Abstract
Background
The effectiveness of glucocorticoids in preventing biphasic reactions in patients with anaphylaxis remains controversial.
Objective
We evaluated the effects of glucocorticoids on rates of biphasic reactions in hospitalized patients with anaphylaxis treated with adrenaline.
Methods
In this retrospective observational study using a national inpatient database in Japan, we identified 31,570 hospitalized patients with anaphylaxis treated with adrenaline on the day of admission. We divided them into two groups: those who were treated with adrenaline plus glucocorticoids and those who received adrenaline only on the day of admission. The primary outcome was the occurrence of a biphasic reaction, defined as requiring two or more ampules of adrenaline within 7 days of admission. We performed a one-to-four propensity score matching analysis to compare the outcomes between the two groups.
Results
Of the 31,570 eligible patients, 28,145 (89.2%) were treated with glucocorticoids on the day of admission. The overall percentage of biphasic reactions within 7 days of admission was 11.2%. One-to-four propensity score matching created matched cohorts of 3,425 patients in the control group and 13,700 patients in the glucocorticoid group. After propensity score matching, there were no significant differences in rates of biphasic reactions (10.7% in the glucocorticoids group vs. 10.5% in the control group; odds ratio, 1.03; 95% confidence interval, 0.85–1.24; p = 0.77) between patients with anaphylaxis treated with and without glucocorticoids on the day of admission.
Conclusion
Our findings do not support the use of glucocorticoids to prevent biphasic reactions in hospitalized patients with severe anaphylaxis requiring adrenaline.
Keywords: Adrenaline, Anaphylaxis, Biphasic reaction, Glucocorticoids, Propensity score
Introduction
Anaphylaxis, a type of hypersensitivity reaction, can be life-threatening at any age. We defined a biphasic reaction as the recurrence of anaphylactic symptoms without reexposure to the allergen within 48–72 h of resolution of the initial reaction [1]. A meta-analysis of biphasic reactions reported a 4.6% rate of biphasic reactions among patients with anaphylaxis [1]. Current anaphylaxis guidelines therefore recommend continuous observation for several hours or longer after resolution of the initial reaction [2, 3, 4, 5]. The guidelines also recommend intramuscular injection of adrenaline as first-line anaphylaxis treatment and glucocorticoids, histamine-1 receptor blockers, and beta-2-adrenergic receptor stimulants as second-line treatments [2, 3, 4, 5]. However, there is limited available evidence for the effects of second-line treatments on symptoms and rates of biphasic reactions in patients with anaphylaxis.
Glucocorticoids inhibit inflammatory responses by suppressing the function of mast cells and are thought to prevent biphasic reactions in patients with anaphylaxis [6]. Recent retrospective cohort studies have failed to show any preventive effects of glucocorticoids on biphasic reactions [7, 8]. However, the study cohorts included patients with various types of allergy, including mild-to-moderate anaphylaxis. Given that glucocorticoids can ameliorate allergic inflammatory responses, it is possible that they prevent biphasic reactions, especially in patients with severe anaphylaxis. Therefore, in this retrospective observational study using a national inpatient database in Japan, we aimed to examine the effect of glucocorticoids on rates of biphasic reactions in hospitalized patients with severe anaphylaxis treated with adrenaline.
Materials and Methods
Data Source
In this nationwide retrospective cohort study, we used data from the Japanese Diagnosis Procedure Combination database, the details of which have been described elsewhere [9]. Briefly, this database includes data of approximately 7 million inpatients per year, which represent more than half of all inpatient admissions to acute care hospitals in Japan. All academic hospitals are obliged to submit data to this database, whereas submission by community hospitals is voluntary. The database includes the following characteristics for each patient: age, sex, body height, weight, Japan Coma Scale score, smoking status, diagnoses, preadmission comorbidities, post-admission complications, medications administered, and discharge status. Diagnoses are recorded using the International Classification of Diseases 10th revision codes. The Institutional Review Board of the University of Tokyo approved this study (Approval Number 3501-3; 25 December 2017). Because the data were anonymized, the requirement for informed consent was waived.
