Abstract
Combining venetoclax, a selective BCL-2 inhibitor, with low-dose navitoclax, a BCL-XL/BCL-2 inhibitor, may allow targeting of both BCL-2 and BCL-XL without dose-limiting thrombocytopenia associated with navitoclax monotherapy. The safety and preliminary efficacy of venetoclax with low-dose navitoclax and chemotherapy was assessed in this phase I dose escalation study (NCT03181126) in pediatric and adult patients with relapsed/refractory acute lymphoblastic leukemia or lymphoblastic lymphoma. Forty-seven patients received treatment. A recommended phase II dose of 50 mg navitoclax for adults and 25 mg for patients <45 kg with 400 mg adult-equivalent venetoclax was identified. Delayed hematopoietic recovery was the primary safety finding. The complete remission rate was 60%, including responses in patients who had previously received hematopoietic cell transplantation or immunotherapy. Thirteen patients (28%) proceeded to transplantation or CAR T-cell therapy on study. Venetoclax with navitoclax and chemotherapy was well tolerated and had promising efficacy in this heavily pretreated patient population.
Keywords: venetoclax, navitoclax, acute lymphoblastic leukemia, lymphoblastic lymphoma, BCL-2
Classifications: Hematological cancers/Leukemias, Hematological cancers/Lymphomas, Clinical trials/Clinical trials, Cell death and senescence/bcl-2 pathways, Biomarkers/Biomarkers
INTRODUCTION
The prognosis for patients with relapsed/refractory acute lymphoblastic leukemia (ALL) remains poor, particularly in those undergoing second or subsequent salvage therapy (1–4). Complete remission (CR) rates as low as 44% and 18% have been reported with second salvage for pediatric and adult patients with relapsed/refractory ALL, respectively (2–4). Similarly poor outcomes are observed in relapsed/refractory precursor B-cell/T-cell lymphoblastic lymphoma (LL) (1,5). The limited curative treatments in relapsed/refractory ALL and LL highlight the need for new therapies irrespective of patient age.
Treatment advances for relapsed/refractory ALL in recent years include the bispecific antibody blinatumomab and the antibody-drug conjugate inotuzumab ozogamicin, both shown to improve response, survival, and undetectable minimal residual disease (MRD) rates in B-cell ALL (B-ALL) (2,6). Chimeric antigen receptor (CAR) T-cell therapy directed to the B-cell antigen CD19 can result in prolonged duration of remission; however, this agent has been approved for use only in patients up to 25 years old and is not effective in all patients (7). Limited advances have been made in treating relapsed/refractory T-cell ALL (T-ALL), where outcomes remain poor (8). The nucleoside inhibitor nelarabine remains the only agent approved by the Food and Drug Administration for children and adults with relapsed/refractory T-ALL, although multi-agent chemotherapy is routinely used. Despite advances in therapies for relapsed/refractory B-ALL and T-ALL, the need for improved treatments remain.
Members of the B-cell lymphoma 2 (BCL-2) protein family function as key regulators of the intrinsic apoptosis pathway via direct protein-protein binding interactions between pro- and antiapoptotic family members (9). Venetoclax, a potent, highly selective and orally bioavailable BCL-2 inhibitor, and navitoclax, an orally bioavailable BCL-XL/BCL-2 inhibitor, promote apoptosis by directly inhibiting their pro-survival targets, releasing pro-apoptotic proteins, and triggering mitochondrial outer membrane permeabilization and caspase activation (10,11). Inhibitors of the BCL-2 family, including venetoclax and navitoclax, have antitumor activity in preclinical models of ALL, with certain ALL subtypes exhibiting profound sensitivity (11–19). BH3 profiling has demonstrated BCL-2 dependence and sensitivity to both venetoclax and navitoclax in ALL cell lines and cell cultures derived from primary ALL tumors (20,21). Finally, synergistic antileukemic effects of venetoclax and navitoclax have been demonstrated in ALL xenograft models (19), suggesting a dependence on BCL-2 family members.
Venetoclax has been approved for patients with chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (10) and has been reported to be clinically active in patients with relapsed/refractory early T-cell precursor ALL (ETP-ALL), an ALL subtype associated with poor prognosis (22,23). Navitoclax has been assessed in early clinical studies across lymphoid malignancies (24,25). In phase I studies of patients with relapsed/refractory lymphoid malignancies, including CLL and non-Hodgkin lymphoma, navitoclax was associated with clinical activity, though thrombocytopenia, due to on-target BCL-XL inhibition, (26) was common and dose limiting (24,25).
Due to the evidence for dependence on both BCL-2 and BCL-XL in ALL (19–21), we hypothesized that combining venetoclax, a selective BCL-2 inhibitor, with low-dose navitoclax, a BCL-XL/BCL-2 inhibitor, may allow targeting of both BCL-2 and BCL-XL to enhance apoptotic cell death of lymphoblasts without the dose-limiting thrombocytopenia associated with navitoclax monotherapy (24,25). Furthermore, the addition of chemotherapy to a regimen with venetoclax and navitoclax may improve the response rates and durability of responses. To explore this hypothesis, this open-label, multicenter, phase I study (NCT03181126) examined the safety, tolerability, pharmacokinetics, and antitumor activity of venetoclax in combination with navitoclax and chemotherapy in pediatric and adult patients with relapsed/refractory ALL or LL.
RESULTS
Patients
From December 5, 2017 to August 29, 2019, 53 patients with relapsed/refractory ALL and LL were assessed for eligibility; 47 were enrolled and received treatment in the dose escalation portion of the study and comprised the intent-to-treat population (Supplementary Fig. S1). A total of 16, 11, and 20 patients received 400 mg venetoclax (or adult-equivalent for patients <45 kg) in combination with navitoclax at dose level 1 (25 mg for ≥45 kg), dose level 2 (50 mg for ≥45 kg; 25 mg for 20 kg to <45 kg), and dose level 3 (100 mg for ≥45 kg; 50 mg for 20 kg to <45 kg), respectively (Supplementary Fig. S2). All 47 patients received chemotherapy, but 8 patients did not receive any PEG-asparaginase. The median age was 29 years (range, 6–72); 12 patients were aged <18 years. Twenty-five patients (53.2%) had B-ALL, 19 (40.4%) had T-ALL, and 3 (6.4%) had precursor B- or T-LL (Table 1). Patients enrolled in the study were heavily pretreated with a median of 4.0 prior lines of therapy (range, 1–10). The study population (N = 47) included patients who were relapsed/refractory following prior blinatumomab (n = 13 [27.7%]) or inotuzumab ozogamicin (n = 7 [14.9%]), or post-CAR T-cell therapy (n = 6 [12.8%]) or hematopoietic cell transplantation (HCT; n = 13 [27.7%]). The median percentage of bone marrow blasts was 51.7% (range, 0%–99%); 7 patients (14.9%) had <5% bone marrow blasts at baseline (including 5 patients with ALL and morphologic CR/MRD positivity and 2 patients with LL and bone marrow involvement [MRD positive marrow]). Patient demographics and baseline characteristics were similar across dose levels with few exceptions (Supplementary Table S1).
Table 1.
Patient demographics and disease characteristics at baseline
| Characteristic | All patients (N = 47) |
|---|---|
| Median age (range), y | 29 (6–72) |
|
| |
| Male sex, n (%)a | 29 (61.7) |
|
| |
| Race, n (%)a | |
| White | 36 (83.7) |
| Black or African American | 3 (7.0) |
| Other | 4 (8.5) |
| Missing | 4 (8.5) |
|
| |
| Type of primary cancer, n (%)a | |
| ALL | 44 (93.6) |
| B-ALL | 25 (53.2) |
| T-ALL | 19 (40.4) |
| LL | 3 (6.4) |
| B-LL | 1 (2.1) |
| T-LL | 2 (4.3) |
|
| |
| ECOG performance status, n (%)a | |
| 0 | 4 (13.8) |
| 1 | 21 (72.4) |
| 2 | 4 (13.8) |
| Missing | 18 (38.3) |
|
| |
| Median prior lines of therapy (range) | 4 (1–10) |
|
| |
| Key prior treatments, n (%) | |
| Asparaginase or PEG-asparaginase | 28 (59.6) |
| Blinatumomab | 13 (27.7) |
| Inotuzumab ozogamicin | 7 (14.9) |
| CAR T-cell therapy | 6 (12.8) |
| Daratumumab | 1 (2.1) |
| Venetoclax | 4 (8.5) |
|
| |
| Prior transplant, n (%) | 13 (27.7) |
|
| |
| Median time from diagnosis to first dose (range), mo | 24.7 (1.4–170.3) |
|
| |
| Median time since last prior therapy (range), mo | 1.5 (0.1–21.2) |
|
| |
| Bone marrow blasts | |
| Median (range), % | 51.7 (0.0–99.0) |
| Patients with <5%, n (%)b | 7 (14.9) |
Percentages calculated based on total number of patients with available data.
