Abstract
Aims
Recently, several therapeutic agents have decreased the progression to critical disease in patients with mild/moderate COVID-19. However, their use is limited to patients with ≥1 clinical risk factor. We aimed to evaluate echocardiographic features that may aid in risk stratification for patients with mild/moderate COVID-19.
Methods
278 consecutive patients with mild/moderate COVID-19 underwent prospective clinical and echocardiographic examination, ≤7 days of symptoms, as part of a predefined protocol. Analysis to identify echocardiographic predictors of outcome was performed.
Results
In the multivariable risk model, E/e′, TAPSE, and pulmonary acceleration time (PAT) were associated with the composite outcome (p = 0.01, 0.005, 0.05, respectively). Stepwise analyses showed that the addition of echocardiography on top of having ≥1 clinical risk factor and even using each parameter separately improved the prediction of outcomes. If patients were re-categorized as high risk only if having both ≥1 clinical and ≥ 1 echocardiography risk parameter (E/e′ > 8, TAPSE<1.8 cm, PAT<90 msec), or even one echo parameter separately, then specificity, positive predictive value, and accuracy improved. If patients were re-classified as high risk if having either ≥1 clinical risk factor or ≥ 1 high-risk echocardiography parameter, all five individuals who were missed by the ≥1 risk factor “rule”, were correctly diagnosed as high risk. Similar analyses, including only patients with mild disease, showed that the addition of TAPSE improved the prediction of outcomes.
Conclusions
In patients with mild/moderate COVID-19, a very limited echocardiographic exam is sufficient for improved outcome prediction, and may improve resource allocation for new anti-COVID-19 agents.
Translational aspect of the work
We show that among patients with mild/moderate COVID-19, several easily obtained echocardiographic findings are strongly correlated with mortality or progression to the need for invasive/non-invasive mechanical ventilation, even when adjusted for the presence or absence of ≥1 clinical risk factor. Furthermore, even a limited echocardiographic exam is sufficient to develop a strategy of risk stratification. We believe that our data have important implications for the clinicians involved in the acute treatment of patients with COVID-19.
Keywords: COVID-19, Echocardiography, Risk stratification
1. Introduction
Although most patients with COVID-19 have mild disease, some may progress to critical illness leading to mechanical ventilation or death [[1], [2], [3], [4], [5]]. Several investigational therapeutic agents are available, for patients with mild/moderate COVID-19 and symptom onset ≤7 days, including intravenous Remdisivir [6], REGEN-COV [7] and Sotrovimab [8], and oral Molnupiravir [9], and Paxlovid [10]. These agents have shown promise in decreasing the progression to severe disease and reducing mortality. However, their use is currently limited to patients with ≥1 clinical risk factor for deterioration to severe COVID-19, excluding patients without clinical risk factors. Limiting these effective treatments based entirely on co-morbidities can result in denying a life-saving drug from patients without clinical risk factors that may still harbor a risk for clinical deterioration or in unnecessary use of these expensive medications in low-income countries.
We previously presented data regarding the cardiac and echocardiographic manifestations of COVID-19 in 530 consecutive patients [[11], [12], [13], [14]], irrespective of the severity of disease or clinical indication, to describe their different echocardiographic and non-invasive hemodynamic profiles. Our cohort included only symptomatic (non-vaccinated) patients, with symptom onset ≤7 days, and mostly (N = 278, 52%) with mild/moderate disease at presentation.
In the present study, we aimed to
-
o
Evaluate the added prognostic value of echocardiographic and hemodynamic parameters for predicting progression to respiratory failure or mortality in patients presenting with mild/moderate disease.
-
o
Evaluate if these parameters may improve patient selection, with special emphasis on increasing specificity and accuracy in patients with ≥1 clinical risk factor for deterioration to severe COVID-19, and increasing sensitivity in those patients without risk factors for severe disease.
2. Material and methods
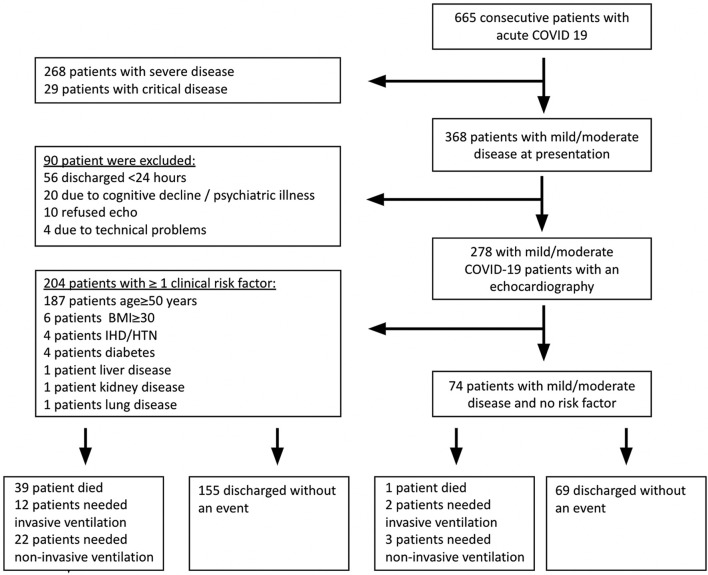
At the beginning of the COVID-19 pandemic, we initiated a prospective program of comprehensive echocardiography on admission for all patients presenting with respiratory illness due to COVID-19 infection, irrespective of clinical indication, using a pre-defined step-by-step protocol, as part of a routine patient care protocol. All patients underwent echocardiography within 48 h of admission and ≤ 7 days of symptoms onset [2 (0, 6) days]. We studied 530 consecutive adult patients (≥18 years old) admitted between 3/23/2020 and 9/25/2020 to the Tel Aviv Medical Center because of COVID-19 infection. The patients appear in previous publications [[11], [12], [13], [14]]. Demographic data, comorbid conditions, medications, physical examination, and laboratory findings were recorded prospectively. We excluded patients with severe and critical diseases. Ninety patients with mild-moderate disease were excluded based on predefined exclusion criteria (Fig. 1 ). Supplementary Table 1 describes the reason for exclusion and the disease severity of the excluded patients. Eventually, this present cohort included 278 patients with mild/moderate disease at presentation. We then divided the patients in accordance with the NIH recommendations [15]. One-hundred thirty-six patients had mild disease (any of the various symptoms and signs of COVID-19 with no radiographic evidence of lower respiratory tract disease by X-ray), and 142 patients had moderate COVID-19 (radiographic evidence of lower respiratory tract disease and PO2 saturation ≥ 94% in room air). The patients were then re-stratified to 204 with mild/moderate disease with ≥1 risk factor for deterioration to severe COVID-19 (age ≥ 50 years, presence of either immunosuppression, obesity, cardiovascular, metabolic, liver, kidney, or lung disease), and 74 patients with no such risk factors (Fig. 1). The stratification was based on risk factors and was performed in a stepwise manner. First, we identified all patients with age ≥ 50 years (187 patients with mild/moderate COVID-19), then patients with BMI ≥30 (6 patients with mild/moderate COVID-19, and age < 50 years), and then by this order, patients with a history of ischemic heart disease, or hypertension (N = 4), diabetes (N = 4), liver disease (N = 1), kidney disease (N = 1), chronic lung disease (N = 1), and immunosuppression (N = 0). The remaining 74 patients had mild/moderate COVID-19 infection with no clinical risk factors for deterioration. Clinical data were collected on a daily basis. Clinical deterioration was defined as either death or respiratory deterioration. Respiratory deterioration was defined as acute new onset of hypoxemia requiring either invasive ventilation or non-invasive ventilation [bilevel positive airway pressure (BIPAP) or high flow inspiratory support (VAPOTERM), or both]. Mortality was ascertained until the end of the follow-up on July 15th, 2021, beyond hospitalization and irrespective of discharge date, for all patients, by telephone calls, and was complete for all the patients. The ethics committee of the Tel Aviv Medical Center approved the study, IRB number 0196–20-TLV.
