Highlights
► We analysed cytokine induction in response to Orthopoxvirus infection of HeLa cells. ► We compared induction by Cowpox virus, Monkeypox virus and Vaccinia virus infection. ► Cowpox virus and Monkeypox virus strongly induced IL-6, IL-8 and CXCL1. ► Vaccinia virus showed no specific induction of cytokines. ► Cowpox virus infection induced chemotaxis of monocytic and macrophage-like cells.
Abbreviations: OPV, Orthopoxvirus; MPXV, Monkeypox virus; VACV, Vaccinia virus; CPXV, Cowpox virus; MVA, Modified Vaccinia Ankara virus; PMA, Phorbol-12-myristate-13-acetate
Keywords: Orthopoxvirus, Cowpox virus, Chemokine, Chemotaxis, Monocyte
Abstract
Orthopoxviruses are large DNA viruses which can cause disease in numerous host species. Today, after eradication of Variola virus and the end of vaccination against smallpox, zoonotic Orthopoxvirus infections are emerging as potential threat to human health. The most common causes of zoonotic Orthopoxvirus infections are Cowpox virus in Europe, Monkeypox virus in Africa and Vaccinia virus in South America. Although all three viruses are genetically and antigenically closely related, the human diseases caused by each virus differ considerably. This observation may reflect different capabilities of these viruses to modulate the hosts’ immune response. Therefore, we aimed at characterizing the specific cytokine response induced by Orthopoxvirus infection in vitro. We analysed the gene expression of nine human pro-inflammatory cytokines and chemokines in response to infection of HeLa cells and could identify an upregulation of cytokine gene expression following Cowpox virus and Monkeypox virus infection but not following Vaccinia virus infection. This was verified by a strong induction of especially IL-6, IL-8 and CXCL1 secretion into the cell culture supernatant following Cowpox virus infection. We could further show that supernatants derived from Cowpox virus-infected cells exhibit an increased chemotactic activity towards monocytic and macrophage-like cells. On the one hand, increased cytokine secretion by Cowpox virus-infected cells and subsequent monocyte/macrophage recruitment may contribute to host defence and facilitate clearance of the infection. On the other hand, given the assumed important role of circulating macrophages in viral spread, this may also point towards a mechanism facilitating delivery of the virus to further tissues in vivo.
1. Introduction
Members of the family Poxviridae are characterized by complex virion morphology and a large linear double-stranded DNA genome of 130–300 kbp (Moss, 2007). Vertebrate Poxviruses include two obligate human pathogens: Molluscum contagiosum virus and the nowadays extinct Variola virus which was eradicated from the human population by a global vaccination campaign (Fenner et al., 1988). In addition, many other Poxviruses can be transmitted to humans zoonotically from various animal hosts. Because of the end of the vaccination campaign and decreasing population immunity, human infections caused by members of the genus Orthopoxvirus (OPV) and especially by Monkeypox virus (MPXV) in Africa (Likos et al., 2005, Rimoin et al., 2010), Vaccinia virus (VACV) in Brazil (de Souza Trindade et al., 2007, Trindade et al., 2007) and Cowpox virus (CPXV) in Europe (Baxby et al., 1994, Vogel et al., 2012, Vorou et al., 2008) have been becoming more common and are now frequently observed (Essbauer et al., 2010). Particularly, CPXV infects a very wide range of different host species and transmission to humans has been observed to originate from infected rodents – which are thought to be the natural reservoir – as well as from various domestic and zoo animals (Baxby et al., 1994, Czerny et al., 1991, Pelkonen et al., 2003, Vorou et al., 2008). Human VACV and CPXV infections of immunocompetent individuals are in general self-limiting. However, severe generalized infection and lethal outcome of CPXV infection has been reported in immunocompromised patients (Czerny et al., 1991, Pelkonen et al., 2003). Both VACV and CPXV infections cause localized skin lesions following infection via skin injuries. The human immune response towards CPXV infection is not well understood. Most efforts to understand the immune responses towards poxvirus infection focused on the mechanisms of protection conferred by the vaccination with VACV (Hammarlund et al., 2003, Hammarlund et al., 2008, Kennedy et al., 2009, Precopio et al., 2007, Tscharke et al., 2005), showing