Patients, Intervention, and Outcomes
We defined severe anaphylaxis as anaphylaxis treated with adrenaline in hospital. Using data from July 2010 to March 2018, we identified hospitalized patients who were diagnosed with anaphylaxis and treated with intramuscular adrenaline on the day of admission. Patients with anaphylaxis were identified on the basis of the following International Classification of Diseases 10th revision codes: T78.0 (Anaphylactic shock due to adverse food reaction), T78.2 (Anaphylactic shock, unspecified), and T88.6 (Anaphylactic shock due to adverse effect of correct drug or medicament properly administered). We only assessed the initial hospitalization for patients who were hospitalized twice or more during the study period. We defined the glucocorticoid group as patients who received oral or intravenous glucocorticoids on the day of admission, the remaining patients comprising the control group.
The primary outcome was a biphasic reaction within 7 days of admission. We defined biphasic reaction as adrenaline reuse within 7 days from the date of admission. Adrenaline reuse was defined as administration of two or more ampules of adrenaline within 7 days from the date of admission because, in Japan, 0.3 mg of adrenaline is commonly administered intramuscularly for the initial reaction of anaphylaxis and repeated up to three times according to the persistence of symptoms by using one 1.0 mg ampule of adrenaline. The secondary outcome was 7-day all-cause mortality and a biphasic reaction, defined as either adrenaline reuse or any readmission within 7 days from the date of the original admission. We also evaluated biphasic reactions on Day 1 (day of admission), Day 2, Days 1–2, and Days 3–7 separately.
Statistical Analyses
We conducted propensity score matching analysis to compare outcomes between the two groups. A multivariable logistic regression model with the following variables as covariates was used to estimate propensity scores for receiving glucocorticoids on the day of admission: age, sex; body mass index category; Japan Coma Scale score (alert, drowsy, somnolent, and comatose) [10]; smoking status (never, past and current smoker, and missing); diagnoses (T78.0, T78.2, T88.6); Charlson Comorbidity Index score (0, 1, 2, and ≥3); history of asthma, atopic dermatitis, and atopic rhinitis; administration of oxygen; use of histamine 1 blockers, histamine 2 blockers, and beta 2-adrenergic receptor stimulants; hospital volume (very low, low, high, and very high); and teaching hospital. We performed one-to-four nearest-neighbor matching with replacement for estimated propensity scores, using a caliper width set at one fifth of the standard deviation of the estimated propensity scores. To assess the accuracy of the matching, we compared the covariates before and after propensity score matching using absolute standardized differences, absolute standardized differences ≤10% being considered to denote negligible imbalances between the two groups [11]. After propensity score matching, we assessed the outcomes through generalized linear models, accompanied by cluster-robust standard errors with hospitals as the clusters. We calculated odds ratios and their 95% confidence intervals (CIs) with generalized linear models using the logit link function.
Sensitivity Analyses
We used the following statistical methods to conduct sensitivity analyses to check the robustness of our findings. First, we performed traditional multivariable regression analysis using a generalized linear model. In this analysis, we created a generalized linear model using outcomes as dependent variable and glucocorticoids on the day of admission and all covariates as independent variables. Second, we performed a propensity score adjustment analysis. In this analysis, we created a generalized linear model using outcomes as dependent variables and estimated propensity score in the main analysis as independent variables. Third, we conducted an inverse probability of treatment weighting analysis [12]. For this, we used a weighted generalized linear model with stabilized average treatment effect weight calculated from the estimated propensity scores in the main analysis.
Continuous variables are presented as mean and standard deviation, and categorical variables as number and percentage. p < 0.05 was defined as denoting statistical significance. We used Stata version 16.0 (StataCorp, College Station, TX, USA) to perform all statistical analyses.
