Abstract
Background
Bud dormancy is a phenological adaptation of temperate perennials that ensures survival under winter temperature conditions by ceasing growth and increasing cold hardiness. SHORT VEGETATIVE PHASE (SVP)-like factors, and particularly a subset of them named DORMANCY-ASSOCIATED MADS-BOX (DAM), are master regulators of bud dormancy in perennials, prominently Rosaceae crops widely adapted to varying environmental conditions.
Results
SVP-like proteins from recently sequenced Rosaceae genomes were identified and characterized using sequence, phylogenetic and synteny analysis tools. SVP-like proteins clustered in three clades (SVP1–3), with known DAM proteins located within SVP2 clade, which also included Arabidopsis AGAMOUS-LIKE 24 (AthAGL24). A more detailed study on these protein sequences led to the identification of a 15-amino acid long motif specific to DAM proteins, which affected protein heteromerization properties by yeast two-hybrid system in peach PpeDAM6, and the unexpected finding of predicted DAM-like genes in loquat, an evergreen species lacking winter dormancy. DAM gene expression in loquat trees was studied by quantitative PCR, associating with inflorescence development and growth in varieties with contrasting flowering behaviour.
Conclusions
Phylogenetic, synteny analyses and heterologous overexpression in the model plant Arabidopsis thaliana supported three major conclusions: 1) DAM proteins might have emerged from the SVP2 clade in the Amygdaloideae subfamily of Rosaceae; 2) a short DAM-specific motif affects protein heteromerization, with a likely effect on DAM transcriptional targets and other functional features, providing a sequence signature for the DAM group of dormancy factors; 3) in agreement with other recent studies, DAM associates with inflorescence development and growth, independently of the dormancy habit.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03856-7.
Keywords: SVP (gene family) evolution, Plant phenology, Bud development, Winter dormancy, Loquat
Background
The adaptation of perennial plants to seasonal variations in temperate climates relies on a tight adjustment of plant phenology to predictable environmental changes, with a drastic impact on plant fitness and survival. In fact, northern and southern boundaries of the geographical distribution of plant species appear to depend on fruit maturation date and chilling required for bud-break, respectively, two important milestones of plant phenology [1]. Environmental conditions, mostly temperature and light, play a key role in such phenological adjustment by entraining a barely known molecular calendar, which shares common genetic and regulatory features in plants and animals [2]. In temperate perennial plants, bud formation and winter dormancy ensure meristematic tissues (vegetative and reproductive) remain in a non-growing safer state, regardless of short temporary warmer periods, until a quantitative perception of environmental chilling fulfils the genetically-encoded chilling requirements of a given cultivar or variety [3]. This true dormancy (or endodormancy) term opposes to paradormancy and ecodormancy, which respectively refer to the quiescent state of buds repressed by correlative inhibition and dormancy-released buds requiring a period of warm temperatures prior to growth resumption and bud-break [4]. Since global warming is expected to reduce available winter chilling for satisfying winter dormancy release requirements, this climatic threat is potentially capable of impairing adaptability and yield in the case of crops [5], and even favouring the summer dormancy trait that enhances plant survival under extreme temperatures and summer droughts [6].
A set of MADS-box domain transcription factors related to SHORT VEGETATIVE PHASE (SVP) and AGAMOUS-LIKE 24 (AGL24), involved in flowering time control in Arabidopsis [7, 8], have been reported to regulate bud formation and dormancy induction in evolutionarily distant perennials [9–12]. A particular group of these SVP-like genes named DORMANCY-ASSOCIATED MADS-BOX (DAM) has been found deleted in the evergrowing (evg) mutant of peach (Prunus persica) showing no growth cessation during winter [13]. Since then, numerous molecular and functional approaches have provided plenty of evidences about the implication of DAM genes in regulating the dormancy cycle in Rosaceae species [14, 15], namely in pear [16–19], apple [20–22], Japanese apricot [23–25], sweet cherry [26, 27] and peach [28, 29]. Also, DAM genes have been found transcriptionally associated with bud dormancy transitions in apricot [30], European plum [31], Chinese plum [32] and almond [33]. These studies depict a master role of DAM genes in promoting winter dormancy, modulated at the transcriptional level by seasonal cues, epigenetic modifications and plant hormones [14, 34].
Previous phylogenetic studies have identified three main clades within SVP-like group, most likely originated in an ancient whole-genome triplication [35]. In Rosaceae, two of these clusters have undergone independent lineage-specific gene expansion events due to whole-genome duplications [36] and tandem gene duplications in the Amygdaloideae subfamily (pears, apple, peach, plum and related crops), with potential effects on SVP-like and DAM-like functional diversification in bud dormancy and other processes [37]. In particular, DAM gene duplication and subsequent subfunctionalization events, leading to different seasonal expression patterns [38] and variable protein-interaction specificity resulting in alternative DNA-binding preferences, have been proposed to support the increased flexibility and complexity required for dormancy regulation in peach and apple [21, 39]. Here, we have scanned available genomes of Rosaceae species, most of them corresponding to crops with high agricultural and economical interest, to search for SVP-like and DAM-like genes and compared their encoded protein sequences in order to identify putative DAM-specific signatures as well as to explore their phylogenetic relationships. In addition, over these evolutionary approaches, we have reported the unexpected presence and expression of DAM-like genes in loquat (Eriobotrya japonica), an evergreen species lacking winter dormancy. Our results support the involvement of DAM-like genes in overall growth responses rather than a more restricted role in dormancy regulation, and provide a useful research model for the study of DAM functions deprived of winter dormancy determinants.
Results
Phylogenetic and synteny analysis supports the evolutionary origin and expansion of Amygdaloideae DAM genes within the AthAGL24 containing SVP2 clade
We built a comprehensive and well curated dataset of 87 SVP and SVP-like genes by scanning the genomes of ten Rosaceae perennial species with phenological and farming interest, including seven Amygdaloideae species, namely wild apple (Malus baccata), apple (Malus x domestica), Chinese pear (Pyrus x bretschneideri) and loquat (Eriobotrya japonica) from the Maleae tribe, and Japanese apricot (Prunus mume), peach (Prunus persica) and European plum (Prunus domestica) from the Amygdaleae tribe, as well as woodland strawberry (Fragaria vesca), black raspberry (Rubus occidentalis) and rose (Rosa chinensis) from the Rosoideae tribes Potentilleae, Roseae and Rubeae, respectively, plus five species from representative lineages of eudicots plants, namely jujube (Ziziphus jujuba), mulberry (Morus notabilis), Arabidopsis thaliana, kiwifruit (Actinidia chinensis), tomato (Solanum lycopersicum), plus the basal angiosperm Amborella trichopoda used as an outgroup (Table 1 and Supplementary Table S1). The list of the 87 detected SVP and SVP-like proteins with their corresponding genomic loci is shown in Supplementary Table S2.
Table 1.