an essential role of adaptive immunity and stable antibody titres for long-term protection (Amanna et al., 2006, Edghill-Smith et al., 2005, Panchanathan et al., 2010, Putz et al., 2006). However, less is known about the human immunological responses elicited during acute OPV infections, although essential roles of the innate and adaptive immunity have been demonstrated (Lauterbach et al., 2010). It has been shown that the innate immunity is necessary to control the infection until adaptive responses arise (Martinez et al., 2010, Moulton et al., 2008, Parker et al., 2007). Afterwards, a combination of cellular and humoral immune responses is crucial to control viral infection and finally eliminate the virus efficiently (Chaudhri et al., 2006, Fang and Sigal, 2005, Xu et al., 2004). However, most of these studies used VACV or the mouse-specific Ectromelia virus as a model system, and little is known about differences of immune recognition and response in CPXV infection which displays some peculiar features. CPXV possesses the broadest host-range observed in OPV, the largest OPV genome and it encodes the most complete set of immunomodulatory proteins of all OPV known so far.
In a previous study we could show that the expression of several innate immunity-associated genes was highly divergent in CPXV or VACV infection of human epithelial cells in vitro (Bourquain et al., 2012). In this study we further investigated the impact of CPXV or VACV infection on cytokine secretion of infected human epithelial HeLa cells and on chemotactic recruitment of U937 cells and PMA-differentiated U937 cells as a model for monocytes and macrophages.
2. Methods
2.1. Cells and culture conditions
HeLa (ATCC ID CCL-2), Hep-2 (ATCC ID CCL-23), Vero E6 (ATCC ID CRL-1586) and U937 (ATCC CRL-1593.2) cell lines were obtained from American Type Culture Collection (ATCC). HaCaT cells (300493) were obtained from CLS Cell Lines Service GmbH. HL-60 cells (ECACC 98070106) were obtained from the European Collection of Cell Cultures (ECACC). HeLa and HaCaT cells were cultivated in EMEM medium supplemented with 10% heat-inactivated foetal calf serum (FCS, PAA) and 2 mM of l-glutamine (PAA). Hep-2 cells and Vero E6 cells were both cultured in standard D-MEM medium containing 10% FCS and 2 mM of l-glutamine. HL-60 cells were cultivated in RPMI 1640 medium supplemented with 15% FCS and 2 mM of l-glutamine. U937 cells (Sundstrom and Nilsson, 1976) were cultivated in RPMI 1640 medium supplemented with 10% FCS and 2 mM of l-glutamine. Differentiation of U937 cells towards the macrophage-like phenotype was achieved by addition of 100 nM Phorbol-12-myristate-13-acetate (PMA) (Sigma–Aldrich) to the culture medium and incubation for 4 days at standard conditions. All cells were routinely screened for absence of mycoplasma contamination.
2.2. Viruses and infection conditions
VACV strain IHD-W (ATCC ID VR-1441) and CPXV strain Brighton Red (BR) (ATCC ID VR-302) were obtained from ATCC. MPXV strain MSF-6, which was obtained from a fatally infected human in the Democratic Republic of Congo, was kindly provided by Prof. Dr. Hermann Meyer (Institut für Mikrobiologie der Bundeswehr, Munich, Germany) (Meyer et al., 2002). All viruses were propagated in Hep-2 cells and cell culture supernatants which were clarified by centrifugation were used as virus stocks. Titres of virus stocks were determined by plaque assay (Tsuchiya and Tagaya, 1970) on Vero E6 cells as described before (Witkowski et al., 2010) and were shown to be comparable for CPXV, VACV and MPXV. All virus stocks were screened for absence of mycoplasma contamination. Inactivation of viruses was achieved via UV-irradiation (2×, 20 min, on ice) using a Stratalinker 2400 UV cross linker (Stratagene). Complete inactivation of UV-irradiated virus stocks was verified by plaque assay. For infection experiments, HeLa cells were grown in 6-well cell culture plates (TPP) and incubated overnight before infection with each virus at a multiplicity of infection (MOI) of 5 plaque forming units (PFU)/cell. Mock infections were performed using fresh culture medium free of any virus. After adsorption of virus for 1 h at 37 °C, the virus-containing medium or mock medium was removed, cells were washed twice with PBS and provided with fresh culture medium. Cells were then incubated at 37 °C for the indicated times. All infection experiments were performed in a biosafety level 3 (S3) laboratory in accordance with the German regulations.