Results
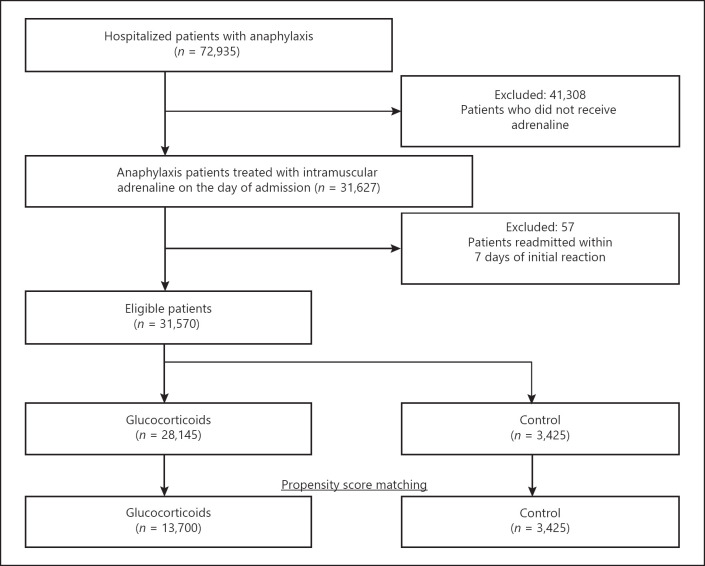
We identified 72,935 patients who had been hospitalized for anaphylaxis during the study period. Of these, 31,627 had been treated with adrenaline on the day of admission and 41,308 patients had not been, leaving 31,570 eligible patients. Of these, 28,145 (89.2%) were treated with glucocorticoids on the day of admission (Fig. 1).
Fig. 1.
Flowchart showing the stratification and selection of patients with severe anaphylaxis.
Table 1 shows the patients' baseline characteristics before and after propensity score matching. Before propensity score matching, significantly higher proportions of patients were using histamine-1 receptor blockers, histamine-2 receptor blockers, and beta-2-adrenergic receptor stimulants in the glucocorticoids group than in the control group.
Table 1.
Baseline characteristics of patients treated with and without glucocorticoids according to unmatched and propensity score matched groups.
| Characteristics | Unmatched |
After 1:4 matching |
||||
|---|---|---|---|---|---|---|
| control (n = 3,425) | glucocorticoids (n = 28,145) | ASD, % | control (n = 3,425) | glucocorticoids (n = 13,700) | ASD, % | |
| Age, mean (SD), years | 37.1 (27.4) | 38.6 (26.5) | 5.4 | 37.1 (27.4) | 36.5 (26.9) | 2.2 |
| Male | 1,829 (53.4) | 15,125 (53.7) | 0.7 | 1,829 (53.4) | 7,241 (52.9) | 1.1 |
| Body mass index, kg/m2 | ||||||
| <18.5 | 861 (25.1) | 6,291 (22.4) | 6.6 | 861 (25.1) | 3,467 (25.3) | 0.4 |
| 18.5 to <25.0 | 1,379 (40.3) | 11,765 (41.8) | 3.1 | 1,379 (40.3) | 5,484 (40.0) | 0.5 |
| 25.0 to <30.0 | 443 (12.9) | 3,973 (14.1) | 3.5 | 443 (12.9) | 1,780 (13.0) | 0.2 |
| ≥30.0 | 132 (3.9) | 1,064 (3.8) | 0.4 | 132 (3.9) | 560 (4.1) | 1.2 |
| Missing | 610 (17.8) | 5,052 (17.9) | 0.4 | 610 (17.8) | 2,409 (17.6) | 0.6 |
| Japan Coma Scale | ||||||
| Alert | 2,941 (85.9) | 24,175 (85.9) | 0.1 | 2,941 (85.9) | 11,827 (86.3) | 1.3 |
| Drowsy | 332 (9.7) | 2,750 (9.8) | 0.3 | 332 (9.7) | 1,279 (9.3) | 1.2 |
| Somnolence | 104 (3.0) | 852 (3.0) | 0.1 | 104 (3.0) | 410 (3.0) | 0.3 |
| Coma | 48 (1.4) | 368 (1.3) | 0.8 | 48 (1.4) | 184 (1.3) | 0.5 |
| Smoking status | ||||||
| Never | 2,341 (68.4) | 18,605 (66.1) | 4.8 | 2,341 (68.4) | 9,498 (69.3) | 2.1 |
