Abstract
Introduction
Statins are effective in preventing vascular disease and are widely recommended and used for the secondary prevention of ischemic stroke. However, there is concern from trials that statins might increase the risk of hemorrhagic stroke, partially reducing their benefit. We sought to systematically review the latest evidence on this question.
Methods
Four electronic databases were searched to identify published randomized controlled trials (RCTs) and observational cohort studies (search date December 2020). Two independent reviewers carried out the eligibility assessment based on predefined inclusion criteria. We examined the outcomes of recurrent stroke (after ischemic stroke) of any type, and separately recurrent ischemic stroke and recurrent hemorrhagic stroke. RCTs and observational cohort studies were meta-analyzed separately. Odds ratios (ORs) were used to assess the effect of statin therapy. Meta-analysis was conducted using RevMan 5.4 software.
Results
We retrieved 559 papers in searches, of which 11 RCTs and 12 observational cohort studies were included. Both RCTs and observational studies found that statins reduced the odds of stroke of any type in those with an initial ischemic stroke (11 RCTs: OR = 0.87, 95% CI [0.77,0.97]; p = 0.02; 12 cohort studies: OR = 0.80, 95% CI [0.66, 0.96]; p = 0.02). Both RCTs and observational studies found that recurrence of ischemic stroke was reduced by statins (6 RCTs: OR = 0.81, 95% CI [0.70, 0.93]; p = 0.002; 3 observational studies: OR = 0.67, 95% CI [0.61, 0.75]; p < 0.00001). Data from 7 RCTs and 8 cohort studies did not find a significant difference in hemorrhagic stroke but could not rule out a substantial increase or reduction (7 RCTs: OR = 1.15, 95% CI [0.62, 2.13]; p = 0.66; 8 cohort studies: OR = 0.93, 95% CI [0.71, 1.21]; p = 0.59).
Conclusions
In people who have experienced an ischemic stroke, statins reduce the risk of recurrent stroke of any type medicated through a reduction of ischemic stroke. We found no increase in the risk of hemorrhagic stroke.
Keywords: Stroke recurrence, Statin treatment, Ischemic stroke, Hemorrhagic stroke
Introduction
Stroke was the second most common death disease worldwide in 2019 [1], which has posed a great challenge to human beings. According to the National Health Service (NHS), there are two major types of strokes: ischemic stroke (IS) and hemorrhagic stroke (HS). IS, occurring when a blood clot interrupts or reduces the flow and oxygen, is the most identified type of stroke that forms approximately 87% of the identified strokes. HS, although less common than IS and transient ischemic attack (TIA), still has a significant public health impact because of the higher mortality and morbidity associated with them. Almost 30% of strokes in recent studies are recurrent events, and recurrent strokes are more likely to result in disability or death [2].
American Heart Association (AHA) and American Stroke Association (ASA) released updated guidelines for recurrent stroke prevention in people with a previous stroke and TIA. They recommended that statin therapy could reduce the risk of cardiovascular and cerebrovascular events [3]. Also, a current meta-analysis study [4] included randomized controlled trials (RCTs) from 1995 to 2016 and reported that statin therapy was related to a reducing tendency in the absolute risk of stroke recurrence for IS patients. However, several studies have detected increased risk of recurrence when implementing statin therapy for IS patients. In a trial, researchers demonstrated that statin therapy was related to an increased chance of intracerebral hemorrhage (ICH). It happened more frequently in the group that received statin therapy, especially in males and elders [5]. Therefore, whether statin therapy is reducing risk of recurrence for IS patients still needs further investigation. Our study aimed to assess the efficacy of statin treatment for stroke recurrence in patients with previous IS: (1) this meta-analysis included studies from 1995 to 2020 and contained more recent studies for the latest and precise results; (2) this study conducted two study designs (RCT and cohort studies) independently and assessed whether these results were consistent; (3) this study classified recurrent stroke into ischemic and HS in both two study designs (RCT and cohort studies) for IS survivors to see if there are any differences.
Methods
Search Strategy and Selection Criteria
A comprehensive search with no language restriction was conducted through four electronic databases, including Medline, Embase, PubMed, and the Cochrane Central Register of Controlled Trials (CEN-TRAL). Relevant studies published between January 1995 and December 2020 were included. Besides, this study carried out a separate search on RCTs and observational cohort studies, and cohort studies were not searched in the CEN-TRAL. A detailed search strategy was provided in the online supplementary Appendix S1 (see www.karger.com/doi/10.1159/000525672 for all online suppl. material). This study followed the PRISMA reporting guidelines in terms of study selection, data collection synthesis, assessment of bias, and quality assessment [6]. To minimize the reporting bias, this systematic review meta-analysis has registered on PROSPERO (CRD42022309030).
Studies were included if (1) study designs were RCTs or observational cohort studies; (2) individuals who were 50 years old and above with previous IS; (3) participants with IS received statin therapy, and studies made a comparison between two groups (statin group and no-statin group); (4) analyses involved different doses of statin therapy intervention; (5) primary outcome was a recurrent stroke, and secondary outcome included patients who developed a recurrent IS or a recurrent HS. Studies were excluded if (1) study designs were commentaries, reviews, or animal models and (2) statin doses were stratified into three groups.