Fig. 1.
Flowchart showing patient selection for the final cohort.
Abbreviations - COVID19, coronavirus disease 2019; BMI, body mass index; IHD, ischemic heart disease; HTN, hypertension.
2.1. Echocardiography
Echocardiography was performed using a dedicated echocardiographic recorder (CX 50, Philips Medical Systems, Bothell, WA). In accordance with the current guidelines [16], the following measures were undertaken to minimize the risk of inadvertent infection: 1) All studies were performed at the designated COVID-19 units; 2) All exams were performed with small dedicated scanners; 3) Personal protection included airborne precautions; 4) Electrocardiographic monitoring during imaging was omitted, and all measurements were performed offline [17,18], Left ventricular (LV) diameters and ejection fraction (LVEF) were measured as recommended [19]. Measurements of mitral inflow included peak early filling (E-wave), late diastolic filling (A-wave) velocities, and E/A ratio. Early diastolic mitral septal and lateral annular velocities (e') were measured in the apical 4-chamber view [20]. Left atrial (LA) volume was calculated using the biplane area length method at end-systole. Forward stroke volume (SV) was calculated from LV outflow tract (LVOT) Doppler time velocity interval multiplied by LVOT cross-sectional area with subsequent stroke volume index and cardiac index calculation. 4-chamber views encompassing the entire right ventricle (RV) were used to calculate end-systolic and end-diastolic RV areas and tricuspid annulus. RV function was evaluated by tricuspid annular plane systolic excursion (TAPSE), systolic tricuspid lateral annular velocity (RV S′) measured in the apical 4-chamber view, and fractional area change (FAC) [19,21]. Hemodynamic right-sided assessment included the measurement of the pulmonic flow acceleration time (PAT) velocity to assess pulmonary vascular resistance and estimated right atrial pressure using the inferior vena cava [22]. Estimation of systolic pulmonary pressure based on tricuspid regurgitation pressure gradient and the estimated right atrial pressure was possible only in 63 (23%) patients.
2.2. Follow-up and outcomes
Clinical follow-up was obtained prospectively. Outcome analysis started at the time of the baseline echocardiographic exam. The study endpoints were: 1) all-cause mortality, 2) need for invasive or non-invasive ventilation, and 3) the composite outcome of death or need for invasive or non-invasive ventilation. Patients who needed non-invasive ventilation and eventually invasive ventilation, or ventilation and eventually died, were censored at the time of the initiating event.
We also included the following analyzes:
-
-
Outcome analysis for the mild patient group only.
-
-
The prediction of short-term outcomes - mortality in 30 days and the combined result.
2.3. Statistical analysis
Continuous normally distributed parameters were presented as means±SD and compared using the Student's t-test. Non-normally distributed data were presented by the median, 1st, and 3rd quartiles, and compared using the Wilcoxon rank-sum test. Categorical data were compared between groups using the χ2 test or Fisher's exact test. Univariate Cox proportional hazards models for mortality or the composite outcome as endpoints allowed the calculation of hazard ratios (HR) and their corresponding 95% confidence interval. The time of follow-up was calculated between baseline echocardiographic and either death, a new need for invasive or non-invasive ventilation, or the last follow-up date. Analyses for clinical endpoints were obtained for all patients. We used multivariable Cox proportional hazard models to assess the independent echocardiographic parameters associated with the composite endpoint. The first step was to group the variables into LV, Doppler, and RV parameters. The second step was to select for each group all the variables with P < 0.05 in a univariate analysis. The third step was to assess correlations between the selected variables within each group to avoid collinearity (R2 > 0.7; p < 0.0001). Covariates were entered in a stepwise forward multivariate analysis. In the fourth step, we performed separate analyses for the composite event adjusted for a nominal clinical parameter: the presence or absence of ≥1 risk factor for deterioration to severe COVID-19. The nominal clinical parameter was entered first, and the echocardiographic parameters second. The statistical significance for the additive value of echocardiographic parameters on top of the clinical dichotomous parameter was examined by: 1) Chi-square test of the loglik reduction; 2) Akaike information criterion (AIC) method.
To determine if models incorporating echocardiographic parameters improve the prediction of outcome, and reclassify more individuals with events as high risk (true positive) and more individuals without events as low risk (true negative) compared with the presence or absence of ≥1 clinical risk factor, we first derived cutoff values for continuous echocardiographic parameters affecting outcome using the maximally selected rank statistics method. We then generated Contingency tables for the nominal clinical parameter alone, or the combination of the nominal clinical parameter, all echocardiographic parameters, or each echocardiographic parameter separately, and calculated sensitivity, specificity, negative predictive value, positive predictive value, and accuracy for each model. In each model, patients with at ≥1 risk factor for deterioration to severe COVID-19 were categorized as high risk. For echocardiographic parameters, the patients were classified as high risk or low risk based on the cutoffs described in the results section. For each event, we assessed models with high sensitivity (patients were categorized as high-risk if having either ≥1 clinical risk factor or high-risk echocardiographic features) or with high specificity (patients were categorized as high risk if having both ≥1 high clinical risk factor, and high-risk echocardiographic features).
