Abstract
Background
To evaluate the association of elevated blood glucose with the risk of acute exacerbations in patients with chronic obstructive pulmonary disease (COPD).
Methods
Totally 526 consecutive patients with COPD recruited between Jan. 2018 and July 2019 were included in this study. Based on the American Diabetes Association’s Standards of Care, these patients were divided into three groups according to HbA1c level: low HbA1c level (HbA1c <5.7%, n=204), moderate HbA1c level (HbA1c 5.7–6.4%, n=165), and high HbA1c level (HbA1c ≥6.5%, n=157). All subjects were followed up for 18 months. Multivariate Cox regression analysis was used to evaluate the predicting value of HbA1c for the time of the next COPD severe exacerbation.
Results
Totally 141 (26.8%) patients in the study had at least 1 severe exacerbation. The proportion of patients suffering from at least 1 severe exacerbation was significantly higher (P<0.01) for patients with high (36.3%) and moderate HbA1c levels (25.5%) compared to those with low HbA1c levels (20.6%). Multivariate Cox regression analysis indicated that high (HR=2.74, 95% CI: 1.70–4.41; P<0.01) and moderate HbA1c levels (HR=2.19, 95% CI: 1.39–3.46; P<0.01) were significantly associated with a higher risk of the next severe exacerbation compared with low HbA1c level, after controlling for potential confounders including age, gender, body mass index (BMI), smoking status, disease duration of COPD, frequency of hospitalization due to acute exacerbation of COPD (AECOPD) in the past 12 months, Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages, COPD assessment test (CAT) score, corticosteroids use, hypertension, and cardiovascular diseases. Subgroup analyses also indicated a significant association between HbA1c levels and risk of the next severe exacerbation in different GOLD stages and diabetes status.
Conclusion
Elevated blood glucose, no matter with or without diabetes, is significantly associated with a higher risk of the next severe exacerbation for patients with COPD.
Keywords: blood glucose, HbA1c, COPD, severe exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive partially reversible airflow obstruction and airways inflammation.1 It is the third leading cause of mortality globally, causing 3.23 million deaths in 2019, and over 80% of these deaths occurred in low- and middle-income countries (LMICs). Acute Exacerbation of COPD (AECOPD) is the leading cause of hospitalization and mortality among COPD patients;2,3 moreover, severe exacerbation is linked to a high risk of early mortality and a median survival of only 3.6 years.4 Thus, identifying and mitigating risk factors for a severe exacerbation is necessary to improve the prognosis of COPD.
AECOPD can be triggered by a multitude of different factors, including respiratory tract infections, various exposures, prior exacerbations, non-adherence to treatment, and associated comorbidities.5 Diabetes mellitus is one of the most frequent and severe comorbidities. The diagnosis rates of Diabetes mellitus among patients hospitalized for AECOPD ranged from 18% in Switzerland6 to 40% in India,7 which were comparatively much higher than those in the general population. Moreover, a large number of studies have reported that COPD patients with diabetes mellitus are at high risk of severe exacerbation and death.8,9 However, little effort has been made to explore the association of hyperglycemia with the risk of severe exacerbation in COPD.
The present study aims to evaluate the effect of hyperglycemia with or without diabetes mellitus on the risk of severe exacerbations in patients with COPD.
Materials and Methods
Patients
This was a prospective, observational study conducted on consecutive patients admitted into our hospital due to exacerbation of COPD between Jan. 2018 and July 2019. Patients aged 40 years and above with an established diagnosis of COPD were included. Diagnosis of COPD and exacerbation of COPD were ascertained according to the definition outlined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines 2018 edition.10 All patients had a post-bronchodilator forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) < 0.7. Exclusion criteria were diagnosis of bronchial asthma or undetermined chronic respiratory conditions, co-existing systemic illness on long-term corticosteroid therapy, malignancy, and pregnancy or within a peripartum period.