Data Collection and Extraction
Investigators Yue Yin and Li Zhang independently used the EndNote Software Version X9 to gather, organize, and record all references with citations and abstracts. Also, the investigators conducted the procedure by hand in case the software failed to detect some of the duplicates.
Two investigators extracted data from studies that met selection criteria, including the study design, participant characteristics (country, age, and sex), the first type of stroke, the second type of stroke, recruitment period, the time from first events to randomization, and the time of follow-up. Disagreements between the two investigators were resolved by consensus with another researcher Yanzhong Wang. As for the secondary outcome, we have extracted the number of any recurrent strokes, recurrent ISs, and recurrent HSs.
Assessment of Study Quality
For RCTs, our study carried out the risk of bias assessment following the criteria of the Cochrane collaboration and classified the risk of bias into three categories, including “low,” “high,” or “unclear” [7]. The CASP Cohort Study Checklist was used for the quality assessment for cohort studies [8]. We classified the risk bias of cohort study into “Yes,” “No,” or “Cannot tell,” and investigators filled the checklist supplement for some questions. I2 was used for heterogeneity assessment, and levels were classified based on the Handbook by Cochrane. Funnel plots for RCTs and cohort studies were created through RevMan 5.4 to assess the publication bias.
Statistical Analysis
We extracted the odds ratio (OR) with 95% confidence intervals (95% CIs) to estimate the treatment effect of statins. RevMan 5.4 was used to analyze data and create forest plots and funnel plots. Statistical heterogeneity among studies was assessed using the Higgins inconsistency index (I2) test. This study conducted subgroup analysis according to different recurrent stroke subtypes (ischemic and HSs). For all analyses, the results were assumed statistically significant when p < 0.05. All statistical tests were two-sided.
Results
The Selection Process and Characteristics of Included Studies
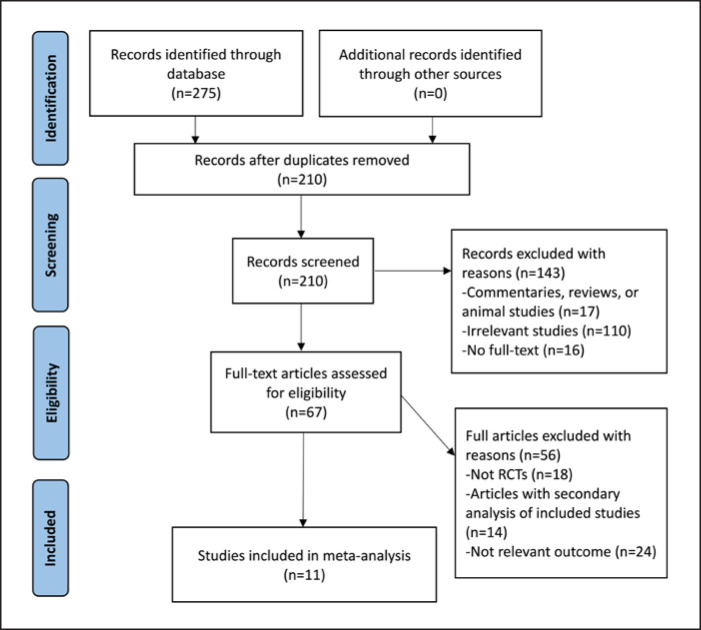
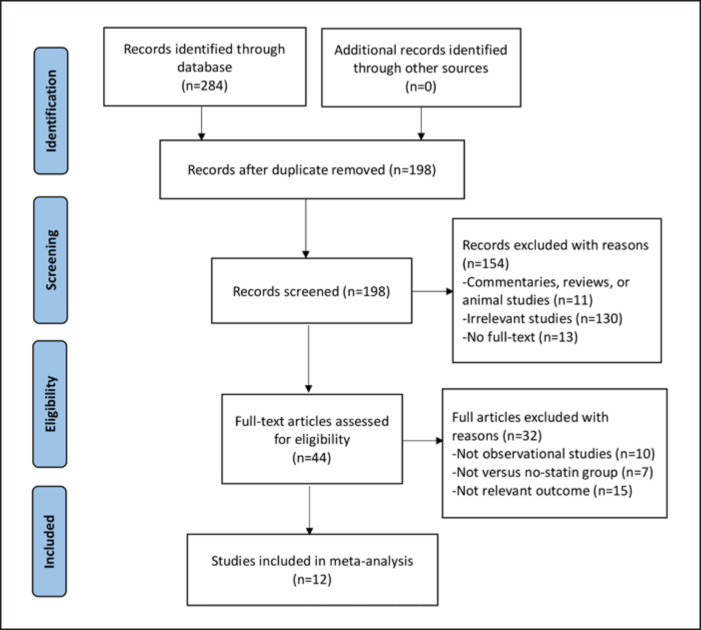
For the RCTs, a total of 275 references were identified by an initial electronic database search and there were no additional records from other sources. We screened 210 articles by titles and abstracts after duplicates removed, and 67 articles with full texts were assessed for eligibility. We included 11 RCTs for the final analysis [5, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18] (shown in Fig. 1). For the observational cohort studies, the initial systematic search identified 284 references. We selected 12 articles [19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30] for the final analysis (shown in Fig. 2) based on the inclusion and exclusion criteria.