Number of SVP-like genes in the species under study
| Family | Subfamily | Tribe | Species | SVP1 | SVP2 | SVP3 | Total |
|---|---|---|---|---|---|---|---|
| Rosaceae | Amygdaloideae | Maleae | Malus baccata | 2 | 5 | 0 | 7 |
| Malus x domestica | 2 | 5 | 0 | 7 | |||
| Pyrus x bretschneideri | 3 | 4 | 0 | 7 | |||
| Eriobotrya japonica | 2 | 6 | 0 | 8 | |||
| Amygdaleae | Prunus mume | 1 | 6 | 1 | 8 | ||
| Prunus persica | 1 | 6 | 1 | 8 | |||
| Prunus domestica | 1 | 6 | 1 | 8 | |||
| Rosoideae | Potentilleae | Fragaria vesca | 1 | 5 | 0 | 6 | |
| Roseae | Rosa chinensis | 1 | 4 | 0 | 5 | ||
| Rubeae | Rubus occidentalis | 1 | 2 | 1 | 4 | ||
| Rhamnaceae | Ziziphus jujuba | 1 | 1 | 1 | 3 | ||
| Moraceae | Morus notabilis | 1 | 1 | 2 | 4 | ||
| Brassicaceae | Arabidopsis thaliana | 1 | 1 | 0 | 2 | ||
| Actinidiaceae | Actinidia chinensis | 3 | 2 | 1 | 6 | ||
| Solanaceae | Solanum lycopersicum | 1 | 1 | 1 | 3 | ||
| Amborellaceae | Amborella trichopoda | – | – | – | 1 |
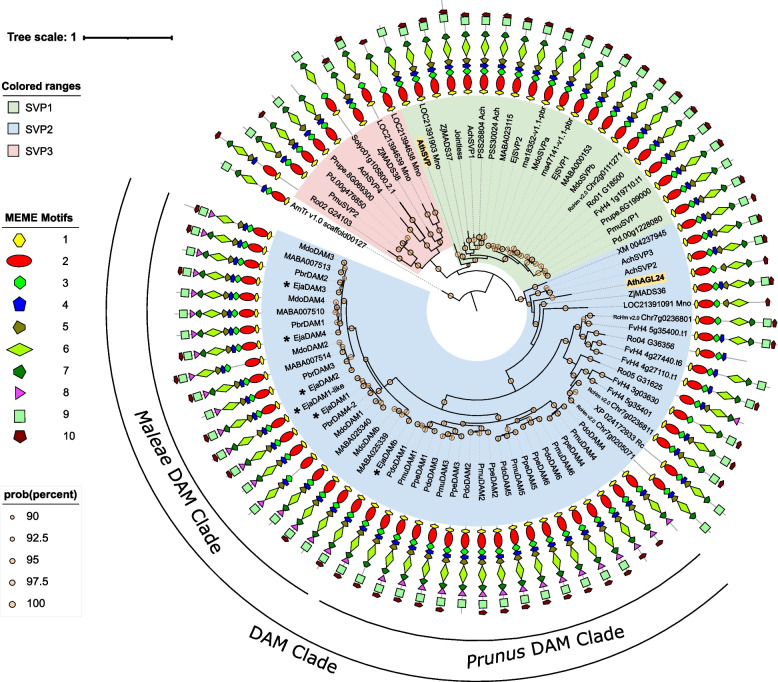
Next, we examined the evolutionary relationships among the compiled set of sequences by constructing a ML phylogenetic tree (Fig. 1). The resulting tree topology returned three major groups of SVP genes previously described [35], named SVP1, SVP2 and SVP3, as independent clades (Fig. 1). SVP1 and SVP2 clades contained the Arabidopsis flowering genes AthSVP and AthAGL24, respectively, whereas no Arabidopsis representatives were found in SVP3 (Table 1). In general, Rosaceae species displayed a higher number of genes in the SVP2 clade (Table 1), particularly in species belonging to the Amygdaloideae subfamily with four to up to six genes in Prunus species located in neighbouring positions of their genomes (Supplementary Table S2), two to five genes in species belonging to the Rosoideae subfamily, and one in non-Rosaceae species, except for the Actinidiaceae kiwifruit, which showed two genes. Expansion in the SVP1 clade was restricted to species from the Maleae tribe, with 2–3 genes for one in the rest of species (Table 1), again except for the Actinidiaceae kiwifruit, which showed three genes (Table 1). In contrast, SVP3 representatives in Rosaceae showed a more scattered distribution, with only one gene found in each of the three Prunus species belonging to the Amygdaleae tribe, and none among Maleae and Rosoideae species, except for the Rubea black raspberry, which showed one gene. All together, these results suggest independent gene duplication and loss events would have promoted lineage-specific expansion and contraction within Rosaceae SVP1 and SVP2 clades.
Fig. 1.
Maximum likelihood phylogenetic tree of SVP-like proteins from 10 Rosaceae species, five other eudicots and Amborella trichopoda. The tree was rooted using the single SVP-like sequence from the early diverging angiosperm A. trichopoda as an outgroup. The tree is drawn to scale, with branch lengths proportional to evolutionary distances between nodes. The scale bar indicates the estimated number of amino acid substitutions per site. Statistical support values on clades based on aLRT tests are represented as orange circles next to each node for those that resulted in posterior probabilities above 0.90, with diameters proportional to the actual value. The three main retrieved clades (SVP1, SVP2 and SVP3) are shown. Loquat SVP-like proteins are labelled with asterisks, and Arabidopsis AthSVP and AthAGL24 are highlighted. A schematic representation drawn to scale of conserved protein motifs detected using MEME is shown next to each sequence
The well-known bud dormancy regulatory DAM proteins from Amygdaloideae clustered within the SVP2 clade, as previously shown [35], leading to two robustly supported independent clades for species belonging to the Maleae and Amygdaleae tribes (Fig. 1). In addition to well-established DAM proteins from Prunus, Malus and Pyrus, six putative loquat proteins without prior molecular data clustered into the DAM clade, being thus plausible candidates to integrate the DAM family in this species.
Phylogenetic analysis suggested the evolutionary origin of DAM genes prior to divergence of two main Amygdaloideae tribes during SVP2 diversification. In order to seek additional evidence of the evolutionary relatedness between Amygdaloideae DAM genes and the Arabidopsis AthAGL24 containing SVP2 clade, we performed in-depth microsynteny analysis among the genomic regions involved. The genomic region clustering the six tandemly-arrayed DAM genes from peach showed a higher number of putatively homologous genes collinearly arranged when compared to the AthAGL24 genomic region than to the AthSVP one (Fig. 2a). Similarly, the two genomic regions, corresponding to linkage groups 3 and 5, which encompassed the six putative DAM genes from loquat identified in this study showed a strong signal of synteny when compared to Arabidopsis AthAGL24 genomic regions, in contrast to what can be observed when compared to AthSVP ones (Fig. 2b,c). In addition, these two genomic regions in loquat had their respective syntenic counterparts in linkage groups 15 and 8 of the apple genome (Fig. 2d,e). A multiplex comparison of peach and loquat DAM loci supports the idea of lineage-specific Maleae DAM clades originating through a whole-genome duplications (WGD) event (Fig. 2f). In summary, i) microsynteny analysis further support the evolutionary origin of Amygdaloideae DAM genes from AthAGL24 like genes in the SVP2 clade (Fig. 2), and ii) both WGD and tandem duplication events might have contributed to the expansion of the Amygdaloideae DAM gene family.
Fig. 2.

High resolution microsynteny analysis of genomic regions containing SVP and SVP-like genes from selected species. Arabidopsis and peach (a), Arabidopsis and loquat (b, c), apple and loquat (d, e), peach and loquat (f). In each pairwise comparison, putatively homologous sequences are connected by red edges. Arabidopsis AthSVP (orange box), Arabidopsis AthAGL24 (blue box), peach DAM locus (green box) and loquat DAM loci (magenta box) are labelled
PpeDAM6 overexpression in Arabidopsis induces an AGL24-like phenotype
In order to get deeper insight into the origin of DAM genes, whether they are closer to AthSVP (clade SVP1) or AthAGL24 (clade SVP2), we overexpressed a well characterized DAM gene in Arabidopsis and compared the phenotype of transformants with already published reports describing AthSVP and AthAGL24 overexpressing lines. For easy availability and in-depth knowledge reasons, we chose the PpeDAM6 gene from peach for that purpose.
The peach PpeDAM6 gene was previously described at regulatory and functional levels [29, 40], being one of the best-known DAM-like genes. PpeDAM6 was fused to c-myc epitope either in N-terminal or in C-terminal position and overexpressed under the control of the 35S promoter in Arabidopsis. At least 20 independent transgenic lines were obtained for each construction, showing qualitatively similar results. The transgenic lines displayed morphological abnormalities in floral structures at different degrees (Fig. 3), resembling floral defects of both the constitutive expression of Arabidopsis AthSVP [41] and AthAGL24 [42]. The presence of the transgene and PpeDAM6 protein production was assessed by PCR and western-blot analysis. Although all the kanamycin-selected plants contained the transgene, PpeDAM6 protein accumulation was variable, in concordance with the severity of the observed phenotypic features (Supplementary Table S3). Accordingly, PpeDAM6 protein was not detected in most of transgenic plants showing wild-type phenotype, whereas plants with moderate protein expression showed mild defects and developed abnormal flowers with vegetative traits (trichomes) leading to defective siliques with no or few viable seeds. Some of them showed leafy sepals and normal petals (e.g. 35S::PpeDAM6-c-myc #15), and other lines had both leafy sepals and petals (e.g. 35S::c-myc-PpeDAM6 #9) (Fig. 3b). On the other hand, plants expressing high levels of PpeDAM6 protein (e.g. 35S::PpeDAM6-c-myc #7) presented a more severe phenotype, with flowers replaced by inflorescences that often developed on the tip a new aberrant inflorescence without siliques (Fig. 3a,b). As noted above, most of these abnormal plants were sterile, with the exception of two lines showing few viable seeds.