Data Collection and Outcomes
A total of 526 patients met the inclusion/exclusion criteria and were divided into three groups according to HbA1c level at admission: low HbA1c level (HbA1c <5.7%, n=204), moderate HbA1c level (HbA1c 5.7–6.4%, n=165), and high HbA1c level (HbA1c ≥6.5%, n=157) based on American Diabetes Association’s Standards of Care.11 Baseline patients’ characteristics including age, sex, body mass index (BMI), smoking habit (never smoker, ex-smoker and smoker), COPD severity (GOLD stages 1–4), COPD assessment test (CAT) score (0–10, 11–20, 21–30, and 31–40), number of prior severe COPD exacerbations in the past 12 months and comorbidities (diabetes, hypertension, and cardiovascular diseases) were recorded. All subjects were followed up for 18 months.
The primary outcome is the occurrence of severe exacerbation during the follow-up, which was measured via telephone interview or medical record review each month. Patients who were lost to follow-up were censored at the moment of the last contact. Severe exacerbation of COPD was defined as requiring a hospital admission of at least 24 h with a discharge diagnosis of COPD exacerbation.
Statistical Analysis
Continuous variables are presented as mean ± SD and compared with the use of One-way Analysis of Variance (ANOVA), followed by post-hoc Turkey analysis. All categorical variables were summarized as counts and percentages and compared by chi-square test or by Fisher’s exact test as appropriate. A Log rank test was used to test differences in time to the next severe exacerbation among three groups which were graphically presented by Kaplan–Meier curves. Multivariate Cox regression analysis was used to evaluate the predicting value of HbA1c for the time of the next COPD severe exacerbation, after controlling for potential confounding factors including age, gender, BMI, smoking status, disease duration of COPD, frequency of hospitalization due to AECOPD in the past 12 months, GOLD stages, CAT score, corticosteroids use, hypertension, and cardiovascular diseases. These covariates have been demonstrated or have the potential to influence the association. The relationship between HbA1c level and the risk of severe exacerbations in different GOLD stages and diabetes status were further evaluated, and additional multivariate Cox regression analyses were performed. All tests were 2-sided and a P value of less than 0.05 was considered significant.
All statistical analyses were performed with the SPSS statistical software program package (SPSS version 20.0 for Windows, Armonk, NY: IBM Corp.).
Results
Among 526 patients, 13 (2.5%) cases were lost to follow-up and regarded as censored data. Clinical characteristics of patients of all the groups are provided in Table 1. It is shown patients with high or moderate HbA1c levels had higher BMI and HbA1c compared with patients with low HbA1c levels (both P<0.01). The proportions of ≥2 hospitalization due to AECOPD in the past 12 months, hypertension, and cardiovascular diseases were significantly more in patients with high or moderate HbA1c levels than those with low HbA1c levels (all P<0.05). Differences in the age, gender, smoking status, disease duration of COPD, GOLD stage, CAT score, and corticosteroid use were not statistically significant among the three groups (all P>0.05).
Table 1.
