Abstract
Background
Previous studies have suggested that metabolic syndrome (MetS) components are associated with renal outcomes, defined as a decline in kidney function or reaching end-stage renal disease (ESRD). Elevated triglycerides (TGs) are a component of MetS that have been reported to be associated with renal outcomes. However, the association of TGs with renal outcomes in chronic kidney disease (CKD) patients independent of the other components of the MetS remains understudied.
Methods
We examined 1,657,387 patients with data on TGs and other components of MetS in 2004–2006 and followed up until 2014. Patients with ESRD on renal replacement therapy were excluded. We examined time to ESRD, estimated glomerular filtration rate (eGFR) slope (renal function decline), and time to incident CKD (eGFR <60 mL/min/1.73 m<sup>2</sup>) among baseline normal kidney function (non-CKD) patients, using Cox or logistic regression, adjusted for clinical characteristics and MetS components. We also stratified analyses by the number of MetS components.
Results
The cohort was on average 64 years old and comprised 5% females, 15% African Americans, and 24% with nondialysis-dependent CKD. Among non-CKD patients, the adjusted relationship of TGs with time to incident CKD was strong and linear. Compared to TGs 120–<160 mg/dL, higher TGs were associated with a faster renal function decline across all CKD stages. Elevated TGs ≥240 mg/dL were associated with a faster time to ESRD among non-CKD and CKD stages 3A–3B, while the risk gradually declined to null or lower in CKD stages 4–5. Models were robust after MetS component adjustment and stratification.
Conclusion
Independent of MetS components, high TGs levels were associated with a higher incidence of CKD and a faster renal function decline, yet showed no or inverse associations with time to ESRD in CKD stages 4–5. Examining the effects of TGs-lowering interventions on incident CKD and kidney preserving therapy warrants further studies including clinical trials.
Keywords: Triglycerides, End-stage renal disease, Chronic kidney disease, Metabolic syndrome, Renal function decline
Introduction
Chronic kidney disease (CKD) and metabolic syndrome (MetS) are major risk factors for cardiovascular disease and mortality, and their burden on public health has grown as the rates of obesity and sedentary lifestyles have also increased [1, 2]. MetS includes abdominal obesity, elevated fasting blood sugar, elevated blood pressure, low high-density lipoprotein, and elevated serum triglycerides (TGs) [1]. A prior study showed a strong linear association between the number of MetS components and the development of CKD [3]. Furthermore, a population-based study from Taiwan showed an impact of MetS on CKD progression only in CKD stage 1–3 patients, yet not in late-stage or diabetic CKD patients [4]. Despite these previous studies, it remains challenging to delineate the individual effect of each MetS component on the risk of CKD.
Dyslipidemia, especially hypertriglyceridemia, is prevalent in CKD patients [5]. A Mendelian randomization study showed that elevated TGs levels were associated with incident CKD [6]. In a small cohort study of middle-aged persons without diabetes, cardiovascular disease, or CKD, the TGs criterion was identified as a risk factor driving the observed association between MetS and renal function decline [7]. However, among nondialysis-dependent CKD patients, cohort studies examining elevated TGs with renal progression have demonstrated conflicting results. Several studies have investigated cohorts of primarily CKD stage 3A–3B patients over short follow-up periods with various definitions of elevated TGs and renal progression. Data from the Chronic Renal Insufficiency Cohort study suggested that high TGs were not associated with CKD progression [8]. In the Modification of Diet in Renal Disease study, TGs were not related to any clinical outcomes (including end-stage renal disease [ESRD]) in nondiabetic kidney disease patients [9]. Yet, Navaneethan et al. [10] showed an association between each component of MetS including high TGs and time to ESRD over a median follow-up of 2 years. Moreover, both serum glucose and impaired fasting glucose have been associated with the progression of CKD and other clinical outcomes [3, 10, 11, 12]. Given that TGs management may be an important therapeutic target in preventing and slowing CKD progression, these contradictory results raise the question of whether TGs alone or in combination of other MetS components are risk factors for renal outcomes across stages of CKD. We sought to examine the association of TGs with renal outcomes independent of other MetS components in a large contemporary cohort of US veterans.
Methods
Primary Study Population
Lipid profiles and management in veterans with CKD is a retrospective cohort study of veteran patients who had at least one serum lipid measurement at one of the United States Veterans Affairs (VAs) Medical Centers between October 1, 2004, and September 30, 2006 [13]. For this study, we excluded 114,031 patients without a TGs measurement during the baseline period, 18,983 patients who were with ESRD on renal replacement therapy at the time of the TGs measurement, 1,750,981 patients missing an estimated glomerular filtration rate (eGFR) measurement within 90 days prior to the TGs measurement, and 60 patients with invalid censoring information. Moreover, we excluded 417,395 patients who were missing any data on MetS components (online suppl. Fig. 1; see www.karger.com/doi/10.1159/000522388 for all online suppl. material). We analyzed three subcohorts for our study. We examined 1,222,108 patients who had post-TGs measurement eGFR data and were non-CKD (eGFR ≥60 mL/min/1.73 m2) at baseline for time to incident CKD analyses, 1,605,911 patients with post-TGs measurement eGFR data for eGFR slope analyses, and 1,657,387 patients for time to ESRD analyses.
Demographics and Clinical Measurements
Ascertainment of clinical characteristics, including demographics, laboratory measurements, comorbidities, and medications, has been previously described [13]. In summary, databases of the VA, Centers for Medicare and Medicaid Services (CMS), and the US Renal Data System were combined to ascertain clinical characteristics. Comorbidities were defined using a two outpatient or one inpatient algorithm of diagnosis codes from VA and CMS databases [14]. Ever smoking and ever alcoholism status until censorship were defined by VA databases [15]. Laboratory measurements were sourced from VA databases only. Low-density lipoprotein (LDL) cholesterol was calculated using the Martin-Hopkins equation [16]. eGFR was calculated from serum creatinine using the CKD-EPI formula and used in CKD staging: non-CKD, 3A, 3B, 4, and 5 [17]. All patients were nondialysis dependent at the time of TGs measurement. For all laboratory measurements, the closest value measured within 90 days prior to the TGs measurement was used in the analyses.