Fig. 3.

PpeDAM6 overexpression affects flower development in Arabidopsis. Plant phenotype of wild-type Col (Columbia) and 35S::PpeDAM6 lines #7, #9 and #15 (a). Flower alterations of these lines (b). Scale bars represent 5 cm (a) or 1 mm (b). A white arrow marks the presence of trichomes
We measured flowering time in genotype 35S::PpeDAM6-c-myc #15, which similarly to 35S::AthAGL24 [8] and contrarily to 35S::AthSVP lines [7] showed early flowering phenotype (Table 2). This suggests that flowering time trait in Arabidopsis could be employed as a functional test for distinguish SVP1 and SVP2 clade proteins from even distant species, and that in close agreement with phylogenetic analyses PpeDAM6 resembles SVP2 clade AthAGL24 in functional overexpression studies.
Table 2.
Flowering time of Arabidopsis transgenic lines with seeds. Flowering time was recorded as the rosette leaf number when the primary inflorescence stem appeared. An asterisk indicates significant difference compared with the control (Col) at a confidence level of 95%
| Genotype | T1 Line | T2 Rossette leaf No. (n = 10) |
|---|---|---|
| Col | 8.1 ± 1.2 | |
| 35S::PpeDAM6-c-myc#15 | 1 | 6.9 ± 1.4* |
| 2 | 6.7 ± 1.0* | |
| 3 | 7.9 ± 0.7 | |
| 4 | 6.8 ± 1.0* | |
| 5 | 7.0 ± 1.3* | |
| 6 | 6.8 ± 1.1* | |
| 7 | 7.5 ± 1.5 | |
| 8 | 6.9 ± 1.1* | |
| 9 | 7.2 ± 1.1* |
A characteristic MEME stretch of DAM proteins modifies protein interaction
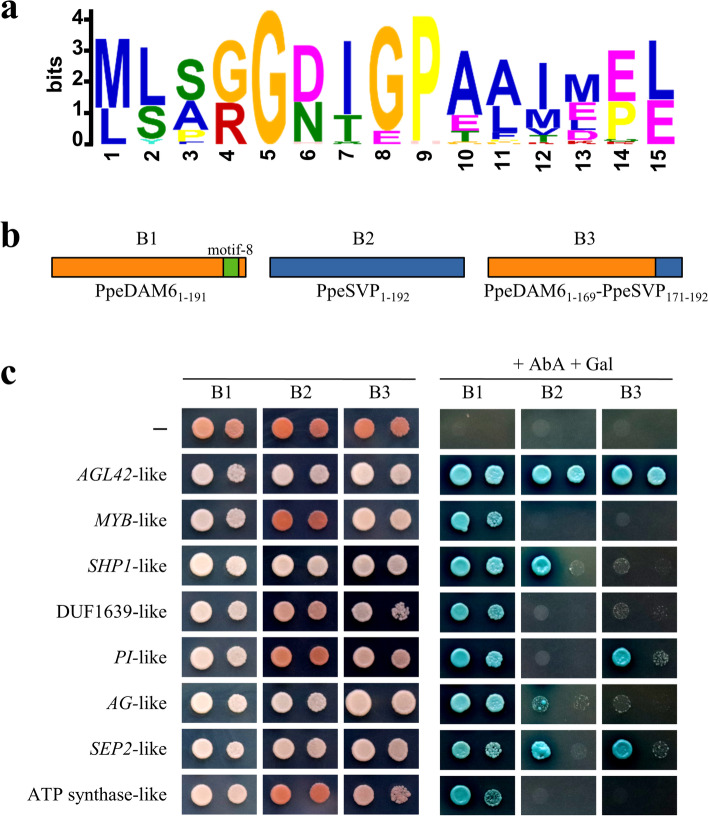
We used MEME for the identification of motifs conserved across our dataset of protein sequences (Supplementary Table S2). Setting the number of motifs to 10 allowed the identification of short sequences different from very well-known domains, such as MADS-box and K-box. A graphic display of MEME motifs distribution along proteins under study is shown in Fig. 1. A 15 amino-acid long motif (motif-8), located between the internal K-box domain and the C-terminal tail, was identified in 37 out of 38 sequences belonging to Amygdaloideae DAM clades (all but PmuDAM1) (Fig. 1), and in one additional SVP2-group protein from black raspberry that has not be described as a DAM member up to now (Ro05_G31625). A logo representation of this motif is shown in Fig. 4a. We next used this motif in FIMO searches against our set of protein sequences. According to FIMO, the whole group of 38 DAM proteins was positive for motif-8 occurrence with a q-value lower than 0.01, including PmuDAM1, and excluding Ro05_G31625 (Supplementary Table S4). Motif-8 could thus be considered as a DAM-specific motif in our set of genomes and be eventually used as a diagnostic motif in searches for DAM genes.
Fig. 4.
Replacement of a DAM-specific motif alters PpeDAM6 protein interactions. Logo representation of the 15 amino acid-long motif identified by MEME as conserved across DAM-like proteins (a). Bait constructs used in yeast two-hybrid (Y2H) experiments (b) including protein PpeDAM61–191 with its natural DAM-specific motif in green (bait B1), PpeSVP1–192 (bait B2) and a chimerical construct of PpeDAM6 with the corresponding C-terminal end from PpeSVP (bait B3). Y2H analysis of protein interactions (c) between combinations of bait vectors (B1, B2 and B3) and prey vectors containing AGL42-like, MYB-like, SHP1-like, DUF1639-like, PI-like, AG-like, SEP2-like and ATP synthase-like genes. Yeast strains were grown on a minimal medium without tryptophan, leucine, histidine and adenine (left) and a chromogenic medium containing Aureobasidin A and X-α-Gal (+AbA +Gal) (right)
Since particular protein-protein interactions have been described to strongly condition the regulatory function of MADS-box transcription factors [43], we wondered whether this DAM-specific motif might contribute to modify the affinity of SVP-like factors for different protein partners, with a potential effect on the evolutionary development of the increased phenological plasticity observed in Rosaceae species. With that aim in view, we chose once more peach and PpeDAM6 gene for a protein interaction assay with and without the DAM-specific motif. Then, we performed a yeast two-hybrid (Y2H) screening using peach PpeDAM6 as bait in a construct lacking the C-terminal tail, due to its transcriptional auto-activating properties, on a peach bud-specific library [44]. The Y2H library contained 1.5 × 106 independent clones, and 1.3 × 107 interactions were tested, leading to 90 positive colonies. After positive rechecking and sequence analysis we obtained the protein interactors listed in Supplementary Table S5. A truncated clone of PpeDAM6 encoding 191 amino acids of the protein, including the 15 residues-long DAM specific motif (bait B1, Fig. 4b), interacted with the MADS-box domain factors encoded by AGAMOUS-LIKE 42 (AGL42)-like, SHATTERPROOF 1 (SHP1)-like, PISTILLATA (PI)-like, AGAMOUS (AG)-like and SEPALLATA 2 (SEP2)-like genes (Supplementary Table S5). Indeed, MADS-box factors are commonly forming heterocomplexes with other related MADS-box proteins [43]. We also identified a MYB-like gene, a gene encoding a domain of unknown function DUF1639, and a putative vacuolar ATPase gene (Supplementary Table S5).
In order to test the ability of the DAM-specific motif to alter the Y2H binding potential of these partners, we assayed two additional bait constructs: Prupe.6G199000 gene encoding peach SVP ortholog PpeSVP (bait B2), and a chimeric fusion of PpeDAM6 (amino acids 1–169) having the motif-8 sequence replaced by the collinear PpeSVP peptide (bait B3, Fig. 4b). The Y2H assay of AGL42-like, PI-like and SEP2-like was not drastically disturbed in B3 (Fig. 4c). However, the interaction of PpeDAM6 with MYB-like, SHP1-like, DUF1639-like, AG-like and ATPsynthase-like was severely reduced after motif-8 replacement, resembling PpeSVP behaviour. These results point to a modification of PpDAM6 structure as a result of motif-8 replacement, with impact on protein-protein interactions and presumably also protein functionality.