Patients’ Baseline Characteristics
| Total | HbA1c Level | ||||
|---|---|---|---|---|---|
| Low (<5.7%) | Moderate (5.7–6.4%) | High (≥6.5%) | P value | ||
| N | 526 | 204 | 165 | 157 | |
| Age, years | 67.4±11.7 | 67.4±11.4 | 68.3±11.9 | 66.5±11.9 | 0.37 |
| Gender, n (%) | |||||
| Male | 415 (78.9%) | 160 (78.4%) | 130 (78.8%) | 125 (79.6%) | 0.96 |
| Female | 111 (21.1%) | 44 (21.6%) | 35 (21.2%) | 32 (20.4%) | |
| BMI, kg/m2 | 25.0±2.3 | 24.3±2.3 | 25.1±2.2 | 25.8±1.9 | <0.01 |
| HbA1c, % | 6.4±1.4 | 5.2±0.5 | 6.1±0.2 | 8.2±1.0 | <0.01 |
| Smoking status, n (%) | |||||
| Never-smoker | 157 (29.8%) | 66 (30.4%) | 49 (29.7%) | 46 (29.3%) | 0.52 |
| Ex-smoker | 266 (50.6%) | 99 (48.5%) | 80 (48.5%) | 87 (55.4%) | |
| Smoker | 103 (19.6%) | 43 (21.1%) | 36 (21.8%) | 24 (15.3%) | |
| Disease duration of COPD, n (%) | |||||
| <3 years | 190 (36.1%) | 81 (39.7%) | 56 (33.9%) | 53 (33.8%) | 0.07 |
| 3–5 years | 218 (41.4%) | 89 (43.6%) | 71 (43.0%) | 58 (36.9%) | |
| >5 years | 118 (22.4%) | 34 (16.7%) | 38 (23.0%) | 46 (29.3%) | |
| Frequency of hospitalization due to AECOPD in the past 12 months, n (%) | |||||
| <2 | 400 (76.0%) | 167 (81.9%) | 124 (75.2%) | 109 (69.4%) | 0.02 |
| ≥2 | 126 (24.0%) | 37 (18.1%) | 41 (24.8%) | 48 (30.6%) | |
| GOLD stage, n (%) | |||||
| Stage 1 | 25 (4.8%) | 7 (3.4%) | 10 (6.1%) | 8 (5.1%) | 0.89 |
| Stage 2 | 143 (27.2%) | 59 (28.9%) | 43 (26.1%) | 41 (26.1%) | |
| Stage 3 | 244 (46.4%) | 91 (44.6%) | 78 (47.3%) | 75 (47.8%) | |
| Stage 4 | 114 (21.7%) | 47 (23.0%) | 34 (20.6%) | 33 (21.0%) | |
| CAT score, n (%) | |||||
| 0–10 | 50 (9.5%) | 20 (9.8%) | 15 (9.1%) | 15 (9.6%) | 0.95 |
| 11–20 | 234 (44.5%) | 93 (45.6%) | 71 (43.0%) | 70 (44.6%) | |
| 21–30 | 208 (39.5%) | 81 (39.7%) | 67 (40.6%) | 60 (38.2%) | |
| 31–40 | 34 (6.5%) | 10 (4.9%) | 12 (7.3%) | 12 (7.6%) | |
| Corticosteroids use, n (%) | 420 (79.8%) | 164 (80.4%) | 131 (79.4%) | 125 (79.6%) | 0.97 |
| Comorbidity, n (%) | |||||
| Diabetes | 180 (34.2%) | 5 (2.5%) | 18 (10.9%) | 157 (100%) | <0.01 |
| Hypertension | 210 (39.9%) | 62 (30.4%) | 67 (40.6%) | 81 (51.6%) | <0.01 |
| Cardiovascular diseases | 300 (57.0%) | 95 (46.6%) | 95 (57.6%) | 110 (70.1%) | <0.01 |
Abbreviations: BMI, body mass index; HbA1c, Hemoglobin A1C; COPD, chronic obstructive pulmonary disease; AECOPD, Acute Exacerbation of COPD; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT, COPD assessment test.
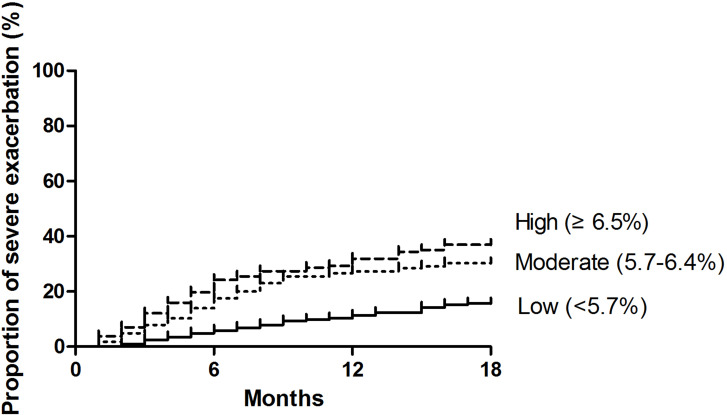
During a follow-up of 18 months, a total of 141 (26.8%) patients in the study had at least 1 severe exacerbation. The proportion of patients suffering from at least 1 severe exacerbation was significantly higher (P<0.01) for patients with high HbA1c levels (36.3%) and moderate HbA1c levels (25.5%) compared to those with low HbA1c levels (20.6%). As shown in Figure 1, the Kaplan–Meier survival curve displayed that compared with those with low HbA1c levels, the risk of the next severe exacerbation of COPD was significantly higher in patients with high HbA1c levels (HR=2.77, 95% CI: 1.80–4.26; P<0.01) or moderate HbA1c levels (HR=2.16, 95% CI: 1.38–3.36; P<0.01).
