurement for each patient was generated. Most patients achieved durable tumor regression following initial response (Fig 2C).
Figure 2.
Clinical Activity. A. Time to response and duration of response and duration of treatment were measured in 30 patients with a clinical response (complete or partial response). * represents progression (20% increase in the sum of diameters of target lesions or appearance of a new lesion) and ♦ represents death. B. Waterfall plot of best response percent change from baseline. Among 50 patients, 2 patients were excluded as the target lesions were irradiated. *, 8 patients experienced clinical progression prior to the first planned restaging. C. Spider plot of change in target lesion diameters and percentage of change in target lesion sum diameters from baseline in individual patients. 2 patients with irradiated target lesion and 8 patients with clinical progression prior to the first planned restaging are not shown.
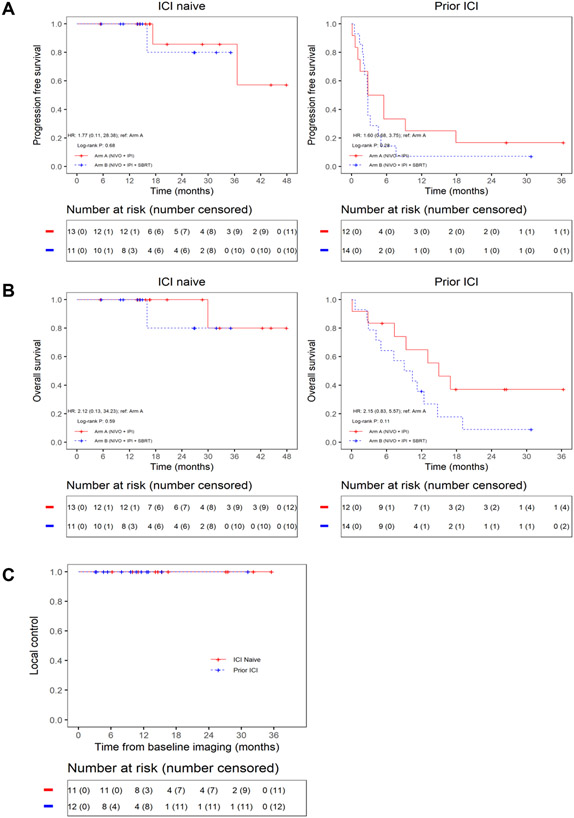
With a median follow-up of 14.6 months, median PFS was not reached for the ICI naïve cohort on either arm. For the prior ICI cohort, median PFS was 4.2 months [95% CI, 1.5 months-NE] (Arm A) and 2.7 months [95% CI, 2.2 months – 7.6 months] (Arm B). (Fig 3A). Median OS was not reached for the ICI naïve cohort in either arm, while median OS for the Prior ICI cohort was 14.9 months [95% CI, 0.3 months-NE] (Arm A) and 9.7 months [95% CI, 5.0 months-NE] (Arm B) (Fig 3B). Local progression of an irradiated lesion was not observed in any patients treated with SBRT (Fig 3C).
Figure 3.
Survival Outcome. A. Kaplan-Meier curves of progression-free survival for ICI naïve and Prior ICI cohort. B. Kaplan-Meier curves of overall survival for ICI naïve and Prior ICI cohort. C. Kaplan-Meier curves of local control of irradiated lesions in Arm B.
SAFETY
Adverse effects were primarily attributable to NIVO/IPI, and minimal SBRT-related toxicities were observed. Treatment-related adverse events of any grade occurred in 90% of the patients. The most common adverse event was fatigue. Grade 3 or 4 treatment-related adverse events were observed in 36% of patients, 40% on Arm A and 32% on Arm B. 5 patients had grade 4 treatment-related adverse events with 4 who had elevated pancreatic enzymes and 1 who had hyponatremia with acute kidney injury (Supplementary Table 1). 8 patients discontinued the protocol treatment due to toxicity. Three patients with rapidly progressive disease after prior ICI treatment expired prior to first planned restaging. In these cases, death was attributed to disease progression and not to treatment-related toxicity; all were considered to have progressive disease as best overall response.