2.3. Quantitative real-time RT-PCR
Total RNA was isolated using Trizol® Reagent (Invitrogen) as described in the manufacturer's protocol. Subsequently, RNA was converted to cDNA with Superscript II reverse transcriptase (Invitrogen). Quantitative real-time PCR analysis of human IL1A, IL1B, IL6, IL8, CXCL1, CXCL2, CXCL3, CCL20 and CSF2 gene expression was performed using commercially available TaqMan® Gene Expression Assays (Applied Biosystems). cMyc gene expression was measured as reference gene for ΔcT normalization as described previously (Kramski et al., 2010). Three independent samples of infected and non-infected cells, respectively, were included. Differential gene expression in CPXV-, VACV- or MPXV-infected cells compared to non-infected cells was calculated via the ΔΔcT method using the formula: change fold = 2−(ΔΔcT). Each PCR setup included no-template controls.
2.4. Human cytokine protein array
Cell culture supernatants were screened with the Proteome Profiler™ Human Cytokine Antibody Array, Panel A (R&D Systems). The assay was performed according to the manufacturer's instructions, and chemiluminescence signals were detected via the ChemiSmart 3000 chemiluminescence imaging system (Vilber Lourmat) using a charge-coupled-device camera. Data were analysed using the GeneSpotter software (MicroDiscovery).
2.5. Determination of cytokine concentration in cell culture supernatants
Quantification of CXCL1, IL-8 and IL-6 concentrations in cell culture supernatants was done by employing commercially available Human CXCL1/GROα Quantikine ELISA Kit (R&D Systems), Human CXCL8/IL-8 Quantikine ELISA Kit (R&D Systems) and Human IL-6 Quantikine ELISA Kit (R&D Systems). The assays were performed according to the manufacturer's instructions and analysed on a Tecan Infinite 200 PRO (Tecan) microplate reader.
2.6. Chemotaxis assay
Chemotactic activity of cell culture supernatants was analysed using fluorimetric 96-well QCM Chemotaxis Cell Migration Assay (Millipore). A membrane with a 5 μm pore size was used to measure U937 cell migration, and one with a 3 μm pore size to measure migration of HL-60 cells. The assay was performed according to the manufacturer's instructions. Briefly, 1 × 105 cells were allowed to migrate towards cell culture supernatants for 16 h. Cells which migrated across the membrane were lysed and subsequently detected by the nucleic acid dye CyQuant GR (Molecular Probes). Data were analysed on a Tecan Infinite 200 PRO (Tecan) microplate reader.
2.7. Data analysis
The GraphPad Prism Software (v5.01) was used for data analysis and statistics.