Includes all patients (ALL and LL). Patients with relapsed/refractory ALL and LL with measurable disease, as defined as any measurable bone marrow blast percentage or detectable minimal residual disease, were allowed to enroll.
Abbreviations: ALL, acute lymphoblastic leukemia; B-ALL, B-cell acute lymphoblastic leukemia; B-LL, B- cell lymphoblastic lymphoma; CAR, chimeric antigen receptor; ECOG, Eastern Cooperative Oncology Group; LL, lymphoblastic lymphoma; T-ALL, T-cell acute lymphoblastic leukemia; T-LL, T-cell lymphoblastic lymphoma.
All patients, except for one, discontinued the study within 9 months (n = 46 [97.9%]); reasons for discontinuation were similar across dose levels (Supplementary Fig. S1). The most common reasons for discontinuation were progressive disease (n = 20 [42.6%]) or proceeding to HCT or CAR T-cell therapy while in remission (n = 14 [29.8%]). Of the latter 14 patients, 13 were in CR/CR with incomplete marrow recovery (CRi) and 1 patient was in partial response (PR) with residual MRD. One of the 14 patients did not receive HCT due to fungal pneumonia followed by vision and hearing impairment, at which time the physician decided on alternative therapy; this patient was alive at last follow-up. Median times on venetoclax and navitoclax were 1.9 months (range, 0–17.5) and 1.5 months (range, 0.2–17.4), respectively; 3 patients received >48 weeks of study drug (Supplementary Table S2).
Safety
The most common treatment emergent adverse events (TEAEs) of any grade were febrile neutropenia (46.8%), diarrhea (46.8%), nausea (46.8%), hypokalemia (44.7%), and abdominal pain (42.6%; Supplementary Table S3). The most common grade 3/4 TEAEs were febrile neutropenia (46.8%), neutropenia (38.3%), and thrombocytopenia (25.5%; Table 2). Common venetoclax- or navitoclax-related hematologic grade 3/4 TEAEs included neutropenia (31.9%), febrile neutropenia (19.1%), and thrombocytopenia (21.3%). Common grade 3/4 toxicities associated with chemotherapy agents were also observed, including neutropenia (17.0%) and hyperbilirubinemia (12.8%; Table 2). Grade 3/4 TEAEs were generally similar across dose levels and the overall study population, with some variation between groups (Supplementary Table S4). Thirty-seven patients (78.7%) experienced serious AEs, and the most common were febrile neutropenia (27.7%) and sepsis (17.0%).
Table 2.
Grade 3/4 TEAEs occurring in >5% of all patients (N = 47), including possible relationship to study drugs
| n (%) | Possible relationship to study drug, n (%) | ||
|---|---|---|---|
| Venetoclax or Navitoclax | Chemotherapy | ||
| Any | 46 (97.9) | 35 (74.5) | 28 (59.6) |
| Hematologic | |||
| Febrile neutropenia | 22 (46.8) | 9 (19.1) | 4 (8.5) |
| Neutropeniaa | 18 (38.3) | 15 (31.9) | 8 (17.0) |
| Anemiaa | 9 (19.1) | 4 (8.5) | 0 |
| Thrombocytopeniaa | 12 (25.5) | 10 (21.3) | 1 (2.1) |
| Leukopeniaa | 8 (17.0) | 5 (10.6) | 2 (4.3) |
| Nonhematologic | |||
| Hypokalemia | 11 (23.4) | 1 (2.1) | 2 (4.3) |
| ALT increased | 9 (19.1) | 2 (4.3) | 3 (6.4) |
| Hyperbilirubinemiaa | 9 (19.1) | 2 (4.3) | 6 (12.8) |
| Sepsis | 9 (19.1) | 2 (4.3) | 1 (2.1) |
| Pneumonia | 7 (14.9) | 1 (2.1) | 1 (2.1) |
| AST increased | 6 (12.8) | 1 (2.1) | 2 (4.3) |
| Hyperglycemia | 6 (12.8) | 0 | 4 (8.5) |
| Hyponatremia | 6 (12.8) | 0 | 0 |
| Diarrhea | 5 (10.6) | 1 (2.1) | 0 |
| Back pain | 4 (8.5) | 0 | 0 |
| Hypotension | 4 (8.5) | 0 | 0 |
| Sinus tachycardia | 4 (8.5) | 0 | 0 |
| Vomiting | 4 (8.5) | 3 (6.4) | 2 (4.3) |
| Abdominal pain | 3 (6.4) | 0 | 1 (2.1) |
| Bacteremia | 3 (6.4) | 0 | 0 |
| Bone pain | 3 (6.4) | 0 | 0 |
| Hypocalcemia | 3 (6.4) | 1 (2.1) | 0 |
| Respiratory failure | 3 (6.4) | 0 | 0 |
Combined preferred terms are presented for neutropenia (neutropenia and neutrophil count decreased), anemia (anemia and hemoglobin decreased), thrombocytopenia (thrombocytopenia and platelet count decreased), leukopenia (leukopenia and white blood cell count decreased), and hyperbilirubinemia (hyperbilirubinemia and blood bilirubin increased).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEAE, treatment-emergent adverse event.
Venetoclax was discontinued due to TEAEs in 11 patients (23.4%) as a result of sepsis (n = 3), septic shock (n = 2), febrile neutropenia (n = 2), cardiac arrest (n = 1), intestinal ischemia (n = 1), death due to disease progression (n = 1), and spinal cord compression due to disease progression (n = 1). Of these, 1 patient reported both sepsis and intestinal ischemia. Navitoclax was discontinued due to TEAEs in 12 patients (25.5%) for the same reasons as those for venetoclax discontinuation, except for one additional patient who discontinued navitoclax due to acute pancreatitis and pulmonary embolism. Both events were related to PEG-asparaginase and unrelated to venetoclax or navitoclax; the venetoclax dose was interrupted and navitoclax was withdrawn due to the need for administration of enoxaparin sodium. In total, 6 patients had events considered related to venetoclax, and 5 had events considered related to navitoclax that resulted in study drug discontinuation (Supplementary Table S4). TEAEs resulted in venetoclax dose reduction in 2 patients (4.3%; pancytopenia and febrile neutropenia, n = 1 each) and navitoclax dose reduction in 2 patients (4.3%; vomiting and febrile neutropenia, n = 1 each).
Two patients experienced AEs related to laboratory tumor lysis syndrome (TLS) that occurred on day 1 and day 2 after treatment with venetoclax. Both events were managed successfully with standard supportive care; venetoclax was held for 1 day for 1 patient and the other patient continued study drugs without interruption. Neither patient had clinically evident TLS.
Thirty patients (63.8%) died on study, with most deaths due to disease progression (n = 26/30 [86.7%]). Fatal TEAEs (defined as occurring within 30 days of last dose of study drug) occurred in 10 patients. One event (intestinal ischemia) was considered related to study drug combination (venetoclax, navitoclax, and asparaginase); 9 patients had fatal TEAEs not related to study drug, including sepsis/septic shock (n = 4); cardiac arrest (n = 1), neurotoxicity (n = 1, due to nelarabine given poststudy), and death/AEs related to disease progression (n = 3).
DLTs were observed in 8 patients: 1 in dose level 1, 2 in dose level 2, and 5 in dose level 3 (Supplementary Table S4). The most common DLT observed across dose levels was delayed count recovery (n = 4), which included AEs of neutropenia/neutrophil count decreased (n = 2), pancytopenia, and grade 4 neutropenia (laboratory value not coded as an AE) persisting beyond day 50/week 8. The other DLTs (n = 1 each) included drug-induced liver injury, hyperbilirubinemia, intestinal ischemia, and sepsis (considered a DLT due to treatment-related prolonged cytopenia). The most DLTs per DLT evaluable patients occurred in dose level 3 (n = 5/13 [38.4%]). Of the 9 DLT evaluable patients in dose level 3 who weighed ≥45 kg, 3 DLTs occurred; thus, 50 mg navitoclax was the recommended phase II dose (RPTD) for patients weighing ≥45 kg. Of the 4 DLT evaluable patients in dose level 3 who weighed <45 kg, 2 DLTs occurred; thus, 25 mg navitoclax was the RPTD for patients weighing <45 kg.
Overall, 14 patients (29.8%) experienced thrombocytopenia as a TEAE on study. Venetoclax and navitoclax were interrupted in 2 of the 14 patients. Development of thrombocytopenia appeared dose dependent, as 18.8% of patients in dose level 1 experienced grade 3/4 thrombocytopenia compared with 27.3% and 30.0% of patients in dose levels 2 or 3, respectively. Laboratory values for platelet and neutrophil counts were assessed on study (Supplementary Table S5). The majority of patients (85.1%) experienced ≥1 grade 3/4 thrombocytopenia on study. Of note, 20 patients (42.6%) had grade 3/4 thrombocytopenia at baseline. Incidence of grade 3/4 thrombocytopenia and neutropenia and duration of low counts for all patients are summarized in Supplementary Table S5. The duration of grade 3/4 thrombocytopenia ranged from 0–7 days in 14 patients to ≥36 days in 15 patients. Cumulative incidence probability of platelet recovery is shown in Supplementary Figure S3. No trends in platelet and neutrophil counts were observed by dose level.