DAM-like gene expression correlates with seasonal growth in loquat
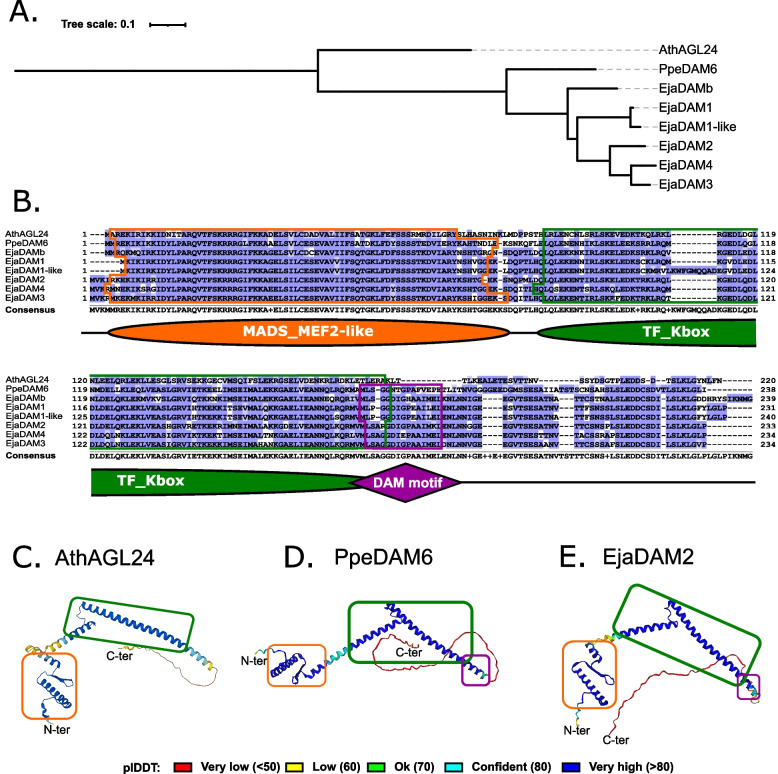
We identified six novel DAM-like genes in loquat, which are homologs of previously described apple DAMs, in agreement with phylogenetic and syntenic studies (Figs. 1 and 2). Accordingly, these genes were named EjaDAM1 (EVM0038001), EjaDAM1-like (EVM0001832), EjaDAM2 (EVM0040812), EjaDAM3 (EVM0017613), EjaDAM4 (EVM0016705) and EjaDAMb (EVM0004045). To further investigate them, the tree shown in Fig. 1 was pruned including AthAGL24, PpeDAM6, and loquat DAMs (Fig. 5a). Multiple sequence analysis in combination with protein domain annotation evidenced clear similarities between loquat DAM proteins, AthAGL24 and PpeDAM6. All proteins exhibited a high degree of similarity, with AthAGL24 being the more distinct. Moreover, all harboured the MADS_MEF2-like and TF_Kbox domains, key components of MADS-box transcription factors (Fig. 5b). This similarity was also showcased by the structural predictions of AthAGL24, PpeDAM6 and EjaDAM2 (Fig. 5c-e).
Fig. 5.
Protein sequence analysis of AthAGL24, PpeDAM6 and loquat DAMs. Pruned phylogenetic tree generated from the data presented in Fig. 1, only showing the proteins of interest (a). Multiple sequence alignment with residues coloured according to BLOSUM62 score. The consensus sequence and logo are shown below. InterPro predicted domains are noted in Orange and Green and the proposed DAM-specific motif is noted in purple (b). Protein structure prediction of AthAGL24 (c), PpeDAM6 (d) and EjaDAM2 (e) using ColabFold. Coloured boxes depict the domains and proteins are coloured according to plDDT that ranges from 0 to 100
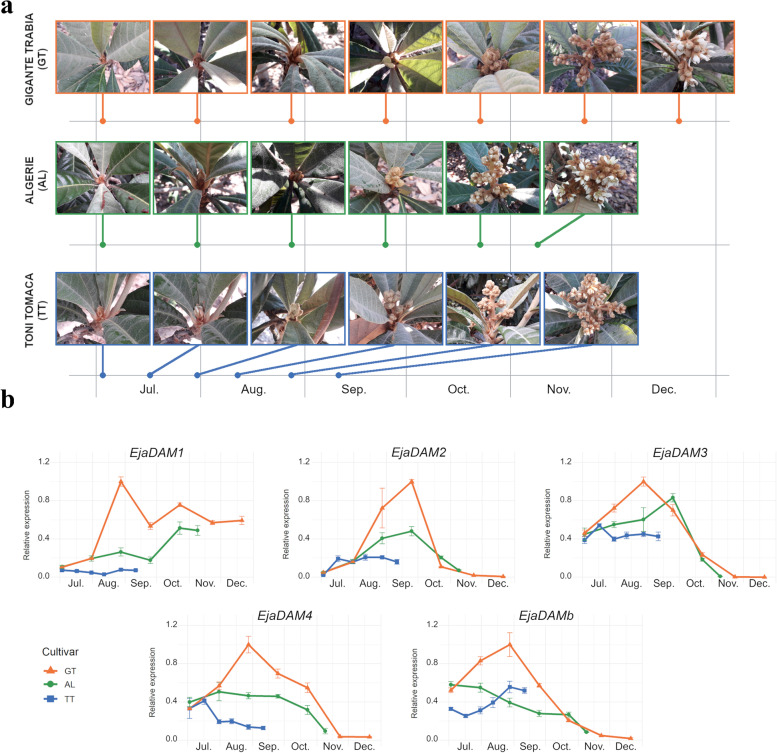
Interestingly, loquat is an evergreen tree crop that flowers from the end of summer until winter, with large genotype-dependent differences. Consequently, loquat plants don’t show winter dormancy, although some authors have used the term summer dormancy to describe a seasonal period of growth rest at high temperatures [45]. Since DAM genes have been widely related to winter dormancy processes in temperate species, and DAM gene expression sharply decreases prior to bud growth resumption [14], we wondered if loquat DAMs were also developmentally regulated during flowering. We selected the varieties Toni Tomaca (TT), Algerie (AL) and Gigante Trabia (GT) as representatives of loquat genotypes with early, medium and late flowering habits, respectively (Fig. 6a). We collected inflorescence samples of them from the beginning of July until full blooming and analysed DAM gene expression by quantitative real time PCR (qRT-PCR). Overall, the expression level of the five DAM genes correlated with flowering dates, with higher peaks in the late GT and lower signals in the early TT, with gene-dependent particularities (Fig. 6b). EjaDAM2, EjaDAM3, EjaDAM4 and EjaDAMb gene expression profile peaked in summer, prior to full inflorescence growth and development, and sharply decreased in the last two collected samples, with the exception of EjaDAMb, which specifically increased in full blooming samples of TT variety. On the other side, EjaDAM1 expression increased in summer inflorescences and stayed stable during the duration of the experiment (Fig. 6b). These results are compatible with a wider role of DAM genes associated with growth repression, allowing flower development resumption under optimal environmental conditions, instead of a more restricted view of DAM as winter dormancy regulators.
Fig. 6.
Flower development expression of DAM-like genes in loquat. Sampling of reproductive buds and inflorescences of three loquat varieties differing in flowering time: Gigante Trabia (GT, late), Algerie (AL, medium) and Toni Tomaca (TT, early) (a). Relative gene expression of EjaDAM1–4 and EjaDAMb genes in these samples (b). An expression value of one is assigned to the highest sample. Data are means from three biological samples with two technical replicates each, with error bars representing standard deviation
Discussion
DAMs are phylogenetically and functionally related to the AGL24 subfamily of SVP proteins
Annual flowering and perennial dormancy processes are affected by environmental inputs by somehow analogous mechanisms [46–48]. Thus, it is not surprising to find the bud dormancy genes DAM within the SVP2 clade of MADS-box domain transcription factors including the Arabidopsis flowering regulator AthAGL24. AthSVP represses flowering by direct inhibition of the floral integrator FT [7, 49], whereas AthAGL24 promotes flowering [8], and both AthSVP and AthAGL24 determine floral meristem identity [50]. Consistently with these data, DAM proteins have been reported to bind the promoter of pear FT2 gene during endodormancy [17]. In view of these precedents, the altered flowering development observed in transgenic Arabidopsis plants overexpressing PpeDAM6 becomes easily understandable. Flower abnormalities observed in 35S::PpeDAM6 plants strongly resemble the phenotype of Arabidopsis plants overexpressing AthSVP [41] and AthAGL24 [8]. In addition, the overexpression in Arabidopsis of PavDAM1–6 and SVP-like genes, which are involved in the dormancy process in Prunus avium and Actinidia deliciosa respectively, result in similar flower phenotypes defects [10, 27].