3. Results
3.1. CPXV and MPXV but not VACV induce upregulation of cytokine gene expression of HeLa cells
We analysed the level of mRNA expression of individual cytokine genes using TaqMan® real-time PCR assays. The selection of genes was based on a previous study in which we profiled the host cell gene expression after in vitro infection with CPXV, VACV or MPXV. All of the cytokine genes included in this study had been found to be upregulated after CPXV infection, but not after VACV infection (Bourquain et al., 2012). Here we infected HeLa cells with an MOI of 5 with CPXV, VACV or MPXV and analysed the expression of pro-inflammatory cytokine and chemokine genes IL1A, IL1B, IL6, IL8, CXCL1, CXCL2, CXCL3, CCL20 and CSF2 in the infected cells at 6 h post infection (p.i.). This point of time was chosen to allow enough time for establishment of infection and progression to late viral gene expression but to avoid the risk of cell lysis and RNA degradation after completion of the first replication cycle (Guerra et al., 2007, Moss, 2007, Rubins et al., 2011). Previous studies also showed that specific induction of host genes in response to infection mainly occurs until 6 h post infection, and is followed by unspecific downregulation of host genes at later stages of infection (Guerra et al., 2003, Guerra et al., 2004). Consistent initiation and establishment of late viral transcription at 6 h following VACV, CPXV and MPXV infection were proven by detection of mRNA expression of the late transcription factor VLTF-1 (VACCP-G8R) and of the late 11 kDa virion core protein (VACCP-F17R), respectively (data not shown).
As shown in Fig. 1 , CPXV-infected cells displayed a strong upregulation of IL8 gene expression (122.4–256.7-fold) when compared to non-infected cells. Similar results were obtained for CXCL1 (265.1–1074.8-fold) and CCL20 (257.1–682-fold). A pronounced upregulation of IL1A (5.9–20.5-fold), IL1B (5.6–21.4-fold), IL6 (17.9–35.3-fold), CXCL2 (13.1–22.1-fold) and CXCL3 (35.4–59.2-fold) gene expression was also detected. A comparable upregulation of these cytokine genes was observed in MPXV-infected cells, but not in VACV-infected cells. CSF2 expression could not be observed in non-infected cells, but weakly after infection with VACV and more strongly after CPXV or MPXV infection. When compared to VACV-infected cells, expression induced by CPXV was 17.3–31-fold increased and expression induced by MPXV was 8.4–80.9-fold increased.
Fig. 1.
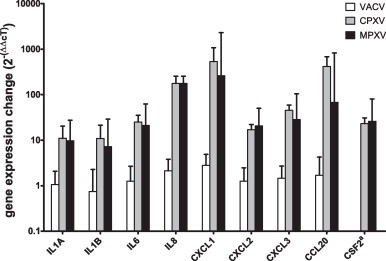
Average change of cytokine gene expression in infected cells compared to non-infected cells. Cytokine gene expression was analysed by TaqMan® real-time PCR and normalized to cMyc gene expression (ΔcT). The fold change values were calculated using the ΔΔcT method by subtraction of average ΔcT values obtained from mock-infected cells from those of infected cells. aAs CSF2 expression was not detectable in non-infected cells, CSF2 expression in VACV-infected cells was used as reference for ΔΔcT calculation of CPXV- (strain BR) or MPXV-infected cells.
3.2. Detection of differentially expressed cytokines in cell culture supernatants of CPXV- or VACV-infected HeLa cells
Since the observed effects were most pronounced in CPXV-infected cells, we compared the strongest cytokine/chemokine inducer CPXV with the poor inducer VACV in further experiments. To confirm the results also at the protein level, we employed a protein array capable of detecting 36 different cytokines, chemokines and acute phase proteins simultaneously and screened cell culture supernatants from CPXV- or VACV-infected HeLa cells at 6 h post infection. We included CPXV HumKre08/01 as a second and phylogenetically different CPXV in this study (∼92.8% genome identity; Radonić et al., 2013).
We could detect several cytokines in the supernatants of infected or non-infected cells. Signal intensities were especially high in the case of interleukin-(IL)-6, -8, -16 and -23, chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-C motif) ligand 2 (CCL-2), CD40 ligand (CD40L), interferon-(IFN)-γ, macrophage migration inhibitory factor (MIF) and serine proteinase inhibitor (Serpin) E1, which are shown in Fig. 2 . Five of these cytokines exhibited ≥2-fold differential regulation of protein secretion following infection. A unique CPXV-induced upregulation of cytokine secretion was detected for IL-6, IL-8 and CXCL1. In the case of CPXV HumKre08/01 infection we could also detect an increased secretion of CCL-2. Notably, in the case of IL-6, IL-8 and CXCL1 the upregulation of cytokine secretion could be observed for two phylogenetically distant CPXV strains. In contrast to this, VACV infection resulted in mild downregulation of IL-6, IL-8 and, even more remarkably, of CXCL1 and CCL-2 secretion. All ratios of protein secretion in infected and non-infected cells are also shown in Table 1 .