Discussion
Antibodies targeting the PD1/PDL1 pathway are active in Merkel cell carcinoma, one of the most immunogenic human cancers, with ORR ranging from 32–62% and durable responses4,8,22. However, Recurrence after or refractoriness to first-line anti-PD1/PDL1 therapy remains a significant clinical challenge. Platinum-based chemotherapy generally only offers transient responses in Merkel cell carcinoma23 and targeted therapies have not been successful24,25. As Merkel cell carcinoma is a relatively rare tumor type, we adopted a randomized study design with two experimental arms, which allowed us to evaluate the efficacy of dual checkpoint inhibitor blockade targeting PD1 and CTLA4 as well as the potential role for subablative immunogenic SBRT, in both ICI naïve patients and patients who previously failed pembrolizumab or avelumab.
To our knowledge, our study is the first to evaluate the use of dual checkpoint inhibitor blockade as first-line therapy in advanced Merkel cell carcinoma. The results to date suggest remarkable clinical activity of combined PD1 and CTLA4 blockade in ICI naïve Merkel cell carcinoma patients. Although NIVO/IPI was not directly compared to anti-PD1/PDL1 monotherapy, the observed ORR at 100% (22 out of 22, 95% CI 82%-100%) with complete response in 41% (9 out of 22) in ICI naïve patients is substantially higher than would be expected with monotherapy4,7. Further, NIVO/IPI demonstrated substantial durability of response in the ICI naïve cohort (Table 2, Fig 2). However, given emerging reports of high rates of Merkel cell carcinoma relapse following ICI discontinuation26, longer follow up will be needed to assess response durability for patients treated on this trial, particularly after treatment discontinuation.
Relapsed or refractory disease following anti-PD1/PDL1 monotherapy remains a clinical challenge. Though far less robust than in the ICI naïve population, our results confirm clinically meaningful activity in the ICI relapsed/refractory population, as has been suggested by recent retrospective reports27,28, but contrasts with one retrospective report which showed zero objective responses in 13 treated patients29. In this latter study, it is notable that the majority of treated patients had poor performance status (ECOG >1), presumably due to advanced disease, and 38% had received cytotoxic chemotherapy between prior ICI and NIVO/IPI. In contrast, in our study all patients had good performance status and only 19% (5 of 26) had also received prior chemotherapy. Taken in context with these prior reports, we believe that our study results strongly suggest that NIVO/IPI has the highest chance of success when implemented early in the disease process for advanced Merkel cell carcinoma, prior to single agent ICI failure and prior to cytotoxic chemotherapy use.
The concept of SBRT as an in situ tumor vaccine harnessing an immunogenic effect has actively been investigated in recent years with some promising signals13,14, but also with negative findings30. In our study, the addition of SBRT failed to improve ORR in comparison to NIVO/IPI alone. Given the extremely high ORR among ICI naïve patients in both arms, it would have been difficult to see any additive or synergistic benefit of SBRT, if it existed. Conversely, in the Prior ICI cohort, any immunogenic effect of SBRT may have been too modest to impact clinical outcomes in the face of a heavily dysfunctional tumor-immune microenvironment31. Though not statistically significant, ORR was numerically lower in the treatment arm that received SBRT (Arm B, 52%) as compared to that arm that did not (Arm A, 72%). Given the small patient sample size, our study was not sufficiently powered to address whether SBRT has a subtle detrimental effect on NIVO/IPI efficacy, and further studies would be required to answer this question. However, having fewer evaluable ICI naïve patients in Arm B (9) versus evaluable ICI naïve patients in arm A (13) could account for much of this difference. Regardless, our data does not support routinely incorporating SBRT with combination ICI therapy in advanced Merkel cell carcinoma unless indicated for palliative reasons. In such cases where SBRT is considered for palliation, our study demonstrates that 24Gy delivered in 3 fractions can be safely administered in combination with NIVO/IPI. Additionally, the excellent local control observed suggests de-escalated radiation dose may be sufficient for local control in Merkel cell carcinoma which warrants further investigation, particularly for early stage Merkel cell carcinoma patients who routine receive radiation doses over 50Gy, as has also been suggested by a previous report32.
Subgroup analysis of baseline PD-L1 expression or Merkel cell polyomavirus status did not reveal any association with clinical response consistent with prior studies with anti-PD1/PDL1 monotherapy (Supplementary Fig 2). Interestingly, subgroup analysis of the prior ICI cohort revealed numerically higher ORR among patients who were previously exposed to PDL1 blockade vs. PD1 blockade (45% vs. 21%) (Supplementary Table 2), although this difference did not translate into survival outcome (Supplementary Fig 3). While our study does not have the statistical power to conclude whether there is a true difference in the efficacy of NIVO/IPI as a salvage regimen following first line failure after anti-PD1 therapy vs. anti-PDL1 therapy, further investigation is warranted to determine whether a non-overlapping mechanism of PD1 vs. PDL1 blockade may lead to an altered therapeutic response to NIVO/IPI.