Fig. 1.
PRISMA flowchart. Study selection for RCTs.
Fig. 2.
PRISMA flowchart. Study selection for observational studies.
Table 1 summarizes the overall characteristics of the included 11 RCTs. Four trials compared the lipid-lowering drug with the nonstatin drug, and the remaining seven trials used a placebo as a control group. The follow-up time varied from 5 days to 5 years within the 11 studies. The 11 RCTs were from the UK (n = 1), Japan (n = 3), USA and Canada (n = 2), Russia (n = 1), Korea (n = 1), Spain (n = 2), and one trial was a worldwide study involving different countries.
Table 1.
Characteristics of 11 included randomized controlled studies
| Authors (year) | Intervention | Control | Region | Length of follow-up | First stroke | Second stroke | Age (mean, years) | Sex (males, %) |
|---|---|---|---|---|---|---|---|---|
| Plehn et al. [12] (1999) | Pravastatin 40 mg | Placebo | USA and Canada | 5 years | IS | Any stroke | 59 | 86 |
| Collins et al. [17] (2004) | Simvastatin 40 mg | Placebo | UK | 5 years | IS | IS and HS | 65 | 75 |
| Amarenco et al. [5] (2006) | Atorvastatin 80 mg | Placebo | International | 4.9 years | IS | Any stroke | 63 | 60 |
| Kennedy et al. [16] (2007) | Simvastatin 40 mg | Placebo | USA and Canada | 90 days | IS | HS | 68 | 48 |
| Montaner et al. [18] (2008) | Simvastatin 40 mg | Placebo | Spain | 90 days | IS | HS | 73 | 52 |
| Yakusevich et al. [13] (2012) | Simvastatin 40 mg | No statin | Russia | 1 year | AIS | IS | 66 | 44 |
| Hosomi et al. [15] (2015) | Pravastatin 10 mg | No statin | Japan | 5 years | IS | IS and HS | 66 | 69 |
| Ueno et al. [14] (2015) | Rosuvastatin 5 mg | No statin | Japan | 6 months | AIS | IS | 71 | 96 |
| Heo et al. [9] (2016) | Rosuvastatin 20 mg | Placebo | Korea | 5 or 14 days | AIS | IS and HS | 65 | 60 |
| Montaner et al. [15] (2016) | Simvastatin 40 mg | Placebo | Spain | 90 days | AIS | HS | 74 | 54 |
| Kitagawa et al. [11] (2019) | Pravastatin 10 mg | No statin | Japan | 2 or 5 years | IS | IS and HS | 66.2 | 68 |
*AIS, acute ischemic stroke; IS, ischemic stroke; HS, hemorrhagic stroke.
Table 2 summarizes the overall characteristics of the included cohort studies. Eleven papers evaluated the efficacy and safety of statin treatment, whereas one paper assessed statin adherence with stroke recurrence. Follow-up time varied from 3 months to 10 years. The 12 studies were from Sweden (n = 1), Japan (n = 1), the USA (n = 2), Denmark (n = 1), Brazil (n = 3), Italy (n = 1), China (n = 2), and the remaining one was a worldwide study involving different countries.
Table 2.
Characteristics of 12 included observational studies
| Authors (year) | Region | Types of statins | Length of follow-up | First stroke | Second stroke | Age (mean, years) | Sex (males, %) |
|---|---|---|---|---|---|---|---|
| Meier et al. [19] (2009) | Sweden | Atorvastatin 23 mg; pravastatin 27 mg; simvastatin 30 mg; fluvastatin 80 mg | 3 months | AIS | HS | 63 | 57 |
| Milionis et al. [23] (2009) | USA | Fluvastatin 40–80 mg; pravastatin 20–40 mg; simvastatin 10–40 mg; atorvastatin 10–40 mg | 10 years | AIS | Any stroke | 67 | 68 |
| Cappellari et al. [20] (2011) | Italy | Atorvastatin 80 mg; rosuvastatin 10 mg; atorvastatin 40 mg; pravastatin 20 mg | 3 months | AIS | HS | 65 | 52 |
| Makihara et al. [30] (2013) | Japan | Not given | 2 years | IS | Any stroke | 70 | 58 |
| Phipps et al. [22] (2013) | USA | Atorvastatin; simvastatin; rosuvastatin; pravastatin; fluvastatin | 3 months | AIS | HS | 85 | 36 |
| Scheitz et al. [26] (2016) | International | Simvastatin; atorvastatin; pravastatin | 3 months | IS | HS | 70 | 54 |
| Lee et al. [21] (2017) | Taiwan | Not given | 1 year | IS | IS and HS | 65 | 57 |
| Lin et al. [28] (2019) | Taiwan | Not given | 1 year | IS | Any stroke | 66 | 54 |
| Vitturi and Gagliardi [24] (2019) | Brazil | Simvastatin 20 mg; simvastatin 40 mg; atorvastatin 40 mg; rosuvastatin 10 mg | 2 years | IS | Any stroke | 61 | 55 |
| Vitturi and Gagliardi [24] (2019) | Brazil | Simvastatin 20 mg; simvastatin 40 mg | 4 years | IS | IS and HS | 56 | 53 |
| Ribe et al. [25] (2020) | Denmark | Not given | 10 years | IS | HS | 50 | 54 |
| Vitturi and Gagliardi [27] (2020) | Brazil | Not given | 4 years | IS | Any stroke | 58.3 | 55 |
* AIS, acute ischemic stroke; IS, ischemic stroke; HS, hemorrhagic stroke.