Fig. 2.
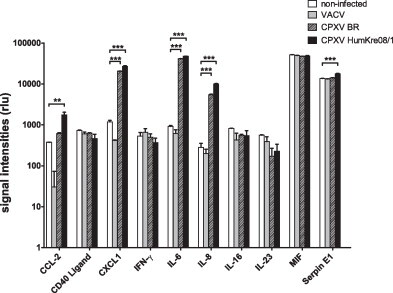
Cytokine secretion by infected cells compared to non-infected cells. Relative quantification of pro-inflammatory cytokines in cell culture supernatants of VACV- or CPXV-(strains BR or CPXV HumKre08/1) infected or mock-infected HeLa cells, as measured via a protein array capable of detecting 36 different cytokines and chemokines simultaneously. The signal intensities (rlu, relative luminescence units) indicated are calculated by using the intensities of the corresponding protein spots after background correction and normalization of the intensities according to the intensity of the positive control. Shown are the 10 cytokines which showed the highest concentration levels. Statistical significance of changes in infected compared to non-infected cells was calculated by one-way ANOVA and Tukey's multiple comparison test. The asterisks indicate statistically significant results. **p = 0.001 to 0.01, ***p < 0.001.
Table 1.
Differential regulation of human cytokine secretion by VACV or CPXV infection.
| Cytokinesb | Average ratio (mock vs. infected)a |
||
|---|---|---|---|
| VACV | CPXV BR | CPXV HumKre08/1 | |
| CCL-2 | 0.08 | 1.64 | 4.64 |
| CD40L | 0.83 | 0.86 | 0.64 |
| CXCL1 | 0.35 | 17.53 | 22.88 |
| IFN-γ | 1.23 | 0.94 | 0.69 |
| IL-6 | 0.67 | 45.12 | 51.99 |
| IL-8 | 0.71 | 19.48 | 35.64 |
| IL-16 | 0.52 | 0.68 | 0.67 |
| IL-23 | 0.70 | 0.31 | 0.41 |
| MIF | 0.96 | 0.93 | 0.94 |
| Serpin E1 | 0.98 | 1.04 | 1.31 |
Relative quantification of human cytokines in the cell culture supernatants as detected by employing a protein array. Ratios were calculated by dividing the average signal intensities of infected cells by those of non-infected cells.
Shown are the ratios of the 10 cytokines which showed the highest concentration levels.
These results confirm our observation of an upregulated IL6, IL8 and CXCL1 gene expression at 6 h post CPXV infection. However, in contrast to the results obtained by quantitative real-time PCR, no increase in secretion of IL-1α, IL-1β and colony stimulating factor 2 (CSF-2) was detected. Secretion of CCL-20 and CXCL2/3 was not yet assessed.
3.3. Quantification of IL-6, IL-8 and CXCL1 secretion following CPXV or VACV infection
We aimed to analyse the cytokine/chemokine secretion in a time-dependent manner to exclude the possibility of temporary differences at 6 h post infection. We decided to quantify concentrations of the most prominently induced cytokines IL-6, IL-8 and CXCL1 in cell culture supernatants of CPXV (strain BR), VACV- or mock-infected cells at successive time-points during the first 24 h post infection (MOI of 5). As shown in Fig. 3 , CPXV induced secretion of IL-6 and IL-8 as early as 1 h post infection, while CXCL1 was detectable at 2 h post infection, with concentrations increasing during the course of infection. Contrastingly, secretion of this cytokines was remarkably lower or even undetectable following VACV or mock infection at any point of time.
Fig. 3.