3. Results
Clinical data were collected for 664 consecutive patients with COVID-19 infection. We excluded 268 patients with severe disease and 29 patients with critical disease. We then excluded 90 patients because they did not undergo echocardiographic assessment. The reasons for not performing the echocardiographic assessment were hospital discharge in <24 h (56 patients), patient refusal (10 patients), patients who had cognitive decline or psychiatric illness (20 patients), and 4 patients were excluded due to technical problems (Fig. 1 and Supplementary Table 1). Baseline characteristics of the final cohort are presented in Table 1 .)
Table 1.
Baseline characteristics.
| Variables | Mild/Moderate disease (all) N = 278 | ≥ 1 risk factors N = 204 | No risk factors N = 74 | p-value |
|---|---|---|---|---|
| Age (years), mean ± SD | 59.2 ± 19 | 67.2 ± 14 | 36.9 ± 8 | <0.0001 |
| Gender (male), n (%) | 161 (58) | 116 (57) | 45 (61) | 0.55 |
| BMI, mean ± SD | 26.6 ± 5.9 | 27.3 ± 6.1 | 24.7 ± 4.0 | 0.002 |
| IHD, n (%) | 41 (15) | 41 (20) | 0 (0) | <0.0001 |
| Atrial Fibrillation, n(%) | 21 (7.5) | 21 (10) | 0 (0) | 0.0002 |
| AICD, n (%) | 2 (0.7) | 2 (1) | 0 (0) | 0.26 |
| Smoking, n (%) | 29 (10) | 24 (11) | 5 (7) | 0.29 |
| Hypercholesterolemia, n (%) | 87 (31) | 85 (42) | 2 (3) | <0.0001 |
| DM, n(%) | 82 (29) | 82 (40) | 0 (0) | <0.0001 |
| HTN, n (%) | 111 (40) | 111 (54) | 0 (0) | <0.0001 |
| COPD, n (%) | 12 (4) | 12 (6) | 0 (0) | 0.006 |
| CRF, n (%) | 18 (6) | 18 (9) | 0 (0) | 0.0007 |
| Liver disease, n (%) | 8 (3) | 8 (4) | 0 (0) | 0.02 |
| Immunosuppression, n (%) | 6 (2) | 6 (3) | 0 (0) | 0.05 |
| Temperature (Celsius), mean ± SD | 37.4 ± 0.9 | 37.4 ± 0.9 | 37.5 ± 1.0 | 0.60 |
| O2%saturation, mean ± SD | 97.5 ± 2.1 | 97.4 ± 2.2 | 98.0 ± 1.6 | 0.11 |
| Heart Rate (beats/min), mean ± SD | 84.5 ± 15 | 84.3 ± 15 | 85.3 ± 15 | 0.63 |
| Systolic blood pressure (mmHg), (mean ± SD) | 134.6 ± 21 | 137.7 ± 22 | 126.2 ± 14 | <0.0001 |
| Diastolic blood pressure (mmHg), mean ± SD | 77.1 ± 13 | 76.4 ± 14 | 78.8 ± 10 | 0.15 |
| Troponin-I (ng/L), median [IQR] [measured in 190 patients] | 8.5 [5, 19] | 6 [3, 9] | 5 [3, 7] | 0.36 |
| BNP (pg/mL), median [IQR] [measured in 192 patients] |
37.5 [14, 102] | 48 [18, 130] | 14 [7, 30] | 0.001 |
| EF (%), mean ± SD | 58.0 ± 6 | 57.5 ± 6 | 59.1 ± 4 | 0.02 |
| LVEDD index (mm/m2), mean ± SD | 23.5 ± 3.4 | 23.3 ± 3.6 | 24.0 ± 2.7 | 0.10 |
| LVESD index (mm/m2), mean ± SD | 15.2 ± 3.2 | 15.3 ± 3.4 | 15.0 ± 2.6 | 0.48 |
| Septal thickness (mm), mean ± SD | 8.7 ± 2.2 | 9.3 ± 2.1 | 7.2 ± 1.6 | <0.0001 |
| Posterior wall thickness (mm), mean ± SD | 9.2 ± 2.2 | 9.5 ± 2.2 | 7.8 ± 1.5 | 0.01 |
| LV mass index (gram/m2), mean ± SD | 69.4 ± 22 | 73.3 ± 23 | 57.3 ± 14 | <0.0001 |
| Stroke volume index (mL/m2), mean ± SD | 31.5 ± 9 | 31.1 ± 9 | 33.5 ± 9 | 0.10 |
| Cardiac index (L/min/m2), mean ± SD | 2.5 ± 2.3 | 2.7 ± 0.6 | 2.4 ± 0.7 | 0.35 |
| LA volume index (mL/m2), mean ± SD | 30.0 ± 12 | 31.7 ± 12 | 25.3 ± 10 | 0.0004 |
| E wave (m/s), mean ± SD | 67.2 ± 19 | 66.1 ± 20 | 71.2 ± 13 | 0.02 |
| A wave (m/s), mean ± SD | 61.0 ± 20 | 66.5 ± 19 | 48.7 ± 16 | <0.0001 |
| E/A | 1.15 ± 0.5 | 1.0 ± 0.4 | 1.5 ± 0.4 | <0.0001 |
| e′ septal (cm/s), mean ± SD | 7.1 ± 2.1 | 6.4 ± 1.9 | 8.9 ± 1.6 | <0.0001 |
| e′ lateral (cm/s), mean ± SD | 9.3 ± 3.2 | 8.1 ± 2.8 | 12.3 ± 2.5 | <0.0001 |
| E/e′ average | 9.4 ± 4.8 | 10.4 ± 5.4 | 7.1 ± 2.2 | <0.0001 |
| RA pressure (mmHg), mean ± SD | 7.2 ± 3.2 | 7.4 ± 3.5 | 6.6 ± 2.5 | 0.05 |
| SPAP (mmHg), mean ± SD [measured in 63 patients] |
30.9 ± 11 | 34.1 ± 12 | 23.3 ± 7 | <0.0001 |
| PAT (ms), mean ± SD | 92.5 ± 28 | 86.1 ± 26 | 107.0 ± 25 | <0.0001 |
| RVEDA index (cm2/m2), mean ± SD | 11.0 ± 2.2 | 11.2 ± 2.3 | 10.7 ± 2.4 | 0.26 |
| RVESA index (cm2/m2), mean ± SD | 6.2 ± 1.6 | 6.3 ± 1.7 | 6.1 ± 1.5 | 0.50 |
| RVFAC (%), mean ± SD | 43.0 ± 11 | 42.6 ± 6 | 44.6 ± 5 | 0.24 |
| TAPSE (cm), mean ± SD | 2.3 ± 0.5 | 2.2 ± 0.5 | 2.4 ± 0.4 | 0.0008 |
| RV S′ (cm/s), mean ± SD | 11.0 ± 2.6 | 11.0 ± 2.9 | 11.0 ± 1.8 | 0.82 |
Abbreviations - SD, standard deviation; IHD, ischemic heart disease; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; DM, diabetes mellitus; HTN, hypertension; BNP, brain natriuretic peptide; LV, left ventricular; EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LA, left atrial; RV, right ventricle; RA, right atrial; SPAP, systolic pulmonary peak pressure; PAT, pulmonary acceleration time; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; RVFAC, right ventricular fractional area change; TAPSE, tricuspid annular plane systolic excursion.