Pharmacokinetics
Mean plasma concentration time profiles are presented in Supplementary Fig. S4A and S5A. Pharmacokinetic parameters for venetoclax and navitoclax at steady state (day 8) are summarized by dose level (Table 3). Maximum observed plasma concentrations (Cmax) of venetoclax and navitoclax were observed approximately 6–8 and 4–8 hours, respectively, post-dose. Estimated pharmacokinetic parameters suggested that navitoclax had no significant impact on venetoclax pharmacokinetics. The weight-based dosing of venetoclax and navitoclax achieved comparable exposures across weight groups (Supplementary Fig. S4B and S5B).
Table 3.
Venetoclax and navitoclax pharmacokinetic parameters at steady state (day 8)
| Parametera | Venetoclax | Navitoclax | ||||
|---|---|---|---|---|---|---|
| Navitoclax dose level | 25 mg | 50 mg | 100 mg | 25 mg | 50 mg | 100 mg |
| n | 15 | 11 | 14 | 15 | 10 | 16 |
| Tmax, hours | 8 (4–8) | 6 (4–8) | 6 (4–6) | 8 (4–8) | 4 (0–8) | 6 (4–8) |
| Cmax, μg/mL | 1.21 (76%) | 1.63 (58%) | 1.04 (57%) | 0.330 (63%) | 0.969 (38%) | 1.57 (55%) |
| AUC0–8, μg × h/mL | 6.64 (81%) | 8.14 (44%) n = 10 | 5.79 (63%) | 1.71 (56%) | 5.01 (39%) n = 9 | 8.99 (59%) |
| AUC0–24, μg × h/mL | 18.7 (74%) | 23.7 (45%) n = 7 | 11.8 (90%) n = 3 | 5.19 (59%) | 13.5 (47%) n = 7 | 9.58 (54%) n = 3 |
Parameters presented as geometric mean (% coefficient of variation) except for Tmax, which is presented as median (range).
Abbreviations: AUC0–8, area under the plasma concentration-time curve from day 0–8; AUC0–24, area under the plasma concentration-time curve from day 0–24; Cmax, maximum observed plasma concentration; Tmax, peak time to Cmax.
Efficacy
The overall CR rate (CR + CRi + CR without platelet recovery [CRp]) was 59.6% (n = 28/47) for all patients and 75.0% (n = 9/12) among pediatric patients. Undetectable MRD was observed in 34% (n = 16) of all patients and 50% (n = 6) of pediatric patients (Table 4). Undetectable MRD was observed in 57% (n = 16/28) of all patients with CR/CRi/CRp and 67% (n = 6/9) of pediatric patients with CR/CRi/CRp. For patients with ALL and morphologic CR (MRD positive) at baseline, 60% (n = 3/5) achieved undetectable MRD after treatment; of the 2 patients who did not maintain CR on study, 1 had stable disease and 1 had progressive disease on study. Efficacy parameters by dose level are provided in Supplementary Table S6.
Table 4.
Summary of investigator-assessed efficacy parameters by histology
| Parameter | B-ALL (n = 25) | T-ALL (n = 19) | LL (n = 3) | All patientsa (N = 47) | Pediatrica (n = 12) |
|---|---|---|---|---|---|
| Responseb, n (%) | 16 (64.0) | 10 (52.6) | 2 (66.7) | 28 (59.6) | 9 (75.0) |
| CR rate (CR/CRi/CRp) | 3 (12.0) | 0 | 0 | 3 (6.4) | 1 (8.3) |
| PR | 2 (8.0) | 6 (31.6) | 0 | 8 (17.0) | 0 |
| SD | 4 (16.0) | 3 (15.8) | 1 (33.3) | 8 (17.0) | 2 (16.7) |
| PD | |||||
|
| |||||
| Patients with ALL and morphologic CR at baseline, n | n = 1 | n = 4 | NA | n = 5 | n = 1 |
| Response, n (%) | |||||
| CR rate (CR/CRi/CRp) | 0 | 3 (75.0) | 3 (60.0) | 1 (100) | |
| SD | 0 | 1 (25.0) | 1 (20.0) | 0 | |
| NEc | 1 (100) | 0 | 1 (20.0) | 0 | |
|
| |||||
| DORd in all responders | |||||
| n | 19 | 10 | 2 | 31 | 10 |
| Median (95% CI), mo | 9.1 (1.4–14.6) | 4.2 (0.8–12.3) | NE (NE–NE) | 4.2 (2.3–11.5) | 3.5 (0.7–3.5) |
|
| |||||
| OS | |||||
| Median (95% CI), mo | 9.7 (4.0–15.7) | 6.6 (3.2–12.5) | NE (2.0–NE) | 7.8 (4.0–12.5) | NE (2.0–NE) |
| 12-month (95% CI), % | 33.8 (13.7–55.2) | 29.7 (10.4–52.2) | 66.7 (5.4–94.5) | 35.6 (20.9–50.7) | 60.8 (25.0–83.6) |
|
| |||||
| Bone marrow MRD, n (%) | |||||
| MRD negative (<10−4) | 9 (36.0) | 6 (31.6) | 1 (33.3) | 16 (34.0) | 6 (50.0) |
| MRD positive | 10 (40.0) | 3 (15.8) | 1 (33.3) | 14 (29.8) | 5 (41.7) |
| Othere | 6 (24.0) | 10 (52.6) | 1 (33.3) | 17 (36.2) | 1 (8.3) |
|
| |||||
| Proceeded to CAR T-cell therapy or HCT, n (%)f | 8 (32.0) | 3 (15.8) | 2 (66.7) | 13 (27.7) | 7 (58.3) |
Includes all patients on study, including those with LL and morphologic CR at baseline.
Includes patients with known bone marrow blasts at baseline ≥5%. Patients with missing baseline blasts were excluded by immunophenotype but included in the overall study population.
Patient was not evaluable for disease assessment (PD occurred prior to start of chemotherapy).
Patients who went to CAR T-cell therapy or transplant were censored at last tumor assessment date.
MRD assessment was missing or not evaluated (e.g., nonresponders).
Only includes patients who received subsequent transplant or CAR T-cell therapy.
Abbreviations: B-ALL, B-cell acute lymphoblastic leukemia; CAR T, chimeric antigen receptor T cell; CR, complete remission; CRi, complete remission with incomplete marrow recovery; CRp, complete remission without platelet recovery; DOR, duration of response; HCT, hematopoietic cell transplantation; LL, lymphoblastic lymphoma; MRD, minimal residual disease; NA, not applicable; NE, not evaluable; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease; T-ALL, T-cell acute lymphoblastic leukemia.
Notably, similar overall CR rates (CR/CRi/CRp) were observed in a post hoc analysis of patient subgroups defined by age, immunophenotype, number of prior therapies, and prior treatment (Supplementary Fig. S6). A best response of CR/CRi/CRp was achieved by 61.5% (n = 8/13), 42.9% (n = 3/7), 50.0% (n = 3/6), and 53.8% (n = 7/13) of patients previously treated with blinatumomab, inotuzumab ozogamicin, CAR T-cell therapy, and HCT, respectively. Of the 4 patients who had received prior venetoclax, 1 patient achieved CRi.
