Objective:
To evaluate whether COVID-19 vaccination status or mode of anesthesia modified the temporal harms associated with surgery following coronavirus disease-2019 (COVID-19) infection.
Background:
Surgery shortly after COVID-19 infection is associated with higher rates of complications, leading to recommendations to delay surgery following COVID-19 infection when possible. However, prior studies were based on populations with low or no prevalence of vaccination.
Methods:
A retrospective cohort study of patients who underwent scheduled surgery in a health system from January 1, 2018 to February 28, 2022 (N=228,913) was performed. Patients were grouped by time of surgery relative to COVID-19 test positivity: 0 to 4 weeks after COVID-19 (“early post-COVID-19”), 4 to 8 weeks after COVID-19 (“mid post-COVID-19”), >8 weeks after COVID-19 (“late post-COVID-19”), surgery at least 30 days before subsequent COVID-19 (“pre-COVID-19”), and surgery with no prior or subsequent test positivity for COVID-19.
Results:
Among patients who were not fully vaccinated at the time of COVID-19 infection, the adjusted rate of perioperative complications for the early post-COVID-19 group was significantly higher than for the pre-COVID-19 group (relative risk: 1.55; P=0.05). No significantly higher risk was identified between these groups for patients who were fully vaccinated (0.66; P=1.00), or for patients who were not fully vaccinated and underwent surgery without general anesthesia (0.52; P=0.83).
Conclusions:
Surgery shortly following COVID-19 infection was not associated with higher risks among fully vaccinated patients or among patients who underwent surgery without general anesthesia. Further research will be valuable to understand additional factors that modify perioperative risks associated with prior COVID-19 infection.
Keywords: COVID-19, health systems, perioperative medicine
As of April 25, 2022, over 80 million people in the United States and 500 million people worldwide have experienced coronavirus disease-2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1
COVID-19 infection has also been shown to adversely impact perioperative morbidity and mortality, with data suggesting that surgery shortly after infection is associated with a significantly elevated risk of complications.2–7 Current guidelines therefore recommend deferring elective surgery for at least 7 weeks after COVID-19 diagnosis among patients who are asymptomatic at the time of surgery, to reduce perioperative morbidity.8,9 On a health systems level, concerns about safely providing surgical care to patients who have recovered from COVID-19 have compounded already significant challenges in providing standard surgical care throughput during COVID-19 surges10 and further impacted the surgical backlogs that resulted from COVID-19 mitigation strategies.11–13
Guidelines regarding the timing of elective surgery following COVID-19 infection are largely informed by 2 retrospective cohort studies that reported higher rates of cardiopulmonary complications and mortality among patients who underwent surgery soon after COVID-19 infection.6,7 However, these earlier studies predated the widespread availability of COVID-19 vaccinations. While vaccination generally reduces postinfection morbidity,14–17 including after surgery,18 the effect of vaccination on the appropriate length of time between infection and surgery is unknown. In addition, while alternatives to general anesthesia such as monitored anesthesia care, regional, or neuraxial anesthesia may mitigate perturbations of respiratory physiology, thus decreasing the risk of pulmonary complications in tenuous patients19 and avoiding unnecessary aerosolization during procedures,20 the effect of general anesthesia on the temporal risk of perioperative harm after COVID-19 infection is not known.
In this retrospective cohort study, we examined scheduled surgeries performed at a large, integrated health care system to evaluate the association between the timing of surgery following COVID-19 infection and adverse surgical outcomes in a contemporary cohort, specifically examining whether vaccination status or mode of anesthesia might impact this association. In addition, we evaluated whether the timing of surgery following COVID-19 infection was associated with measures of health care utilization, including readmission and emergency department (ED) use.
METHODS
This retrospective study was deemed exempt by the Kaiser Permanente Institutional Review Board.
Identification of Scheduled Surgical Cases
Within Kaiser Permanente Northern California, all hospitals and clinics employ the same information systems and share a single electronic health record system. Our study population consisted of scheduled surgical cases for adult patients (≥18 y of age), excluding labor and delivery, at 21 Kaiser Permanente Northern California medical centers between January 1, 2020 to February 28, 2022. We identified cases based on electronic health record data entered by the surgeon into the surgical “case request,” which is a prerequisite for scheduling surgical procedures, excluding cases if the surgeon indicated it was an “add on” case or “emergent” and “urgent” cases needing to be performed within 24 or 48 hours from the time of the case request submission. We excluded ophthalmological and interventional pain management procedures due to incomplete sampling within our data systems and cardiac, maxillofacial, podiatry, gynecologic oncology robotics, and transgender procedures due to the small number of such cases among patients with prior COVID-19 infection.