MetS Components
The presence of any MetS components was defined by the combination of laboratory measurements, prescription medication, and/or comorbid condition diagnosis, where applicable [18]. Definitions of MetS components are presented in online supplementary Table 1. Data on waist circumference were highly missing in this cohort, and we used body mass index (BMI) as a surrogate for abdominal obesity. Patients with available laboratory data, yet not meeting MetS component criteria, were considered as without said MetS component.
Exposure and Outcome Assessment
The exposure was a single measurement of serum TGs and categorized as <80, 80–<120, 120–<160 (reference), 160–<200, 200–<240, and ≥240 mg/dL [13]. We examined three outcomes in this study. First, incident CKD among non-CKD patients was defined as having at least two eGFR measurements at least 90 days apart where the eGFR dropped below 60 mL/min/1.73 m2 and never rose above that threshold again during follow-up [19]. There were few patients (N = 1,759) who reached ESRD, yet they did not meet incident CKD outcome criteria. These patients were considered as having the incident CKD outcome. Second, eGFR decline was assessed by using all eGFR measurements after the baseline TG measurement until the end of follow-up to compute an eGFR slope via a mixed-effects model. Our outcome was defined as a steep eGFR slope (≤−3 mL/min/1.73 m2/year) to represent kidney function decline [20]. Finally, we evaluated progression to ESRD with receipt of renal replacement therapy. The date of transition to ESRD was obtained from the US Renal Data System records.
Patients were followed from the baseline TGs measurement until ESRD with receipt of renal replacement therapy, lost to follow-up, death, or end of the study period (December 31, 2014), whichever occurred first. For incident CKD analyses, participants were further censored at the onset of incident CKD.
Statistical Analysis
Data are presented as mean ± standard deviation, median [interquartile range], or percentage, as appropriate. Our analysis utilized Cox proportional hazards models to investigate the association of TGs with time to ESRD, stratified by CKD stage. Cox proportional hazards models were also used to investigate time to incident CKD among baseline non-CKD patients. Logistic regression was used to evaluate the association of TGs with steep eGFR slope decline, stratified by CKD stage, with the exception of CKD stage 5 due to poor model convergence.
We examined the following models. (1) Unadjusted, (2) Age Adjusted, (3) Case-Mix Adjusted, which included age, gender, race, ethnicity, ever smoker, ever alcoholism, Charlson comorbidity index, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disorder, dementia, liver disease, cancer, diabetes, atrial fibrillation, hypertension, depression, ischemic heart disease, prescription of statins, and prescription of nonstatins, (4) Case-Mix + Lab Adjusted, which included the same variables as the Case-Mix model, and albumin and eGFR, and finally (5) Case-Mix + Lab + Metabolic Components Adjusted, which included the covariates from the Case-Mix + Lab model and the four MetS components other than TGs. We also stratified our analyses by the number of MetS components (≤2 vs. ≥3) to evaluate effect measure modification under the Case-Mix + Lab + Metabolic Components Adjusted model. The Case-Mix + Lab + Metabolic Components Adjusted model was our model of interest. In sensitivity analyses, we examined a small subpopulation with available data on urine albumin to creatinine ratio (UACR) within a year prior to the TGs measurement. We included UACR in adjustment with our main model as well as in stratification by number of MetS components.
For the main cohort, demographic data were missing for 1% of the cohort, while ever smoking and alcoholism statuses were missing for 5% and 20% of the cohort, respectively. These variables were imputed with a missing category. Serum albumin was missing for 25% of the cohort and was imputed by means. Other analyses had similar rates of missingness of covariate data and were handled using the same imputation methods. Statistical analyses were performed with SAS Enterprise Guide (7.1) (Cary, NC, USA) and STATA (15) (College Station, TX, USA). The study was approved by the Institutional Review Board of the Tibor Rubin VA Medical Center of Long Beach, CA, USA. Written consent was waived given the research's nonintrusive nature, large sample size, and patient anonymity.
Results
The cohort of 1,657,387 patients had an age (mean ± SD) of 64 ± 14 years and included 5% females, 15% African Americans, and 30% diabetics (Table 1). Only 24% of patients had nondialysis-dependent CKD at baseline, including 0.2% of patients with CKD stage 5. The median [IQR] TGs level was 129 [88, 193] mg/dL and 76% of patients had at least two other MetS components. The most prevalent MetS conditions were high blood pressure and elevated serum glucose. Patients with higher TGs level tended to be younger, white, diabetic have a history of depression, smoking, and likely to be on a nonstatin medication, specifically fibrate, yet less likely to be anemic or have a history of alcoholism.
Table 1.