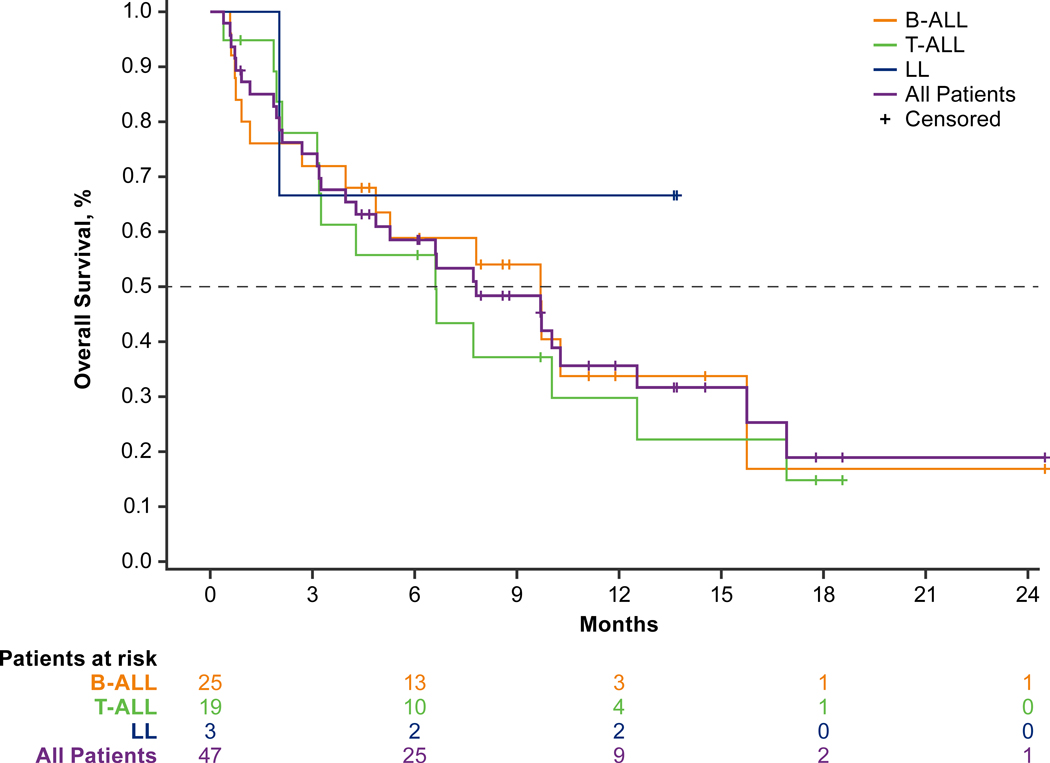
The Kaplan-Meier estimated median duration of response (DOR) was 4.2 months (range, 2.3–11.5), and the estimated median overall survival (OS) was 7.8 months (range, 4.0–12.5; Table 4). The estimated median OS was longer in patients with B-ALL versus T-ALL (9.7 months [range, 4.0–15.7] versus 6.6 months [range, 3.2–12.5]; Fig. 1). The 12-month OS estimate for pediatric patients was 60.8% (95% CI, 25.0–83.6) and was 35.6% (95% CI, 20.9–50.7) overall. Among all patients, 27.7% (n = 13) proceeded to transplantation (n = 8) or CAR T-cell therapy (n = 5; Supplementary Fig. S7); of these 13 patients, the Kaplan-Meier estimated median DOR was 13.4 months (minimum 0.03+, maximum 14.6, where “+” indicates the patient was censored), and 11 were alive at completion of follow-up.
Figure 1.
Kaplan-Meier plot of overall survival. Overall survival was defined as the number of months from the date of first dose to the date of death. Abbreviations: B-ALL, B-cell acute lymphoblastic leukemia; LL, lymphoblastic lymphoma; T-ALL, T-cell acute lymphoblastic leukemia.
Biomarker Analysis
Subtype Analysis
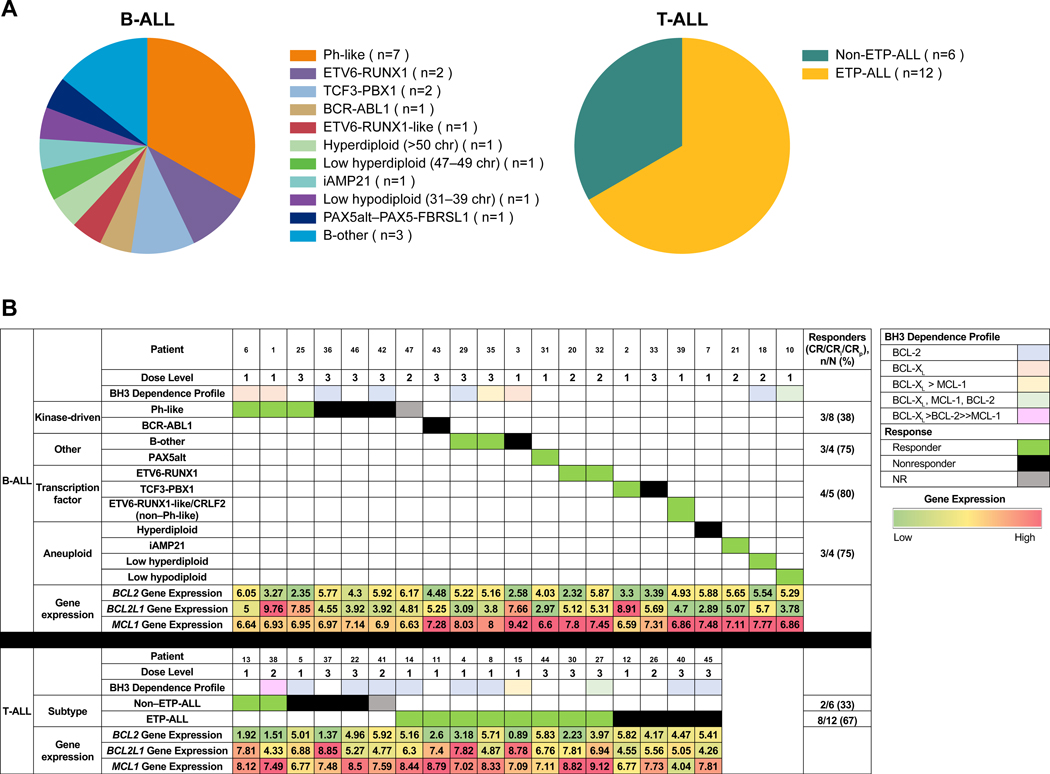
RNA sequencing (RNA-Seq) analysis was performed for 39 patients (B-ALL [n = 21]; T-ALL [n = 18]) with available baseline blood or bone marrow samples. B-ALL subtyping analysis was performed based on genetic alterations and dysregulated expression of transcription factor genes, gene expression profiling (predictive analysis of microarrays, t-weighted stochastic neighbor embedding), and identification of aneuploidy (27) (Fig. 2A). The 21 patients with B-ALL were classified into 11 subgroups; 7 patients had Philadelphia chromosome (Ph)-like B-ALL. Variable levels of BCL2 and BCL2L1 (BCL2 like 1) gene expression were observed across subtypes; however, given the small number of patients in each subgroup, no significant associations between subtype and BCL2/BCL2L1 expression and response were identified. In all subtypes, MCL1 (myeloid cell leukemia 1) gene expression was generally higher than BCL2 and BCL2L1 gene expression (Supplementary Fig. S8A, S8B, and S8C). To assess response by subtype, patients with B-ALL were grouped into 4 broader subtypes (aneuploid, kinase-driven, transcription factor-deregulated, and other). Responses were observed in all B-ALL subtype groups at 61.9% (n = 13/21), but only 37.5% of patients (n = 3/8) in the kinase-driven subtype group achieved a response (Fig. 2B). Given the small number of patients in the subtype analysis, further investigation with more patients will be required to confirm these observations.
Figure 2.
Subtype analysis and response. A, Subtype profiles in patients with B-ALL and T-ALL. B, Subtypes assessed based on response. Abbreviations: B-ALL, B-cell acute lymphoblastic leukemia; BCL-2, B-cell lymphoma 2; BCL2L1, BCL2 like 1; CRi, complete remission with incomplete marrow recovery; CRp, complete remission without platelet recovery; ETP-ALL, early T-cell precursor acute lymphoblastic leukemia; MCL-1, myeloid cell leukemia 1; NR, not reached; Ph, Philadelphia chromosome; T-ALL, T-cell acute lymphoblastic leukemia.
Eighteen samples were available for patients with T-ALL and were classified as ETP-ALL (n = 12/18 [66.7%]; cCD3 and CD7 positive, CD1a negative, CD5 negative or dim, and variably positive for myeloid and stem cell markers)(28) or non–ETP-ALL (n = 6/18 [33.3%]). Gene expression levels of BCL2, BCL2L1, and MCL1 were similar in non–ETP-ALL and ETP-ALL (Supplementary Fig. S8). Ten patients (55.6%) with T-ALL achieved CR/CRi/CRp, including ETP-ALL (n = 8/12 [66.7%]) and non–ETP-ALL (n = 2/6 [33.3%]) subgroups (Fig. 2B).