The NIVO/IPI regimen used in our trial demonstrated an expected safety profile. Because most Merkel cell carcinoma patients are elderly, a low dose of ipilimumab (1mg/kg every 6 weeks) was selected for this study. Grade 3 and 4 treatment-related toxicities were observed in 36% of the patients enrolled, comparable to that observed with phase III studies in NSCLC (CheckMate-227) and malignant mesothelioma (CheckMate-743) using the same dose and schedule of NIVO/IPI10,33, and substantially lower than that observed with higher doses of ipilimumab in combination with anti-PD1/PDL134. Whether a regimen including a higher dose or greater frequency of administration of ipilimumab would be more useful in the refractory disease setting merits further evaluation.
This study does have limitations. This is a phase II study from 2 centres with a small patient sample size. Additionally, this was a non-registrational trial and independent assessment was not performed. However, Merkel cell carcinoma is one of the rarest malignancies affecting elderly population often with substantial comorbidities, which renders large phase III studies difficult. Despite these limitations, our results suggest that first-line therapy with NIVO/IPI in patients with recurrent or metastatic Merkel cell carcinoma is effective and durable with a manageable safety profile. NIVO/IPI has also shown clinical activity in patients who have previously failed anti-PD1/PDL1 monotherapy, albeit with a far lower response than in the first-line setting. With further validation with followup studies, NIVO/IPI could be considered in the first-line and salvage setting.
Supplementary Material
Research in context.
Evidence before this study
Merkel cell carcinoma is a rare but aggressive skin cancer driven by Merkel cell polyomavirus or ultraviolet light exposure with high rate of metastasis and poor prognosis. In recent years, a major breakthrough with anti-PD1/PDL1 therapy has shifted paradigms in the management of Merkel cell carcinoma. With an ORR ranging from 33% - 58% with durable responses, avelumab and pembrolizumab were approved by Food and Drug Administration as first-line therapy, and are currently the standard of care as per the National Comprehensive Cancer Network and European consensus-based interdisciplinary guidelines. Despite the immense success of anti-PD1/PDL1 therapy, patients who are refractory to these agents represent a major clinical challenge with few therapeutic options. Multiple chemotherapy regimens are clinically active in Merkel cell carcinoma, but responses are short-lived. Further, there have been limited success with targeted therapies. Therefore, strategies to improve upon the efficacy of anti-PD1/PDL1 monotherapy is of crucial importance. Targeting CTLA4 in combination with anti-PD1/PDL1 therapy has been tested in several cancers with promising outcome. Recent retrospective institutional case series have reported some clinical activity of NIVO/IPI among Merkel cell carcinoma patients who have previously progressed on anti-PD1/PDL1 monotherapy, but prospective studies of this combination have not been previously conducted. Additionally, the capacity of radiation therapy to induce systemic anti-tumor immune responses has been under intense research with conflicting outcome in recent years. While radiation therapy has an integral role in locoregional management of early stage Merkel cell carcinoma, its potential impact in augmenting systemic immunotherapy has not been previously investigated in advanced Merkel cell carcinoma.
Added value of this study
This randomized trial with two experimental arms investigated the efficacy of NIVO/IPI and NIVO/IPI plus SBRT in a population of patients with unresectable, recurrent, or metastatic Merkel cell carcinoma. Patients were stratified by prior exposure to immune checkpoint inhibitor (ICI) treatment. Among 22 ICI naïve patients, 100% ORR was achieved, 41% with complete response. The median duration of response, PFS and OS had not been reached with a median follow-up time of 14.6 months in this cohort. A substantial clinical activity was also seen among patients who have previously received anti-PD1/PDL1 monotherapy with 31% ORR, 15% with complete response. There was no significant difference in ORR in patients treated with NIVO/IPI plus SBRT versus those treated with NIVO/IPI alone. To our knowledge, this is the first prospective study demonstrating the efficacy of combined anti-PD1/PDL1 and anti-CTLA4 in Merkel cell carcinoma.