3.1. Univariate analyses
Results of the univariate analyses for the prediction of mortality and the composite outcome using clinical and echocardiographic parameters are shown in Table 2 . The median follow-up was 446 days, with an interquartile range of 389 to 476 days. Forty patients (14.3%) died, 14 (5%) needed invasive ventilation, 25 (9%) needed non-invasive ventilation, and 54 (19.5%) reached the composite outcome. Multiple co-morbidities were associated with adverse outcomes. The presence of ≥1 clinical risk factor for deterioration to severe COVID-19 was significantly associated with excess mortality [HR 14.7(3.2–162); p < 0.0001] and combined adverse events [HR 4.68 (1.91–15.4); p = 0.0002]. The echocardiographic parameters significantly associated with a higher risk of either mortality or the composite end-point were LV mass index, left atrial volume index, E and A wave velocities, e’ septal and lateral, E/e′ ratio, RV S′, TAPSE, right atrial pressure (RAP), and PAT (Table 2). In the 74 patients without any clinical risk factor for deterioration, five patients had at least one adverse clinical outcome [one patient died (1.3%), 2 (2.7%) needed invasive mechanical ventilation, 3 (4%) needed non-invasive mechanical ventilation]. Results of univariate analyses for the composite outcome, stratified for patients with or without ≥1 clinical risk factor, are shown in Supplementary Table 2 and Supplementary Fig. 1. In the group of patients with ≥1 clinical risk factors, echocardiographic parameters significantly associated with the composite outcome were: left atrial volume index, E and A wave velocities, e’ lateral, E/e′ ratio, RV S′, TAPSE, and PAT. However, in the group of patients without any clinical risk factors, the only echocardiographic parameters significantly associated with the composite endpoint were E and A wave velocities, E/e′ ratio, RAP, and PAT.
Table 2.
Univariate analyses for the prediction of mortality and the composite outcome, using the clinical and echocardiographic parameters.
| Parameter | HR mortality (CI 95%) | P-value | HR composite outcome (CI 95%) | P-value |
|---|---|---|---|---|
| Age (years) | 1.07 (1.05–1.09) | <0.0001 | 1.05 (1.03–1.07) | <0.0001 |
| Gender (male) | 1.23 (0.66–2.3) | 0.50 | 1.16 (0.68–1.99) | 0.57 |
| Obesity | 1.52 (0.46–9.3) | 0.54 | 1.53 (0.56–6.2) | 0.44 |
| IHD | 1.79 (0.80–3.6) | 0.14 | 1.49 (0.73–2.8) | 0.25 |
| COPD | 2.48 (0.74–6.2) | 0.12 | 3.77 (1.55–7.8) | 0.005 |
| CRF | 4.7 (2.1–9.5) | 0.005 | 3.81 (1.80–7.3) | 0.001 |
| DM | 2.3 (1.26–4.4) | 0.008 | 2.21 (1.28–3.78) | 0.004 |
| HTN | 1.9 (1.02–3.6) | 0.04 | 1.83 (1.07–3.1) | 0.02 |
| Liver disease | 1.86 (0.30–6.1) | 0.43 | 1.26 (0.25–4.0) | 0.75 |
| Immunosuppression | 1.22 (0.20–3.9) | 0.78 | 1.21 (0.37–7.4) | 0.78 |
| ≥1 risk score | 14.7 (3.2–162) | <0.0001 | 4.68 (1.91–15.4) | 0.0002 |
| O2 saturation, % | 1.08 (0.93–1.31) | 0.31 | 1.0 (0.87–1.13) | 0.88 |
| Troponin I, ng/L | 0.98 (0.73–1.03) | 0.74 | 1.06 (1.01–1.09) | 0.01 |
| BNP, pg/mL | 1.008 (1.004–1.02) | 0.0008 | 1.006 (1.002–1.009) | 0.005 |
| Echocardiography | ||||
| EF (%) | 0.98 (0.94–1.05) | 0.69 | 1.00 (0.95–1.05) | 0.95 |
| LVEDD index, mm/m2 | 1.05 (0.95–1.15) | 0.25 | 1.04 (0.96–1.12) | 0.31 |
| LVESD index, mm/m2 | 1.04 (0.93–1.15) | 0.47 | 1.05 (0.96–1.15) | 0.28 |
| LV mass index, gram/m2 | 1.01 (1.00–1.03) | 0.02 | 1.01 (1.00–1.03) | 0.01 |
| LA volume index, ml/m2 | 1.03 (1.01–1.06) | 0.007 | 1.03 (1.01–1.05) | 0.002 |
| RVEDA index, cm2/m2 | 1.1 (0.93–1.29) | 0.24 | 1.01 (0.89–1.16) | 0.78 |
| RVESA, cm2/m2 | 0.98 (0.73–1.28) | 0.90 | 0.84 (0.64–1.08) | 0.18 |
| TAPSE, cm | 0.22 (0.11–0.45) | <0.0001 | 0.26 (0.15–0.47) | <0.0001 |
| RV S′, cm/s | 0.84 (0.73–0.96) | 0.01 | 0.90 (0.80–1.01) | 0.09 |
| RVFAC, % | 1.02 (0.98–1.06) | 0.32 | 1.02 (0.98–1.06) | 0.23 |
| Stroke volume index, cc/m2 | 0.98 (0.94–1.02) | 0.41 | 0.98 (0.95–1.02) | 0.45 |
| Cardiac index, L/m2 | 0.97 (0.66–1.08) | 0.74 | 0.98 (0.77–1.07) | 0.85 |
| E wave velocity, cm/s | 1.01 (1.00–1.03) | 0.01 | 1.01 (1.00–1.03) | 0.02 |
| A wave velocity, cm/s | 1.02 (1.00–1.04) | 0.01 | 1.02 (1.01–1.04) | 0.0001 |
| E/A ratio | 1.00 (0.42–2.1) | 0.98 | 0.61 (0.26–1.27) | 0.19 |
| e′ septal, cm/s | 0.76 (0.64–0.90) | 0.001 | 0.81 (0.69–0.93) | 0.003 |
| e′ lateral, cm/s | 0.79 (0.69–0.89) | <0.0001 | 0.82 (0.74–0.9) | <0.0001 |
| E/e′ average ratio | 1.08 (1.04–1.12) | 0.0005 | 1.07 (1.03–1.10) | 0.001 |
| RA pressure, mmHg | 1.12 (1.04–1.2) | 0.007 | 1.10 (1.03–1.18) | 0.009 |
| SPAP, mmHg͌ | 1.06 (1.02–1.11) | 0.006 | 1.03 (0.99–1.07) | 0.07 |
| PAT, msec | 0.97 (0.96–0.98) | <0.0001 | 0.97 (0.96–0.98) | <0.0001 |
Abbreviations - HR, hazard ratio; CI, confidence interval; IHD, ischemic heart disease; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; DM, diabetes mellitus; HTN, hypertension; BNP, brain natriuretic peptide; LV, left ventricular; EF, ejection fraction; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; LA, left atrial; RV, right ventricle; RVEDA, right ventricular end diastolic area; RVESA, right ventricular end systolic area; TAPSE, tricuspid annular plane systolic excursion; RVFAC, right ventricular fractional area change; RA, right atrial; SPAP, systolic pulmonary peak pressure; PAT, pulmonary acceleration time.