COVID-19 Status Designation
COVID-19 status was based on the first positive Reverse Transcription Polymerase Chain Reaction test taken by the patient within the study period. The date of this first test was assumed to represent the start of COVID-19 infection for the patient, even though the actual date of infection was unknown. We classified patients as severe if they had a diagnosis of COVID-19 and pneumonia (ICD-10 codes U07.1 and J12.89).
We categorized patients into 5 groups based on the time lag between COVID-19 positivity and surgery date (Fig. 1): (1) “early post-COVID-19” if surgery occurred between 0 to <4 wk after COVID-19 infection; (2) “mid post-COVID-19” if surgery occurred between 4 and <8 weeks after COVID-19 infection; (3) “late post-COVID-19” if surgery occurred after 8 wk of COVID-19 infection; and (4) “pre-COVID-19” if occurred at least 30 days prior to COVID-19 infection. A fifth group included patients with no COVID-19 test positivity during the study period (“no COVID-19”). The exposure groups included patients with early, mid, or late post-COVID-19 surgery, while the pre-COVID-19 group was the primary control. The no COVID-19 group was included as a secondary control.
FIGURE 1.
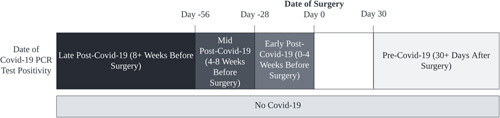
Categories of scheduled surgical cases defined by timing between COVID-19 infection and surgery. PCR indicates polymerase chain reaction.
COVID-19 Vaccination Status
We designated patients as “fully vaccinated” if they were >14 days following receipt of 1 dose of Ad.26.COV2.S or at least 2 doses of BNT162b2 or mRNA-1273 vaccines, based on the definition used by the United States Centers for Disease Control and Prevention.21 Otherwise, patients were designated as “not fully vaccinated.” Vaccine status was based on a lookback from the date of the positive COVID-19 test for patients who underwent surgery after a COVID-19 infection; for control patients, we used the surgery date to establish vaccination status.
Covariates
To control for confounding we used age at time of surgery, sex, admission month, COVID-19 pneumonia, scalar measures of comorbid disease burden and illness severity, mode of anesthesia, and intended postoperative disposition, surgeon specialty, and case class. We quantified comorbid disease burden with a previously validated risk score, the Comorbidity Points Score, Version 2 (COPS2),22 which is based on patients’ medical diagnoses within the 12 months preceding the surgery admission date. We quantified severity of illness with the abbreviated Laboratory-based Acute Physiology Score (abLAPS), which was based on the most physiologically deranged value of 14 laboratory tests over the month before the date surgery admission date.23 The abLAPS score was categorized into 4 groups: unavailable, low (0–4), medium (5–10), and high (>10).24 The mode of anesthesia was determined by the anesthesia provider. Cases were categorized as “general anesthesia” if a general anesthetic was used, even if accompanied by other adjunctive modes of anesthesia or analgesia, including secondary regional or neuraxial techniques, or if a case was converted to general anesthesia during the procedure. Finally, we also included risk factors noted within the case request submission form, including the intended postoperative disposition (ie, inpatient or outpatient), surgical specialty of the primary surgeon, and case class designations that inform scheduling workflows within our system.
Surgical Adverse Outcomes
The primary outcome of interest was a major postsurgical complication within 30 days of surgery including: arrythmia, deep vein thrombosis, pulmonary embolism, pneumonia, respiratory failure, renal failure, sepsis, urinary tract infection.6 We used a previously published method for ascertaining these complications6 by identifying select ICD diagnosis codes (Table, Supplemental Digital Content 1, http://links.lww.com/SLA/E37) across any care setting (hospital, ED, clinic, skilled nursing facility, home health) during hospitalization (if not present on admission) or within 30 days of hospital discharge. Secondary outcomes included a nonelective hospital admission, defined as any hospital admission starting in the ED, or an ED treat-and-release visit, defined as an ED visit without hospital admission, within 30 days of hospital discharge.