Stratified patient characteristics by serum TGs group in 1,657,387 patients
| Total | Serum TGs, mg/dL |
||||||
|---|---|---|---|---|---|---|---|
| <80 | 80–<120 | 120–<160 | 160–<200 | 200–<240 | ≥240 | ||
| N (%) | 1,657,387 | 318,967 (19) | 420,710 (25) | 320,067 (19) | 209,750 (13) | 132,019 (8) | 255,874 (15) |
| eGFR, mL/min/1.73 m2 | 75 [61, 91] | 79 [64, 94] | 75 [61, 90] | 74 [60, 89] | 74 [59, 89] | 74 [59, 89] | 76 [60, 92] |
| CKD Stage, % | |||||||
| Non-CKD | 76 | 80 | 76 | 74 | 74 | 74 | 76 |
| CKD 3A | 15 | 13 | 16 | 16 | 16 | 16 | 15 |
| CKD 3B | 7 | 5 | 7 | 7 | 7 | 7 | 7 |
| CKD 4 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| CKD 5 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 |
| Age, years | 64±14 | 64±15 | 65±14 | 64±13 | 64±13 | 63±13 | 60±12 |
| Gender, female, % | 5 | 6 | 5 | 4 | 4 | 4 | 4 |
| Race, % | |||||||
| White | 82 | 74 | 80 | 84 | 85 | 86 | 86 |
| African American | 15 | 23 | 16 | 13 | 11 | 10 | 9 |
| Other | 4 | 3 | 3 | 4 | 4 | 4 | 4 |
| Hispanic ethnicity, % | 4 | 3 | 4 | 4 | 4 | 4 | 4 |
| CCI | 1 [0, 2] | 1 [0, 2] | 1 [0, 2] | 1 [0, 2] | 1 [0, 2] | 1 [0, 2] | 1 [0, 2] |
| Comorbid conditions, % | |||||||
| Ml | 6 | 6 | 7 | 7 | 7 | 7 | 6 |
| CHF | 10 | 11 | 10 | 10 | 10 | 10 | 10 |
| PVD | 9 | 9 | 10 | 10 | 10 | 9 | 9 |
| Cerebrovascular disease | 9 | 8 | 9 | 9 | 9 | 8 | 8 |
| Dementia | 3 | 3 | 3 | 2 | 2 | 2 | 2 |
| COPD | 18 | 20 | 19 | 18 | 18 | 17 | 16 |
| Liver disease | 3 | 4 | 3 | 3 | 3 | 3 | 3 |
| Diabetes | 30 | 22 | 26 | 30 | 33 | 36 | 41 |
| Cancer | 12 | 12 | 13 | 13 | 12 | 11 | 10 |
| Anemia | 11 | 14 | 12 | 11 | 10 | 9 | 9 |
| Atrial fibrillation | 7 | 8 | 7 | 7 | 6 | 6 | 5 |
| Hypertension | 66 | 60 | 66 | 68 | 69 | 70 | 69 |
| ISHD | 27 | 25 | 28 | 29 | 28 | 28 | 27 |
| Depression | 18 | 15 | 16 | 17 | 19 | 20 | 23 |
| Substance abuse | 7 | 8 | 7 | 6 | 6 | 6 | 7 |
| Ever smoking | 64 | 61 | 63 | 64 | 65 | 66 | 68 |
| Ever alcoholism | 24 | 29 | 25 | 23 | 22 | 22 | 22 |
| Laboratory measurements | |||||||
| Albumin, g/dL | 4.1 ±0.4 | 4.0±0.5 | 4.0±0.4 | 4.1 ±0.4 | 4.1 ±0.4 | 4.1 ±0.4 | 4.1 ±0.4 |
| AST, U/L | 23 [19, 29] | 23 [19, 29] | 23 [19, 28] | 23 [19, 28] | 23 [19, 28] | 23 [19, 29] | 24 [19, 30] |
| ALT, U/L | 25 [18, 35] | 23 [17, 32] | 23 [17, 33] | 25 [18, 35] | 26 [19, 36] | 26 [19, 37] | 28 [20, 40] |
| Glucose, mg/dL | 115.3±44.3 | 105.7±32.1 | 109.9±35.9 | 114.0±40.0 | 117.6±44.3 | 121.1 ±48.4 | 132.8±63.3 |
| Hemoglobin, g/dL | 14.4±1.6 | 14.1 ±1.7 | 14.3±1.6 | 14.5±1.6 | 14.6±1.6 | 14.7±1.6 | 14.8±1.6 |
| SBP, mm Hg | 135±19 | 133±20 | 135±20 | 135+19 | 136±19 | 136±19 | 137+19 |
| DBP, mm Hg | 76±12 | 74±12 | 75±12 | 75±12 | 76±12 | 76±12 | 77±12 |
| BMI, kg/m2 | 29±6 | 27±5 | 29±6 | 30±6 | 30±6 | 31±6 | 31±6 |
| Lipid panel, mg/dL | |||||||
| TGs | 129 [88, 193] | 63 [53, 72] | 99 [89, 109] | 138 [128, 148] | 177 [168, 188] | 217 [208, 228] | 313 [269, 398] |
| HDL | 42 [35, 51] | 50 [41, 61] | 44 [37, 53] | 41 [35, 49] | 39 [33, 46] | 38 [32, 44] | 35 [30, 42] |
| Cholesterol | 177 [152, 205] | 163 [140, 187] | 170 [146, 196] | 176 [152, 203] | 182 [157, 210] | 187 [162, 215] | 200 [173, 233] |
| LDL | 106 [86, 130] | 95 [76, 116] | 104 [83, 127] | 108 [88, 133] | 112 [92, 136] | 114 [94, 139] | 115 [93, 140] |
| Lipid-modulating therapy use, % | |||||||
| Statin | 33 | 28 | 33 | 36 | 36 | 36 | 34 |
| Ezetimibe | 0.4 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 |
| Nonstatin | 6 | 3 | 4 | 6 | 7 | 8 | 12 |
| Fibrate | 4 | 1 | 2 | 3 | 4 | 5 | 8 |
| Niacin | 2 | 1 | 1 | 2 | 2 | 2 | 3 |
| Fish oil | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.3 |
| Bile acid sequestrants | 0.4 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 |
| Metabolic components, % Reduced HDL | 46 | 23 | 36 | 48 | 56 | 63 | 72 |
| Elevated blood pressure | 86 | 81 | 86 | 88 | 89 | 89 | 90 |
| Elevated glucose | 61 | 50 | 57 | 62 | 65 | 67 | 72 |
| Obesity | 39 | 23 | 33 | 41 | 46 | 50 | 55 |
| Number of components, % | |||||||
| 0 | 5 | 9 | 5 | 4 | 3 | 2 | 1 |
| 1 | 19 | 31 | 23 | 17 | 14 | 11 | 8 |
| 2 | 31 | 36 | 35 | 32 | 29 | 27 | 23 |
| 3 | 29 | 18 | 27 | 32 | 35 | 36 | 37 |
| 4 | 16 | 5 | 10 | 15 | 20 | 24 | 31 |
Data presented as mean±standard deviation, median [interquartile range], or percentage, as appropriate. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CCI, Charlson comorbidity index; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disorder; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HDL, high-density lipoprotein; ISHD, ischemic heart disease; LDL, low-density lipoprotein; MI, myocardial infarction; PTSD, post-traumatic stress disorder; PVD, peripheral vascular disease; SBP, systolic blood pressure; TGs, triglycerides.