3.2. Multivariate analysis and contingency tables
In multivariate Cox hazard analysis, E/e′, PAT and TAPSE were the only echocardiographic parameters independently associated with the combined outcome (Table 3 ). The prevalence of abnormal TAPSE (<1.8 cm), E/e′ (>8), and PAT (<90 msec) were 18%, 60%, and 45%, respectively. Stepwise addition of these echocardiographic parameters to the nominal clinical risk factor improved the multivariate model's prediction (Akaike information criterion decreased from 578 to 450, P = 0.005). The stepwise addition of either E/e′, TAPSE, or PAT, separately, to the nominal clinical risk factor, resulted in improved prediction of the multivariate model as well (p = 0.05, p < 0.0001, and p = 0.001, respectively; Table 3). The results of contingency tables for models incorporating the nominal clinical risk factors alone, or combined with echocardiography, are shown in Supplementary Table 3. The addition of echocardiography, so patients were categorized as high-risk only if having both ≥1 risk factor for clinical deterioration, and either TAPSE, PAT, or E/e′ high-risk imaging features, reclassified more individuals without events as low-risk and improved specificity, positive predictive value, and accuracy of the models compared with the nominal clinical score alone. The addition of either TAPSE, PAT, or E/e′, so patients were categorized as high-risk if having either ≥1 risk factor for clinical deterioration, or any of the high-risk imaging features, reclassified more individuals with events as high-risk and thus increasing sensitivity (4 patients, 4 patients, but only 1 patient, for TAPSE, PAT, and E/e′, respectively), increasing sensitivity. The addition of either PAT or E/e′, but not TAPSE, came with the expense of decreasing accuracy, specificity, and positive predictive value. Results of analyses of only the 136 patients with mild COVID-19 for the composite outcome, using clinical and echocardiographic parameters, are shown in the supplementary text, and Tables 4 and 5. Results for short-term outcome analyses are shown in the supplementary text, and in supplementary tables 6 and 7.
Table 3.
Univariate and multivariate Cox hazard analyses for the prediction of the combined outcome using different combinations of clinical and echocardiographic risk factors.
| Variable | Univariate analysis (HR) |
Multivariate analysis (HR) |
|---|---|---|
| Composite outcome- ≥ 1 risk factor | ||
| ≥1 clinical risk factor for deterioration | 4.68 (1.91–15.4); p = 0.0002 | |
| χ2 for model | 13.7 | |
| P-value for model | 0.0002 | |
| AIC | 578.1 | |
| Composite outcome- Echocardiography alone | ||
| LV mass index, gram/m2 | 1.01 (1.00–1.03); p = 0.01 | NS |
| LA volume index, ml/m2 | 1.03 (1.01–1.05); p = 0.002 | NS |
| E/e′ | 1.07 (1.03–1.10); p = 0.001 | 1.05 (1.007–1.09); p = 0.01 |
| RA pressure, mmHg | 1.10 (1.03–1.18); p = 0.009 | NS |
| PAT, msec | 0.97 (0.96–0.98); p < 0.0001 | 0.98 (0.97–1.00); p = 0.05 |
| TAPSE | 0.26 (0.15–0.47); p < 0.0001 | 0.40 (0.21–0.76); p = 0.005 |
| χ2 for model | 27.8 | |
| P-value for model | <0.0001 | |
| AIC | 451.0 | |
| P-value for LogLik | 0.0001 | |
| Composite outcome- ≥ 1 risk factor | ||
| ≥1 clinical risk factor for deterioration | 4.68 (1.91–15.4); p = 0.0002 | 2.41 (0.89–8.5); p = 0.08 |
| E/e′ | 1.07 (1.03–1.10); p = 0.001 | 1.04 (1.00–1.09); p = 0.05 |
| PAT | 0.97 (0.96–0.98); p < 0.0001 | NS |
| TAPSE | 0.26 (0.15–0.47); p < 0.0001 | 0.43 (0.23–0.81); p = 0.009 |
| χ2 for model | 30.9 | |
| P-value for model | <0.0001 | |
| AIC | 450.0 | |
| P-value for LogLik | 0.005 | |
| Composite outcome- ≥ 1 risk factor and E/e′ | ||
| ≥1 clinical risk factor for deterioration | 4.68 (1.91–15.4); p = 0.0002 | 3.36 (1.28–11.5); p = 0.02 |
| E/e′ > 8 | 2.47 (1.42–4.3); p = 0.001 | 1.80 (1.01–3.26); p = 0.04 |
| χ2 for model | 17.9 | |
| P-value for model | 0.0002 | |
| AIC | 537.1 | |
| P-value for LogLik | 0.05 | |
| Composite outcome- ≥ 1 risk factor and PAT | ||
| ≥1 clinical risk factor for deterioration | 4.68 (1.91–15.4); p = 0.0002 | 3.35 (1.34–11.2); p = 0.0004 |
| PAT <90 msec | 6.8 (3.0–19.8); p < 0.0001 | 5.4 (2.3–15.6); p < 0.0001 |
| χ2 for model | 34.2 | |
| P-value for model | <0.0001 | |
| AIC | 512.1 | |
| P-value for LogLik | <0.0001 | |
| Composite outcome- ≥ 1 risk factor and TAPSE | ||
| ≥1 clinical risk factor for deterioration | 4.68 (1.91–15.4); p = 0.0002 | 3.08 (1.22–10.3); p = 0.03 |
| TAPSE <1.8 cm | 3.7 (2.0–6.7); p < 0.0001 | |
| χ2 for model | 27.9 | |
| P-value for model | <0.0001 | |
| AIC | 481.3 | |
| P-value for LogLik | 0.001 | |
| Composite outcome- ≥ 1 risk factor and Troponin | ||
| ≥1 clinical risk factor for deterioration | 4.68 (1.91–15.4); p = 0.0002 | 3.79 (0.04–0.86); p = 0.02 |
| Troponin, ng/L | 1.06 (1.01–1.09); p = 0.01 | 1.08 (1.03–1.12); p = 0.004 |
| χ2 for model no Troponin | 3.1 | |
| AIC model no Troponin | 495 | |
| χ2 for the entire model | 11.2 | |
| P-value for model | 0.003 | |
| AIC | 489.2 | |
| P-value for LogLik | 0.005 | |
| Composite outcome- ≥ 1 risk factor and BNP | ||
| ≥1 clinical risk factor for deterioration | 4.68 (1.91–15.4); p = 0.0002 | 2.34 (0.93–7.8); p = 0.07 |