Figure 1.
Kaplan-Meier survival curves for the time to the next severe exacerbation of chronic obstructive pulmonary disease in patients with high (≥6.5%), median (5.7%-6.4%), and low (≤5.7%) HbA1 levels.
Multivariate Cox proportional hazards regression analysis was performed to explore the association of HbA1c levels with the risk of the next severe exacerbation of COPD (Table 2). It is shown that after adjusting for potential confounding factors, high HbA1c level (HR=2.74, 95% CI: 1.70–4.41; P<0.01) and moderate HbA1c level (HR=2.19, 95% CI: 1.39–3.46; P<0.01) were significantly associated with the risk of the next severe exacerbation compared with low HbA1c level.
Table 2.
Multivariate Cox Regression Analysis Exploring the Association of HbA1c Levels with the Risk of the Next Severe Exacerbation of COPD
| HR | 95% CI | P value | |
|---|---|---|---|
| HbA1c level | |||
| Low (<5.7%) | Reference | ||
| Moderate (5.7–6.4%) | 2.19 | 1.39–3.46 | <0.01 |
| High (≥6.5%) | 2.74 | 1.70–4.41 | <0.01 |
| Age | 1.00 | 0.99–1.02 | 0.57 |
| Gender | |||
| Male | Reference | ||
| Female | 1.20 | 0.80–1.80 | 0.38 |
| BMI | 0.94 | 0.87–1.02 | 0.13 |
| Smoking status | |||
| Never-smoker | Reference | ||
| Ex-smoker | 1.18 | 0.78–1.78 | 0.43 |
| Smoker | 1.09 | 0.66–1.81 | 0.73 |
| Disease duration of COPD, years | |||
| <3 | Reference | ||
| 3–5 | 1.17 | 0.79–1.74 | 0.42 |
| >5 | 0.94 | 0.59–1.48 | 0.78 |
| Frequency of hospitalization due to AECOPD in the past 12 months | |||
| <2 | Reference | ||
| ≥2 | 3.42 | 2.43–4.82 | <0.01 |
| GOLD stage | |||
| Stage 1 | Reference | ||
| Stage 2 | 1.19 | 0.56–2.52 | 0.65 |
| Stage 3 | 0.90 | 0.44–1.84 | 0.77 |
| Stage 4 | 0.79 | 0.36–1.71 | 0.54 |
| CAT score | |||
| 0–10 | Reference | ||
| 11–20 | 0.83 | 0.48–1.43 | 0.50 |
| 21–30 | 0.82 | 0.46–1.46 | 0.50 |
| 31–40 | 0.73 | 0.29–1.81 | 0.50 |
| Corticosteroids use | 1.13 | 0.73–1.77 | 0.59 |
| Comorbidity | |||
| Hypertension | 1.13 | 0.80–1.61 | 0.50 |
| Cardiovascular diseases | 1.10 | 0.77–1.57 | 0.60 |
Abbreviations: BMI, body mass index; HbA1c, Hemoglobin A1C; COPD, chronic obstructive pulmonary disease; AECOPD, Acute Exacerbation of COPD; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT, COPD assessment test.