Interestingly, AthSVP and AthAGL24 show opposite effects on flowering time in Arabidopsis by virtue of their different partners and targets [7, 8]. In this respect, the early flowering phenotype observed in a fertile 35S::PpeDAM6 line in this study emplaces PpeDAM6 closer to AthAGL24 than to AthSVP. Such functional evidence corroborates phylogenetic and syntenic analyses arguing for a common origin of AthAGL24 and DAM genes, both belonging to the SVP2 clade, in close agreement with previous phylogenetic studies of the SVP family [35, 37]. In line with these observations, the heterologous expression of apple SVP genes, but not the expression of apple DAMs, rescues the early flowering phenotype of the Arabidopsis svp-41 mutant [21].
Functional and structural specialization of DAM proteins in Rosaceae
Lineage-specific gene duplications events have been proposed to cause gene expansion and functional diversification in the SVP in Rosaceae [37]. Whereas SVP2 clade is expanded in the ten Rosaceae genomes included in this study, showing 2–6 members, SVP1 clade is specifically expanded in the four species of the Maleae tribe (Table 1). Such expansion and diversification of SVP-like factors has been proposed to support functional requirements for the perennial habit of growth of temperate climate trees, such as the formation of buds, the regulation of dormancy and the juvenile to mature transition [39]. The authors suggest the presence of subfunctionalization and/or neofunctionalization events in peach DAM genes [39], consistent with their different seasonal expression patterns [38]. In this respect, different heteromeric complexes of MdSVPa with MdDAM1, MdDAM4 and MdFLC resulted in different sets of transcriptional target genes, suggesting that these proteins performed non-redundant roles in dormancy [21]. Under this perspective, the lineage-specific expansion and subsequent functional diversification across Rosaceae genes belonging to the SVP1 and SVP2 clades might constitute the elemental basis of a finely tuned mechanism for a plastic genomic response under changing environments.
We have identified a short 15-residues motif specific to DAM and other related proteins from species belonging the Amygdaloideae subfamily including the genera Malus, Pyrus, Eriobotrya and Prunus, which formed a well-supported subclade within the SVP2 clade. This motif was found by the FIMO tool to be conserved in every DAM protein included in our dataset of examined sequences, and consequently can be considered as a DAM-specific signature, which can be tested on new species of Amygdaloideae as long as proteome annotations become available. Interestingly, the replacement of this DAM motif in PpeDAM6 by the collinear PpeSVP sequence affects its protein interaction capabilities (Fig. 4). Since the interaction of MADS-box domain proteins involved in dormancy in apple with different partners modifies their DNA-binding specificity and the set of downstream transcriptional targets [21], we may analogously infer that DAM-motif effect on PpeDAM6 interactions involves functional specialization with impact on gene regulatory networks.
DAMs are not strictly linked to winter dormancy
Such previous phylogenetic and biochemical considerations contribute to shape a restricted DAM clade in Amygdaloideae subfamily (Fig. 1) with common structural and functional features, supposedly involved in bud dormancy regulation during winter time. This implies that certain SVP-like proteins from non-Amygdaloideae species, previously reported as DAM proteins, by virtue of their effect on bud growth and dormancy, could not strictly belong to this clade, such as DAM factors from leafy spurge [9], in spite of evident molecular and regulatory resemblances with DAMs in the clade [51]. On the other hand, loquat (and conceivably other related species with no available genomic data) shows consistent DAM proteins at the phylogenetic and molecular levels (Figs. 1, 6), but its physiological behaviour diverges from winter bud dormancy observed in perennial temperate plants belonging to the Amygdaloideae subfamily. Loquat is an evergreen tree that interrupts growth and flower development during the high temperatures of summer, and resumes normal growth and blooming in autumn and winter. Despite the fact that some authors describe this behaviour as summer bud dormancy [45], evidences supporting this rest as a true dormancy process, that is independent on environmental conditions until a given intrinsic regulated requirement is fulfilled, are lacking. Summer dormancy has been more extensively studied in herbaceous perennials than in woody ones, leading to the conclusion that phenological cycles of winter and summer dormant species are remarkably similar, but induced by symmetrical photoperiod and temperature environmental conditions [6]. Loquat could become an interesting woody model for comparatively studying the biochemical and molecular resemblances and differences between winter and summer dormancy adaptive strategies, but a deeper insight about its dormant behaviour is required beforehand. Regardless of such physiological details, DAM genes in loquat behave like genuine genotype-specific bud-growth repressor factors, characterized by a high gene expression in resting buds and also in cultivars with deeper or longer resting periods (Fig. 6). Such transcriptional patterns truly support the growing hypothesis that DAM genes are not strictly associated with winter dormancy events, but instead are more general growth regulatory factors impinging on meristematic cell division and hormonal balances, with impact on the growth-stress survival trade-off, as suggested by recent studies [25, 29].
Conclusions
We provide an extensive compilation of SVP-like proteins deduced from recently sequenced genomes of the Rosaceae, a family of successful perennial plant species well adapted to temperate and tropical climatic conditions, which show a plastic and diverse response to winter temperatures by adjusting their bud dormancy requirements. Our data support a key role of SVP-like gene expansion and diversification, leading to the appearance of the DAM group in the subfamily Amygdaloideae within SVP2 clade, on this adaptive response. Amygdaloideae subfamily contains crops with a remarkable economic relevance such as apple, pear, peach, plum and almond, among others. We have identified a 15-amino acid long DAM-specific motif that constitutes a signature of known DAM factors. The absence of this motif impairs protein heteromerization with other regulatory factors, affecting thus to DAM transcriptional target specificity and function. We have analyzed DAM-like gene expression in the evergreen loquat, described as a summer dormancy species by some authors, to conclude that DAM expression associates with flower meristem activity and development in the important Amygdaloideae subfamily independently of the winter/summer dormancy habit.
Methods
Plant material
Loquat trees (Eriobotrya japonica) analysed in this study were grown at Instituto Valenciano de Investigaciones Agrarias, IVIA, Moncada, Spain, at 39° 34′ N, 0° 24′ W and 55 m above the sea level with drip irrigated silty-sandy soil at pH = 7,8. Flower buds from 3 varieties ranging in flowering time were harvested from the 3rd of July of 2020. The early blooming variety Toni Tomaca (TT) was harvested on 3/7, 17/7, 31/7, 12/8, 28/8 and 11/9. The intermediate blooming variety Algerie (AL) was harvested on 3/7, 31/7, 28/8, 25/9, 23/10 and 09/11. Finally, the latest blooming variety Gigante Trabia (GT) was harvested on 3/7, 31/7, 28/8, 25/9, 23/10, 23/11 and 21/12. Temperature data recorded during the timespan of the study is shown in Supplementary Fig. S1.
Identification of SVP and SVP-like sequences
In order to obtain a reliable set of the SVP and SVP-like genes in representative plant species, their genomes (Supplementary Table S1) were scanned using well-characterized SVP-like protein sequences from Arabidopsis thaliana, namely AthSVP and AthAGL24, as queries in independent BLASTP searches. Sequences retrieved as best reciprocal hits [52] using one or another query were used to build a list of candidate gene (Supplementary Table S2). The list was completed using SVP-like and DAM-like genes previously reported in selected species and not included in our preliminary dataset [53], leading to a definitive list with their corresponding genomic loci shown in Supplementary Table S2. The retrieved sequences were further examined by means of sequence analysis tools [54] and their predicted gene models individually curated using GeneWise (https://www.ebi.ac.uk/Tools/psa/genewise/), with both their genomic DNA and protein sequences as input and settings left as default [55].
Phylogenetic and protein motif analyses
Maximum Likelihood (ML) phylogenetic analysis was performed using PhyMLv3.1 [56] on the basis of multiple amino acid sequence alignments obtained using MUSCLE [57]. Prior to the analysis, the best fit amino acid substitution model by the AIC test was inferred using ProtTest v3.4.2 [58] to be JTT + G [59], i.e., modelling heterogeneity in nucleotide substitution rates across positions in the alignment by means of a Gamma distribution with eight categories and an alpha shape parameter of 1.315. To optimize the search for the most likely tree topology, the best of NNI & SPR option (NNI, nearest-neighbor inter-change; SPR, subtree pruning and regrafting) was selected. Statistical significance on the retrieved topology was assessed by means of the Shimodaira–Hasegawa-like approximate likelihood ratio test [60].