| BNP, pg/mL | 1.006 (1.002–1.009); p = 0.005 | 1.006 (1.001–1.009); p = 0.008 |
| χ2 for model no BNP | 4.3 | |
| AIC model no BNP | 405.7 | |
| χ2 for the entire model | 11.2 | |
| P-value for model | 0.003 | |
| AIC | 400.8 | |
| P-value for LogLik | 0.01 | |
Abbreviations - HR, hazard ratio; AIC, Akaike information criterion; LV, left ventricular; LA, left atrial; NS, non-significant; RA, right atrial; PAT, pulmonary acceleration time; TAPSE, tricuspid annular plane systolic excursion; Loglik, log-likelihood.
4. Discussion
We analyzed the additive predictive value of echocardiography on top of clinical risk factors for deterioration to death or respiratory failure in hospitalized patients presenting with mild/moderate COVID-19 infection. Our main findings are: (1) In patients presenting with mild/moderate COVID-19, multiple echocardiographic parameters at presentation are predictors of mortality or respiratory failure; (2) E/e′, PAT, and TAPSE have additive predictive value on top of clinical risk factors; (3) Although rare, some patients presenting with mild/moderate disease and no clinical risk factors, may still deteriorate to a need for ventilation or death; (4) A very limited echocardiographic examination is sufficient to develop an improved strategy for risk stratification in patients presenting with mild/moderate COVID-19 infection; (5) In patients presenting with mild COVID-19, TAPSE has additive predictive value on top of clinical risk factors.
4.1. Echocardiographic evaluation in patients with COVID-19
Although the American and European societies recognize the importance of echocardiographic assessment of patients with COVID-19, the amount of data collected prospectively for patients with mild/moderate infection is very limited [[23], [24], [25], [26], [27], [28], [29]]. Half of the patients in the present cohort appeared in our previous publication [14], in which we reported on the non-invasive hemodynamic and echocardiographic results of all the 530 patients with COVID-19 admitted to our institution. Nevertheless, in the present study, we focus only on patients presenting with mild/moderate disease and with a longer follow-up period, allowing us to evaluate the independent predictive ability of echocardiography combined with clinical parameters for mortality or respiratory deterioration in these selected patients. Numerous echocardiographic parameters were associated with adverse outcomes in non-adjusted analyses. All these parameters are known associates of disturbed RV hemodynamics (elevated preload or afterload), RV contraction, or elevated left filling pressure. We have previously shown that worsening echocardiographic RV functional parameters and increasing RV preload and afterload are associated with worsening pulmonary involvement [14]. Furthermore, in contrast to LV dysfunction, all indexes of RV dysfunction were poorer in COVID-19 patients, especially with elevated troponin, worsening disease grade, or clinical deterioration. The most common abnormal echocardiographic patterns in deteriorating patients were RV dilatation and dysfunction and shortened AT, while LV systolic and diastolic functions were normal. Several recently published seminal studies showed similar findings. Li et al., in their work on 120 consecutive COVID-19 patients, have shown that RV parameters such as TAPSE and RV fractional area change were associated with mortality, but also showed that RV longitudinal strain was even more accurate for prognostic assessment [30]; D'Andrea et al., found that mean pulmonary artery pressure and TAPSE were the only independent echocardiography parameters to predict in-hospital death in COVID-19 patients [31]; and Pagnesi et al. showed that in non-ICU COVID-19 patients TAPSE was associated with pulmonary hypertension; And that, interestingly, pulmonary hypertension and not isolated right ventricular dysfunction was associated with all-cause death or ICU admission in this study [32]. Interestingly, Silverio et al., also showed that echocardiography can be a useful tool for risk stratification of COVID-19 patients and that abnormal TAPSE is associated with higher in-hospital mortality. However, unlike the other studies reviewed, they also demonstrated a prognostic significance for poor LVEF among COVID-19 patients [33]. In the present study, including only patients with mild/moderate disease, thus by definition without de-saturation at presentation, these parameters were still predictive of adverse outcomes, suggesting that echocardiographic parameters may be used as early markers for pulmonary deterioration, preceding the decrease in oxygen saturation (the hallmark of severe disease). We also showed that TAPSE was associated with outcome, even in patients with mild COVID-19 at presentation (with no radiographic evidence of lower respiratory tract disease and normal O2 saturation). In fact, TAPSE was superior to the nominal clinical risk parameter, suggesting that it may be the earliest sign of pulmonary deterioration, preceding even radiographic evidence of lower tract disease [34].
