Abstract
We present two cases of spontaneous coronary artery dissection (SCAD). Both examples encourage a broad differential and open mind for chest pain in a young woman. We also highlight a case of SCAD where the patient presented following ventricular fibrillation arrest, a less common though potentially fatal consequence of SCAD.
Keywords: spontaneous coronary artery dissection, myocardial infarction, myocardial infarction with non-obstructive coronary arteries, women, cardiovascular disease, ventricular fibrillation arrest
1. Introduction
Spontaneous coronary artery dissection (SCAD) is an infrequent cause of myocardial infarction where there is a separation of the layers of the coronary artery leading to an intramural hematoma. 1,2 The event is unrelated to trauma, iatrogenic causes, or atherosclerotic disease.1 Possible mechanisms can be explained further. For example, coronary artery tortuosity can serve as a marker or a potential mechanism for SCAD.2 Other mechanisms include an intimal tear with the subsequent formation of an intramural hematoma. Another is a spontaneous hemorrhage within the arterial wall likely within the vasa vasorum.1 This hematoma then compresses or obstructs the true lumen of the coronary arteries.1 It has been theorized that a double hit phenomenon may lead to SCAD. Patients may have some underlying arteriopathy that makes the arterial wall susceptible when a certain trigger occurs.1
The etiology of SCAD is not fully understood. The strongest link is to fibromuscular dysplasia (FMD).1,3 Other predisposing factors for SCAD are thought to be connective tissue disorders, systemic inflammatory states such as inflammatory bowel disease, and hormonal therapy.1 Potential triggers can include intense emotional stress, intense exercise, labor and delivery, and recreational drugs.1 In one study by Saw et al., 40.5% reported significant emotional stress and 24.5% reported intense exercise prior to the onset of chest pain3.
2. Case presentation
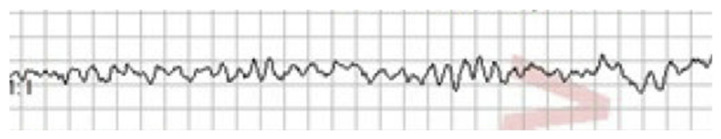
Our first patient is a 22 year-old female with a history of depression and obesity. She called emergency medical services (EMS) because of sudden onset, substernal, sharp chest pain, without any radiation. This happened in the setting of an emotionally distressing situation. Upon arrival of EMS, she was noted to be in normal sinus rhythm with frequent premature ventricular beats, blood pressure 180/111, and pulse oximetry 98% on ambient air. Patient subsequently went into sustained ventricular fibrillation (Fig. 1) and was defibrillated three times.
Fig. 1.
Rhythm strip showing ventricular fibrillation.
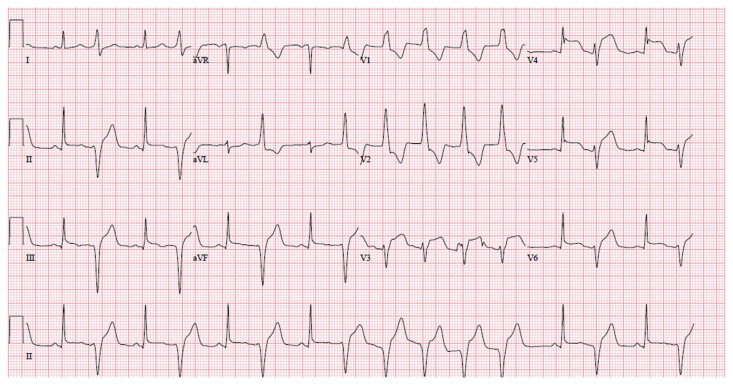
After return of spontaneous circulation ECG showed ST segment elevations in anterolateral and inferior leads (Fig. 2). The patient arrived at the hospital. She continued to have non-sustained ventricular tachycardia. Blood pressure was 117/ 102 mmHg, heart rate was 109 beats per minute, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on room air.
Fig. 2.
ECG with anterolateral and inferior ST segment elevation.