BCL-2 and BCL-XL dependence in B-ALL and T-ALL
BH3 profiling is a functional assay that measures the threshold at which a commitment to apoptosis occurs (ie, apoptotic priming) and identifies the dependence on individual anti-apoptotic proteins (BCL-2, BCL-XL, or MCL-1). By treating cellular mitochondria with activator (BID) and sensitizer (BAD, NOXA, HRK) BH3 peptides that mimic their endogenous counterparts, the level of cytochrome c release from the mitochondria is assessed as a surrogate marker for MOMP (mitochondrial outer membrane permeabilization) and subsequently, the dependence on either BCL-2, BCL-XL, or MCL-1 is determined. BH3 profiling was performed on 20 patients (B-ALL [n = 9]; ETP-ALL [n = 7]; non–ETP-ALL [n = 4]) with available pretreatment blood or bone marrow samples; 8 of 20 patients also had samples while on study (Table 5). At baseline, patients with B-ALL exhibited a heterogenous mix of dependencies on BCL-2 (n = 4/9), BCL-XL (n = 3/9), or dependency on both (n = 2/9). These patients maintained their BCL-XL dependency or switched to a BCL-2/BCL-XL dependency (from a BCL-2 dependency) while on study. Interestingly, 72.7% (n = 8/11) of T-ALL patients were BCL-2–dependent at baseline. All patients with T-ALL with on study BH3 profiling (n = 11) later switched to BCL-XL or a mix of BCL-2/BCL-XL dependency. A consistency in the dependency detected in bone marrow and blood was observed for patients with samples in both tissue compartments, with the exception of 1 patient with BCL-2 dependency in the bone marrow but BCL-XL dependency in the blood. Baseline RNA-Seq data were available for all 20 patients who had pretreatment BH3 profiling. Correlation of BCL2, BCL2L1, and MCL1 gene expression with BH3 profiling dependencies was assessed (Supplementary Fig. S9). Generally, median BCL2 gene expression was higher than BCL2L1 in patients who had a predominant BCL-2 dependency (n = 14) and median BCL2L1 gene expression tended to be higher than BCL2 gene expression in patients who were predominantly BCL-XL–dependent at baseline (n = 6). Notably, although no patients were predominantly MCL-1–dependent (Table 5), median MCL1 gene expression was relatively high in all patients compared with BCL2 and BCL2L1, suggesting that MCL1 transcript levels may not translate into a functional dependency in these patients.
Table 5.
BH3 profiling
| Patient | Disease | Dose Level | BH3 Dependency | Best Response | |
|---|---|---|---|---|---|
| Baseline | On Study | ||||
| 6 | Pre–B-ALL | 1 | BCL-XL | BCL-XL | CR |
| 3 | Pre–B-ALL | 1 | BCL-XL | BCL-XL | PR |
| 1 | Pre–B-ALL | 1 | BCL-XL | BCL-XL | CRi |
| 10 | Pre–B-ALL | 1 | BCL-XL (PB), BCL-2 (BM)a | — | CRp |
| 36 | Pre–B-ALL | 3 | BCL-2 | BCL-2, BCL-XL | PR |
| 29 | Pre–B-ALL | 3 | BCL-2 | — | CR |
| 35 | Pre–B-ALL | 3 | BCL-2 > BCL-XL | — | NR |
| 18 | Pro–B-ALL | 2 | BCL-2 | — | CR |
| 42 | B | 3 | BCL-2 | — | PD |
| 22 | Pre–T-ALL | 2 | BCL-2 | BCL-2, BCL-XLb | SD |
| 41 | Pre–T-ALL | 3 | BCL-2 | — | NR |
| 38 | Pro–T-ALL | 3 | BCL-XL > BCL-2 >> MCL-1 | — | CR |
| 5 | T-ALL: medullary | 1 | BCL-2 | BCL-XLb | SD |
| 14 | ETP-ALL | 1 | BCL-2 | — | CR |
| 8 | ETP-ALL | 1 | BCL-2 | BCL-XLb | CR |
| 4 | ETP-ALL | 1 | BCL-2 | — | CRi |
| 15 | ETP-ALL | 1 | BCL-XL > MCL-1 | — | CR |
| 27 | ETP-ALL | 2 | BCL-XL, MCL-1, BCL-2 | — | CRi |
| 40 | ETP-ALL | 3 | BCL-2 | BCL-2, BCL-XL | SD |
| 45 | ETP-ALL | 3 | BCL-2 | — | PD |
Note: The em dash denotes patients that did not have on study samples available for BH3 profiling.
For all samples with matched BM and PB available, only 1 patient had discordant BH3 profiles between BM and PB.
On study samples collected at time of disease progression.
Abbreviations: B-ALL, B-cell acute lymphoblastic leukemia; BCL-2, B-cell lymphoma 2; BM, bone marrow; CR, complete remission; CRi, complete remission with incomplete marrow recovery; CRp, complete remission without platelet recovery; ETP-ALL, early T-cell precursor acute lymphoblastic leukemia; NR, not reached; MCL-1, myeloid cell leukemia 1; PB, peripheral blood; PD, progressive disease; PR, partial response; SD, stable disease; T-ALL, T-cell acute lymphoblastic leukemia.
DISCUSSION
Limited treatment options and unfavorable long-term outcomes drive the search for improved therapies in relapsed/refractory ALL. Preclinical studies have demonstrated dependence of leukemic cells on BCL-2 and BCL-XL, as well as antitumor activity of BCL-2/BCL-XL inhibitors in ALL models (19–21). The hypothesis that led to the examination of venetoclax in combination with low-dose navitoclax in this phase I study was that inhibition of both BCL-2 and BCL-XL are required for clinical efficacy in relapsed/refractory ALL/LL and that combining low-dose navitoclax with standard doses of venetoclax could mitigate consequences of the previously observed side effect of thrombocytopenia with navitoclax, while optimizing responses by permitting dual pathway targeting (25). Results of the present study demonstrate the feasibility of using venetoclax and low-dose navitoclax in combination with standard chemotherapy regimens in patients with B-ALL, T-ALL, and LL to simultaneously inhibit BCL-2 and BCL-XL, with an achievable balance between maximizing efficacy and clinical tolerability. One limitation of this study is that it is a single arm phase I study, and additional studies with direct comparator arms are needed to confirm these findings.
Across all dose levels, 14 patients (29.8%) reported any grade TEAEs of thrombocytopenia and 12 patients (25.5%) reported grade 3/4 TEAEs of thrombocytopenia, which is considerably less than the rate of grade 3/4 thrombocytopenia (52.7%) previously reported in an earlier trial of navitoclax in lymphoid malignancies with maximum tolerated dose of 325 mg/day (25). However, the lower counts at baseline in our cohort, as is expected for patients with ALL, (https://pubmed.ncbi.nlm.nih.gov/29083572/) likely resulted in fewer grade 3/4 thrombocytopenia events reported on study. Collectively, it may be difficult to determine whether low platelet counts are related to treatment or secondary to underlying disease; given the mechanism of action of navitoclax and known effects on BCL-XL-dependent platelet function, it is likely multifactorial. Furthermore, a recent report of venetoclax without navitoclax in combination with chemotherapy in relapsed/refractory T-ALL patients also reported prolonged thrombocytopenia, with a median time to count recovery (≥100 × 109/L) of 44 days (23). Nevertheless, thrombocytopenia did not result in venetoclax or navitoclax dose reduction or discontinuation on study. Development of thrombocytopenia appeared to be dose dependent. While the majority of patients experienced grade 3/4 thrombocytopenia (either at baseline or on study), few patients experienced serious AEs of hemorrhage, suggesting that sustained reductions in platelet counts were largely manageable, despite many patients receiving study treatment with asparaginase. Notably, previous reports of navitoclax in relapsed/refractory lymphoid malignancies demonstrated platelet count recovery with intermittent dosing (target dose of 250 mg navitoclax) versus continuous dosing (24). Analyses of venetoclax and navitoclax exposure and cytopenias are ongoing.
Markers of hepatotoxicity (increased alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) and hepatic function (hyperbilirubinemia) are well-known toxicities with asparaginase treatment and occur more commonly in adult versus pediatric patients (29,30). TEAEs related to hepatotoxicity or hepatic function were reported in this study, though grade 3/4 events were reported in <20% of all patients, including 19.1% with grade 3/4 increased ALT, 19.1% with grade 3/4 hyperbilirubinemia, and 12.8% with grade 3/4 increased AST; notably, 8 patients (17.0%) did not receive any PEG-asparaginase on study. Rates of PEG-asparaginase–related grade 3/4 transaminitis, hyperbilirubinemia, and pancreatitis ranged from 54%–63%, 17%–31%, and 5%–14%, respectively, in other ALL studies that used PEG-asparaginase doses ranging from 1000–2500 IU/m2 (31–33). Comparatively, the dose of PEG-asparaginase used in this study (1250 IU/m2) is generally lower than standard pediatric and younger adult protocols (30–32).
Infections are a known complication of ALL and its treatment. Grade 3/4 infections reported in >5% of patients included sepsis (n = 9 [19.1%]), pneumonia (n = 7 [14.9%]), and bacteremia (n = 3 [6.4%]). The infections were fatal in 4 patients (8.5%) due to bacterial sepsis. Study drug-related neutropenia may increase susceptibility to infection, though the overall rates of grade 3/4 or serious infections observed in this study were comparable with infection rates in other studies of heavily pretreated leukemia (6). Furthermore, despite the use of high-dose corticosteroids in this study, only 4 patients (8.5%) experienced grade 3/4 fungal infections, including 2 with Candida infection, 1 with fungaemia, and 1 with fungal pneumonia. Finally, despite the quick ramp-up to 400 mg venetoclax treatment across dose levels, only 2 events of laboratory TLS were observed on study drug and patients were able to continue treatment after no or short venetoclax interruption.