Assessment of Primary Outcome
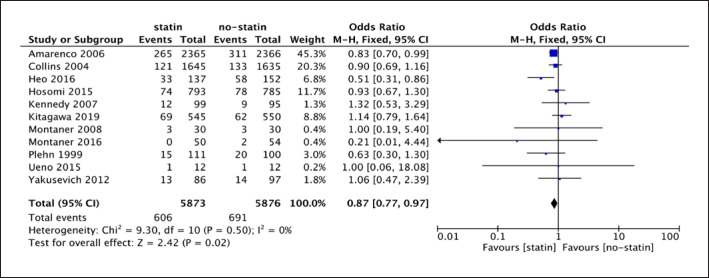
The forest plot of 11 RCTs (shown in Fig. 3) illustrated that I2 was 0% (p = 0.50 > 0.1), and therefore, the fixed-effect model was appropriate. Any type of recurrent stroke was the primary outcome of this meta-analysis. The analysis of 11 RCTs showed a difference between statin drug and placebo or no-statin treatment for any type of recurrent stroke (11 studies; 11,749 participants; OR = 0.87, 95% CI [0.77, 0.97]; p = 0.02). According to the result (shown in Fig. 3), statin therapy reduced the risk of recurrent stroke with any type by 13% on average for IS patients.
Fig. 3.
Forest plot of 11 RCTs for any type of recurrent stroke.
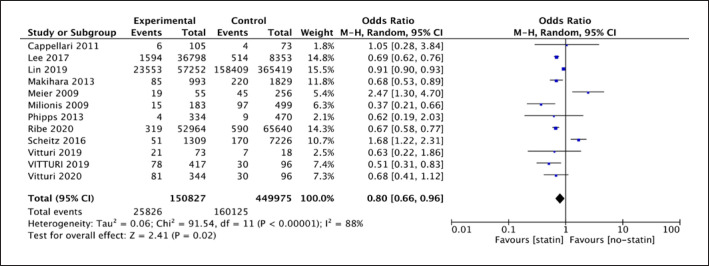
The random-effect model was used due to the high heterogeneity (I2 = 88%, p < 0.001) in the analysis of 12 observational cohort studies. Results showed a difference between statin therapy and no-statin therapy for any type of recurrent stroke (12 studies; 600,802 participants; OR = 0.80; 95% CI [0.66, 0.96]; p = 0.02). The OR of 0.80 (shown in Fig. 4) demonstrated statin therapy could be a protective factor for reducing recurrent stroke, and it reduced the risk by 20% on average for IS patients.
Fig. 4.
Forest plot of 12 cohort studies for any type of recurrent stroke.
Assessment of Secondary Outcome
Recurrent IS as the Outcome (after Initial IS)
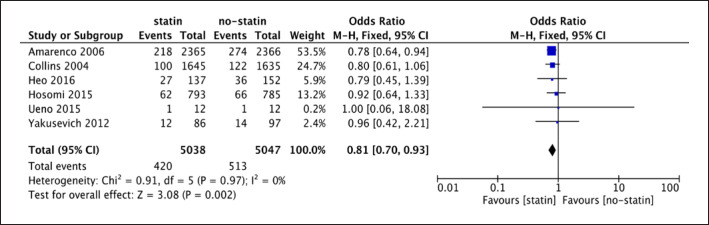
The forest plot of 6 RCTs (shown in Fig. 5) illustrated that I2 was 0% (p = 0.97 > 0.1), and therefore, the fixed-effect model was appropriate. The analysis of 6 RCTs indicated a difference between statin use and placebo or no-statin treatment for recurrent IS (6 studies; 10,085 participants; OR = 0.81; 95% CI [0.70, 0.93]; p = 0.002). The OR of 0.81 referred that statin therapy could be a protective treatment for recurrent IS in IS patients, and it reduced the risk by 19% on average (shown in Fig. 5).
Fig. 5.
Forest plot of 6 RCTs for recurrent IS.