| Past and current | 717 (20.9) | 6,262 (22.2) | 3.2 | 717 (20.9) | 2,796 (20.4) | 1.3 |
| Missing | 367 (10.7) | 3,278 (11.6) | 3.0 | 367 (10.7) | 1,406 (10.3) | 1.5 |
| Diagnosis | ||||||
| T78.0† | 730 (21.3) | 5,284 (18.8) | 6.3 | 730 (21.3) | 2,845 (20.8) | 1.3 |
| T78.2‡ | 2,343 (68.4) | 20,039 (71.2) | 6.1 | 2,343 (68.4) | 9,549 (69.7) | 2.8 |
| T88.6§ | 352 (10.3) | 2,822 (10.0) | 0.8 | 352 (10.3) | 1,306 (9.5) | 2.5 |
| Charlson Comorbidity Index | ||||||
| 0 | 2,771 (80.9) | 21,834 (77.6) | 8.2 | 2,771 (80.9) | 11,214 (81.9) | 2.4 |
| 1 | 441 (12.9) | 4,160 (14.8) | 5.5 | 441 (12.9) | 1,681 (12.3) | 1.8 |
| 2 | 135 (3.9) | 1,288 (4.6) | 3.1 | 135 (3.9) | 513 (3.7) | 1.0 |
| ≥3 | 78 (2.3) | 863 (3.1) | 4.9 | 78 (2.3) | 292 (2.1) | 1.0 |
| Medical history | ||||||
| Asthma | 197 (5.8) | 2,139 (7.6) | 7.4 | 197 (5.8) | 725 (5.3) | 2.0 |
| Atopic dermatitis | 80 (2.3) | 634 (2.3) | 0.6 | 80 (2.3) | 292 (2.1) | 1.4 |
| Atopic rhinitis | 50 (1.5) | 686 (2.4) | 7.1 | 50 (1.5) | 202 (1.5) | 0.1 |
| Administration of oxygen | 1,477 (43.1) | 13,740 (48.8) | 11.4 | 1,477 (43.1) | 5,751 (42.0) | 2.3 |
| Use of drugs | ||||||
| H1 blocker | 2,385 (69.6) | 24,227 (86.1) | 40.4 | 2,385 (69.6) | 9,560 (69.8) | 0.3 |
| H2 blocker | 1,398 (40.8) | 15,167 (53.9) | 26.4 | 1,398 (40.8) | 5,694 (41.6) | 1.5 |
| β2 agonist | 392 (11.4) | 4,531 (16.1) | 13.5 | 392 (11.4) | 1,582 (11.5) | 0.3 |
| Hospital volume | ||||||
| Very low | 756 (22.1) | 7,268 (25.8) | 8.8 | 756 (22.1) | 2,892 (21.1) | 2.3 |
| Low | 868 (25.3) | 7,014 (24.9) | 1.0 | 868 (25.3) | 3,610 (26.4) | 2.3 |
| High | 852 (24.9) | 6,982 (24.8) | 0.2 | 852 (24.9) | 3,480 (25.4) | 1.2 |
| Very high | 949 (27.7) | 6,881 (24.4) | 7.4 | 949 (27.7) | 3,718 (27.1) | 1.3 |
| Clinical training hospital | 3,167 (92.5) | 26,027 (92.5) | 0.0 | 3,167 (92.5) | 12,735 (93.0) | 1.9 |
Data are presented as n (%) except for age. ASD, absolute standardized difference; H1 blocker, histamine-1 receptor blockers; H2 blocker, histamine-2 receptor blockers; SD, standardized difference; β2 agonist, beta 2-adrenergic receptor stimulants.
T78.0, Anaphylactic shock due to adverse food reaction.
T78.2, Anaphylactic shock, unspecified.