Search for conserved motifs shared across protein sequences was performed with the Multiple Em for Motif Elicitation tool (MEME) suite v5.4.1 [61]., using the “zoops” site distribution (Zero or One Occurrence Per Sequence) set to 10 motifs and the rest of settings as default. Selected motifs resulting from MEME were used to scan the set of sequences using the Find Individual Motif Occurrences (FIMO) tool from the MEME suite [62]. Trees were edited and further annotated with the detected MEME protein motifs using interactive Tree Of Life (iTOL) v5 [63].
Microsynteny analysis
Microsynteny analysis was conducted using the SynFind and Genome Evolution analysis (GEvo) tools from the Comparative Genomics platform (CoGe) [64]. First, syntenic regions between Arabidopsis AthSVP and AthAGL24 and peach, apple and loquat genomes were searched using SynFind. The detected syntenic regions were further examined using GEvo. Non-coding regions were masked to include only protein-coding sequences and to ease visualization among comparisons. We used the default setting to define the minimum number of collinear genes for two regions to be called syntenic.
Arabidopsis vectors and transformation
To overexpress PpeDAM6 in Arabidopsis, a fragment containing full-length PpeDAM6 fused to a N-terminal c-myc epitope was obtained from PpeDAM6 cloned in pGBKT7 plasmid with specific primers (Supplementary Table S6) and then inserted into the pROK2 vector under the 35S promoter (35S::c-myc-PpeDAM6). A construct with the c-myc epitope fused to the C-terminal end of PpeDAM6 (35S::PpeDAM6-c-myc) was obtained using pGBKT7-PpeDAM6 as a template in a PCR with specific primers (Supplementary Table S6). Both plasmids were introduced into Agrobacterium tumefaciens strain EHA105. Arabidopsis transformation was carried out by the floral dipping method [65]. Transformed seeds were selected on Murashige and Skoog (MS) medium supplemented with 50 μg/ml of kanamycin. Floral alterations were evaluated directly in T0 plants since many of them were sterile. For flowering time measurements, 10 T2 plants from 35S::PpeDAM6 line #15 with abnormal phenotype but viable seeds were used. Seedlings were cultured in a chamber at 24 °C with a 16 h:8 h light-dark cycle.
Western blot analysis
Protein extraction, western blot and immunological detection was performed according to [29]. Briefly, Arabidopsis leaves were boiled in Laemmli buffer during 10 min at 95 °C. Protein samples were resolved on sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) on 15% resolving gel [66], and transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare-Life sciences). Membranes were incubated with Anti-myc Tag clone 4A6 (EMD Millipore) for 1.5 h, washed and then incubated for 1 h with anti-mouse IgG POD-secondary antibody (Roche). The BM chemiluminescence western blotting kit (Mouse/Rabbit) (Roche) was used for chemiluminescent detection.
Yeast two-hybrid assay (Y2H)
A preliminary Y2H library screening using truncated PpeDAM6 led to the isolation of several putative protein interactors [44]. The library construction and the Y2H screening was performed following Make Your Own “Mate & PlateTM” Library System and Matchmaker® Gold Yeast Two-Hybrid System (Clontech-Takara Bio) previously described in [67]. PpeDAM6, PpeSVP and a chimeric PpeDAM6-PpeSVP gene were cloned into pGBKT7 using primers shown in Supplementary Table S6, and introduced into yeast strain Y2HGold, using the Yeastmaker yeast Transformation System 2 (Takara Bio). Two-hybrid interactions were tested in minimal medium without tryptophan, leucine, histidine and adenine, and supplemented with Aureobasidin A (125 ng/ml) and X-α-Gal (125 μg/ml).
Protein sequence analysis
Selected protein sequences (AthAGL24, PpeDAM6 and loquat DAMs) were subjected to Multiple Sequence Alignment (MSA) using ClustalW algorithm with default settings [54]. MSA results were processed for a proper interpretation using Jalview [68]. Protein structure prediction of the selected proteins was computed using ColabFold: a combination of MMseqs2 with AlphaFold2 [69].
Analysis of gene expression by real-time quantitative PCR (RT-qPCR)
RNA extraction from loquat floral loquat buds was performed using a quick cetyltrimethylammonium bromide (CTAB) based procedure [70]. Potential contaminants were removed using RNase-Free DNase Set (Qiagen) following the manufacturer instruction. 500 ng of each sample were used for retrotranscription using the PrimeScript RT reagent kit (Takara Bio) in a total volume of 10 μL. RT-qPCR was conducted with 20x diluted samples on a StepOnePlus Real-Time PCR System (Life Technologies) using SYBR premix Ex Taq (Tli RNaseH Plus) (Takara Bio) with an initial incubation of 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C each. Reaction specificity was tested by amplicon size estimation in electrophoresis and by melting curve analysis. For every datapoint three biological replicates with two technical replicates each were measured. EjaActin was used as the reference gene for all the experiments as previously described [53, 71]. The primers used in this study are listed in Supplementary Table S6.
Supplementary Information
Additional file 1: Fig. S1. Environmental temperature variation corresponding to loquat sampling. Table S1. Plant genomes used in this study. Table S2. Proteins and genes shown in the phylogenetic tree. Table S3. Summary of PpeDAM6 overexpressing Arabidopsis lines. Table S4. FIMO display of motif-8 occurrence in the set of SVP-like proteins. Table S5. Yeast 2-Hybrid (Y2H) interactors. Table S6. Primers used in this study.
Acknowledgements
GR acknowledges the continuous support of Elpidio Ríos.
Abbreviations
- AbA
Aureobasidin A
- AG
Agamous
- AGL24
Agamous-like 24
- AL
Algerie
- aLRT
Approximate likelihood-ratio test for branches
- BLAST
Basic Local Alignment Search Tool
- CoGe
Comparative Genomics platform
- Col
Columbia
- CTAB
Cetyltrimethylammonium bromide
- DAM
Dormancy-Associated MADS-box
- evg
Evergrowing
- FIMO
Find Individual Motif Occurrences tool
- FLC
Flowering Locus C
- FT
Flowering Locus T
- GEvo
Genome Evolution analysis tool
- GT
Gigante Trabia
- iTOL
Interactive Tree Of Life
- MEME
Multiple Em for Motif Elicitation tool
- ML
Maximum likelihood
- MS
Murashige and Skoog medium
- MSA
Multiple Sequence Alignment
- MYB
Myeloblastosis
- NNI
Nearest-neighbor inter-change
- PI
Pistillata
- PVDF
Polyvinylidene difluoride
- qRT-PCR
Quantitative real time PCR
- SDS-PAGE
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- SEP2
Sepallata 2
- SHP
Shatterproof
- SPR
Subtree pruning and regrafting
- SVP
Short Vegetative Phase
- TT
Toni Tomaca
- WGD
whole-genome duplication
- X-α-Gal
5-Bromo-4-chloro-3-indoxyl-α-D-galactopyranoside
- Y2H
Yeast two-hybrid
Authors’ contributions
CQ-T and AL performed the experiments; LC-P and CQ-T did the phylogenetic analyses; LC-P, MLB and GR conceived and designed the research; GR drafted the manuscript. The author(s) read and approved the final manuscript.
Funding
This research was funded by MCIN/AEI/10.13039/501100011033, the European Union “NextGenerationEU”/PRTR and IVIA-FEDER with project references PCI2020–120686-2, PID2020-114380RB-I00, 52201 and 51308 to GR, a “Proyectos I+D Generación de Conocimiento” grant from the Spanish Ministry of Science and Innovation (grant code: PID2020-113277GB-I00) to LCP and by funds received by the “Sistema de Información Científica de Andalucía” Research Group id BIO359. AL was funded by a fellowship from Ministerio de Ciencia (Spanish Government). CQ-T was funded by a fellowship co-financed by the European Social Fund and the IVIA. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study were downloaded from the links and references listed in Table S1. Protein and DNA accession numbers are shown in Table S2, corresponding to the following databases and repositories: Genome Database for Rosaceae (GDR, https://www.rosaceae.org/), Genome Warehouse (GWH, http://bigd.big.ac.cn/gwh/), GenBank at the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/), The Arabidopsis Information Resource (TAIR, https://www.arabidopsis.org/), Kiwifruit Genome Database (KGD, https://kiwifruitgenome.org/), Solanaceae Genomics Network (https://solgenomics.net/) and Phytozome (https://phytozome-next.jgi.doe.gov/).