4.2. Contingency tables
The cutoff values for TAPSE (<18 mm), E/e′ (>8), and PAT (<90 msec) were similar to those described for the general population [19,20,35]. The results of contingency tables for models incorporating the nominal clinical risk parameter, with or without echocardiography, are presented in Supplementary Table 3. They show that with the addition of echocardiography, so patients were categorized as high risk only if having both high-risk ≥1 clinical risk factor, and ≥1 echocardiographic risk factor, reclassified more individuals without events as low risk (from 69 to 106 patients), and improved specificity, positive predictive value, and accuracy of the models compared with the nominal clinical risk factor alone. In simple words, the addition of echocardiography in patients with ≥1 clinical risk factor decreases the rate of falsely identifying patients as high risk to deteriorate by approximately three quarters (from 154 to 36) and can improve resource allocation, which may become especially important once expensive new anti-COVID-19 agents are approved in low-income countries. In fact, the addition of TAPSE alone, so patients were categorized as high risk only if having both high-risk ≥1 clinical risk factor and TAPSE<1.8 cm, was similar to the model using all echocardiographic parameters, suggesting that using only TAPSE is sufficient.
The addition of echocardiography, so patients were categorized as high risk if having either high-risk ≥1 clinical risk factor or ≥1 high-risk imaging feature, reclassified another five individuals with events as high risk, increasing the sensitivity of the combined model to 100% (all 55 patients with clinical deterioration were classified as high risk by the combined model). Importantly, this was done without decreasing accuracy, specificity, or positive predictive value. In clinical terms, it seems that performing echocardiography in patients without risk factors for deterioration adds to further identification of those at high risk. In fact, the addition of either TAPSE, or PAT alone, so patients were categorized as high risk only if having both high-risk ≥1 clinical risk factor, and TAPSE<1.8 cm or PAT<90 msec, was almost similar to the model using all echocardiographic parameters (only one patient deteriorating to non-invasive ventilation was classified as low risk in such models), suggesting that using only TAPSE, or PAT is probably sufficient.
As for now, the use of the novel oral therapeutic agents is recommended only in patients with mild-moderate disease and clinical risk factors for deterioration [14]. The clinical risk factors mentioned in these reports [6,9,10] include older age, diabetes, hypertension, ischemic heart disease, renal dysfunction, COPD, liver disease, immunosuppression, and obesity. Our data suggest that by adding a simple, focused echocardiography exam, one can better stratify patients' risk with improved sensitivity and specificity and offer a better allocation of resources. For example, high-risk echocardiography parameters in a patient with no clinical risk factor for clinical deterioration suggest that, although not indicated by current guidelines, novel oral therapeutic agents should be considered to prevent the development of severe COVID-19 disease. On the other side, in patients with ≥1 clinical risk factor and normal echocardiography the risk of deterioration is markedly lower, thus can improve resource allocation in low-income countries. Also, this important information, gained from a very limited and focused echocardiography exam, suggests bedside echocardiography as an effective tool to better triage ambulatory patients, who are the main target of these therapeutic agents [14].
4.3. Study limitations
Our study included only patients with COVID-19 infection who were hospitalized. The fact that, at the time of the study, only 10% of identified patients and 50% of patients presenting to the emergency room with COVID-19 infection were admitted to the hospital may lead to an overestimation of the impact of echocardiography on patient selection. Given the low number of events in the mild cohort and in patients without clinical risk factors, the algorithms presented in the manuscript are liable to overfitting; thus, their prognostic value will need external validation prior to clinical use. The fact that, in some cases, echocardiographic parameters were measured by the cardiologist caring for the patient may lead to bias.
5. Conclusions
We describe a cohort of echocardiographic studies in mild/moderate COVID-19 patients. A very limited echocardiogram evaluation in patients with ≥1 clinical risk factor is sufficient to achieve maximal clinical value for risk stratification.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' statement
All author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Authors' contributions
Y.T is the guarantor of this study and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. L.L, A.B and Y.T researched data and wrote the manuscript; L.L, A.B, Y·S, Y.L, P.T, O·S, Y.G, I.M, E.G, A.B, S·S, E.L contributed to data curation. Y.T, A.H- Statistics; M.L.P and S·B contributed to the discussion and reviewed and edited the manuscript. All authors reviewed and provided edits and comments on the manuscript, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2022.09.079.
Appendix A. Supplementary data
Supplementary material
References
- 1.CDC COVID-19 and Your Health, Cent. Dis. Control Prev. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed November 17, 2021)
- 2.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 3.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., Tie Y., Fullerton K.E. Coronavirus disease 2019 Case surveillance - United States, January 22-May 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S.J.W., Smeeth L., Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., Oguchi G., Ryan P., Nielsen B.U., Brown M., Hidalgo A., Sachdeva Y., Mittal S., Osiyemi O., Skarbinski J., Juneja K., Hyland R.H., Osinusi A., Chen S., Camus G., Abdelghany M., Davies S., Behenna-Renton N., Duff F., Marty F.M., Katz M.J., Ginde A.A., Brown S.M., Schiffer J.T., Hill J.A. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Xiao J., Hooper A.T., Hamilton J.D., Musser B.J., Rofail D., Hussein M., Im J., Atmodjo D.Y., Perry C., Pan C., Mahmood A., Hosain R., Davis J.D., Turner K.C., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Roque-Guerrero L., Acloque G., Aazami H., Cannon K., Simón-Campos J.A., Bocchini J.A., Kowal B., DiCioccio A.T., Soo Y., Geba G.P., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D., Trial investigators REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Falci D.R., Sarkis E., Solis J., Zheng H., Scott N., Cathcart A.L., Hebner C.M., Sager J., Mogalian E., Tipple C., Peppercorn A., Alexander E., Pang P.S., Free A., Brinson C., Aldinger M., Shapiro A.E. COMET-ICE investigators, early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 9.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., Du J., Pedley A., Assaid C., Strizki J., Grobler J.A., Shamsuddin H.H., Tipping R., Wan H., Paschke A., Butterton J.R., Johnson M.G., De Anda C., MOVe-OUT Study Group Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfizer's Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study | Pfizer, (n.d.). https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate (accessed November 17, 2021).