Implications of all the available evidence
Our findings provide evidence that nivolumab plus ipilimumab exhibit an exceptionally high objective response rate with durable responses in ICI naïve advanced Merkel cell carcinoma. Nivolumab plus ipilimumab also demonstrated clinically meaningful activity in patients who have previously received anti-PD1/PDL1 monotherapy. The protocol treatment was well tolerated with the toxicity profile comparable to that previously observed for the study drug dose and schedule. These data support the use of combination immune checkpoint inhibitor therapy for the treatment of advanced Merkel cell carcinoma.
Acknowledgement
The trial was primarily supported by funding from the Bristol Myers Squibb Rare Population Malignancy Program. Bristol Myers Squibb provided scientific accuracy review of the protocol and publication. The company played no other role in the study or report. This work was also supported in part by K08 CA194273, R37CA248298, NCI Cancer Center Support Grant (P30-CA076292), and the Moffitt Foundation.
Role of funding source
This investigator initiated clinical trial was sponsored by the Moffitt Cancer Center and funded through the Bristol Myers Squibb Rare Population Malignancy Program. Bristol Myers Squibb provided scientific accuracy review of the protocol and publication, but otherwise had no role in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements
SK reports Research Support (Bristol Myers Squibb) for the submitted work and Research Support (Bristol Myers Squibb, AstraZeneca) outside the submitted work. EW reports Advisory Board (Viewray, Varian, Alphatau, Castle Biosciences, Teiko) outside the submitted work. ZE reports Advisory Boards (Array, Pfizer, OncoSec, Regeneron, Genentech, Novartis, Natera, Eisai) and Research Support (Novartis, Pfizer, Boehringer-Ingelheim) outside the submitted work. JJC reports Research Support (Varian, Galera) outside the submitted work. AT reports Research Support (Bristol Myers Squibb, Genentech-Roche, Regeneron, Sanofi-Genzyme, Nektar, Clinigen, Merck, Acrotech, Pfizer, Checkmate, OncoSec); Personal Fees (Bristol Myers Squibb, Merck, Eisai, Instil Bio, Clinigen, Regeneron, Sanofi-Genzyme, Novartis, Partner Therapeutics, Genentech/Roche, BioNTech) outside the submitted work. JM reports Research Support (Microba, Jackson Laboratories, Merck, and Morphogenesis) outside the submitted work. BP reports Advisory Board (Bristol Myers Squibb, AstraZeneca, G1 therapeutics) outside the submitted work. VKS reports Research Support (Neogene, Turnstone); Advisory Board (Bristol Myers Squibb, Eisai, Iovance, Merck, Novartis, Regeneron, Statking) outside the submitted work. NK reports Advisory Board (Bristol Myers-Squibb, Regeneron, Merck, Jounce Therapeutics, Iovance Biotherapeutics, Genzyme, Novartis, Castle Biosciences, Nektar, Instill Bio); Steering or Scientific Committee Member (Nektar, Regeneron, Replimune, Bristol Myers-Squibb, National Comprehensive Cancer Network via Pfizer); Data Safety Monitoring Committee (AstraZeneca, Incyte); Research Support (All to institution – Bristol Myers-Squibb, Merck, Celgene, Regeneron, Replimune, Novartis, HUYA Bioscience, GlaxoSmithKline); Common stock (Bellicum, Amarin, Asensus Surgical) outside the submitted work. AB reports Advisory Board (Deciphera and Bayer) outside the submitted work.
Ethics committee approval
The protocol was approved by the institutional review board at each participating center, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Data Sharing Statement
Individual participant data including data dictionaries will be available. All of the individual participant data collected during the trial will be shared after deidentification. Study protocol and statistical analysis plan will also be available. Data will be available beginning 3 months and ending 5 years following article publication to the researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to Sungjune.kim@moffitt.org. To gain access, data requestors will need to sign a data access agreement.