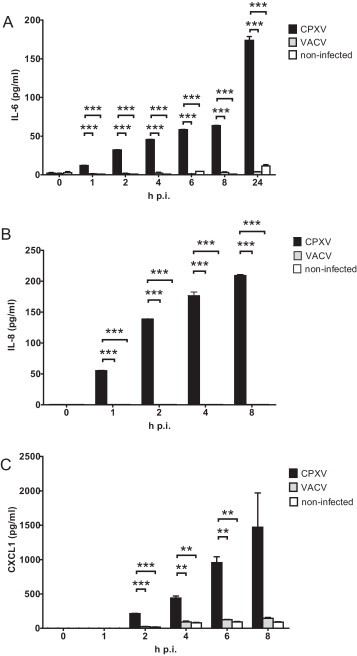
Secretion of IL-6, IL-8 and CXCL1 by infected cells compared to non-infected cells. Concentration of the cytokines IL-6 (A), IL-8 (B) and CXCL1 (C) in cell culture supernatants of CPXV- (strain BR) or VACV-infected or mock-infected HeLa cells at different times post infection, as measured by ELISA. Statistics: one-way ANOVA and Tukey's multiple comparison test. The asterisks indicate statistically significant results. **p = 0.001 to 0.01, ***p < 0.001.
3.4. Attraction of monocytes and macrophages by supernatants of CPXV infected cells
Since we could show that CPXV infection in contrast to VACV infection triggers the secretion of IL-6, IL-8 and CXCL1, with IL-8 and CXCL1 being chemokines that especially attract neutrophil granulocytes (Baggiolini and Clark-Lewis, 1992, Schumacher et al., 1992) and IL-6 to attract monocytes and macrophages (Clahsen and Schaper, 2008), we therefore tested if supernatants from CPXV-infected cells possess an increased chemotactic activity towards these cell types via chemotaxis assays. Cell culture supernatants derived from VACV- or non-infected cells served as control. Human monocytic U937 cells or Phorbol-12-myristate-13-acetate (PMA)-differentiated macrophage-like U937 (U937+PMA) cells were used to analyse recruitment of monocytes and macrophages, respectively. Human HL-60 cells which resemble neutrophilic promyelocyte precursor cells were chosen to analyse recruitment of neutrophils. As shown in Fig. 4 , we could observe a significantly increased migration of U937 cells and U937+PMA cells towards supernatants of CPXV-infected cells in contrast to those derived from VACV- or mock-infected cells which caused a comparably low degree of cell migration. In the case of HL-60 cells no significant increase in cell recruitment could be observed when using supernatants of CPXV-infected cells as stimulant (data not shown).
Fig. 4.
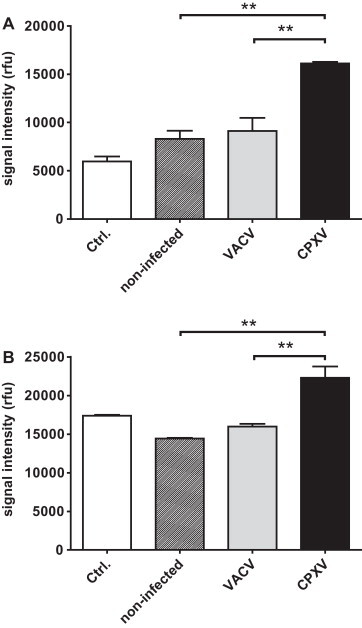
Chemotactic recruitment of monocytic U937 cells and macrophage-like PMA-differentiated U937 cells by cell culture supernatants of infected and non-infected cells. Attraction of U937 cells (A) or PMA-differentiated U937 cells (B) towards cell culture supernatants of VACV-, CPXV- (strain BR) or mock-infected HeLa cells (6 h p.i.). Fresh media served as control (Ctrl.). Cell migration towards the cell culture supernatants was assessed by chemotaxis assay (5 μm pore size) over a period of 12 h (rfu, relative fluorescence units). Statistics: one-way ANOVA and Tukey's multiple comparison test. The asterisks indicate statistically significant results. **p = 0.001 to 0.01.