T88.6, Anaphylactic shock due to adverse effect of correct drug or medicament properly administered.
The online supplementary file (for all online suppl. material, see www.karger.com/doi/10.1159/000524612) shows the baseline characteristics of hospitalized patients with anaphylaxis treated with and without adrenaline. Patients treated with adrenaline were more likely to have received histamine-1 receptor blockers, histamine-2 receptor blockers, beta 2-adrenergic receptor stimulants, and oxygen, and to have been hospitalized in clinical training hospitals than patients treated without adrenaline.
The overall percentage of biphasic reactions within 7 days of admission was 11.2%, and 7-day all-cause mortality was 0.4% (Table 2). The percentages of biphasic reactions that occurred on days 1, 2, and 3–7 of the initial reaction were 88.8%, 9.1%, and 2.1%, respectively.
Table 2.
Proportions of biphasic reactions, adrenaline reuse, or any readmission within 7 days of admission, and 7-day all-cause mortality in unmatched and propensity score matched groups
| Unmatched |
After 1:4 matching |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| control (n = 3,425) | glucocorticoids (n = 28,145) | total (n = 31,570) | control (n = 3,425) | glucocorticoids (n = 13,700) | odds ratio | 95% CI | p value | ||||
| Biphasic reaction | 358 (10.5) | 3,172 (11.3) | 3,530 (11.2) | 358 (10.5) | 1,469 (10.7) | 1.03 | 0.85–1.24 | 0.77 | |||
| Day 1 | 304 (8.9) | 2,831 (10.1) | 3,135 (9.9) | 304 (8.9) | 1,320 (9.6) | 1.09 | 0.89–1.35 | 0.39 | |||
| Day 2 | 46 (1.3) | 274 (1.0) | 320 (1.0) | 46 (1.3) | 126 (0.9) | 0.68 | 0.46–1.00 | 0.05 | |||
| Days 1–2 | 350 (10.2) | 3,105 (11.0) | 3,455 (10.9) | 350 (10.2) | 1,446 (10.6) | 1.04 | 0.85–1.26 | 0.71 | |||
| Days 3–7 | 8 (0.2) | 67 (0.2) | 75 (0.2) | 8 (0.2) | 23 (0.2) | 0.72 | 0.29–1.77 | 0.47 | |||
| Adrenaline reuse or any readmission | 378 (11.0) | 3,399 (12.1) | 3,777 (12.0) | 378 (11.0) | 1,572 (11.5) | 1.04 | 0.87–1.25 | 0.63 | |||
| All-cause mortality | 19 (0.6) | 93 (0.3) | 112 (0.4) | 19 (0.6) | 61 (0.4) | 0.80 | 0.45–1.41 | 0.45 | |||
Data are presented as n (%).
One-to-four propensity score matching created matched cohorts of 3,425 patients in the control group and 13,700 patients in the glucocorticoid group. After propensity score matching, the distribution of patient characteristics was well-balanced between the matched groups. There were no statistically significant differences in rates of biphasic reactions (odds ratio 1.03; 95% CI: 0.85–1.24; p = 0.77), adrenaline reuse, or any readmission within 7 days from the date of the original admission (odds ratio 1.04; 95% CI: 0.87–1.25; p = 0.63), or 7-day all-cause mortality (odds ratio 0.80; 95% CI: 0.45–1.41; p = 0.45) between the two groups (Table 2). There were no significant differences in rates of biphasic reactions for Day 1, Day 2, Days 1–2, and Days 3–7. The results of three sensitivity analyses of traditional multivariable regression analyses, propensity score adjustment, and inverse probability of treatment weighting were similar to those using propensity score matching for biphasic reactions (Table 3).
Table 3.