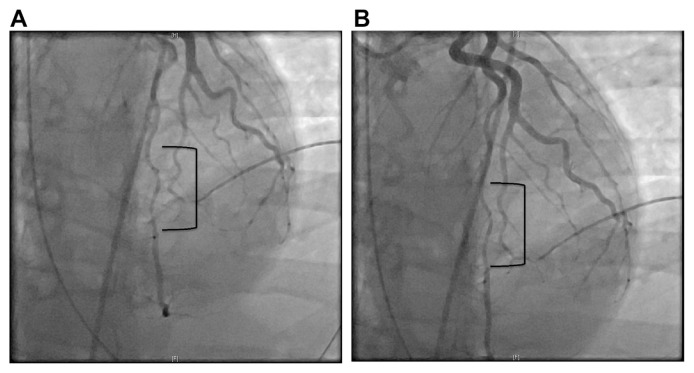
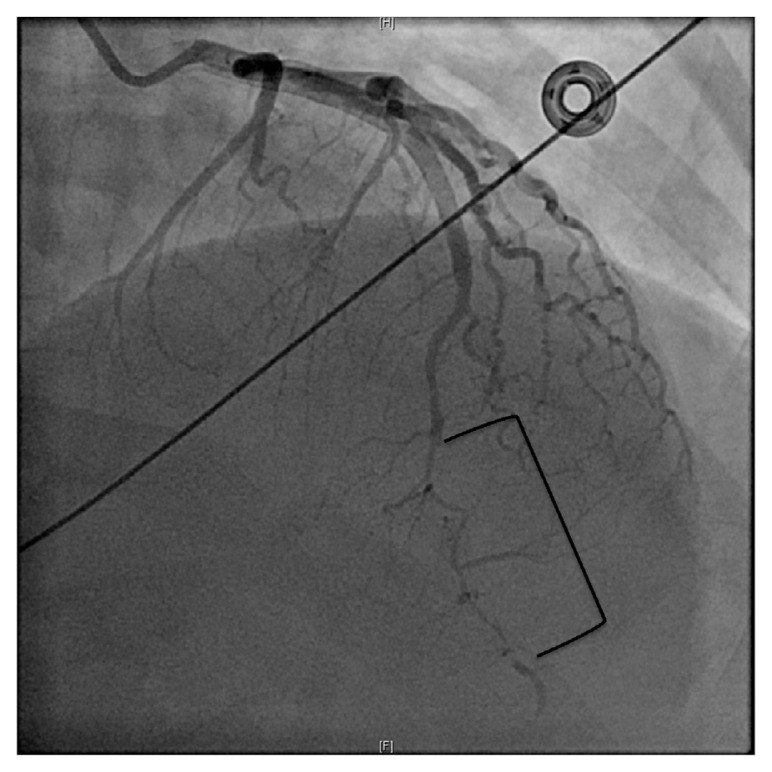
Troponin was 0.04 ng/mL on presentation. She underwent emergent cardiac catheterization that showed probable SCAD of the mid to distal LAD (left anterior descending artery) (Fig. 3). No intervention was performed. Five hours after initial troponin, repeat troponin was 2.00 ng/mL. This was also drawn after cardiac catheterization. Troponin 6 h later was 3.78 ng/mL. Transthoracic echocardiogram was performed the next day. Reduced ejection fraction 45–50%. Segmental wall motion abnormalities noted. Severe hypokinesis of the apex and periapical segments were appreciated. Because of the ventricular fibrillation arrest, subcutaneous ICD was implanted.
Fig. 3.
A: Type 2A SCAD coronary angiography showing abrupt narrowing of the LAD with distal widening of the artery. B: Type 2A SCAD coronary angiography showing abrupt narrowing of the LAD with distal widening of the artery.
During the hospitalization she had recurrence of the chest pain. She was begun on nitroglycerin infusion. Prior to discharge, she was without chest pain. She was discharged on metoprolol tartrate 25 mg twice daily, isosorbide mononitrate 60 mg daily, and aspirin 81 mg daily.