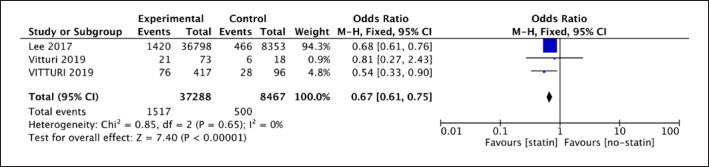
The analysis of 3 observational studies showed a difference between statin therapy and no-statin therapy for IS recurrence (3 studies; 45,744 participants; OR = 0.67; 95% CI [0.61, 0.75]; p < 0.001). The OR of 0.67 (shown in Fig. 6) represented that statin therapy played a protective role for IS recurrence, and it reduced the risk by 33% on average.
Fig. 6.
Forest plot of 3 cohort studies for recurrent IS.
Recurrent HS as the Outcome (after Initial IS)
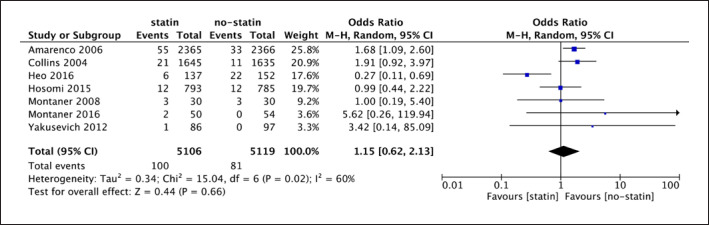
A random-effect model was used (shown in Fig. 7) due to the high heterogeneity among the seven trials (I2 = 60%, p = 0.02 < 0.05). The analysis of 7 RCTs showed that there was no evidence of a difference between statin and no-statin therapy for HS recurrence in IS patients (7 studies; 10,255 participants; OR = 1.15; 95% CI [0.62, 2.13]; p = 0.66).
Fig. 7.
Forest plot of 7 RCTs for recurrent hemorrhagic stroke.
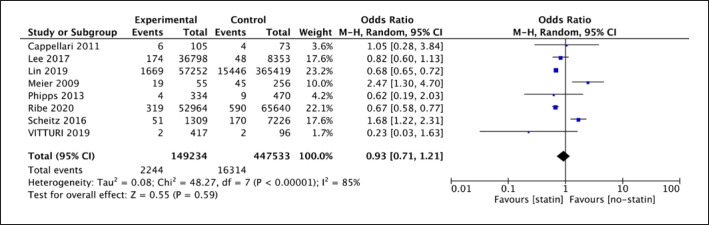
The forest plot of the analysis showed that high heterogeneity was obvious as I2 = 85% and p < 0.00001; therefore, a random-effect model was selected (shown in Fig. 8) for the analysis of 8 cohort studies. The results showed that there was no evidence of a difference between statin therapy and no-statin therapy in reducing risks of recurrent HS for IS patients (8 studies; 596,767 participants; OR 0.93; 95% CI [0.71, 1.21], p = 0.59).
Fig. 8.
Forest plot of 8 cohort studies for recurrent hemorrhagic stroke.
Risk of Bias of Included Studies
Two trials were graded as high risk of bias, and three studies were graded as moderate risk and six trials as low risk (shown in Fig. 9 in the online Appendix S2). The details of each domain for risk of bias have been graphically depicted and summarized (shown in Fig. 9, 10 in the online Appendix S2). CASP cohort study checklists [31] were used for quality assessment of cohort studies. As was shown in the checklist (shown in Table 3 in the online Appendix S3), most of the studies failed to identify all the confounding factors and six studies reported missing data during the follow-up duration. However, it was still obvious that nearly all studies showed a high quality of validity.
Assessment of Publication Bias
The details of the assessment have been displayed graphically (shown in Fig. 11, 12 in the online Appendix S2). It could be seen (shown in Fig. 11 in the online Appendix S2) that this graph was nearly symmetrical; thus, there was no publication bias or other bias. However, the funnel plot of 12 observational studies (shown in Fig. 13 in the online Appendix S2) showed that the graph was asymmetrical, and some dots (6 studies) lied out of the dashed lines triangular region, indicating that there may exist publication bias among the 12 observational studies.
Discussion
This systematic review and meta-analysis analyzed 11 RCTs (11,749 participants) and 12 cohort studies (600, 802 patients) independently. Both studies demonstrated that statin use was associated with statistically significant reduced risk for stroke recurrence of any stroke subtype. Also, the results from both RCTs and cohort studies illustrated that statin therapy could prevent recurrent IS in IS patients. However, both studies showed that statin therapy was not associated with apparent risk reduction for recurrent HS in IS patients.
According to the guideline released by the AHA and the ASA [3], statin therapy is useful for stroke secondary prevention, especially for stroke survivors with high LDL cholesterol levels; that statin therapy could reduce their risk by at least 50%. However, evidence is still insufficient for clinical practice in that the guideline is initially based on secondary data analysis from the SPARCL trial [5]. In this meta-analysis of 11 RCTs, only two studies showed that statin therapy increased the risk of recurrent stroke compared to the placebo. The remaining seven studies showed statin therapy reduced the risk of recurrent stroke as compared to the placebo or no-statin group. The meta-analysis comprised 12 cohort studies illustrated that statin treatment positively reduced the risk for any recurrent stroke in the IS survivors. The overall effect showed that statin therapy reduced the risk of any recurrent stroke by 20% on average (p = 0.02). A 2019 meta-analysis of IS confirmed that the positive impact of statin treatment for stroke recurrence seemed evident for patients with atherosclerotic IS [32]. Hence, the analysis results of 12 cohort studies were consistent with 11 RCTs and previous papers that reported a similar effect.