Overall, the study regimen was well tolerated in most patients, with low rates of venetoclax and navitoclax discontinuation (n = 6 [12.8%] and n = 5 [10.6%], respectively) due to study drug related AEs. Rates of gastrointestinal AEs may be increased with the combination of venetoclax and navitoclax; this could become more concerning if this combination therapy were moved into earlier lines of therapy. The most common DLT was delayed count recovery (n = 4), and the number of DLTs increased at each successive dose level. Given the increased number of DLTs in dose level 3, without evidence of increased efficacy at this dose, the recommended phase II dose for navitoclax in combination with 400 mg venetoclax is 50 mg for patients ≥45 kg and 25 mg for patients <45 kg, which is the weight adjusted equivalent to a 50 mg dose for patients ≥45 kg. A safety expansion cohort is enrolling patients to explore a 21-day of 28-day dosing schedule of venetoclax with 50 mg navitoclax (25 mg for patients <45 kg) to determine if prolonged myelosuppression is mitigated.
Promising response rates were observed in this heavily pretreated patient population. The median OS for B-ALL (9.7 months) and T-ALL (6.6 months) patients on study compared favorably with the survival reported in studies of blinatumomab (7.7 months), inotuzumab ozogamicin (7.7 months), and nelarabine (4.7 months) (6,34) (https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021877s010lbl.pdf). Of note, CR rates were similar across patient subgroups, including in patients who had relapsed or were refractory to blinatumomab, inotuzumab ozogamicin, and CAR T-cell therapy, suggesting utility of the study combination treatment despite heavy pretreatment. Notably, undetectable MRD, an important determinant of patient outcome in ALL, was achieved by approximately one-third of all patients, and nearly one-quarter of all patients proceeded to HCT or CAR T-cell therapy. These findings highlight that a small subset of patients were able to remain on this treatment regimen for a prolonged duration, while others were able to use the treatment combination as a bridge to potentially curative treatment options. Additionally, anthracycline was not included in this study’s chemotherapy regimen, and the study results provide further impetus to potentially remove more chemotherapy in the future. There is also potential to study venetoclax and low-dose navitoclax in combination with other novel agents, such as inotuzumab ozogamicin, or in a frontline setting.
RNA-Seq-based subtyping was performed in patients with B-ALL. Patients were classified into both favorable (hyperdiploid, ETV6-RUNX1, and TCF3-PBX1) and unfavorable (hypodiploid, BCR-ABL1, Ph-like, and intrachromosomal amplification of chromosome 21) subtypes (35); the greatest number of patients were classified into the Ph-like group. Responses were seen in all genomic subtypes, with fewer responses in patients with alterations driving kinase signaling (Ph-like and BCR-ABL1), which is not surprising considering these patients usually have inferior prognosis (36). Of note, use of tyrosine kinase inhibitors (TKIs) was permitted, though not required for patients identified as having a Ph-like or BCR-ABL subtype of B-ALL. Immunophenotype-based classification was performed in patients with T-ALL. Responses were seen in patients with the ETP-ALL subtype, in addition to patients with non–ETP-ALL, which supports previous findings showing that EPT-ALL is sensitive to venetoclax (37). In addition to RNA-Seq-based subtyping, ongoing mutational profiling efforts seek to further define response in key molecular subgroups of ALL, which may guide our understanding of potential innate and adaptive resistance mechanisms.
BH3 profiling demonstrated similar BH3 protein dependencies in the bone marrow and peripheral blood among ALL patients with matched samples. However, 1 patient showed a BCL-2 dependency in the bone marrow, but a BCL-XL dependency in the blood at baseline, which may be attributed to a preferential upregulation of certain antiapoptotic proteins (e.g., BCL-2) by the bone marrow stroma, as previously described in leukemic cells (38,39). Interestingly, BCL-2–dependent patients with T-ALL switched to a BCL-XL or BCL-2/BCL-XL dependency on treatment, while patients with B-ALL who were BCL-XL dependent at baseline generally maintained their dependency. For the 3 patients with paired samples collected at baseline and at time of disease progression, a shift from BCL-2 dependency to either BCL-XL or mixed BCL-2/BCL-XL dependency was observed. It may be that venetoclax inhibition of BCL-2 causes cells to acquire or maintain a BCL-XL dependency as a resistance mechanism, which could be further suppressed with the addition of navitoclax. Nevertheless, objective responses were observed in patients with ALL dependent on either BCL-2 or BCL-XL, supporting the use of venetoclax and navitoclax combination therapy in these patients.
Irrespective of disease type, patients with a predominant BCL-2 or BCL-XL dependency at baseline displayed higher median BCL2 or BCL2L1 gene expression, respectively. These findings suggest that transcript expression may correlate with functional dependency and ultimately, sensitivity to venetoclax or navitoclax. Overall, these results demonstrate that patients with relapsed/refractory ALL are dependent on BCL-2 and BCL-XL, supporting use of study treatment combination. With respect to MCL-1, none of the 20 patients tested exhibited a sole dependency on MCL-1, although 3 patients who were more dependent on BCL-2 or BCL-XL also showed partial dependence on MCL-1 at baseline. High MCL1 gene expression was observed across all patient samples assessed. As MCL-1 protein expression is regulated by a variety of post-transcriptional mechanisms (40), gene expression may not translate into high protein levels of MCL-1 and may not correlate with functional dependence.
In conclusion, dual inhibition of BCL-2 and BCL-XL with the combination of venetoclax and navitoclax with chemotherapy was associated with marked response rates and a well-tolerated safety profile in adult and pediatric patients with relapsed/refractory B-ALL, T-ALL, and LL, many of whom had failed multiple therapies, including stem cell transplant, targeted agents, and immunotherapies. Neutropenia and thrombocytopenia occurred frequently and suggest that discontinuous dosing in conjunction with growth factor support may be needed when venetoclax and navitoclax are used in combination with chemotherapy. Additional analyses on study drug exposure and its relation to blood cytopenias, in addition to data from the safety expansion cohort, will inform the optimal dosing strategy to further maximize efficacy while mitigating cytopenias, the main side effects of this combination.
METHODS
Study Design and Patients
This open-label, phase I, dose escalation study in pediatric and adult patients with relapsed/refractory ALL or LL was conducted in 15 sites in 2 countries. Patients received venetoclax, adjusted by weight, in combination with navitoclax at 3 dose levels, and chemotherapy (Supplementary Fig. S2). Eligible patients were aged ≥4 years (those aged <18 years had no standard of care available), weighed ≥20 kg, and had relapsed/refractory ALL or LL. Refractory status was defined as persistent disease after ≥2 courses of chemotherapy. Patients with Ph positive ALL or with a targetable ABL class fusion were eligible and could receive a TKI. Patients with LL had radiographic evidence of disease. Patients were required to have adequate performance status, defined for those aged ≤16 years as Lansky score ≥50 and for those aged >16 years as Karnofsky score ≥50 or Eastern Cooperative Oncology Group score ≤2. Additional inclusion and exclusion criteria are provided in the Supplementary Methods.
The study protocol and amendments were reviewed and approved by Institutional Review Boards. The trial was conducted in compliance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent.
Procedures
Patients received oral venetoclax daily, adjusted by weight, to match the exposure of the adult equivalent target doses of 200 mg on day 1 and 400 mg on day 2 onwards. Oral navitoclax was administered daily on day 3 onwards based on dose level and patient weight (≥45 kg versus <45 kg): dose level 1 was 25 mg for ≥45 kg only; dose level 2 was 50 mg for ≥45 kg and 25 mg for 20 kg to <45 kg; dose level 3 was 100 mg for ≥45 kg and 50 mg for 20 kg to <45 kg (Supplementary Tables S7–S8). In the original protocol, chemotherapy could be started on day 9; following protocol amendment 3, chemotherapy could begin on day 1. The recommended chemotherapy schedule consisted of PEG-asparaginase 1250 IU/m2 intravenous (IV) or intramuscular equivalent on days 1 and 15, vincristine 1.5 mg/m2 (maximum 2 mg) IV weekly on days 1, 8, 15, and 22, and dexamethasone 20 mg/m2/day orally divided twice daily on days 1–5 and 15–19. The chemotherapy regimen could be repeated for a second cycle and beyond, if the patient remained on study and continued to receive clinical benefit. Chemotherapy (any of the three drugs) and/or a TKI could be omitted, reduced, or delayed at the investigator’s discretion.
Dose escalation decisions were guided by Bayesian optimal interval design, which utilized a decision rule within each weight group (<45 kg and ≥45 kg) based on cumulative numbers of patients who experienced a DLT. Dose escalation decisions based on DLTs were assessed in the first 28 days and could be attributable to venetoclax, navitoclax, or chemotherapy. The DLT window for patients enrolled through study protocol amendment 2 was 35 days. AEs and toxicities that occurred beyond the DLT window were evaluated by the investigator and could be considered as dose limiting. Delayed count recovery was considered a DLT and was defined as absolute neutrophil count <500/μL and platelets <25,000/μL that persisted during the first 50 days (or at the week 8 visit) that was not attributable to another identifiable factor (eg, concomitant medication, documented myelosuppressive infection, residual leukemia). To be DLT evaluable, patients needed to have received at least 75% of study drug during the DLT window; only DLT-evaluable patients were considered for dose escalation decisions, which were performed separately for each weight group. Additional details regarding DLTs are provided in the Supplementary Methods.