Besides a history of obesity and depression, she had no other past medical history. She had no known family history of heart disease. She had a significant psychiatric history. She had already presented to the emergency department three times in the two months prior for anxiety attacks, need for psychiatric medications, and following an episode of domestic violence.
Following her discharge, she has been followed by cardiology. She had a transthoracic echocardiogram approximately one year after the SCAD occurrence. Ejection fraction improved to 55–60%. No regional wall motion abnormalities. She has not had recurrence of SCAD or ventricular arrhythmias.
Our second patient is a 39-year-old female with a history of depression and ongoing tobacco use who presented to the emergency department with intermittent substernal chest pain, burning in quality. The chest pain had started five days prior to presentation. The most recent episode had begun 2 h prior to presentation. The pain radiated to her left shoulder and arm. Associated symptoms included dyspnea and nausea. The patient had been under significant emotional stress related to family and work matters. She had no known family history of coronary artery disease.
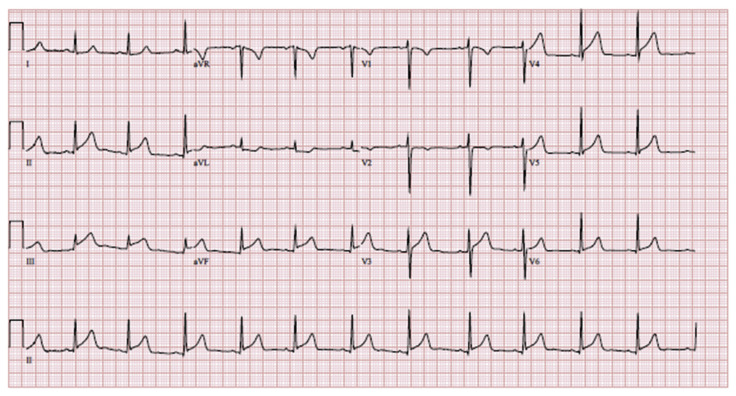
ECG showed ST segment elevation in inferior and anterolateral leads (Fig. 4). Initial blood pressure 143/128 mmHg, heart rate 70–90 beats per minute, respiratory rate was 20 breaths per minute, and oxygen saturation 100% on room air.
Fig. 4.
ECG ST segment elevations most pronounced in inferior leads also with anterolateral ST segment elevations.
Initial troponin was <0.03 ng/mL. Cardiac catheterization showed mid to distal spontaneous coronary artery dissection of the LAD. The left circumflex and right coronary artery were free of disease (Fig. 5). Troponin collected 4 h after initial presentation was 2.32 ng/mL. Troponin peaked at 27.49 ng/mL 18 h after initial presentation. Transthoracic echocardiogram appreciated an ejection fraction of 59% with apical akinesis to dyskinesis.
Fig. 5.
Type 2A SCAD coronary angiography showing abrupt narrowing of the LAD with distal widening of the artery.
She continued to have chest pain after catheterization and was started on nitroglycerin infusion. The patient’s pain resolved the following day and nitroglycerin infusion was discontinued. She was started on aspirin 81 mg, clopidogrel 75 mg daily, atorvastatin 10 mg nightly, metoprolol tartrate 25 mg twice daily, and isosorbide mononitrate 30 mg daily.
Following her discharge, she has also been followed by cardiology. She had a repeat transthoracic echocardiogram three months after her initial presentation. The ejection fraction was 61% with similar apical dyskinesis. She also underwent CTA head and neck, which did not appreciate hemodynamically significant stenosis or aneurysmal dilation. She has not had recurrence of SCAD.