Declarations
Ethics approval and consent to participate
This study complies with relevant institutional, national, and international guidelines and legislation. The experiments did not involve endangered or protected species. Loquat tree samples were obtained from the loquat germplasm collection located at the Instituto Valenciano de Investigaciones Agrarias (Moncada, Spain).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carles Quesada-Traver and Alba Lloret contributed equally to this work.
Contributor Information
Carles Quesada-Traver, Email: carles.quesadatraver@usys.ethz.ch.
Alba Lloret, Email: alloret@mpipz.mpg.de.
Lorenzo Carretero-Paulet, Email: lpaulet@ual.es.
María Luisa Badenes, Email: badenes_mlu@gva.es.
Gabino Ríos, Email: rios_gab@gva.es.
References
- 1.Chuine I. Why does phenology drive species distribution? Philos Trans R Soc Lond Ser B Biol Sci. 2010;365:3149–3160. doi: 10.1098/rstb.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloret A, Quesada-Traver C, Ríos G. Models for a molecular calendar of bud-break in fruit trees. Sci Hortic. 2022;297:110972. doi: 10.1016/j.scienta.2022.110972. [DOI] [Google Scholar]
- 3.Rohde A, Bhalerao RP. Plant dormancy in the perennial context. Trends Plant Sci. 2007;12:217–223. doi: 10.1016/j.tplants.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Lang GA. Dormancy: a new universal terminology. HortScience (USA) 1987;22:817–820. doi: 10.21273/HORTSCI.22.5.817. [DOI] [Google Scholar]
- 5.Campoy JA, Ruiz D, Egea J. Dormancy in temperate fruit trees in a global warming context: a review. Sci Hortic. 2011;130:357–372. doi: 10.1016/j.scienta.2011.07.011. [DOI] [Google Scholar]
- 6.Gillespie LM, Volaire FA. Are winter and summer dormancy symmetrical seasonal adaptive strategies? The case of temperate herbaceous perennials. Ann Bot. 2017;119:311–323. doi: 10.1093/aob/mcw264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann U, Höhmann S, Nettesheim K, Wisman E, Saedler H, Huijser P. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J. 2000;21:351–360. doi: 10.1046/j.1365-313x.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- 8.Michaels SD, Ditta G, Gustafson-Brown C, Pelaz S, Yanofsky M, Amasino RM. AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J. 2003;33:867–874. doi: 10.1046/j.1365-313X.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 9.Horvath DP, Sung S, Kim D, Chao W, Anderson J. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Mol Biol. 2010;73:169–179. doi: 10.1007/s11103-009-9596-5. [DOI] [PubMed] [Google Scholar]
- 10.Wu R-M, Walton EF, Richardson AC, Wood M, Hellens RP, Varkonyi-Gasic E. Conservation and divergence of four kiwifruit SVP-like MADS-box genes suggest distinct roles in kiwifruit bud dormancy and flowering. J Exp Bot. 2012;63:797–807. doi: 10.1093/jxb/err304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh RK, Maurya JP, Azeez A, Miskolczi P, Tylewicz S, Stojkovič K, et al. A genetic network mediating the control of bud break in hybrid aspen. Nat Commun. 2018;9:4173. doi: 10.1038/s41467-018-06696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergara R, Noriega X, Pérez FJ. VvDAM-SVPs genes are regulated by FLOWERING LOCUS T (VvFT) and not by ABA/low temperature-induced VvCBFs transcription factors in grapevine buds. Planta. 2021;253:31. doi: 10.1007/s00425-020-03561-5. [DOI] [PubMed] [Google Scholar]
- 13.Bielenberg DG, Wang Y(E), Li Z, Zhebentyayeva T, Fan S, Reighard GL, et al. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet Genomes. 2008;4:495–507. doi: 10.1007/s11295-007-0126-9. [DOI] [Google Scholar]
- 14.da Falavigna VS, Guitton B, Costes E, Andrés F. I want to (bud) break free: the potential role of DAM and SVP-like genes in regulating dormancy cycle in temperate fruit trees. Front Plant Sci. 2019;9:1990. doi: 10.3389/fpls.2018.01990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q, Gao Y, Wu X, Moriguchi T, Bai S, Teng Y. Bud endodormancy in deciduous fruit trees: advances and prospects. Hortic Res. 2021;8:139. doi: 10.1038/s41438-021-00575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T, Bai S, Imai T, Ito A, Nakajima I, Moriguchi T. Histone modification and signalling cascade of the dormancy-associated MADS-box gene, PpMADS13-1, in Japanese pear (Pyrus pyrifolia) during endodormancy. Plant Cell Environ. 2015;38:1157–1166. doi: 10.1111/pce.12469. [DOI] [PubMed] [Google Scholar]
- 17.Niu Q, Li J, Cai D, Qian M, Jia H, Bai S, et al. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifolia white pear group) flower bud. J Exp Bot. 2016;67:239–257. doi: 10.1093/jxb/erv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Yan X, Ahmad M, Yu W, Song Z, Ni J, et al. Alternative splicing of the dormancy-associated MADS-box transcription factor gene PpDAM1 is associated with flower bud dormancy in ‘Dangshansu’ pear (Pyrus pyrifolia white pear group) Plant Physiol Biochem. 2021;166:1096–1108. doi: 10.1016/j.plaphy.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Yang Q, Yan X, Wu X, Yang F, Li J, et al. High-quality genome assembly of ‘Cuiguan’ pear (Pyrus pyrifolia) as a reference genome for identifying regulatory genes and epigenetic modifications responsible for bud dormancy. Hortic Res. 2021;8:197. doi: 10.1038/s41438-021-00632-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moser M, Asquini E, Miolli GV, Weigl K, Hanke MV, Flachowsky H, et al. The MADS-box gene MdDAM1 controls growth cessation and bud dormancy in apple. Front Plant Sci. 2020;11:1003. doi: 10.3389/fpls.2020.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Falavigna VS, Severing E, Lai X, Estevan J, Farrera I, Hugouvieux V, et al. Unraveling the role of MADS transcription factor complexes in apple tree dormancy. New Phytol. 2021;232:2071–2088. doi: 10.1111/nph.17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu R, Cooney J, Tomes S, Rebstock R, Karunairetnam S, Allan AC, et al. RNAi-mediated repression of dormancy-related genes results in evergrowing apple trees. Tree Physiol. 2021;41:1510–1523. doi: 10.1093/treephys/tpab007. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki R, Yamane H, Ooka T, Jotatsu H, Kitamura Y, Akagi T, et al. Functional and expressional analyses of PmDAM genes associated with endodormancy in Japanese apricot. Plant Physiol. 2011;157:485–497. doi: 10.1104/pp.111.181982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao K, Zhou Y, Ahmad S, Yong X, Xie X, Han Y, et al. PmCBFs synthetically affect PmDAM6 by alternative promoter binding and protein complexes towards the dormancy of bud for Prunus mume. Sci Rep. 2018;8:4527. doi: 10.1038/s41598-018-22537-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamane H, Wada M, Honda C, Matsuura T, Ikeda Y, Hirayama T, et al. Overexpression of Prunus DAM6 inhibits growth, represses bud break competency of dormant buds and delays bud outgrowth in apple plants. Plos One. 2019;14:e0214788. doi: 10.1371/journal.pone.0214788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothkegel K, Sánchez E, Montes C, Greve M, Tapia S, Bravo S, et al. DNA methylation and small interference RNAs participate in the regulation of MADS-box genes involved in dormancy in sweet cherry (Prunus avium L.) Tree Physiol. 2017;37:1739–1751. doi: 10.1093/treephys/tpx055. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Gao Z, Li H, Jiu S, Qu Y, Wang L, et al. Dormancy-associated MADS-box (DAM) genes influence chilling requirement of sweet cherries and co-regulate flower development with SOC1 gene. Int J Mol Sci. 2020;21:E921. doi: 10.3390/ijms21030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H, Chen P-Y, Zhong S, Dardick C, Callahan A, An YQ, et al. Thermal-responsive genetic and epigenetic regulation of DAM cluster controlling dormancy and chilling requirement in peach floral buds. Hortic Res. 2020;7:114. doi: 10.1038/s41438-020-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloret A, Quesada-Traver C, Conejero A, Arbona V, Gómez-Mena C, Petri C, et al. Regulatory circuits involving bud dormancy factor PpeDAM6. Hortic Res. 2021;8:261. doi: 10.1038/s41438-021-00706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balogh E, Halász J, Soltész A, Erös-Honti Z, Gutermuth Á, Szalay L, et al. Identification, structural and functional characterization of dormancy regulator genes in apricot (Prunus armeniaca L.) Front Plant Sci. 2019;10:402. doi: 10.3389/fpls.2019.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quesada-Traver C, Guerrero BI, Badenes ML, Rodrigo J, Ríos G, Lloret A. Structure and expression of bud dormancy-associated MADS-box genes (DAM) in European plum. Front Plant Sci. 2020;11:1288. doi: 10.3389/fpls.2020.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang ZZ, Lin-Wang K, Dai H, Zhou DR, Jiang CC, Espley RV, et al. The genome of low-chill Chinese plum “Sanyueli” (Prunus salicina Lindl.) provides insights into the regulation of the chilling requirement of flower buds. Mol Ecol Resour. 2022;22:1919–1938. doi: 10.1111/1755-0998.13585. [DOI] [PubMed] [Google Scholar]