Subgroup analyses were employed to further evaluate the association of HbA1c levels with the risk of the next severe exacerbation of COPD in different GOLD stages and diabetes status (Table 3). It is shown that higher HBA1c levels were significantly associated with a higher risk of the next severe exacerbation in each subgroup analysis (all P<0.05).
Table 3.
Multivariate Cox Regression Analysis Exploring the Association of HbA1c Levels with the Risk of Next Severe Exacerbation of COPD in Different GOLD Stages and Diabetes Status
| HbA1c Level | HR | 95% CI | P value | |
|---|---|---|---|---|
| GOLD stages* | ||||
| Stage 1–2 (n=168) | Low (<5.7%) | Reference | ||
| Moderate (5.7–6.4%) | 2.20 | 1.01–4.78 | 0.04 | |
| High (≥6.5%) | 2.73 | 1.19–6.29 | 0.02 | |
| Stage 3 (n=244) | Low (<5.7%) | Reference | ||
| Moderate (5.7–6.4%) | 1.84 | 1.11–3.74 | 0.03 | |
| High (≥6.5%) | 2.26 | 1.10–4.62 | 0.03 | |
| Stage 4 (n=114) | Low (<5.7%) | Reference | ||
| Moderate (5.7–6.4%) | 2.26 | 1.16–7.76 | 0.02 | |
| High (≥6.5%) | 3.80 | 1.05–13.8 | 0.04 | |
| Diabetes Status# | ||||
| Diabetes (n=180) | HbA1c as a continuous variable | 1.73 | 1.14–3.39 | 0.01 |
| Non-Diabetes (n=346) | HbA1c as a continuous variable | 1.58 | 1.05–2.38 | 0.03 |
Notes: *Potential confounders including age, gender, BMI, smoking status, disease duration of COPD, frequency of hospitalization due to AECOPD in the past 12 months, CAT score, corticosteroids use, hypertension, and cardiovascular diseases were adjusted. #Potential confounders including age, gender, BMI, smoking status, disease duration of COPD, frequency of hospitalization due to AECOPD in the past 12 months, GOLD stages, CAT score, corticosteroids use, hypertension, and cardiovascular diseases were adjusted.
Discussion
Our study showed that the proportions of patients suffering from at least one severe exacerbation were 36.3% and 25.5% for patients with high and moderateHbA1c levels, respectively, which were significantly higher than those with low HbA1c levels (20.6%). Moreover, compared with low HbA1c levels, both high and moderate HbA1c levels were significantly associated with a higher risk of the next severe exacerbation of COPD.
Compared to the general population, patients with COPD are reported to be at a higher risk of developing diabetes, and the risk increases along with disease severity.12 Elevated glucose concentrations, in turn, can directly stimulate bacteria growth and/or promote interaction between bacteria and the epithelium of the airways.13,14 In patients with COPD, the presence of diabetes may promote unfavorable evolution concerning the resolution of infection. An increasing number of studies have reported that COPD patients with diabetes mellitus are at high risk of severe exacerbation and death. In a prospective, observational study with a maximum follow-up of 18 months, the presence of diabetes mellitus increased the risk of hospital admission due to COPD exacerbations was detected (OR=2.64, 95% CI: 1.09–6.56; P=0.033).9 Another prospective cohort study indicated that COPD patients with diabetes were significantly associated with a higher risk of death (OR=3.38, 95% CI: 1.08–13.89; P=0.037).8
Though the association of diabetes with severe exacerbation in COPD has been well established, the relationship between hyperglycemia and adverse outcome after COPD is still controversial. Our results are in contrast with a previous study,15 in which the Cox regression analysis showed that the levels of HbA1c on admission were not a predictor neither of the time to the next moderate or severe COPD exacerbation (HR=1.00, 95% CI: 0.80–1.25; P=0.98) nor of the time to the next severe COPD exacerbation (HR=0.90, 95% CI: 0.74–1.33; P=0.96). In addition, HbA1c on admission was not a predictor of mortality (HR=0.97, 95% CI: 0.59–1.58; P=0.9). It should be noted that the sample size in this study is just 156, which may be not enough to detect the association of HbA1c levels with adverse outcomes in COPD. In addition, continuous HbA1c levels rather than stratification variables were used as the independent variable, which increased the difficulty of yielding significant association.