3. Discussion
Recognition of SCAD as a significant etiology of myocardial infarction in women, particularly younger women, continues to grow.4,5 In the general population, SCAD is estimated to be the precipitator of acute coronary syndrome in only 1.7%–4.0% of cases.1 Approximately 90% of these involve women.1,2 The mean age of SCAD patients is 51.8 years.5 And in women younger than 50 years old, SCAD is the culprit for 25%–35% of myocardial infarctions.1
The correct diagnosis of SCAD is crucial as treatment differs from that of myocardial infarction due to atherosclerotic disease.2 Not recognizing SCAD can lead to worse outcomes.2 Deploying a stent in a coronary artery affected by SCAD can lead to complications such as cannulation of the false lumen or worsening of the dissection.2
The diagnostic gold standard for SCAD remains conventional coronary angiography.1 Using intravascular ultrasound or optical coherence tomography, though not necessary, can be helpful at better visualizing the intramural hematoma.9 However, it does come with increased risk such as dissection or creating a false lumen.2
There are four types of SCAD detected with angiography.2 Caused by intimal tear, type 1 leads to the contrast dye flowing through multiple lumens or the contrast resting in the intimal tear.2 Occurring in 65–70% of cases, type 2 is most common and is the result of intramural hematoma.2 On angiography the artery can be visualized as abrupt narrowing of the lumen either with the distal lumen widening again (2 A) or remaining narrow (2 B) for >20 mm.2 Type 3 is also caused by intramural hematoma, but the length of the lesion is < 20 mm.2 Type 4 is complete occlusion of the artery that looks like thromboembolic disease.2
Furthermore, coronary computed tomographic angiography (CCTA) can be used as a diagnostic test.2 It has the potential to visualize intramural hematomas, dissection flaps, and stenosis.2 While it has the advantage of being non-invasive, it also might not detect SCAD in smaller, more distal vessels.2 Furthermore, the imaging modality cannot always delineate non-calcified aortic plaque from intramural hematoma.2 Therefore, angiography remains preferred for the diagnosis of SCAD.2
Treatment for SCAD is conservative management. 4 In general, the arteries affected will spontaneously heal.1 In one study, 79 medically managed patients had repeat angiography greater than 26 days after event and 100% of these cases showed spontaneous angiographic healing.3 Furthermore, if SCAD were to happen again, it could occur in a different artery.2 Medical treatment for SCAD includes initiating beta-blocker.1 Beta-blockers have been linked with a reduction in the recurrence of SCAD.2 Dual antiplatelet therapy is also used for at least the first year after the initial SCAD event.2 Anticoagulation should be avoided as this can worsen the intramural hemorrhage.1
There is also concern that strenuous physical activity could lead to recurrence of SCAD.2 While patients with SCAD should not be discouraged from exercise, they should do so in moderation.2 They should avoid very strenuous or very high intensity work out regimens.2 They may benefit from a cardiac rehabilitation program. It stands to reason that there will be much variability in what each individual patient has the capacity to do. A patient more physically fit prior to the SCAD event will have a higher capacity than someone who is not.2
The most common artery involved is the LAD.1,2 Furthermore, in most SCAD cases only one artery is affected although multiple noncontiguous arteries can be involved in up to 10–15% of cases.2 Both of our patients had coronary angiographies concerning for type 2 A SCAD in the mid to distal LAD. Both were medically managed and eventually discharged on a beta-blocker and antiplatelet therapy. Angiography provided a diagnosis that was supported by the clinical picture in each case.
High clinical suspicion at the time of presentation also remains as patients may present with nonspecific symptoms2,7 and biomarkers and ECGs may at first be unremarkable.3,5,6–8
Early cardiac biomarkers can remain normal in up to 28% of cases as seen in the study performed by Lindor et al.7 Though all of these patients did eventually have troponin elevation within 6 h of presentation.7 Although SCAD can also be characterized by the presence of ST elevation MI, any-where from 50 to 74% of patients will not present with ST elevation MI (STEMI).3,5,6,8
Most SCAD patients do not have the traditional risk factors of myocardial ischemia.3,7 In Saw et al., the average BMI was 25, only 5.4% had diabetes, 24% had dyslipidemia, 38.7% had hypertension, 12.5% were current smokers, 1.8% had previous history of MI, and 1.8% had previous history of stroke.3
Furthermore, symptoms may be nonspecific as well. Of patients found to have SCAD, the pre-dominant symptom was chest pain in 85–96% of patients.2 The chest pain can vary in nature from crushing to heaviness or pressure.7 Additional symptoms included nausea, diaphoresis, light-headedness, and dyspnea.7
Though uncommon, other complications are associated with SCAD. 3%–7% can present with sustained ventricular arrhythmias.3,4 It has been shown in a previous study by Lettieri et al. that cardiogenic shock occurred in 2.2% of patients and that cardiac arrest in 2.8% of patients who presented with SCAD.6
Both of our patients were women less than 50 years old who experienced chest pain in the midst of significant emotional distress. The first had a history of obesity. The second had a history of tobacco use. Neither had other commonly identified cardiac risk factors such as hypertension, hyperlipidemia, diabetes, nor family history of coronary artery disease.