Statistical Analysis
Data are presented as mean (SD) and number (percentage). For description, Current Procedural Terminology codes for surgical cases were classified into clinically meaningful categories using the Clinical Classification Software for Services and Procedures, developed by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project.25 We used a generalized linear regression model with a log link and Poisson distribution to evaluate the relative risk of developing any postoperative complication, adjusting for the covariates described above. Our primary analyses compared the early, mid, and late post-COVID-19 groups with the pre-COVID-19 control group. For sensitivity analyses, we compared early, mid, and late post-COVID-19 groups with the no COVID-19 control group. As an additional sensitivity analysis, we limited our population to only surgeries performed after January 1, 2021, given that vaccines were not widely available prior to that date.
This manuscript followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline when summarizing results.26 All statistics were 1-sided with the hypothesis that surgeries occurring after a COVID-19 infection would have a higher risk of complications. Statistical significance was accepted at the P<0.05 level. We used the Dunnett-Hsu multiple comparison adjustment method for comparing the 3 intervention groups against the same control.27 Statistical analyses were performed using SAS version 9.4 (SAS Institute Cary, North Carolina.) and R version 4.0.2 (The R Foundation).
RESULTS
The analytic cohort consisted of 228,913 scheduled surgical cases, 30,316 (4.8%) had COVID-19 before or after their surgery. Among all surgeries, 765 (0.3%) cases occurred within 0 to 4 weeks after COVID-19 (“early post-COVID-19”), 961 (0.4%) occurred within 4 to 8 weeks after COVID-19 (“mid post-COVID-19”), and 9170 (4.0%) occurred >8 weeks after COVID-19 (“late post-COVID-19”). For the control groups, 19,420 (8.5%) of cases were performed in patients who developed COVID-19 30 days or more after surgery (“pre-COVID-19”), while the remaining 198,597 (86.8%) cases were performed in patients who had no prior or subsequent positive COVID-19 test. COVID-19 pneumonia occurred less frequently in the early post-COVID-19 group (2, 0.3%) than the mid post-COVID-19 (9, 0.9%) and late post-COVID-19 (431, 4.7%) groups (Table 1). A cancer case class was more frequent among the early COVID-19 group (139, 18.2%) than with the pre-COVID-19 (2038, 10.5%) or the no COVID-19 (28,092, 14.1%) control groups. Mean age was 51.9 years for early post-COVID-19, 51.1 for mid post-COVID-19, 51.4 for late post-COVID-19, and 51.3 for pre-COVID-19. Patients within the no COVID-19 group were older with a mean age of 57.1. The most common procedure categories across all groups were “Other OR therapeutic procedure on bone” and “Cholecystectomy and common duct exploration,” and “Arthroplasty knee” (Table, Supplemental Digital Content 2, http://links.lww.com/SLA/E37).
TABLE 1.
Characteristics of Scheduled Surgical Cases
| Characteristic | Early Post-COVID-19 (Surgery 0–4 wk After COVID-19), N (%) | Mid Post-COVID-19 Surgery (4–8 wk After COVID-19), N (%) | Late Post-COVID-19 (Surgery at Least 8 wk After COVID-19), N (%) | Pre-COVID-19 (COVID-19 After Surgery), N (%) | No COVID-19, N (%) |
|---|---|---|---|---|---|
| N (%) | 765 (0.3) | 961 (0.4) | 9170 (4.0) | 19,420 (8.5) | 198,597 (86.8) |
| Age, mean (SD) | 51.9 (16.9) | 51.1 (16.6) | 51.4 (16.0) | 51.3 (16.0) | 57.1 (16.7) |
| Male | 318 (41.6) | 396 (41.2) | 3729 (40.7) | 7761 (40.0) | 89,107 (44.9) |
| Case class | |||||
| Cancer | 139 (18.2) | 144 (15.0) | 850 (9.3) | 2038 (10.5) | 28,092 (14.1) |