Subgroup analysis of 6 RCTs showed that in the IS patients, statin treatment was related to significant risk reduction in recurrent IS (average reduction of the risk by 19%). A subgroup of stroke patients in the HPS trial illustrated that statin therapy exerted a protective influence on recurrent IS to IS patients [17]. Additionally, results from three cohort studies showed similar findings with RCTs. The overall effect, which included 45,744 participants, showed that statin therapy reduced the risk of IS recurrence by 33% in IS participants (p < 0.00001).
ICH is a crucial issue related to statin therapy, and many studies found that statin therapy increases the risk of this subtype of HS. Our analysis of 7 RCTs containing 10,255 participants showed no difference between statin use and placebo or no-statin treatment for preventing recurrent HS. Similarly, analysis from 8 cohort studies that included 596,767 participants showed that statin use was not related to recurrence of HS in previous IS patients [33]. Nevertheless, a recent meta-analysis which included 15 RCTs and targeted at ICH after IS found that statin therapy increased the occurrence of ICH for patients with IS or HS (RR 1.42; 95% CI [1.07–1.87]) [33]. Our finding that there was no increase in the risk of HS may be controversial. However, if there is any risk of HS from statins, it would be far outweighed by reduction of stroke and myocardial infarction [34]. Additionally, based on the SPARCL trial, an article showed that many patients taking atorvastatin stopped treatment due to adverse events, and approximately 25% of them in the placebo group crossed over to statin. Therefore, intention-to-treat analysis may not be reliable, and it underestimated the advantages of atorvastatin and also incorrectly reported an increased risk of ICH [33, 35, 36, 37, 38, 39]. Thus, the association between statin treatment and HS recurrence remains unclear in IS participants.
Implications for Practice and Research
According to the AHA/ASA Guidelines [3], statin therapy with extensive lipid-lowering effects is suggested for primary prevention of cardiovascular accidents and secondary prevention of stroke in IS or TIA patients. The results of this study were consistent with the recommendation of guidelines; however, there still existed some limitations to the AHA/ASA guidelines. The guidelines only demonstrate that statin is beneficial for preventing recurrent stroke but does not clarify specific subtypes of recurrent stroke. This study found that statin therapy has a beneficial effect not only on recurrent stroke of any type but also on recurrent IS in IS survivors (reducing the risk by 19% and 33% from analysis of RCTs and cohort studies, respectively). Thus, it is necessary to state clearly in the guidelines that statin therapy is recommended for the secondary prevention of recurrent IS in IS patients. Regarding HS, no substantial evidence showed a relationship between statin therapy and recurrent HS in IS patients. Thus, it could be clarified clearly in the guidelines there is not sufficient evidence to prove statin treatment exerts a positive impact on preventing recurrent HS.
Second, although statin use has been established for preventing recurrent stroke, whether patients have good adherence to statin therapy is still unclear. Study estimated that statin adherence might be low, with a proportion of 54% currently [37], and most patients who showed low adherence to statins were linked with worse outcomes than those with good adherence. Considering the current low rate of statin adherence and poor statin adherence risk, it is necessary to promote statin therapy in the clinical practice and improve patients' statin adherence. Also, the side effects of statins are undeniable and reaching a favorable outcome of statin therapy the side effect is a crucial issue that remains to be investigated and solved.
Third, there still exists some limitation for this study. Ethnicity is a significant factor to consider in that stroke epidemiology is different due to various ethnic populations. As cited in a review [40], east Asians showed a higher incidence of stroke. Therefore, it is necessary to consider a further analysis to compare the east and west population. Additionally, this study only included patients who are 50 years old and above; however, it could not be denied that there indeed exist HS patients who show younger onset less than 50 years; hence, investigation targeted at those populations is necessary as well.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded by the NIHR (Programme Grants for Applied Research [NIHR202339]) and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author Contributions
Conceptualization was conceived by Yue Yin and Yanzhong Wang. Data collection was conducted by Yue Yin and Li Zhang. Data synthesis was conducted by Yue Yin. Supervision was performed by Yanzhong Wang. Writing − original draft − was performed by Yue Yin. Writing − review and editing − was performed by Li Zhang, Iain Marshall, Charles Wolfe, and Yanzhong Wang.
Data Availability Statement
All data generated or analyzed during this study are included in this article or its supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
The authors acknowledge support from the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) South London at King's College Hospital (KCH) NHS Foundation Trust, and the NIHR Biomedical Research Centre (BRC), Guy's and St Thomas' NHS Foundation Trust and King's College London, UK.