In the first case, we highlight the initial presentation of ventricular fibrillation, which occurs in a minority of SCAD cases. In both cases, our patients did not initially have troponin elevation, again uncommon in SCAD. However, four to 5 h after initial presentation both had significant troponin elevation. In a more typical fashion, both presented with ST segment elevation MI.
4. Conclusion
SCAD is the most common cause of myocardial infarction in young women. Correct diagnosis is important both to be prepared for potential complications associated with the disease such as ventricular arrhythmias and also to correctly treat these patients. A high index of suspicion is necessary as some patients can have negative troponin on initial presentation. Without any of the traditional risk factors, and with stress as a major trigger, these patients may be overlooked. Therefore, for a woman with chest pain, however young and otherwise healthy, SCAD deserves a place on the differential. Furthermore, when the diagnosis of SCAD has been made the work up is not necessarily complete. An evaluation for FMD should also be included as there is a strong link between this disease and SCAD.
Supplementary Information
Acknowledgements
The authors would like to thank Dr. Macciocca, Department of Cardiology, Reading Hospital, Tower Health System, for providing angiography images.
Footnotes
Conflict of interest
We have no conflicts of interest to disclose.
References
- 1. Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016 Jul 19;68(3):297–312. doi: 10.1016/j.jacc.2016.05.034. . Erratum in: J Am Coll Cardiol 2016; Oct 4; 68(14)1606. [DOI] [PubMed] [Google Scholar]
- 2. Kim ESH. Spontaneous coronary-artery dissection. N Engl J Med. 2020 Dec 10;383(24):2358–2370. doi: 10.1056/NEJMra2001524. [DOI] [PubMed] [Google Scholar]
- 3. Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014 Oct;7(5):645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. . Epub 2014 Oct 7. [DOI] [PubMed] [Google Scholar]
- 4. García-Guimaraes M, Bastante T, Macaya F, et al. Spontaneous coronary artery dissection in Spain: clinical and angiographic characteristics, management, and in-hospital events. Rev Esp Cardiol (Engl Ed) 2021 Jan;74(1):15–23. doi: 10.1016/j.rec.2020.04.002. English, Spanish. . Epub 2020 May 14. [DOI] [PubMed] [Google Scholar]
- 5. Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188–1197. doi: 10.1093/eurheartj/ehz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lettieri C, Zavalloni D, Rossini R, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116:66–73. doi: 10.1016/j.amjcard.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 7. Lindor RA, Tweet MS, Goyal KA, et al. Emergency department presentation of patients with spontaneous coronary artery dissection. J Emerg Med. 2017 Mar;52(3):286–291. doi: 10.1016/j.jemermed.2016.09.005. . Epub 2016 Oct 8. [DOI] [PubMed] [Google Scholar]
- 8. Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012 Jul 31;126(5):579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. . Epub 2012 Jul 16. [DOI] [PubMed] [Google Scholar]
- 9. Lebrun S, Bond RM. Spontaneous coronary artery dissection (SCAD): the underdiagnosed cardiac condition that plagues women. Trends Cardiovasc Med. 2018 Jul;28(5):340–345. doi: 10.1016/j.tcm.2017.12.004. . Epub 2017 Dec 11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.