Results using other analytic methods
| Traditional multivariable regression analyses |
Propensity score adjustment |
Inverse probability of treatment weighting |
||||
|---|---|---|---|---|---|---|
| odds ratio (95% CI) | p value | odds ratio (95% CI) | p value | odds ratio (95% CI) | p value | |
| Biphasic reaction | 1.06 (0.94–1.20) | 0.32 | 1.06 (0.94–1.19) | 0.33 | 1.10 (0.97–1.25) | 0.15 |
| Day 1 | 1.11 (0.98–1.27) | 0.10 | 1.11 (0.98–1.26) | 0.11 | 1.13 (0.99–1.30) | 0.08 |
| Day 2 | 0.77 (0.56–1.06) | 0.11 | 0.77 (0.56–1.06) | 0.12 | 0.92 (0.65–1.29) | 0.62 |
| Days 1–2 | 1.07 (0.94–1.20) | 0.30 | 1.06 (0.94–1.20) | 0.32 | 1.11 (0.98–1.26) | 0.11 |
| Days 3–7 | 0.94 (0.44–1.98) | 0.86 | 0.93 (0.44–1.96) | 0.85 | 0.75 (0.32–1.78) | 0.52 |
| Adrenaline reuse or any readmission | 1.08 (0.96–1.21) | 0.19 | 1.08 (0.96–1.21) | 0.20 | 1.12 (0.99–1.27) | 0.07 |
| All-cause mortality | 0.75 (0.42–1.35) | 0.33 | 0.80 (0.48–1.33) | 0.39 | 0.97 (0.55–1.71) | 0.93 |
Discussion
In this study, about 90% of the 31,570 patients with anaphylaxis treated with adrenaline were also treated with glucocorticoids. The overall proportion of biphasic reactions was 11.2%. There were no significant differences in rates of biphasic reactions or 7-day all-cause mortality between those treated with and without glucocorticoids.
Previous studies have failed to show that glucocorticoids reduce the rate of biphasic reactions in patients with anaphylaxis of varying severity [7, 8]. In the present study, which included only hospitalized patients with severe anaphylaxis treated with adrenaline, we also found no evidence that glucocorticoids reduce the rate of biphasic reactions.
In the study, 90% of the biphasic reactions occurred on the day of the initial reaction. A previous study reported a median time between initial symptom resolution and onset of biphasic reaction of 11 h (range 0.2–72 h) [1]. The antiallergic effects of glucocorticoids, which are thought to be responsible for any effect on biphasic reactions, occur within 4–6 h and persist for 8–12 h [6, 13]. Therefore, the failure of glucocorticoids to reduce the rate of biphasic reactions may be attributable to the difference between the period during which glucocorticoids are effective and the time of onset of biphasic reactions.
Long-term steroid administration is associated with adverse effects, including infection, osteoporosis, hypertension, mood disorder, peptic ulcer, and adrenal insufficiency. Even short-term administration may result in avascular necrosis [14, 15]. Short-term steroid use can also be associated with development of hyperglycemia within a few hours [16]. Given that this study showed no significant association between glucocorticoid use and rate of biphasic reactions, routine use of glucocorticoids to prevent biphasic reactions may not be indicated, not even in patients with severe anaphylaxis requiring intramuscular adrenaline.
This study has several limitations. First, it was a retrospective observational study. Although we used propensity score matching to adjust for confounding factors, our results may have been affected by unmeasured confounding factors, including symptoms of anaphylaxis, time from onset of anaphylaxis to treatment, use of epinephrine autoinjectors, and the results of laboratory tests. Future research is expected to be prospective or to use registries that include detailed clinical data. Second, only about half of all acute care hospitals in Japan are included in the database. Hospitals that choose not to contribute to the database may have different characteristics, possibly reducing the internal validity of the results of our study. Third, in this study, severe anaphylaxis was defined as anaphylaxis requiring admission to hospital and treatment with adrenaline. However, some patients without severe clinical symptoms receive adrenaline, potentially resulting in misclassification and underestimation of the effects of glucocorticoids. Fourth, some patients who required more than one ampule of adrenaline for the initial anaphylactic reaction may have been misclassified as having had a biphasic reaction. Given that multiple ampules are rarely required to manage initial anaphylactic reactions, this possibility would have had minimal impact on our findings. Fifth, we excluded hospitalized patients with anaphylaxis treated without adrenaline on the day of admission. It is possible that these patients did not meet the diagnostic criteria for anaphylaxis or had mild to moderate symptoms. However, it is also possible that these patients did have anaphylaxis and did not receive the correct treatment. The results of this study do not apply to such patients.