| Elective | 401 (52.4) | 575 (59.8) | 7066 (77.1) | 14,376 (74.0) | 141,596 (71.3) |
| Occupational medicine | 14 (1.8) | 13 (1.4) | 87 (0.9) | 191 (1.0) | 2040 (1.0) |
| Within 2 wk | 211 (27.6) | 229 (23.8) | 1167 (12.7) | 2815 (14.5) | 26,869 (13.5) |
| Inpatient | 96 (12.5) | 112 (11.7) | 949 (10.3) | 2319 (11.9) | 25,378 (12.8) |
| COVID-19 pneumonia | 2 (0.3) | 9 (0.9) | 431 (4.7) | — | — |
| General anesthesia | 585 (76.5) | 764 (79.5) | 7251 (79.1) | 15,007 (77.3) | 145,833 (73.4) |
| Comorbidity Points Score, Version 2, mean (SD) | 23.3 (29.9) | 23.8 (29.8) | 25.7 (35.1) | 21.5 (28.2) | 23.0 (28.4) |
| Abbreviated laboratory-based acute physiology score | |||||
| Low | 109 (14.2) | 155 (16.1) | 1355 (14.8) | 2779 (14.3) | 29,335 (14.8) |
| Medium | 116 (15.2) | 139 (14.5) | 879 (9.6) | 1787 (9.2) | 17,783 (9.0) |
| High | 85 (11.1) | 142 (14.8) | 846 (9.2) | 1803 (9.3) | 19,830 (10.0) |
| Missing | 455 (59.5) | 525 (54.6) | 6090 (66.4) | 13,051 (67.2) | 131,649 (66.3) |
| Case service | |||||
| Bariatrics | 7 (0.9) | 15 (1.6) | 210 (2.3) | 370 (1.9) | 1986 (1.0) |
| General surgery | 198 (25.9) | 276 (28.7) | 2377 (25.9) | 4987 (25.7) | 51,569 (26.0) |
| Gynecology | 129 (16.9) | 121 (12.6) | 1353 (14.8) | 2855 (14.7) | 22,303 (11.2) |
| Head and neck | 41 (5.4) | 67 (7.0) | 869 (9.5) | 1706 (8.8) | 16,967 (8.5) |
| Neurosurgery | 22 (2.9) | 20 (2.1) | 185 (2.0) | 460 (2.4) | 4940 (2.5) |
| Orthopedics | 198 (25.9) | 217 (22.6) | 2182 (23.8) | 4743 (24.4) | 53,898 (27.1) |
| Plastics | 26 (3.4) | 43 (4.5) | 397 (4.3) | 894 (4.6) | 8518 (4.3) |
| Spine | 12 (1.6) | 22 (2.3) | 161 (1.8) | 441 (2.3) | 4623 (2.3) |
| Thoracic | 10 (1.3) | 14 (1.5) | 105 (1.1) | 204 (1.1) | 2402 (1.2) |
| Urology | 103 (13.5) | 136 (14.2) | 1027 (11.2) | 2116 (10.9) | 25,891 (13.0) |
| Vascular | 19 (2.5) | 30 (3.1) | 304 (3.3) | 644 (3.3) | 5500 (2.8) |
The rates of any perioperative complication in the cohort was 5.0% (Table, Supplemental Digital Content 3, http://links.lww.com/SLA/E37). The rates of nonelective hospitalization and ED treat-and-release within 30 days of discharge were 3.1% and 8.4%, respectively. The overall adjusted rate of perioperative complications in the early, mid, and late post-COVID-19 groups were 4.5%, 3.6%, and 3.8% respectively, which were not significantly different from the pre-COVID-19 group (4.1%) (Fig. 2). Among not fully vaccinated patients, the adjusted rate of perioperative complications was higher among the early post-COVID-19 compared with the pre-COVID-19 group (relative risk: 1.55, P=0.05), though no significant differences were seen for the mid post-COVID-19 (relative risk: 0.94, P=0.95) or late post-COVID-19 (relative risk: 0.85, P=1.00) groups (Table 2). Among not fully vaccinated patients, the adjusted rate of perioperative complications was not significantly different for the mid post-COVID-19 group (0.94, P=0.95) or late post-COVID-19 group (0.85, P=1.00) compared with the pre-COVID-19 group. Among fully vaccinated patients, there was no significant difference in the adjusted rate of perioperative complications between the early post-COVID-19 (relative risk: 0.66, P=1.00), mid post-COVID-19 (0.74, P=1.00), late post-COVID-19 (1.00, P=0.91) compared with the pre-COVID-19 group. Similar results were found after limiting the analysis to cases that were performed after January 2021 (Table, Supplemental Digital Content 4, http://links.lww.com/SLA/E37), with the not fully vaccinated early post-COVID-19 group demonstrating higher risk of perioperative complications compared with the pre-COVID-19 group (relative risk: 1.83, P=0.02).
FIGURE 2.