Association of Serum TGs with Time to Incident CKD among Non-CKD Patients
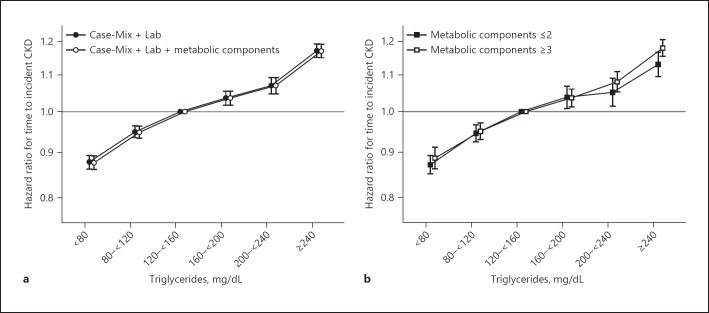
Among 1.2 million non-CKD patients at the time of the TGs measurement, we observed a total of 145,812 incident CKD events over follow-up yielding a crude rate of 15.0 events per 1,000 person-years. We observed a linear association between TGs and time to incident CKD after adjustment for Case-Mix + Lab + Metabolic Components (Fig. 1a). A TGs level <80 mg/dL was protective for incident CKD, while a TGs level ≥240 mg/dL had the greatest effect estimate (hazard ratio [95% confidence interval]: HR [95% CI]: 1.17 [1.15, 1.19]) (online suppl. Table 2). Likewise, parallel relationships were observed after stratification for a number of MetS components (Fig. 1b, online suppl. Table 3).
Fig. 1.
Association of serum TGs with time to incident CKD (<60 mL/min/1.73 m2) among non-CKD patients across levels of adjustment (a) and stratified by the number of metabolic components under Case-Mix + Lab + Metabolic Components Adjustment (b).
Association of Serum TGs with Odds of Steep eGFR Decline
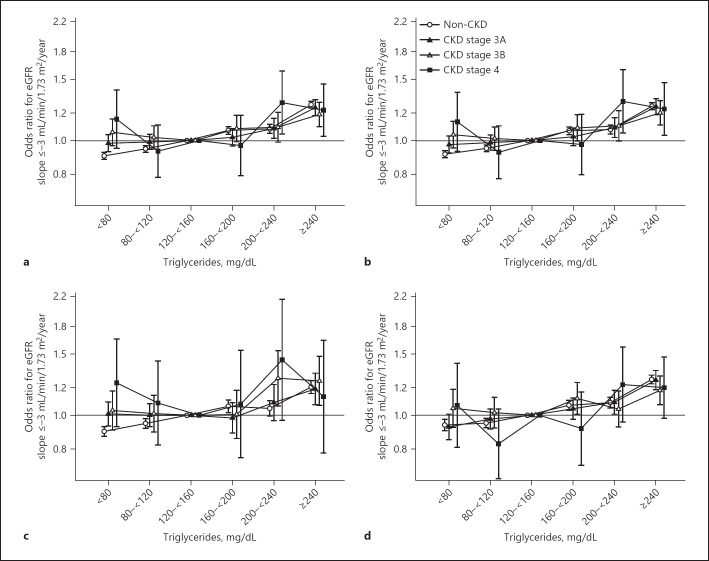
During follow-up, patients had a median [IQR] of 16 [8, 21] eGFR measurements, and an eGFR slope of −0.5 [−1.2, 0.4] mL/min/1.73 m2/year. Under our definition of a steeper slope of −3 mL/min/1.73 m2/year, unadjusted analyses showed a slight linear association between higher TGs levels (≥160 mg/dL) and odds of eGFR slope decline across all CKD strata (online suppl. Table 4). The highest TGs level (≥240 mg/dL) had the greatest odds of an eGFR slope decline in all CKD stages. Further adjustment for clinical characteristics and laboratory variables showed a similar pattern in non-CKD and CKD stage 3A patients. CKD stage 4 patients exhibited a U-shaped association between TGs and odds of eGFR slope decline (Fig. 2a). The effect estimates for high TGs levels and odds of eGFR slope decline very gradually diminished with advancing CKD stage. Further adjustment for MetS components did not alter associations (Fig. 2b).
Fig. 2.
Association of serum TGs with odds of eGFR slope ≤−3 mL/min/1.73 m2/year by CKD stage among Case-Mix + Lab (a) and Case-Mix + Lab + Metabolic Component Adjusted models (b) and stratified by ≤2 (c) and ≥3 (d) metabolic components under the Case-Mix + Lab + Metabolic Components Adjusted model.
We observed a lower odds of steep eGFR slope decline with low TGs levels (<120 mg/dL) irrespective of the number of MetS components in non-CKD patients, yet these relationships were null in CKD stage 3A–3B patients (Fig. 2c, d; online suppl. Table 3). In patients with ≥3 MetS components, we observed a generally linear association between TGs and odds of eGFR slope decline in all CKD strata. While attenuated, in patients with ≤2 MetS components, a largely linear association between TGs and eGFR slope decline was observed for most stages except for CKD stage 4. A TGs level ≥240 mg/dL had higher odds of eGFR slope decline in both MetS strata among non-CKD and CKD 3A–3B patients (online suppl. Table 3).