This study presents some limitations. Firstly, although the same device was used by the same nurse for all spirometries, variability in spirometry results cannot completely be ruled out. Second, we were unable to collect information on potential confounders, such as inflammation rate, physical activity, functional mobility status, muscle strength, and sociocultural aspects and the influence of further residual confounders cannot be excluded. In addition, HbA1c was evaluated at a single time point on admission and the glucose variation was not considered, which may cause bias in evaluating the association of blood glucose with long-term AECOPD outcome.
In conclusion, the present study showed that elevated blood glucose at admission, no matter with or without diabetes, is significantly associated with a higher risk of the next severe exacerbation for patients with COPD. However, more studies considering the glucose variation and involving more confounders are required to confirm the conclusion.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Affiliated Hospital of Putian University. All procedures were conducted according to the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients gave written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wu L, Lan N, Yang X. Effects of empirical glucocorticoid use on severe acute exacerbation of COPD during hospitalization. Int J Chron Obstruct Pulmon Dis. 2021;16:2419–2431. doi: 10.2147/COPD.S300789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez D, Agusti A, Tabernero E, et al. Effect of a pulmonary embolism diagnostic strategy on clinical outcomes in patients hospitalized for COPD exacerbation: a randomized clinical trial. JAMA. 2021;326(13):1277–1285. doi: 10.1001/jama.2021.14846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogea SP, Tudorache E, Fildan AP, Fira-Mladinescu O, Marc M, Oancea C. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J. 2020;14(3):183–197. doi: 10.1111/crj.13129 [DOI] [PubMed] [Google Scholar]
- 6.Flattet Y, Garin N, Serratrice J, Perrier A, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467–475. doi: 10.2147/COPD.S122382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rambaran K, Bhagan B, Ali A, et al. High prevalence of diabetes mellitus in a cohort of patients with chronic obstructive pulmonary disease in Trinidad, West Indies. Turk Thorac J. 2019;20(1):12–17. doi: 10.5152/TurkThoracJ.2018.18036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castan-Abad MT, Montserrat-Capdevila J, Godoy P, et al. Diabetes as a risk factor for severe exacerbation and death in patients with COPD: a prospective cohort study. Eur J Public Health. 2020;30(4):822–827. doi: 10.1093/eurpub/ckz219 [DOI] [PubMed] [Google Scholar]
- 9.Figueira Goncalves JM, Garcia Bello MA, Golpe R, Alonso Jerez JL, Garcia-Talavera I. Impact of diabetes mellitus on the risk of severe exacerbation in patients with chronic obstructive pulmonary disease. Clin Respir J. 2020;14(12):1208–1211. doi: 10.1111/crj.13255 [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Fang Y, Chen X, et al. The impacts of obstructive sleep apnea hypopnea syndrome severity and surgery intervention on psychological and behavioral abnormalities and postoperative recovery in pediatric patients. Med Sci Monit. 2014;20:1474–1480. doi: 10.12659/MSM.890532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes-2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14–37. doi: 10.2337/cd17-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin HL, Yin SQ, Lin QY, Xu Y, Xu HW, Liu T. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: a meta-analysis. Medicine. 2017;96(19):e6836. doi: 10.1097/MD.0000000000006836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284–289. doi: 10.1136/thx.2005.051029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15(2):256–260. doi: 10.2337/diacare.15.2.256 [DOI] [PubMed] [Google Scholar]
- 15.Papathanassiou E, Papaioannou AI, Papanikolaou I, et al. Glycated hemoglobin (HbA1c) as a predictor of outcomes during acute exacerbations of chronic obstructive pulmonary disease. COPD. 2021;18(2):219–225. doi: 10.1080/15412555.2021.1902491 [DOI] [PubMed] [Google Scholar]