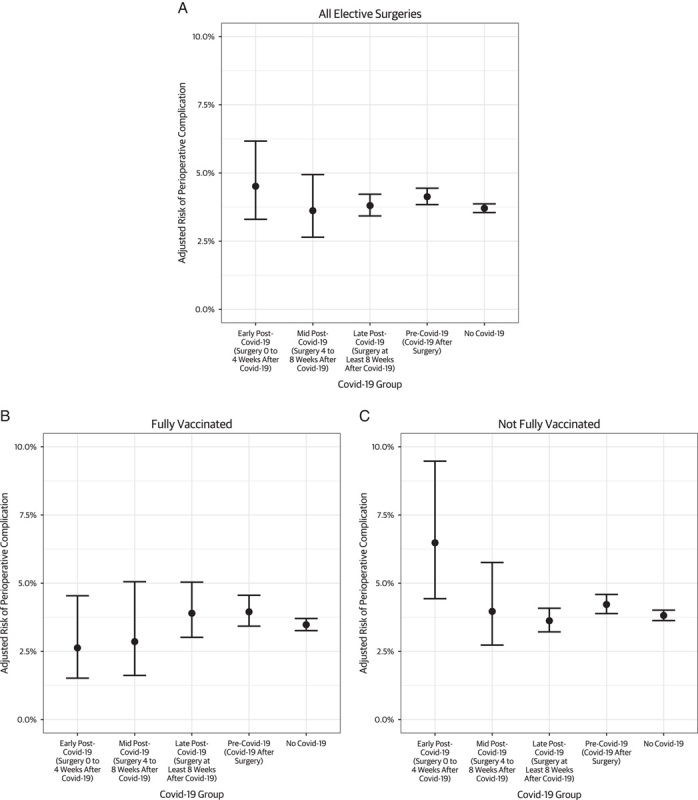
Adjusted risk of perioperative complication, by timing of surgery relative to COVID-19 diagnosis and vaccination status.
TABLE 2.
Comparison of Adjusted Risk of Perioperative Complications of Scheduled Surgeries Performed After COVID-19 Infection With Scheduled Surgeries Performed Before COVID-19 Infection, by COVID-19 Vaccination Status and Mode of Anesthesia
| All | General Anesthesia | No General Anesthesia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Relative Risk | Lower Bound of 1-Sided 95% Confidence Interval | P | Relative Risk | Lower Bound of 1-Sided 95% Confidence Interval | P | Relative Risk | Lower Bound of 1-Sided 95% Confidence Interval | P | |
| Fully vaccinated | ||||||||||
| Early post-COVID-19 (surgery 0–4 wk after COVID-19) | 392 | 0.66 | 0.35 | 1.00 | 0.69 | 0.35 | 1.00 | 0.53 | 0.11 | 0.53 |
| Mid post-COVID-19 (surgery 4–8 wk after COVID-19) | 365 | 0.74 | 0.38 | 1.00 | 0.54 | 0.23 | 1.00 | 1.44 | 0.51 | 1.44 |
| Late Post-COVID-19 (surgery at least 8 wk after COVID-19) | 1210 | 1.00 | 0.72 | 0.91 | 0.98 | 0.68 | 0.91 | 0.98 | 0.51 | 0.98 |
| Pre-COVID-19 (COVID-19 after surgery) | 3916 | Reference group | ||||||||
| Not fully vaccinated | ||||||||||
| Early post-COVID-19 (surgery 0–4 wk after COVID-19) | 373 | 1.55 | 1.00 | 0.05 | 1.84 | 1.17 | 0.01 | 0.52 | 0.11 | 0.83 |
| Mid post-COVID-19 (surgery 4–8 wk after COVID-19) | 596 | 0.94 | 0.61 | 0.95 | 0.87 | 0.54 | 0.98 | 1.32 | 0.52 | 0.93 |
| Late post-COVID-19 (surgery at least 8 wk after COVID-19) | 7960 | 0.85 | 0.73 | 1.00 | 0.89 | 0.74 | 1.00 | 0.75 | 0.52 | 0.29 |
| Pre-COVID-19 (COVID-19 after surgery) | 15,504 | Reference group | ||||||||
Among not fully vaccinated patients, early post-COVID-19 cases involving general anesthesia had a significantly higher adjusted rate of perioperative complications compared with the pre-COVID-19 group involving general anesthesia (relative risk: 1.84, P=0.01) (Table 2). Among not fully vaccinated patients who underwent surgery without general anesthesia, there were no significant differences in the adjusted rates of perioperative complications between the early post-COVID-19 (relative risk: 0.52, P=0.83), mid post-COVID-19 (1.32, P=0.93), late post-COVID-19 (0.75, P=0.29) compared with the pre-COVID-19 group. No significantly elevated risks were detected across groups amongst surgeries with outpatient or surgeries with inpatient intended dispositions (Table, Supplemental Digital Content 5, http://links.lww.com/SLA/E37). Similar results were found in comparisons with the no COVID-19 control group, with the not fully vaccinated early post-COVID-19 group demonstrating higher risk of perioperative complications compared with the no COVID-19 group overall (relative risk: 1.70, P=0.01), particularly amongst those that underwent surgeries involving general anesthesia (2.02, P<0.001) (Table, Supplemental Digital Content 6, http://links.lww.com/SLA/E37). Among both fully vaccinated patients and not fully vaccinated patients, there were no significant differences in the rates of nonelective hospitalization or ED treat-and-release for the early, mid, and late post-COVID-19 groups, compared with the pre-COVID-19 group (Table 3).