Reference
- 1.Harms PW, Harms KL, Moore PS, et al. The biology and treatment of Merkel cell carcinoma: current understanding and research priorities. Nature reviews Clinical oncology 2018; 15(12): 763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schadendorf D, Lebbe C, Zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European journal of cancer 2017; 71: 53–69. [DOI] [PubMed] [Google Scholar]
- 3.Knepper TC, Montesion M, Russell JS, et al. The Genomic Landscape of Merkel Cell Carcinoma and Clinicogenomic Biomarkers of Response to Immune Checkpoint Inhibitor Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2019; 25(19): 5961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology 2016; 17(10): 1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Angelo SP, Lebbe C, Mortier L, et al. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J immunother Cancer 2021; 9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SP D'Angelo, Bhatia S, Brohl AS, et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer 2020; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nghiem P, Bhatia S, Lipson EJ, et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J Immunother Cancer 2021; 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 2016; 374(26): 2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet Oncology, 17(7): 883–95. [DOI] [PubMed] [Google Scholar]
- 10.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019; 381(21): 2020–31. [DOI] [PubMed] [Google Scholar]
- 11.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369(2): 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis R Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nature reviews Cancer 2012; 12(12): 860–75. [DOI] [PubMed] [Google Scholar]
- 13.Altorki NK, McGraw TE, Borczuk AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. The Lancet Oncology 2021; 22(6): 824–35. [DOI] [PubMed] [Google Scholar]
- 14.Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. The Lancet Oncology 2015; 16(7): 795–803. [DOI] [PubMed] [Google Scholar]
- 15.Strom T, Naghavi AO, Messina JL, et al. Improved local and regional control with radiotherapy for Merkel cell carcinoma of the head and neck. Head & neck 2017. 39(1): 48–55. [DOI] [PubMed] [Google Scholar]
- 16.Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol 2015; 1(9): 1325–32. [DOI] [PubMed] [Google Scholar]
- 17.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520(7547): 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen DT, Huang PY, Lin HY, et al. A Bayesian pick-the-winner design in a randomized phase II clinical trial. Oncotarget 2017; 8(51): 88376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015; 33(31): 3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institutes of Health NCI. Common terminology criteria for adverse events v4.0. May 28, 2009. (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf). 2009.
- 21.Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics 1982; 38(4): 921–32. [PubMed] [Google Scholar]
- 22.D'Angelo SP Russell J, Lebbe C, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol 2018; 4(9): e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nghiem P, Kaufman HL, Bharmal M, Mahnke L, Phatak H, Becker JC. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol 2017; 13(14): 1263–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samlowski WE, Moon J, Tuthill RJ, et al. A phase II trial of imatinib mesylate in merkel cell carcinoma (neuroendocrine carcinoma of the skin): A Southwest Oncology Group study (S0331). American journal of clinical oncology 2010; 33(5): 495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinowits G, Lezcano C, Catalano PJ, et al. Cabozantinib in Patients with Advanced Merkel Cell Carcinoma. The oncologist 2018; 23(7): 814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stege HM, Haist M, Schultheis S, et al. Response durability after cessation of immune checkpoint inhibitors in patients with metastatic Merkel cell carcinoma: a retrospective multicenter DeCOG study. Cancer Immunol Immunother 2021; 70(11): 3313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glutsch V, Kneitz H, Gesierich A, et al. Activity of ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma. Cancer Immunol Immunother 2021; 70(7): 2087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LoPiccolo J, Schollenberger MD, Dakhil S, et al. Rescue therapy for patients with anti-PD-1-refractory Merkel cell carcinoma: a multicenter, retrospective case series. J Immunother Cancer 2019; 7(1): 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalhout SZ, Emerick KS, Kaufman HL, Silk AW, Thakuria M, Miller DM. A Retrospective Study of Ipilimumab Plus Nivolumab in Anti-PD-L1/PD-1 Refractory Merkel Cell Carcinoma. J Immunother 2022; 45(7): 299–302. [DOI] [PubMed] [Google Scholar]
- 30.McBride S, Sherman E, Tsai CJ, et al. Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2021; 39(1): 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma V, Shrimali RK, Ahmad S, et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1(+)CD38(hi) cells and anti-PD-1 resistance. Nature immunology 2019; 20(9): 1231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyer JG, Parvathaneni U, Gooley T, et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer medicine 2015; 4(8): 1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021; 397(10272): 375–86. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017; 377(14): 1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data including data dictionaries will be available. All of the individual participant data collected during the trial will be shared after deidentification. Study protocol and statistical analysis plan will also be available. Data will be available beginning 3 months and ending 5 years following article publication to the researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to Sungjune.kim@moffitt.org. To gain access, data requestors will need to sign a data access agreement.