Patients received full supportive care during study participation, and anti-infective prophylaxis could be administered at investigator discretion following consideration of drug-drug interactions. All patients received TLS prophylaxis, including allopurinol or rasburicase and oral and/or IV hydration before the first venetoclax dose, prior to escalation of venetoclax plus navitoclax, and prior to the start of chemotherapy. Hospitalization was required on days 1–4 at a minimum and included real-time TLS laboratory monitoring at 0, 4, 8, and 24 hours at a minimum.
Assessments
Primary outcome measures included safety assessments and pharmacokinetics. Safety assessments included numbers of patients with DLTs, AEs, and changes from baseline in clinical laboratory parameters. Pharmacokinetic assessments of venetoclax and navitoclax included Cmax, peak time to Cmax (Tmax), and area under the plasma concentration-time curve (AUC). Secondary outcome measures included efficacy assessments: CR rate, progression-free survival (PFS), OS, and proportion of patients who proceeded to HCT or CAR T-cell therapy.
Tumor assessments consisted of bone marrow aspiration (and biopsy if clinically indicated) for patients with bone marrow involvement. For patients with ALL, disease was assessed pre-study, on day 8 (for those starting chemotherapy on day 9) and after completing cycle 1 of chemotherapy (either on day 29 for those starting chemotherapy on day 1 or day 36 for those starting chemotherapy on day 9), and as clinically indicated. For ALL patients, CR was defined as bone marrow blasts <5% and hematological recovery (absolute neutrophil count [ANC] ≥500/μl and platelet counts ≥75,000/μl), CRi as bone marrow blasts <5% without hematological recovery (ANC <500/μl or ANC <500/μl and platelet counts <75,000/μl), CRp as bone marrow blasts <5% without platelet recovery (platelet counts <75,000/μl), and PR as bone marrow blasts 5–25% with >50% decrease in leukemic blast percentage. For patients with LL, disease was assessed by radiographic imaging at pre-study, week 9, and week 25, and bone marrow assessments were performed concurrently for those with bone marrow involvement. Efficacy criteria for LL patients was based on the Lugano criteria (41). MRD in peripheral blood and/or bone marrow was assessed at a sensitivity threshold of 10−4 per standard of care using flow cytometry, quantitative PCR, or sequencing of immunoglobulin heavy chain/T-cell receptor rearrangements as an exploratory measure at time of disease assessment if clinically indicated. Pharmacokinetic assessments were performed on days 1, 2, 3, 8 and 9 for venetoclax and on days 3, 8, and 9 for navitoclax. Pharmacokinetic samples were collected pre-dose, and at 2, 4, 6, and 8 hours post-dose.
BH3 Profiling by Intracellular Staining (iBH3)
Cryopreserved blood or bone marrow cells were stained with B-, T-, or ETP-ALL-specific surface antibodies and permeabilized as previously described (42–44). BAD, BID, HRK, and NOXA peptides were tested for induction of cytochrome c loss from the mitochondria of digitonin-permeabilized blasts. Dependence on BCL-2, BCL-XL, and MCL-1 were determined by the relative release of cytochrome c due to mitochondrial outer membrane permeabilization in the presence of these agents as measured by multicolor flow cytometry.
Transcriptome Sequencing and Pathway Analysis
Total stranded transcriptome sequencing (RNA-Seq; 100 base pair paired-end reads) was performed using the TruSeq Stranded Total RNA library preparation kit and sequencing performed using HiSeq 4000 and NovaSeq 6000 platforms (Illumina). Sequencing was performed retrospectively, and these results were not returned to patients. The low input RNA library preparation kit (NuGen Ovation V2) was used for samples with limiting material (2–100 ng). Genetic subtypes were determined by integrating gene expression, rearrangement, copy number and SNV/indel as previously described (27). Sequencing reads from total stranded protocol were mapped to the GRCh37 human genome reference by STAR (version 2.5.3a) (45) through the suggested two-pass mapping pipeline, with reads mapped to more than 20 loci considered as unmapped. Gene expression was quantified using RSEM (version 1.3.0) (46) against Ensembl gene annotation (version 75; http://www.ensembl.org/). All samples were sequenced with RefSeq coding region covered with 30-fold coverage ≥15% (median ± standard deviation, 38.3% ± 6%). Sequencing reads from low quality or low input protocols were preprocessed by removing reads with low complexity (Prinseq version 0.20.4) (47) or mapped to ribosome RNAs and trimmed with trimgalore (version 0.4.4; https://github.com/FelixKrueger/TrimGalore), followed by STAR mapping and RSEM gene quantification as applied to total stranded libraries. B-ALL subtyping was accomplished using a previously described pipeline (27). FusionCatcher (48) was used to detect fusions, and all the reported rearrangements were manually reviewed. Due to the complexity of DUX4 rearrangements, some of the fusions were manually rescued by checking the aligned reads within the Integrative Genomics Viewer (IGV) browser (49). In addition, expression of the highly repetitive gene DUX4 was also quantified with kallisto (version 43) (50) using all transcript sequences regardless of gene location in the genome. DUX4 rearrangement was supported by high DUX4 expression.
Statistical Analysis
The date of data cutoff was January 21, 2020. Summary statistics were computed for each sampling time and pharmacokinetic parameter for venetoclax and navitoclax. Safety analyses included all patients who received ≥1 dose of the study drug. AE analyses included only TEAEs, defined as those with onset on or after the day of the first dose of study drug. Events with an onset ≥30 days after the last dose of study drug were excluded. TEAEs were summarized using the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1; toxicity grade and relationship to study drugs were reported using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Changes from baseline in clinical laboratory parameters were summarized using descriptive statistics.
All efficacy analyses were based on the intent-to-treat population. The CR and PR rates were calculated, and the corresponding 95% CIs were constructed using the Clopper-Pearson exact method. CR rates were also assessed by patient subgroups. PFS and OS were analyzed by Kaplan-Meier methodology. For PFS, patients who had not died or experienced disease progression were censored at the date of last disease assessment. For OS, patients who had not died were censored at the last known date the patient was alive. The proportion of patients with MRD negativity, defined as less than the 10−4 threshold, was calculated, and the corresponding 95% CI was constructed using the Clopper-Pearson exact method. Pharmacokinetic parameters were determined using noncompartmental analysis.
Gene expression of BCL2, BCL2L1, and MCL1 were compared across biomarker subgroups and disease subtypes. Medians were calculated.
Supplementary Material
Statement of Significance.
In this phase I study, venetoclax with low-dose navitoclax and chemotherapy was well tolerated and had promising efficacy in patients with relapsed/refractory acute lymphoblastic leukemia or lymphoblastic lymphoma. Responses were observed in patients across histological and genomic subtypes and in those who failed available therapies including stem cell transplant.
Acknowledgments:
We thank the patients and their families, study coordinators, and support staff. Venetoclax is being developed in collaboration between AbbVie and Genentech. AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided by Ashley Skorusa, PhD, Robert Rydzewski, MS, CMPP, and Grace Lewis, PharmD of Bio Connections, LLC, funded by AbbVie.
Conflicts of Interest:
VA Pullarkat: Honoraria from AbbVie; consultancy/advisory role for AbbVie; and speakers’ bureau participation for AbbVie.
NJ Lacayo, SI Colace, KG Roberts, RJ Mattison, Y Zhao, C Qu: No relevant financial relationship(s) to disclose.
E Jabbour: Honoraria from AbbVie, Amgen, Pfizer, Takeda, and Adaptive Biotechnologies; research support from AbbVie, Amgen, Pfizer, Takeda, and Adaptive Biotechnologies.
JE Rubnitz: Research funding from AbbVie.
A Bajel: Honoraria from AbbVie, Amgen, Novartis, and Pfizer; speaker’s bureau participation for Amgen.
TW Laetsch: Consultancy/advisory role for Bayer, Cellectis, Lilly, Loxo, and Novartis; research funding from AbbVie, Amgen, Atara Biotherapeutics, Bayer, Bristol-Myers Squibb, Epizyme, GlaxoSmithKline, Janssen, Jubilant Pharmaceuticals, Lilly, Loxo, Novartis, Novella Clinical, Pfizer, and SERVIER.
J Leonard: Research funding from Amgen and AbbVie. Consultancy/advisory role for Takeda and Adaptive Biotechnologies.
SL Khaw: Travel support from Amgen and Novartis; research funding from AbbVie, Amgen, Bristol-Myers Squibb, and Jazz Pharmaceuticals. Recipient of a share in royalty payments paid to the Walter and Eliza Hall Institute of Medical Research.