TABLE 3.
Comparison of Adjusted Rates of Utilization Measures of COVID-19 Surgery Groups, by COVID-19 Vaccination Status
| Nonelective Hospitalizations | Emergency Department Treat-and-Release | |||||
|---|---|---|---|---|---|---|
| Relative Risk | Lower Bound of 1-Sided 95% Confidence Interval | P | Relative Risk | Lower Bound of 1-Sided 95% Confidence Interval | P | |
| Fully vaccinated | ||||||
| Early post-COVID-19 (surgery 0–4 wk after COVID-19) | 0.78 | 0.38 | 0.99 | 0.60 | 0.37 | 1.00 |
| Mid post-COVID-19 (surgery 4–8 wk after COVID-19) | 0.69 | 0.29 | 0.99 | 0.76 | 0.47 | 1.00 |
| Late post-COVID-19 (surgery at least 8 wk after COVID-19) | 1.05 | 0.70 | 0.79 | 0.97 | 0.76 | 0.95 |
| Pre-COVID-19 (COVID-19 after surgery) | Reference group | |||||
| Not fully vaccinated | ||||||
| Early post-COVID-19 (surgery 0–4 wk after COVID-19) | 1.31 | 0.76 | 0.40 | 1.09 | 0.78 | 0.67 |
| Mid post-COVID-19 (surgery 4–8 wk after COVID-19) | 1.11 | 0.69 | 0.72 | 0.92 | 0.69 | 0.98 |
| Late post-COVID-19 (surgery at least 8 wk after COVID-19) | 0.69 | 0.56 | 1.00 | 0.85 | 0.76 | 1.00 |
| Pre-COVID-19 (COVID-19 after surgery) | Reference group | |||||
DISCUSSION
In this study, we identified an elevated risk of adverse surgical outcomes among patients who were not fully vaccinated at the time of COVID-19 infection and subsequently underwent surgery within 4 weeks. Though limited by the small sample of patients who underwent surgery shortly after COVID-19 infection, we neither detected such an elevated risk among surgical patients who were fully vaccinated at the time of COVID-19 infection, nor did we observe elevated risk among patients who were not fully vaccinated but underwent surgeries without general anesthesia. We also failed to detect any difference in readmissions or ED utilization associated with the timing of COVID-19 infection relative to surgery. To our knowledge, this is the first study of the impact of COVID-19 vaccination and mode of anesthesia on outcomes among patients with prior COVID-19 infection.
Two prior retrospective cohort studies have demonstrated increased risks of adverse perioperative outcomes among patients who underwent surgery soon after COVID-19 diagnosis.6,7 A multicountry and multicenter study of 3127 patients who had COVID-19 infections before elective or urgent surgery found increased risks or mortality and morbidity to be present at 5 to 6 weeks regardless of being symptoms, age, operative severity, or urgency. However, the study period was October 2020, which was prior to the availability of COVID-19 vaccines. A second United States study of 5479 patients who underwent major elective surgery following COVID-19 infection reported higher perioperative complications when surgery was performed within 8 weeks of COVID-19 diagnosis. This study excluded patients with COVID-19 vaccination before surgery or within 90 days after surgery, but did not assess immunization status at the time of COVID-19 infection. Notably the study period of March 2020 to May 2021 ranged from a time of no vaccination availability to until a period fewer than 40% of Americans had achieved full vaccination by US CDC standards.28 We were able to examine data from a recent cohort of scheduled surgical patients through February 28, 2022. The study population captures the variety of adult patients who undergo minor and major elective surgery within a large community health system, and the timeline encompasses the widespread domestic availability of COVID-19 vaccines, as well as the emergence of several variants of interest.29 While our findings were largely consistent with the prior studies, we failed to identify an increased risk of perioperative complications among surgical patients who underwent surgery 4 to 8 weeks after COVID-19 infection, which we suspect may be related to the rapidly evolving nature of the virus.30,31
Determining the optimal timing for scheduled surgery for patients who are recovering from COVID-19 is a clinical and operational challenge for providers and for health care systems at large. Millions of elective surgical procedures were deferred worldwide during the initial waves of the COVID-19 pandemic,11,32 which resulted in systemic backlogs of patient waiting for indicated surgical procedures. As the cumulative prevalence of patients with a previous COVID-19 infection increases, many health systems have struggled to manage the existent population of patients with deferred surgical care and known COVID-19. As the incidence of new COVID-19 infections remain high, individual providers and health systems are further stymied by the clinical and operation challenge of currently scheduled patients with an interval COVID-19 diagnosis. While current national and international guidelines recommend deferring elective surgery for at least 7 weeks following COVID-19 infection, even among asymptomatic patients, our analysis suggests that full vaccination status may negate the association between the timing of COVID-19 infection and risk of perioperative harm with subsequent surgery. Furthermore, among patients who are not fully vaccinated, our study suggests that avoidance of general anesthesia might further mitigate a patient’s temporal risk of harm. In conjunction with considerations for transmission-based precautions,33,34 our data may support liberalizing restrictions on the timing of scheduled surgical care for fully vaccinated patients, and for procedures that can be accomplished without the use of general anesthesia.