Association of Serum TGs with Time to ESRD
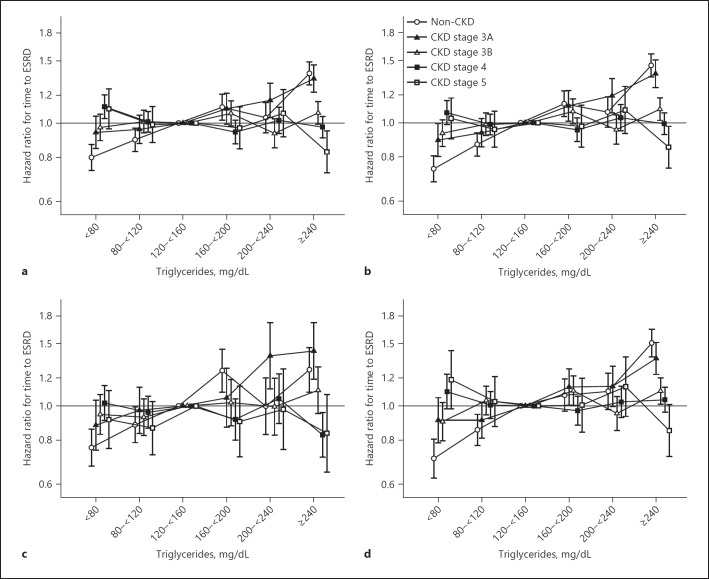
Over a median [IQR] follow-up of 9.2 [6.4, 9.9] years, 29,383 veterans transitioned to ESRD for a crude rate of 2.3 events per 1,000 person-years (online suppl. Table 5). In unadjusted analyses, we observed a linear association between TGs and time to ESRD, for non-CKD and CKD stage 3A–3B patients. However, there was a U-shaped association between TGs and time to ESRD in patients with CKD stage 4 and an almost inverse association for CKD stage 5 patients. CKD stage 4–5 patients with TGs levels ≥240 mg/dL had a lower unadjusted risk of ESRD compared to TGs levels ranging 120–<160 mg/dL (online suppl. Table 6). After adjustment for Case-Mix + Lab variables, the association between TGs and time to ESRD was linear among non-CKD and CKD stage 3A patients (Fig. 3a). However, the relationship between TGs and time to ESRD in CKD stage 4 or 5 was relatively flat with the exception of high TGs levels. The high TGs levels and ESRD risk gradually cascaded across CKD stages. Those with CKD stage 5 and a TGs level ≥240 mg/dL were associated with a lower risk of ESRD (HR [95% CI]: 0.83 [0.72, 0.95]). These relationships remained robust after adjustment for MetS components (Fig. 3b).
Fig. 3.
Association of serum TGs with time to ESRD by CKD stage among Case-Mix + Lab (a) and Case-Mix + Lab + Metabolic Component Adjusted models (b) and stratified by ≤2 (c) and ≥3 (d) metabolic components under the Case-Mix + Lab + Metabolic Components Adjusted model.
To further examine for effect modification, we stratified our associations by number of MetS components (Fig. 3c, d; online suppl. Table 3). Among those with ≤2 MetS components, we observed similar albeit largely attenuated trends between TGs and ESRD risk across CKD stages. Compared to the reference (TGs levels 120–<160 mg/dL), low TGs levels were associated with a lower risk of ESRD for non-CKD and CKD 3A patients. High TGs levels with ESRD risk gradually attenuated across worse CKD stages. Among those with ≥3 MetS components, we also observed this attenuation in high TGs levels and ESRD risk across CKD stages. Patients with elevated TGs levels with ≥3 MetS components, and in CKD stage 5, had the lowest risk of time to ESRD (HR [95% CI]: 0.85 [0.72, 1.01]) (online suppl. Table 3).
Sensitivity Analyses for UACR
Only 156,541 patients had data on UACR in the year prior to the TGs measurement. The median [IQR] UACR level was 11 [5, 22] mg/g and the majority of patients had normal albuminuria (<30 mg/g). Patients with an UACR measurement had a greater prevalence of MetS components, including glucose intolerance (92% vs. 57% in patients without UACR data).
Among 113,328 non-CKD patients, we still observed a strong and linear relationship between TGs and time to incident CKD after additional adjustment for UACR (online suppl. Table 7). Among 156,453 patients with post-TGs eGFR data, the relationship between eGFR and a steep slope was also similar yet largely attenuated after UACR adjustment. Notably, CKD stage 3B patients with elevated TGs levels had a null relationship with odds of a steep slope, whereas our main analyses demonstrated higher odds for all stages. Finally, analyses between TGs and time to ESRD were attenuated. The TGs-ESRD relationship among CKD stage 3A–3B patients were U or J shaped. A generally lower risk of ESRD was observed for those with high TGs levels and CKD stage 4. For most analyses, we were unable to evaluate stratification by number of MetS components and CKD stage 5 to sufficiently infer conclusions given the small sample sizes.
Discussion
This study demonstrated that high TGs levels are an independent predictor of the time to incident CKD, progression of CKD defined by eGFR decline, and time to ESRD with renal replacement therapy in non-CKD and CKD stage 3A–3B patients. In addition, we observed that the relationship between high TGs levels and time to ESRD was attenuated in CKD stage 4–5 patients.
Hypertriglyceridemia is a component of MetS, which itself is a risk factor for CKD. Thomas et al. [3] published a meta-analysis investigating a total of 30,146 patients and showed that MetS components were associated with incident CKD. The strength of the association was stronger as the number of MetS components increased and each component including TGs was independently associated with the outcome. Subsequently, other studies explored these findings and showed that TGs and other metabolic risk factors were associated with incident CKD in the elderly, type 2 diabetes, and type 1 diabetes populations [23, 24, 25, 26, 27, 28]. Other studies have also shown an association between high TGs levels and a higher rate of CKD progression [21, 29]. Our data are in agreement with prior studies showing an association between hypertriglyceridemia and ESRD [10, 30]. However, several studies did not evaluate TGs as a predictor of CKD incidence and progression independent of other metabolic risk factors. In addition, many studies were restricted to certain populations, thus reducing the generalizability of their findings. We believe that ours is the first study demonstrating the association between TGs and CKD incidence and progression in patients across CKD stages, reporting the time to occurrence of the events and adjusting for all other components of MetS. Our results highlight the importance of investigating and targeting TGs as a potential means of hindering CKD development and progression. This concept is also supported by a Mendelian randomization showing TGs as the only lipid factor associated with incident CKD [6].