- 11.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I., Gal Oz A., Rothschild E., Baruch G., Peri Y., Arbel Y., Topilsky Y. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szekely Y., Lichter Y., Hochstadt A., Taieb P., Banai A., Sapir O., Granot Y., Lupu L., Merdler I., Ghantous E., Borohovitz A., Sadon S., Oz A.G., Ingbir M., Arbel Y., Laufer-Perl M., Banai S., Topilsky Y. The predictive role of combined cardiac and lung ultrasound in coronavirus disease 2019. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021;34:642–652. doi: 10.1016/j.echo.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banai A., Taieb P., Furie N., Hochstadt A., Merdler I., Sapir O., Granot Y., Lupu L., Ghantous E., Borohovitz A., Gal-Oz A., Ingbir M., Arbel Y., Banai S., Topilsky Y., Lichter Y., Szekely Y. COVID-19, a tale of two peaks: patients’ characteristics, treatments, and clinical outcomes. Intern. Emerg. Med. 2021;16:1629–1639. doi: 10.1007/s11739-021-02711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taieb P., Szekely Y., Lupu L., Ghantous E., Borohovitz A., Sadon S., Lichter Y., Ben-Gal Y., Banai A., Hochstadt A., Merdler I., Sapir O., Granot Y., Laufer-Perl M., Banai S., Topilsky Y. Risk prediction in patients with COVID-19 based on haemodynamic assessment of left and right ventricular function. Eur. Heart J. Cardiovasc. Imaging. 2021;22:1241–1254. doi: 10.1093/ehjci/jeab169. [DOI] [PubMed] [Google Scholar]
- 15.Information on COVID-19 Treatment, Prevention and Research, COVID-19 Treat. Guidel. (n.d.). https://www.covid19treatmentguidelines.nih.gov/ (accessed June 27, 2022).
- 16.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J. Am. Coll. Cardiol. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell C., Collins K., Hua L., McClanahan C., Shea E., Umland M., Wasserman M. Specific considerations for sonographers when performing echocardiography during the 2019 Novel coronavirus outbreak: supplement to the American Society of Echocardiography Statement. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:654–657. doi: 10.1016/j.echo.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johri A.M., Galen B., Kirkpatrick J.N., Lanspa M., Mulvagh S., Thamman R. ASE statement on point-of-care ultrasound during the 2019 novel coronavirus pandemic. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:670–673. doi: 10.1016/j.echo.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., Dokainish H., Edvardsen T., Flachskampf F.A., Gillebert T.C., Klein A.L., Lancellotti P., Marino P., Oh J.K., Popescu B.A., Waggoner A.D. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Topilsky Y., Khanna A.D., Oh J.K., Nishimura R.A., Enriquez-Sarano M., Jeon Y.B., Sundt T.M., Schaff H.V., Park S.J. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 22.Kitabatake A., Inoue M., Asao M., Masuyama T., Tanouchi J., Morita T., Mishima M., Uematsu M., Shimazu T., Hori M., Abe H. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–309. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 23.Jain S.S., Liu Q., Raikhelkar J., Fried J., Elias P., Poterucha T.J., DeFilippis E.M., Rosenblum H., Wang E.Y., Redfors B., Clerkin K., Griffin J.M., Wan E.Y., Abdalla M., Bello N.A., Hahn R.T., Shimbo D., Weiner S.D., Kirtane A.J., Kodali S.K., Burkhoff D., Rabbani L.E., Schwartz A., Leon M.B., Homma S., Di Tullio M.R., Sayer G., Uriel N., Anstey D.E. Indications for and findings on transthoracic echocardiography in COVID-19. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:1278–1284. doi: 10.1016/j.echo.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Wang B., Zhou J., Kirkpatrick J., Xie M., Johri A.M. Bedside focused cardiac ultrasound in COVID-19 from the Wuhan epicenter: the role of cardiac point-of-care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:676–682. doi: 10.1016/j.echo.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyls C., Bohbot Y., Huette P., Abou-Arab O., Mahjoub Y. Tricuspid longitudinal annular displacement for the assessment of right ventricular systolic dysfunction during prone positioning in patients with COVID-19. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:1055–1057. doi: 10.1016/j.echo.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchill T.W., Bertrand P.B., Bernard S., Namasivayam M., Churchill J., Crousillat D., Davis E.F., Hung J., Picard M.H. Echocardiographic features of COVID-19 illness and association with cardiac biomarkers. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sud K., Vogel B., Bohra C., Garg V., Talebi S., Lerakis S., Narula J., Argulian E. Echocardiographic findings in patients with COVID-19 with significant myocardial injury. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:1054–1055. doi: 10.1016/j.echo.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon S.R., De Francis G., Schwartz S., Duvall W.L., Arora B., Silverman D.I. Tablet-based limited echocardiography to reduce sonographer scan and decontamination time during the COVID-19 pandemic. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020;33:895–899. doi: 10.1016/j.echo.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., Kritek P.A., West T.E., Luks A., Gerbino A., Dale C.R., Goldman J.D., O’Mahony S., Mikacenic C. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Li H., Zhu S., Xie Y., Wang B., He L., Zhang D., Zhang Y., Yuan H., Wu C., Sun W., Zhang Y., Li M., Cui L., Cai Y., Wang J., Yang Y., Lv Q., Zhang L., Xie M. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc. Imaging. 2020;13:2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Andrea A., Scarafile R., Riegler L., Liccardo B., Crescibene F., Cocchia R., Bossone E. Right ventricular function and pulmonary pressures as independent predictors of survival in patients with COVID-19 pneumonia. JACC Cardiovasc. Imaging. 2020;13:2467–2468. doi: 10.1016/j.jcmg.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagnesi M., Baldetti L., Beneduce A., Calvo F., Gramegna M., Pazzanese V., Ingallina G., Napolano A., Finazzi R., Ruggeri A., Ajello S., Melisurgo G., Camici P.G., Scarpellini P., Tresoldi M., Landoni G., Ciceri F., Scandroglio A.M., Agricola E., Cappelletti A.M. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart Br. Card. Soc. 2020;106:1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverio A., Di Maio M., Scudiero F., Russo V., Esposito L., Attena E., Pezzullo S., Parodi G., D’Andrea A., Damato A., Silvestro A., Iannece P., Bellino M., Di Vece D., Borrelli A., Citro R., Vecchione C., Galasso G. Clinical conditions and echocardiographic parameters associated with mortality in COVID-19. Eur. J. Clin. Investig. 2021;51 doi: 10.1111/eci.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N. Engl. J. Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 35.Tossavainen E., Söderberg S., Grönlund C., Gonzalez M., Henein M.Y., Lindqvist P. Pulmonary artery acceleration time in identifying pulmonary hypertension patients with raised pulmonary vascular resistance. Eur. Heart J. Cardiovasc. Imaging. 2013;14:890–897. doi: 10.1093/ehjci/jes309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material