Our study has important limitations. First, the adjusted risk estimates had wide confidence intervals, particularly for the group of patients who underwent surgery within 0 to 4 weeks of COVID-19 infection. While we did not find evidence of substantial differences in adjusted complications between certain groups, our study was underpowered to detect more modest differences. While our sample size and methods were similar to the 2 previous retrospective cohort studies on the timing of surgery relative to COVID-19 infection,6,7 further evaluation will be needed as COVID-19 becomes endemic and data on more surgical patients with prior COVID-19 becomes available. Second, although we accounted for many confounders related to severity of illness and surgery, as with all observational studies, our study likely suffers from some residual confounding. Unmeasured factors that influenced the decision to proceed with or delay surgery, such as the urgency of the indication for surgery or substantial residual symptoms following COVID-19 infection such as fatigue, shortness of breath, and chest pain, could not be fully adjusted for.35,36 Similarly, there were likely unmeasured confounders that influenced patients’ decision to receive vaccination, which may be associated with patients’ health seeking behaviors. While a randomized clinical trial would provide the optimal measure of the causal impact of vaccination, such a study is not feasible. Third, while our study investigated how vaccination status and mode of anesthesia influenced risks at different timepoints following COVID-19 infection, there may be other patient or procedure factors that modify these risks, such as a history of chronic obstructive pulmonary disease or lung resections. Our study did not have adequate power to investigate individual comorbidities or procedure types, but we view this as a highly important area for further investigation, as more data becomes available. Fourth, our study used data from a regional integrated health care delivery organization in Northern California. While the findings from our study are broadly consistent with the 2 prior studies, additional validation in other settings are needed.37,38 Fifth, we stratified our analysis between fully vaccinated patients, which likely also included some boosted patients, as well as not fully vaccinated patients, which likely included some partially vaccinated patients. Further study may be needed to evaluate how adverse outcomes differ among partially vaccinated or among boosted populations. Finally, while we used the timing COVID-19 test positivity relative to surgery to define our exposure, the true timing of disease onset may differ from the timing of test positivity. While this may result in some misclassification, the timing of test positivity may be a more objective standard for clinicians to base decisions about the timing of surgery.
In conclusion, surgery performed after COVID-19 infection was not associated with an increased risk of perioperative harm among fully vaccinated patients. While unvaccinated or partially vaccinated patients remained vulnerable to increased rates of perioperative complications within 4 weeks of infection, this risk was not seen among patients who underwent surgeries that were performed without general anesthesia. Provided infection prevention considerations have been satisfied, these data suggest that scheduled surgical interventions can proceed without delay following COVID-19 infection among fully vaccinated patients or among not fully vaccinated patients for whom general anesthesia can be avoided. As the pandemic continues and outcomes data accrue for surgical patients with a history of COVID-19 infection, further research will be valuable to understand other patient or procedure factors that modify the perioperative risks associated with prior COVID-19 infection, and whether these associations persist as immunity wanes and different variants emerge.
Supplementary Material
Footnotes
This work was supported by The Permanente Medical Group. S.T.L. received funding from The Permanente Medical Group Delivery Science Fellowship Program. V.X.L. was supported in part by NIH R35GM128672.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Sidney T. Le, Email: sidney.le@kp.org.