The possible mechanism of kidney injury by hypertriglyceridemia could be explained by an increase in oxidative stress leading to vessel injury which accelerates the rate of glomerulosclerosis [31]. Animal models have shown that both high cholesterol and TGs are associated with podocyte injury, proteinuria, and interstitial injury [31]. Recent studies have shown that the intracellular accumulation of TGs might also account for the formation of lipid-laden cells (foam cells). In diabetic mouse models, the increase in sterol regulatory element-binding proteins in the renal cortex was associated with high renal TGs content and an increase in mesangial matrix expansion and glomerulosclerosis. These findings suggest that increased intracellular accumulation of TGs may play a central role in progression of renal disease in diabetic nephropathy [32]. As the TGs levels increase, the levels of small dense very LDL particles also increase. These very LDL particles can be pro-inflammatory and mediate maladaptive responses which lead to mesangial sclerosis [22, 33].
Within the USA, pharmacologic interventions used to target TGs include fibrates, omega-3 fatty acids, and niacin. Fibrates, including fenofibrate and, to a lesser extent, gemfibrozil, increase the serum creatinine level [34, 35]. However, in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, fenofibrate reduced albuminuria and slowed eGFR loss over a follow-up period of 5 years, despite an increase in the overall creatinine level after initiation of the medication [36]. In rodent experimental models of CKD, both fenofibrate and niacin were shown to have renoprotective effects, whereas omega-3 fatty acids only had a renoprotective effect after a myocardial infarction [37, 38, 39]. These data point to a potential utility of TGs-lowering agents for prevention and treatment of CKD [40].
Another aspect of our study demonstrated the diminishing association of hypertriglyceridemia and progression to ESRD with later CKD stages. Patients with CKD stage 4 and hypertriglyceridemia did not have a higher risk of progression to ESRD, while hypertriglyceridemia was associated with a slower rate of progression to ESRD in CKD stage 5 patients. These findings are consistent with previous studies evaluating the association of elevated TGs levels and mortality in ESRD patients. It was shown that higher TGs levels were associated with reduced risk of all-cause and cardiovascular mortality in hemodialysis patients [41]. It has been postulated that in advanced CKD including ESRD patients, altered energy metabolism, malnutrition, inflammation, cachexia, and protein energy wasting may play a more prominent role in disease progression and poor outcomes [5, 42, 43, 44, 45]. Therefore, higher serum TGs levels may indicate a more favorable metabolic state with increased energy production in a setting where patients are more prone to energy dysmetabolism and wasting. In such conditions, including advanced CKD, the efficacy of TGs-lowering therapies is less clear.
The strengths of this study include a large cohort of veteran patients with the ability to evaluate individual strata of CKD stages. The use of large and combined electronic medical records afforded the detailed measurements of potential confounders including medication and laboratory data, as well as repeated serial measurements of eGFR to evaluate renal function decline.
Several limitations should be noted. First, UACR data were highly missing. Given the impact of UACR on lipid metabolism, the risk of higher TGs levels with progression of CKD may have been confounded by UACR. However, data on UACR were only measured in a small and select VA population, rather than routinely in the general VA population. While we adjusted for UACR in a subpopulation with data and observed similar relationships as our main analyses, we acknowledge that our results may be subjected to these biases. Thus, future studies are needed to delineate the role of UACR in the association of TGs and renal outcomes. Furthermore, CKD staging, incident CKD, and impaired renal function can be defined by other measurements beyond eGFR, such as urine sediment abnormalities or abnormal imaging of the kidney; however, these data were limited or unavailable in our administrative database. In addition, while waist circumference is a standard measurement for MetS, we also had little available data and used BMI ≥30 kg/m2 as a surrogate marker [46]. Among the patients with waist circumference data, we found it to strongly correlate with BMI (rho = 0.78). Another limitation is that our laboratory data could not identify fasting status. While we can surmise that lipids and glucose were drawn in a fasting state given clinical practice, we cannot make a definite confirmation. Prior studies have demonstrated only small differences between fasting and nonfasting lipid levels, yet these MetS components may be subject to potential misclassification and measurement error [47]. Given the observational study design, we cannot infer a causal relationship between the observed associations. There remains a risk for residual confounding due to laboratory markers like uric acid, diet, or physical activity risk factors. Finally, the veteran population is composed of primarily white males and may not be externally valid to the general population.
We evaluated the associations of TGs with several definitions of renal outcomes, stratified by CKD stages. Higher TGs levels were associated with higher risk of decline in kidney function, while also associated with a lower or null relationship with time to ESRD in CKD stage 4 and 5 patients. Further studies are needed to examine the mechanistic pathways in which TGs manifest in CKD patients independent of MetS components and whether lipid management therapy can prevent CKD development and ameliorate progression across stages of CKD.
Statement of Ethics
The study was approved by the institutional review board of the Tibor Rubin VA Medical Center of Long Beach, CA, USA. The paper is exempt from Ethical Committee approval and the written consent was waived given the research's nonintrusive nature, large sample size, and patient anonymity.
Conflict of Interest Statement
K.K.Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO Oncology, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, International Federation of Kidney Foundations, International Society of Hemodialysis, International Society of Renal Nutrition & Metabolism, Japanese Society of Dialysis Therapy, Hospira, Kabi, Keryx, Novartis, National Institutes of Health, National Kidney Foundation, OPKO, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, and ZSPharma. C.P.K. has received honoraria from Abott, Akebia, Astra-Zeneca, Bayer, Boehringer-Ingelheim, Cara Therapeutics, CSL Behring, Rockwell, and Vifor. H.M. has received research funding from Novartis and Amgen. E.S. has received support from Astra Zeneca, and Edwards Lifesciences. All other authors have no conflicts of interest to declare.
Funding Sources
K.K.Z. has been supported by the NIH/NIDDK mid-career award K24-DK091419. ES is supported by a career development award from the Office of Research and Development of the Department of VAs (IK2- CX 001266-01). HM is supported by a career development award from the Office of Research and Development of the Department of VAs (1IK- CX 001043-01A2).