- 33.Prudencio ÁS, Dicenta F, Martínez-Gómez P. Monitoring dormancy transition in almond [Prunus dulcis (miller) Webb] during cold and warm mediterranean seasons through the analysis of a DAM (dormancy-associated MADS-box) gene. Horticulturae. 2018;4:41. doi: 10.3390/horticulturae4040041. [DOI] [Google Scholar]
- 34.Hsiang T-F, Chen W, Yamane H. The MADS-box gene family involved in the regulatory mechanism of dormancy and flowering in Rosaceae fruit trees. Ann Plant Rev Online. 2021;4:649–686. doi: 10.1002/9781119312994.apr0759. [DOI] [Google Scholar]
- 35.Liu X, Sun Z, Dong W, Wang Z, Zhang L. Expansion and functional divergence of the SHORT VEGETATIVE PHASE (SVP) genes in eudicots. Genome Biol Evol. 2018;10:3026–3037. doi: 10.1093/gbe/evy235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiang Y, Huang C-H, Hu Y, Wen J, Li S, Yi T, et al. Evolution of Rosaceae fruit types based on nuclear phylogeny in the context of geological times and genome duplication. Mol Biol Evol. 2017;34:262–281. doi: 10.1093/molbev/msw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Ren M, Chen H, Wu S, Yan H, Jalal A, et al. Evolution of SHORT VEGETATIVE PHASE (SVP) genes in Rosaceae: implications of lineage-specific gene duplication events and function diversifications with respect to their roles in processes other than bud dormancy. Plant Genome. 2020;13:e20053. doi: 10.1002/tpg2.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Reighard GL, Abbott AG, Bielenberg DG. Dormancy-associated MADS genes from the EVG locus of peach [Prunus persica (L.) Batsch] have distinct seasonal and photoperiodic expression patterns. J Exp Bot. 2009;60:3521–3530. doi: 10.1093/jxb/erp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiménez S, Lawton-Rauh AL, Reighard GL, Abbott AG, Bielenberg DG. Phylogenetic analysis and molecular evolution of the dormancy associated MADS-box genes from peach. BMC Plant Biol. 2009;9:81. doi: 10.1186/1471-2229-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leida C, Conesa A, Llácer G, Badenes ML, Ríos G. Histone modifications and expression of DAM6 gene in peach are modulated during bud dormancy release in a cultivar-dependent manner. New Phytol. 2012;193:67–80. doi: 10.1111/j.1469-8137.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 41.Masiero S, Li M-A, Will I, Hartmann U, Saedler H, Huijser P, et al. INCOMPOSITA: a MADS-box gene controlling prophyll development and floral meristem identity in Antirrhinum. Development. 2004;131:5981–5990. doi: 10.1242/dev.01517. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Ito T, Wellmer F, Meyerowitz EM. Repression of AGAMOUS-LIKE 24 is a crucial step in promoting flower development. Nat Genet. 2004;36:157–161. doi: 10.1038/ng1286. [DOI] [PubMed] [Google Scholar]
- 43.de Folter S, Immink RGH, Kieffer M, Parenicová L, Henz SR, Weigel D, et al. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell. 2005;17:1424–1433. doi: 10.1105/tpc.105.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloret A. A dynamic snapshot of bud dormancy in peach; PhD thesis. Valencia: Universitat Politècnica de València; 2020. [Google Scholar]
- 45.Cuevas J, Del Grosso L. Loquat response to experimental defoliation: shoot growth, bud dormancy and flowering. Acta Hortic. 2011;887:185–190. doi: 10.17660/ActaHortic.2011.887.30. [DOI] [Google Scholar]
- 46.Horvath D. Common mechanisms regulate flowering and dormancy. Plant Sci. 2009;177:523–531. doi: 10.1016/j.plantsci.2009.09.002. [DOI] [Google Scholar]
- 47.Hemming MN, Trevaskis B. Make hay when the sun shines: the role of MADS-box genes in temperature-dependant seasonal flowering responses. Plant Sci. 2011;180:447–453. doi: 10.1016/j.plantsci.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Lloret A, Badenes ML, Ríos G. Modulation of dormancy and growth responses in reproductive buds of temperate trees. Front Plant Sci. 2018;9:1368. doi: 10.3389/fpls.2018.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007;21:397–402. doi: 10.1101/gad.1518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregis V, Sessa A, Colombo L, Kater MM. AGAMOUS-LIKE24 and SHORT VEGETATIVE PHASE determine floral meristem identity in Arabidopsis. Plant J. 2008;56:891–902. doi: 10.1111/j.1365-313X.2008.03648.x. [DOI] [PubMed] [Google Scholar]
- 51.Hao X, Chao W, Yang Y, Horvath D. Coordinated expression of FLOWERING LOCUS T and DORMANCY ASSOCIATED MADS-BOX-like genes in leafy spurge. Plos One. 2015;10:e0126030. doi: 10.1371/journal.pone.0126030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y, Peng J, Zhang Z, Lin S, Lin S, Yang X. The role of EjSVPs in flower initiation in Eriobotrya japonica. Int J Mol Sci. 2019;20:E5933. doi: 10.3390/ijms20235933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 57.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 60.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 61.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015;43:W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Letunic I, Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyons E, Freeling M. How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 2008;53:661–673. doi: 10.1111/j.1365-313X.2007.03326.x. [DOI] [PubMed] [Google Scholar]
- 65.Mara C, Grigorova B, Liu Z. Floral-dip transformation of Arabidopsis thaliana to examine pTSO2::beta-glucuronidase reporter gene expression. J Vis Exp. 2010;40:e1952. doi: 10.3791/1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 67.Lloret A, Conejero A, Leida C, Petri C, Gil-Muñoz F, Burgos L, et al. Dual regulation of water retention and cell growth by a stress-associated protein (SAP) gene in Prunus. Sci Rep. 2017;7:332. doi: 10.1038/s41598-017-00471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold: making protein folding accessible to all. Nat Methods. 2022;19:679–682. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gambino G, Perrone I, Gribaudo I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal. 2008;19:520–525. doi: 10.1002/pca.1078. [DOI] [PubMed] [Google Scholar]
- 71.Shan LL, Li X, Wang P, Cai C, Zhang B, Sun CD, et al. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta. 2008;227:1243–1254. doi: 10.1007/s00425-008-0696-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Environmental temperature variation corresponding to loquat sampling. Table S1. Plant genomes used in this study. Table S2. Proteins and genes shown in the phylogenetic tree. Table S3. Summary of PpeDAM6 overexpressing Arabidopsis lines. Table S4. FIMO display of motif-8 occurrence in the set of SVP-like proteins. Table S5. Yeast 2-Hybrid (Y2H) interactors. Table S6. Primers used in this study.
Data Availability Statement
The datasets used and/or analysed during the current study were downloaded from the links and references listed in Table S1. Protein and DNA accession numbers are shown in Table S2, corresponding to the following databases and repositories: Genome Database for Rosaceae (GDR, https://www.rosaceae.org/), Genome Warehouse (GWH, http://bigd.big.ac.cn/gwh/), GenBank at the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/), The Arabidopsis Information Resource (TAIR, https://www.arabidopsis.org/), Kiwifruit Genome Database (KGD, https://kiwifruitgenome.org/), Solanaceae Genomics Network (https://solgenomics.net/) and Phytozome (https://phytozome-next.jgi.doe.gov/).