Patricia Kipnis, Email: patricia.kipnis@kp.org.
Bradley Cohn, Email: brad.r.cohn@kp.org.
Vincent X. Liu, Email: vincent.x.liu@kp.org.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBrun DG, Konnaris MA, Ghahramani GC, et al. Hip fracture outcomes during the COVID-19 pandemic: early results from New York. J Orthop Trauma. 2020;34:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonker PKC, van der Plas WY, Steinkamp PJ, et al. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: a Dutch, multicenter, matched-cohort clinical study. Surgery. 2021;169:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng JZ, Chan JS, Potter AL, et al. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann Surg. 2022;275:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborative C, Collaborative G. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76:748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Boghdadly K, Cook TM, Goodacre T, et al. SARS-CoV-2 infection, COVID-19 and timing of elective surgery: a multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. Anaesthesia. 2021;76:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anesthesia Patient Safety Foundation. American Society of Anesthesiologists and Anesthesia Patient Safety Foundation Joint Statement on Elective Surgery and Anesthesia for Patients After COVID-19 Infection. 2022. Available at: https://www.apsf.org/news-updates/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection/. Accessed March 3, 2022.
- 10.Rouillard S, Liu VX, Corley DA. COVID-19 and long-term planning for procedure-based specialties during extended mitigation and suppression strategies. Gastroenterology. 2021;160:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaborative ISR, Surgery ISoC, Training AoSi. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain A, Dai T BK, Myers C. Covid-19 created an elective surgery backlog: how can hospitals get back on track. Harv Bus Rev. 2020;10:2–7. [Google Scholar]
- 13.AbuRahma AF, Campbell J, Stone PA, et al. The correlation of aortic neck length to early and late outcomes in endovascular aneurysm repair patients. J Vasc Surg. 2009;50:738–748. [DOI] [PubMed] [Google Scholar]
- 14.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal JL, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021;385:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386:494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad NK, Lake R, Englum BR, et al. COVID-19 vaccination associated with reduced postoperative SARS-CoV-2 infection and morbidity. Ann Surg. 2022;275:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren J, Sundaram K, Anis H, et al. Spinal anesthesia is associated with decreased complications after total knee and hip arthroplasty. J Am Acad Orthop Surg. 2020;28:e213–e221. [DOI] [PubMed] [Google Scholar]
- 20.Uppal V, Sondekoppam RV, Lobo CA, et al. Practice recommendations on neuraxial anesthesia and peripheral nerve blocks during the COVID-19 pandemic. ASRA/ESRA COVID-19 Guidance for Regional Anesthesia. Anesthesia. 2020;31:1350–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Immunization and Respiratory Diseases. Science Brief: background rationale and evidence for public health recommendations for fully vaccinated people. CDC COVID-19 Science Briefs. Centers for Disease Control and Prevention (US); 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html.
- 22.Escobar GJ, Gardner MN, Greene JD, et al. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51:446–453. [DOI] [PubMed] [Google Scholar]
- 23.Escobar GJ, Greene JD, Scheirer P, et al. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46:232–239. [DOI] [PubMed] [Google Scholar]
- 24.Le ST Corbin D Myers LC et al.. Development and validation of an electronic health record-based score for triage to perioperative medicine [published online November 9, 2021]. Ann Surg. doi: 10.1097/SLA.0000000000005284. [DOI] [PMC free article] [PubMed]
- 25.Sample NI. Healthcare Cost and Utilization in Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2003. [PubMed] [Google Scholar]
- 26.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu J. Multiple Comparisons: Theory and Methods. Boca Raton, Florida: CRC Press; 1996. [Google Scholar]
- 28.Demographic characteristics of people receiving COVID-19 vaccinations in the United States: percent of people receiving COVID-19 by race/ethnicity and date administered, December 14, 2020-May, 2021. Centers for Disease Control and Prevention, eds. Altanta, GA, 2021 Available at: https://www.cdc.gov/mmwr/volumes/70/wr/mm7005e1.htm.
- 29.Parums DV. Revised World Health Organization (WHO) terminology for variants of concern and variants of interest of SARS-CoV-2. Med Sci Monit. 2021;27:e933622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022;327:583–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. J Br Surg. 2020;107:1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Control CfD, Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. 2020.
- 34.Control CfD, Prevention. Ending isolation and precautions for people with COVID-19: Interim guidance. 2021.
- 35.Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. Morbid Mortal Wkly Rep. 2020;69:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. [DOI] [PubMed] [Google Scholar]
- 38.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