Author Contributions
Study design and conduct: E.S., C.P.K., and K.K.Z. Data collection: K.K.Z. and C.P.K. Data analysis: M.S. and J.T.H. Data interpretation: M.S., H.M., C.P.K., K.K.Z., and E.S. Drafting manuscript: M.S., L.H., and E.S. Revising manuscript content: M.S., J.T.H., H.M., M.J.B., C.P.K., K.K.Z., and E.S. Approving final version of the manuscript: M.S., L.H., J.T.H., H.M., M.J.B., C.P.K., K.K.Z., and E.S. Responsibility for the integrity of the data analysis: E.S.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study. The United States Department of VAs places legal restrictions on access to veteran's health care data, which includes both identifying data and sensitive patient information. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
Supplementary Material
Supplementary data
Acknowledgments
This study was supported by Dr. Streja's Career Development Award IK2- CX 001266-01 from the US Veterans Administration Clinical Sciences Research and Development Program. The data reported here have been supplied by the US Veterans Administration. Support for VA/CMS data is provided by the Department of VAs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). Opinions expressed in this presentation are those of the authors and do not represent the official opinion of the US Department of VAs or the US Government. Results have been presented at the 2018 American Heart Association Scientific Sessions, Chicago, IL, USA as well as the virtual 2020 National Lipid Association Scientific Sessions.
Funding Statement
K.K.Z. has been supported by the NIH/NIDDK mid-career award K24-DK091419. ES is supported by a career development award from the Office of Research and Development of the Department of VAs (IK2- CX 001266-01). HM is supported by a career development award from the Office of Research and Development of the Department of VAs (1IK- CX 001043-01A2).
References
- 1.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015 May 19;313((19)):1973–4. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 2.Carney EF. The impact of chronic kidney disease on global health. Nat Rev Nephrol. 2020 May;16((5)):251. doi: 10.1038/s41581-020-0268-7. [DOI] [PubMed] [Google Scholar]
- 3.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011 Oct;6((10)):2364–73. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CC, Sun CY, Wu IW, Wang SY, Wu MS. Metabolic syndrome loses its predictive power in late-stage chronic kidney disease progression: a paradoxical phenomenon. Clin Nephrol. 2011 Feb;75((2)):141–9. doi: 10.5414/cnp75141. [DOI] [PubMed] [Google Scholar]
- 5.Soohoo M, Moradi H, Obi Y, Kovesdy CP, Kalantar-Zadeh K, Streja E. Serum triglycerides and mortality risk across stages of chronic kidney disease in 2 million U.S. veterans. J Clin Lipidol. 2019;13((5)):744–53.e15. doi: 10.1016/j.jacl.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YB, Sheng LT, Wei W, Guo H, Yang H, Min X, et al. Association of blood lipid profile with incident chronic kidney disease: a mendelian randomization study. Atherosclerosis. 2020 May;300:19–25. doi: 10.1016/j.atherosclerosis.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Stefansson VTN, Schei J, Solbu MD, Jenssen TG, Melsom T, Eriksen BO. Metabolic syndrome but not obesity measures are risk factors for accelerated age-related glomerular filtration rate decline in the general population. Kidney Int. 2018 May;93((5)):1183–90. doi: 10.1016/j.kint.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Rahman M, Yang W, Akkina S, Alper A, Anderson AH, Appel LJ, et al. Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2014;9((7)):1190–8. doi: 10.2215/CJN.09320913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawla V, Greene T, Beck GJ, Kusek JW, Collins AJ, Sarnak MJ, et al. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin J Am Soc Nephrol. 2010 Sep;5((9)):1582–7. doi: 10.2215/CJN.01450210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navaneethan SD, Schold JD, Kirwan JP, Arrigain S, Jolly SE, Poggio ED, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol. 2013 Jun;8((6)):945–52. doi: 10.2215/CJN.09870912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obermayr RP, Temml C, Knechtelsdorfer M, Gutjahr G, Kletzmayr J, Heiss S, et al. Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant. 2008 Apr;23((4)):1265–73. doi: 10.1093/ndt/gfm790. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal S, Shlipak MG, Kramer H, Jain A, Herrington DM. The association of chronic kidney disease and metabolic syndrome with incident cardiovascular events: multiethnic study of atherosclerosis. Cardiol Res Pract. 2012;2012:806102. doi: 10.1155/2012/806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soohoo M, Moradi H, Obi Y, Kovesdy CP, Kalantar-Zadeh K, Streja E. Serum triglycerides and mortality risk across stages of chronic kidney disease in 2 million U.S. veterans. J Clin Lipidol. 2019;13((5)):744–53.e15. doi: 10.1016/j.jacl.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 14.VIReC . Calculating a comorbidity index for risk adjustment using VA or medicare data. Hines, IL: U.S. Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2014. [Google Scholar]
- 15.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, et al. Validating smoking data from the veteran's affairs health factors dataset, an electronic data source. Nicotine Tob Res. 2011 Dec;13((12)):1233–9. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013 Nov;310((19)):2061–8. doi: 10.1001/jama.2013.280532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May;150((9)):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005 Oct;112((17)):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009 Nov;76((10)):1089–97. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 20.Kovesdy CP, Coresh J, Ballew SH, Woodward M, Levin A, Naimark DM, et al. Past decline versus current eGFR and subsequent ESRD risk. J Am Soc Nephrol. 2016;27((8)):2447–55. doi: 10.1681/ASN.2015060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Jiang Y, Jia J, He D, Sun P, Li J, et al. Metabolic syndrome is associated with rapid estimated glomerular filtration rate decline in a Chinese community-based population. Diabetes Metab Syndr Obes. 2019;12:2085–93. doi: 10.2147/DMSO.S217326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008 Jul;28((7)):1225–36. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 23.De Cosmo S, Viazzi F, Pacilli A, Giorda C, Ceriello A, Gentile S, et al. Predictors of chronic kidney disease in type 2 diabetes: a longitudinal study from the AMD annals initiative. Medicine. 2016 Jul;95((27)):e4007. doi: 10.1097/MD.0000000000004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo GT, De Cosmo S, Viazzi F, Pacilli A, Ceriello A, Genovese S, et al. Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: the AMD annals initiative. Diabetes Care. 2016 Dec;39((12)):2278–87. doi: 10.2337/dc16-1246. [DOI] [PubMed] [Google Scholar]