SA Fleming: Consultancy/advisory role for Amgen, Pfizer, BMS/Celgene, Ariad, and Astellas; research funding from Amgen; and speakers’ bureau participation for Amgen, BMS/Celgene and Pfizer.
R Norris: Travel support from Merck.
JT Opferman: Research funding from AbbVie.
M Badawi, M Schmidt, B Tong, JC Pesko, JA Ross, D Vishwamitra, L Rosenwinkel, Y Sun, SY Kim: Employment with AbbVie; may hold stock or other options.
A Jacobson: Former employment with AbbVie; may hold stock or other ownership in AbbVie.
CG Mullighan: Honoraria from Pfizer and Illumina; consultancy/advisory role for Illumina; speakers’ bureau participation for Amgen, Pfizer, and Illumina; and research funding from AbbVie and Pfizer.
TB Alexander: Travel support from AbbVie.
W Stock: Honoraria from UpToDate and Research To Practice; speaker honoraria from AbbVie and Pfizer; and consultancy/advisory role for Servier, Kite, Pfizer, Daiichi, Astellas, and Agios.
Footnotes
Data Sharing Statement
RNASeq data can be accessed online through Gene Expression Omnibus (GEO) with accession GSE162894. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1.Burkhardt B, Reiter A, Landmann E, Lang P, Lassay L, Dickerhoff R, et al. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the berlin-frankfurt-muenster group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27(20):3363–9 doi 10.1200/jco.2008.19.3367. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med 2016;375(8):740–53 doi 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28(4):648–54 doi 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien S, Thomas D, Ravandi F, Faderl S, Cortes J, Borthakur G, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer 2008;113(11):3186–91 doi 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Fattah MA. Prognostic Factors and Outcomes of Adult Lymphoblastic Lymphoma in the United States. Clin Lymphoma Myeloma Leuk 2017;17(8):498–505 e6 doi 10.1016/j.clml.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med 2017;376(9):836–47 doi 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378(5):439–48 doi 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadia TM, Gandhi V. Nelarabine in the treatment of pediatric and adult patients with T-cell acute lymphoblastic leukemia and lymphoma. Expert Rev Hematol 2017;10(1):1–8 doi 10.1080/17474086.2017.1262757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death & Differentiation 2017;25(1):65–80 doi 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013;19(2):202–8 doi 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 11.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008;68(9):3421–8 doi 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 12.High LM, Szymanska B, Wilczynska-Kalak U, Barber N, O’Brien R, Khaw SL, et al. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Molecular pharmacology 2010;77(3):483–94 doi 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
- 13.Kang MH, Kang YH, Szymanska B, Wilczynska-Kalak U, Sheard MA, Harned TM, et al. Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood 2007;110(6):2057–66 doi 10.1182/blood-2007-03-080325. [DOI] [PubMed] [Google Scholar]
- 14.Lock R, Carol H, Houghton PJ, Morton CL, Kolb EA, Gorlick R, et al. Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr Blood Cancer 2008;50(6):1181–9 doi 10.1002/pbc.21433. [DOI] [PubMed] [Google Scholar]
- 15.McBride A, Houtmann S, Wilde L, Vigil C, Eischen CM, Kasner M, et al. The Role of Inhibition of Apoptosis in Acute Leukemias and Myelodysplastic Syndrome. Front Oncol 2019;9:192 doi 10.3389/fonc.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz-Flores E, Comeaux EQ, Kim KL, Melnik E, Beckman K, Davis KL, et al. Bcl-2 Is a Therapeutic Target for Hypodiploid B-Lineage Acute Lymphoblastic Leukemia. Cancer Res 2019;79(9):2339–51 doi 10.1158/0008-5472.Can-18-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard JT, Rowley JS, Eide CA, Traer E, Hayes-Lattin B, Loriaux M, et al. Targeting BCL-2 and ABL/LYN in Philadelphia chromosome-positive acute lymphoblastic leukemia. Sci Transl Med 2016;8(354):354ra114 doi 10.1126/scitranslmed.aaf5309. [DOI] [PubMed] [Google Scholar]
- 18.Suryani S, Carol H, Chonghaile TN, Frismantas V, Sarmah C, High L, et al. Cell and molecular determinants of in vivo efficacy of the BH3 mimetic ABT-263 against pediatric acute lymphoblastic leukemia xenografts. Clin Cancer Res 2014;20(17):4520–31 doi 10.1158/1078-0432.CCR-14-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaw SL, Suryani S, Evans K, Richmond J, Robbins A, Kurmasheva RT, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood 2016;128(10):1382–95 doi 10.1182/blood-2016-03-707414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alford SE, Kothari A, Loeff FC, Eichhorn JM, Sakurikar N, Goselink HM, et al. BH3 Inhibitor Sensitivity and Bcl-2 Dependence in Primary Acute Lymphoblastic Leukemia Cells. Cancer Res 2015;75(7):1366–75 doi 10.1158/0008-5472.CAN-14-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood 2008;111(4):2300–9 doi 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Numan Y, Alfayez M, Maiti A, Alvarado Y, Jabbour EJ, Ferrajoli A, et al. First Report of Clinical Response to Venetoclax in Early T-Cell Precursor Acute Lymphoblastic Leukemia. JCO precision oncology 2018(2):1–6 doi 10.1200/po.18.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard-Carpentier G, Jabbour E, Short NJ, Rausch CR, Savoy JM, Bose P, et al. Clinical Experience With Venetoclax Combined With Chemotherapy for Relapsed or Refractory T-Cell Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk 2020;20(4):212–8 doi 10.1016/j.clml.2019.09.608. [DOI] [PubMed] [Google Scholar]
- 24.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30(5):488–96 doi 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Safety, Pharmacokinetics, Pharmacodynamics, and Activity of Navitoclax, a Targeted High Affinity Inhibitor of BCL-2, in Lymphoid Malignancies. The Lancet Oncology 2010;11(12):1149–59 doi 10.1016/s1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell 2007;128(6):1173–86 doi 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Gu Z, Churchman ML, Roberts KG, Moore I, Zhou X, Nakitandwe J, et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat Genet 2019;51(2):296–307 doi 10.1038/s41588-018-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol 2009;10(2):147–56 doi 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J Oncol Pharm Pract 2018;24(4):299–308 doi 10.1177/1078155217701291. [DOI] [PubMed] [Google Scholar]
- 30.Incidence Earl M. and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol 2009;7(9):600–6. [PubMed] [Google Scholar]
- 31.Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood 2019;133(14):1548–59 doi 10.1182/blood-2018-10-881961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia 2018;32(3):606–15 doi 10.1038/leu.2017.265. [DOI] [PubMed] [Google Scholar]
- 33.Douer D, Aldoss I, Lunning MA, Burke PW, Ramezani L, Mark L, et al. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(9):905–11 doi 10.1200/jco.2013.50.2708. [DOI] [PubMed] [Google Scholar]
- 34.Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gokbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019;125(14):2474–87 doi 10.1002/cncr.32116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nature reviews Clinical oncology 2015;12(6):344–57 doi 10.1038/nrclinonc.2015.38. [DOI] [PubMed] [Google Scholar]
- 36.Jain N, Roberts KG, Jabbour E, Patel K, Eterovic AK, Chen K, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood 2017;129(5):572–81 doi 10.1182/blood-2016-07-726588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer discovery 2014;4(9):1074–87 doi 10.1158/2159-8290.Cd-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia 2002;16(9):1713–24 doi 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 39.Schneider P, Vasse M, Sbaa-Ketata E, Lenormand B, Hong L, Soria C, et al. The growth of highly proliferative acute lymphoblastic leukemia may be independent of stroma and/or angiogenesis. Leukemia 2001;15(7):1143–5 doi 10.1038/sj.leu.2402141. [DOI] [PubMed] [Google Scholar]
- 40.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cellular and molecular life sciences : CMLS 2009;66(8):1326–36 doi 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(27):3059–68 doi 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karol SE, Alexander TB, Budhraja A, Pounds SB, Canavera K, Wang L, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol 2020;21(4):551–60 doi 10.1016/S1470-2045(20)30060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 2012;151(2):344–55 doi 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan J, Montero J, Rocco J, Letai A. iBH3: simple, fixable BH3 profiling to determine apoptotic priming in primary tissue by flow cytometry. Biol Chem 2016;397(7):671–8 doi 10.1515/hsz-2016-0107. [DOI] [PubMed] [Google Scholar]
- 45.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29(1):15–21 doi 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011;12:323 doi 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011;27(6):863–4 doi 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicorici D, Satalan M, Edgren H, Kangaspeska S, Murumagi A, Kallioniemi O, et al. FusionCatcher – a tool for finding somatic fusion genes in paired-end RNA-sequencing data. bioRxiv 011650 2014. doi 10.1101/011650. [DOI] [Google Scholar]
- 49.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol 2011;29(1):24–6 doi 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 2016;34(5):525–7 doi 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.