Funding Statement
This study was funded by the NIHR (Programme Grants for Applied Research [NIHR202339]) and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
References
- 1.World Health Organisation The top 10 causes of death [Internet] 2021. [cited 25 October 2021]. Available from https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JB, Culebras A, et al. An updated definition of stroke for the 21st century. Stroke. 2013 Jul;44((7)):2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palacio S, Hart RG. Regarding article “guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart association/American stroke association”. Stroke. 2011 Dec;42((2)):e23. doi: 10.1161/STROKEAHA.110.602474. [DOI] [PubMed] [Google Scholar]
- 4.Tramacere I, Boncoraglio GB, Banzi R, Del Giovane C, Kwag KH, Squizzato A, et al. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta-analysis. BMC Med. 2019 Mar;17((1)):67. doi: 10.1186/s12916-019-1298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amarenco P, Bogousslavsky J, Callahan A. High-dose atorvastatin after stroke or transient ischemic attack. J Vasc Surg. 2006 Aug;44((6)):1374. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Jul;6((7)):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Oxford: Wiley-Blackwell; 2008. [Google Scholar]
- 8.Long HA., French DP, Brooks JM. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res Methods Med Health Sci. 2020 Feb;1((1)):31–42. [Google Scholar]
- 9.Heo JH, Song D, Nam HS, Kim EY, Kim YD, Lee KY, et al. Effect and safety of rosuvastatin in acute ischemic stroke. J Stroke. 2016 Jan;18((1)):87–95. doi: 10.5853/jos.2015.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, et al. The Japan statin treatment against recurrent stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. 2015 Aug;2((9)):1071–8. doi: 10.1016/j.ebiom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Maruyama H, et al. Cumulative effects of LDL cholesterol and CRP Levels on recurrent stroke and TIA. J Atheroscler Thromb. 2019 May;26((5)):432–41. doi: 10.5551/jat.45989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plehn JF, Davis BR, Sacks FM, Rouleau JL, Pfeffer MA, Bernstein V, et al. Reduction of stroke incidence after myocardial infarction with pravastatin. Circulation. 1999 Jan;99((2)):216–23. doi: 10.1161/01.cir.99.2.216. [DOI] [PubMed] [Google Scholar]
- 13.Yakusevich VV, Malygin AY, Lychenko SV, Petrochenko AS, Kabanov AV. The efficacy of high dose simvastatin in acute period of ischemic stroke. Racional'naa farmakoterapia v kardiologii. 2012 Jan;8((1)):4–16. [Google Scholar]
- 14.Ueno Y, Yamashiro K, Tanaka Y, Watanabe M, Miyamoto N, Shimada Y, et al. Rosuvastatin may stabilize atherosclerotic aortic plaque: transesophageal echocardiographic study in the EPISTEME trial. Atherosclerosis. 2015 Apr;239((2)):476–82. doi: 10.1016/j.atherosclerosis.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Montaner J, Bustamante A, García-Matas S, Martínez-Zabaleta M, Jiménez C, de la Torre J, et al. Combination of thrombolysis and statins in acute stroke is safe. Stroke. 2016 Nov;47((11)):2870–3. doi: 10.1161/STROKEAHA.116.014600. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007 Nov;6((11)):961–9. doi: 10.1016/S1474-4422(07)70250-8. [DOI] [PubMed] [Google Scholar]
- 17.Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20 536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004 Mar;363((9411)):757–67. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 18.Montaner J, Chacón P, Krupinski J, Rubio F, Millán M, Molina CA, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J Neurol. 2007 Dec;15((1)):82–90. doi: 10.1111/j.1468-1331.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 19.Meier N, Nedeltchev K, Brekenfeld C, Galimanis A, Fischer U, Findling O, et al. Prior statin use, intracranial hemorrhage, and outcome after intra-arterial thrombolysis for acute ischemic stroke. Stroke. 2009 Mar;40((5)):1729–37. doi: 10.1161/STROKEAHA.108.532473. [DOI] [PubMed] [Google Scholar]
- 20.Cappellari M, Deluca C, Tinazzi M, Tomelleri G, Carletti M, Fiaschi A, et al. Does statin in the acute phase of ischemic stroke improve outcome after intravenous thrombolysis? A retrospective study. J Neurol Sci. 2011 Sep;308((1-2)):128–34. doi: 10.1016/j.jns.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Saver JL, Wu YL, Tang SC, Lee JD, Rao NM, et al. Utilization of statins beyond the initial period after stroke and 1-year risk of recurrent stroke. J Am Heart Assoc. 2017 Aug;6((8)):e005658. doi: 10.1161/JAHA.117.005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phipps MS, Zeevi N, Staff I, Fortunato G, Kuchel GA, McCullough LD, et al. Stroke severity and outcomes for octogenarians receiving statins. Arch Gerontol Geriatr. 2013 Nov-Dec;57((3)):377–82. doi: 10.1016/j.archger.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Milionis HJ, Giannopoulos S, Kosmidou M, Panoulas V, Manios E, Kyritsis AP, et al. Statin therapy after first stroke reduces 10-year stroke recurrence and improves survival. Neurology. 2009 May;72((21)):1816–22. doi: 10.1212/WNL.0b013e3181a711cb. [DOI] [PubMed] [Google Scholar]