- 25.Piscitelli P, Viazzi F, Fioretto P, Giorda C, Ceriello A, Genovese S, et al. Predictors of chronic kidney disease in type 1 diabetes: a longitudinal study from the AMD annals initiative. Sci Rep. 2017 Jun 12;7((1)):3313. doi: 10.1038/s41598-017-03551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y, Sun G, Liu R, Sun A, Zhang Q, Li Y, et al. Plasma triglyceride levels and central obesity predict the development of kidney injury in Chinese community older adults. Ren Fail. 2019 Nov;41((1)):946–53. doi: 10.1080/0886022X.2019.1655451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkins BA, Bebu I, de Boer IH, Molitch M, Tamborlane W, Lorenzi G, et al. Risk factors for kidney disease in type 1 diabetes. Diabetes Care. 2019 May;42((5)):883–90. doi: 10.2337/dc18-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang W, Wang J, Shen X, Lu W, Wang Y, Li W, et al. Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta-analysis of 20 cohorts. Diabetes Care. 2020 Apr;43((4)):925–33. doi: 10.2337/dc19-1897. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Wang B, Yang J, Wang J, Yu Y, Jiang C, et al. Serum lipids and risk of rapid renal function decline in treated hypertensive adults with normal renal function. Am J Hypertens. 2019 Mar 16;32((4)):393–401. doi: 10.1093/ajh/hpz001. [DOI] [PubMed] [Google Scholar]
- 30.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2010 Jan;55((1)):31–41. doi: 10.1053/j.ajkd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Joles JA, Kunter U, Janssen U, Kriz W, Rabelink TJ, Koomans HA, et al. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J Am Soc Nephrol. 2000 Apr;11((4)):669–83. doi: 10.1681/ASN.V114669. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002 May 24;277((21)):18919–27. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 33.Lynn EG, Siow YL, O K. Very low-density lipoprotein stimulates the expression of monocyte chemoattractant protein-1 in mesangial cells. Kidney Int. 2000 Apr;57((4)):1472–83. doi: 10.1046/j.1523-1755.2000.00992.x. [DOI] [PubMed] [Google Scholar]
- 34.Tonelli M, Collins D, Robins S, Bloomfield H, Curhan GC. Effect of gemfibrozil on change in renal function in men with moderate chronic renal insufficiency and coronary disease. Am J Kidney Dis. 2004 Nov;44((5)):832–9. [PubMed] [Google Scholar]
- 35.Forsblom C, Hiukka A, Leinonen ES, Sundvall J, Groop PH, Taskinen MR. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes: the FIELD Helsinki substudy. Diabetes Care. 2010 Feb;33((2)):215–20. doi: 10.2337/dc09-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the fenofibrate intervention and event lowering in diabetes (FIELD) study. Diabetologia. 2011 Feb;54((2)):280–90. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 37.Cho KH, Kim HJ, Rodriguez-Iturbe B, Vaziri ND. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am J Physiol Renal Physiol. 2009 Jul;297((1)):F106–13. doi: 10.1152/ajprenal.00126.2009. [DOI] [PubMed] [Google Scholar]
- 38.Hoogeveen EK, Geleijnse JM, Kromhout D, Stijnen T, Gemen EF, Kusters R, et al. Effect of omega-3 fatty acids on kidney function after myocardial infarction: the alpha omega trial. Clin J Am Soc Nephrol. 2014 Oct 7;9((10)):1676–83. doi: 10.2215/CJN.10441013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaribeygi H, Mohammadi MT, Rezaee R, Sahebkar A. Fenofibrate improves renal function by amelioration of NOX-4, IL-18, and p53 expression in an experimental model of diabetic nephropathy. J Cell Biochem. 2018 Sep;119((9)):7458–69. doi: 10.1002/jcb.27055. [DOI] [PubMed] [Google Scholar]
- 40.Streja E, Kovesdy CP, Streja DA, Moradi H, Kalantar-Zadeh K, Kashyap ML. Niacin and progression of CKD. Am J Kidney Dis. 2015 May;65((5)):785–98. doi: 10.1053/j.ajkd.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 41.Moradi H, Abhari P, Streja E, Kashyap ML, Shah G, Gillen D, et al. Association of serum lipids with outcomes in Hispanic hemodialysis patients of the West versus East Coasts of the United States. Am J Nephrol. 2015;41((4-5)):284–95. doi: 10.1159/000381991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K, Anker SD. Inflammation, cholesterol levels, and risk of mortality among patients receiving dialysis. JAMA. 2004 Apr 21;291((15)):1834–5. doi: 10.1001/jama.291.15.1834-a. [DOI] [PubMed] [Google Scholar]
- 43.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007 Jan;18((1)):293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 44.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol. 2007 Jan;18((1)):304–11. doi: 10.1681/ASN.2006060674. [DOI] [PubMed] [Google Scholar]
- 45.Tonelli M, Muntner P, Lloyd A, Manns B, Klarenbach S, Pannu N, et al. Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol. 2013 May;24((6)):979–86. doi: 10.1681/ASN.2012080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson PM, Tuomilehto J, Rydén L. The metabolic syndrome: what is it and how should it be managed? Eur J Prev Cardiol. 2019 Dec;26((2_Suppl)):33–46. doi: 10.1177/2047487319886404. [DOI] [PubMed] [Google Scholar]
- 47.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008 Nov;118((20)):2047–56. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study. The United States Department of VAs places legal restrictions on access to veteran's health care data, which includes both identifying data and sensitive patient information. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.