In conclusion, our nationwide database study of patients with severe anaphylaxis showed no significant reduction in rates of biphasic reactions by glucocorticoids. Our findings do not justify routine administration of glucocorticoids to prevent biphasic reaction in patients with severe anaphylaxis.
Statement of Ethics
The Institutional Review Board of the University of Tokyo approved this study (Approval No. 3501-3; 25 December 2017). Because the data were anonymized, the requirement for informed consent was waived.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 20AA2005) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907).
Author Contributions
Shimpei Nagata, Hiroyuki Ohbe, Taisuke Jo, and Hideo Yasunaga designed the research; Shimpei Nagata, Hiroyuki Ohbe, Taisuke Jo, Hiroki Matsui, Kiyohide Fushimi, and Hideo Yasunaga conducted the research; Shimpei Nagata and Hiroyuki Ohbe analyzed the data; Shimpei Nagata, Hiroyuki Ohbe, Taisuke Jo, and Hideo Yasunaga wrote the paper; Shimpei Nagata had primary responsibility for the final content. All the authors have read and approved the final manuscript.
Data Availability Statement
The dataset analyzed in the current study is not publicly available because of contracts with the hospitals providing data to the database.
Supplementary Material
Supplementary data
Edited by: H.-U. Simon, Bern.
Funding Statement
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 20AA2005) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907).
References
- 1.Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3((3)):408–2. doi: 10.1016/j.jaip.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Society of Allergology Anaphylaxis guideline. 2014. Available from https://anaphylaxis-guideline.jp/pdf/anaphylaxis_guideline (accessed October 2, 2021.
- 3.Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis: a 2020 practice parameter update, systematic review, and grading of recommendations, assessment, development and evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145((4)):1082–123. doi: 10.1016/j.jaci.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 1920 Oct 30;13((10)):100472. doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, et al. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy. 2014;69((8)):1026–45. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 6.Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49((5)):196–207. doi: 10.23822/EurAnnACI.1764-1489.15. [DOI] [PubMed] [Google Scholar]
- 7.Grunau BE, Wiens MO, Rowe BH, McKay R, Li J, Yi TW, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66((4)):381–9. doi: 10.1016/j.annemergmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Bellolio MF, Hess EP, Campbell RL. Predictors of biphasic reactions in the emergency department for patients with anaphylaxis. J Allergy Clin Immunol Pract. 2014;2((3)):281–7. doi: 10.1016/j.jaip.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Yasunaga H. Real world data in Japan: chapter II the diagnosis procedure combination database. Ann Clin Epidemiol. 2019;1((3)):76–9. [Google Scholar]
- 10.Ono K, Wada K, Takahara T, Shirotani T. Indications for computed tomography in patients with mild head injury. Neurol Med Chir. 2007;47((7)):291–8. doi: 10.2176/nmc.47.291. [DOI] [PubMed] [Google Scholar]
- 11.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28((25)):3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168((6)):656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9((1)):30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilisio MF. Osteonecrosis following short-term low-dose oral. corticosteroids: a Population-Based Study of 24 million patients. Orthopedics. 2014;37((7)):e631–6. doi: 10.3928/01477447-20140626-54. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy P, Bassiouni A, Psaltis A, Antisdel J, Brunworth J. Avascular necrosis after oral corticosteroids in otolaryngology: case report and review of the literature. Allergy Rhinol. 2016;7((1)):50–4. doi: 10.2500/ar.2016.7.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts A, James J, Dhatariya K. Joint British Diabetes Societies (JBDS) for inpatient care. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for inpatient care group. Diabet Med. 2018;35((8)):1011–7. doi: 10.1111/dme.13675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The dataset analyzed in the current study is not publicly available because of contracts with the hospitals providing data to the database.