- 24.Vitturi BK, Gagliardi RJ. Effects of statin therapy on outcomes of ischemic stroke: a real-world experience in Brazil. Arq Neuropsiquiatr. 2020 Jun;78((8)):461–7. doi: 10.1590/0004-282X20200027. [DOI] [PubMed] [Google Scholar]
- 25.Ribe AR, Vestergaard CH, Vestergaard M, Pedersen HS, Prior A, Lietzen LW, et al. Statins and risk of intracerebral hemorrhage in individuals with a history of stroke. Stroke. 2020 Mar;51((4)):1111–9. doi: 10.1161/STROKEAHA.119.027301. [DOI] [PubMed] [Google Scholar]
- 26.Scheitz JF, MacIsaac RL, Abdul-Rahim AH, Siegerink B, Bath PM, Endres M, et al. Statins and risk of poststroke hemorrhagic complications. Neurology. 2016 Apr;86((17)):1590–6. doi: 10.1212/WNL.0000000000002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitturi BK, Gagliardi RJ. The influence of statin withdrawal and adherence on stroke outcomes. Neurol Sci. 2020 Jun;42((6)):2317–23. doi: 10.1007/s10072-020-04790-y. [DOI] [PubMed] [Google Scholar]
- 28.Lin HC, Lin JR, Tsai WC, Lu CH, Chang WN, Huang CC, et al. The outcomes of statin therapy in patients with acute ischemic stroke in Taiwan: a nationwide epidemiologic study. QJM. 2019 Dec;112((12)):891–9. doi: 10.1093/qjmed/hcz189. [DOI] [PubMed] [Google Scholar]
- 29.Kusznir Vitturi B, José Gagliardi R. The role of statins in cardioembolic stroke. J Clin Neurosci. 2020 Jan;72:174–9. doi: 10.1016/j.jocn.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Makihara N, Kamouchi M, Hata J, Matsuo R, Ago T, Kuroda J, et al. Statins and the risks of stroke recurrence and death after ischemic stroke: the Fukuoka Stroke Registry. Atherosclerosis. 2013 Dec;231((2)):211–5. doi: 10.1016/j.atherosclerosis.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 31.CASP: Critical Appraisal Skills Programme Brice R. CASP checklists: CASP: critical appraisal skills programme [Internet] 2021. [cited 25 October 2021]. Available from https://casp-uk.net/casp-tools-checklists/
- 32.Christophe B, Karatela M, Sanchez J, Pucci J, Connolly ES. Statin therapy in ischemic stroke models: a meta-Analysis. Transl Stroke Res. 2020 Aug;11((4)):590–600. doi: 10.1007/s12975-019-00750-7. [DOI] [PubMed] [Google Scholar]
- 33.Teoh RJJ, Huang CJ, Chan CP, Chien LY, Chung CP, Sung SH, et al. Does statin increase the risk of intracerebral hemorrhage in stroke survivors? A meta-analysis and trial sequential analysis. Ther Adv Neurol Disord. 2019 Jul;12:175628641986483. doi: 10.1177/1756286419864830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Cholesterol Treatment Trialists' CTT Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170, 000 participants in 26 randomised trials. Lancet. 2010;376((9753)):1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CH, Lin CH, Caffrey JL, Lee YC, Liu YC, Lin JW, et al. Risk of intracranial hemorrhage from statin use in asians. Circulation. 2015 Jun;131((23)):2070–8. doi: 10.1161/CIRCULATIONAHA.114.013046. [DOI] [PubMed] [Google Scholar]
- 36.Birtcher K. When compliance is an issue: how to enhance statin adherence and address adverse effects. Curr Atheroscler Rep. 2014 Dec;17((1)):471. doi: 10.1007/s11883-014-0471-8. [DOI] [PubMed] [Google Scholar]
- 37.Hernan MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med. 2017 Oct 5;377((14)):1391–8. doi: 10.1056/NEJMsm1605385. [DOI] [PubMed] [Google Scholar]
- 38.Sheiner LB, Rubin DB. Intention-to-treat analysis and the goals of clinical trials. Clin Pharmacol Ther. 1995 Jan;57((1)):6–15. doi: 10.1016/0009-9236(95)90260-0. [DOI] [PubMed] [Google Scholar]
- 39.Spence JD. The need for clinical judgement in the application of evidence-based medicine. BMJ Evid Based Med. 2020 Oct;25((5)):172–7. doi: 10.1136/bmjebm-2019-111300. [DOI] [PubMed] [Google Scholar]
- 40.Burke TA, Venketasubramanian RN. The epidemiology of stroke in the east asian region: a literature-based review. Int J Stroke. 2006 Nov;1((4)):208–15. doi: 10.1111/j.1747-4949.2006.00060.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article or its supplementary material files. Further inquiries can be directed to the corresponding author.