In summary, we could show that CPXV infection of human epithelial HeLa cells triggers the expression of numerous pro-inflammatory cytokine and chemokine genes and enhances the secretion of several of these cytokines, especially of IL-6, IL-8 and CXCL1, into the cell culture supernatant. Enhanced secretion of IL-6, IL-8 and CXCL1 was observed early following infection and continued to rise at later times of infection. Furthermore, we could prove that – at least in our in vitro model system – enhanced cytokine secretion by CPXV-infected cells is associated with an increased attraction of monocyte- and macrophage-like cells. None of these effects could be observed following VACV infection.
4. Discussion
In this study we report on differences in the cytokine response of a human epithelial cell line towards in vitro infection with different zoonotic OPV. Despite the great success of the eradication of Variola virus, today OPV are again raising public health concerns as zoonotic infections are increasingly reported among the immunologically naïve population (Essbauer et al., 2010). Fortunately, the human diseases caused by the three common zoonotic OPV CPXV, VACV and even MPXV are less severe than smallpox and in the case of CPXV and VACV often benign. However, we do know little about the mechanisms underlying OPV pathogenesis and the reasons for these different phenotypes.
Most likely the ability to control the hosts’ immune response is critical for successful viral replication and pathogenesis. OPV are well known to possess numerous immune evasion proteins which target a wide range of antiviral host responses (Seet et al., 2003). However, most of our knowledge on the mechanisms utilized by OPV to do so originates from studies using VACV. But our knowledge of the different strategies of host modulation exploited by CPXV, VACV or MPXV is still limited. CPXV is particularly interesting, as it is supposed to be closest to the common ancestor of all OPV (Shchelkunov et al., 1998). Furthermore, it possesses the most complete set of immunomodulatory genes among OPV (Alzhanova and Fruh, 2010, Carroll et al., 2011, Shchelkunov et al., 1998), as it not only shares several immunomodulatory genes with highly pathogenic VARV but also encodes several unique genes (Seet et al., 2003).
In this study we describe an induction of pro-inflammatory cytokines and chemokines by CPXV and MPXV infection. Cytokines and chemokines are produced by the immune system in response to infection and orchestrate migration of immune cells to the sites of infection and induction of antiviral defence. The importance of the chemokine network for poxvirus replication is highlighted by the manifold homologues of chemokine ligands (virokines), chemokine receptors (viroceptors) and secreted chemokine-binding proteins (CKBPs) encoded by many poxviruses to modulate chemokine activity (Alcami and Lira, 2010). To our knowledge, all OPV interfere with NFκB, TNF and IFN signalling, which regulate cytokine expression, and inactivate caspase-1 via CrmA and its homologues (Seet et al., 2003). Caspase-1 is required for processing of IL-1β and IL-18 into its active form. In relation to our results, inhibition of caspase-1 via CrmA may therefore explain the contrasting observation of IL-1β mRNA upregulation following CPXV infection without enhanced IL-1β protein secretion. In addition, all OPV encode viral IL-1β and IL-18 receptors and the viral chemokine inhibitor vCCI which specifically inhibits certain CC chemokines, especially MIP-1α (CCL-3) and MIP-1β (CCL-4), and thereby can inhibit monocyte chemotaxis (Buatois et al., 2010, Zhang et al., 2006). Therefore, we assume that vCCI was present in the cell culture supernatants of virus-infected cells which were tested for induction of immune cell chemotaxis in this study. However, vCCI is not expected to affect chemotaxis induced by CXC chemokines IL-8 and CXCL1 or by IL-6 (Clahsen and Schaper, 2008) which were most prominently induced in our study.
We could identify strong induction of IL-6, IL-8 and CXCL1 gene expression by CPXV and MPXV infection and prove secretion of these cytokines by CPXV-infected HeLa cells. Comparable results, showing an increased secretion of IL-6, IL-8 and CXCL1 – with the effect on the former being most pronounced – following CPXV infection in comparison to VACV infection, were also obtained for infection of human HaCaT cells (data not shown). This indicates that the observed results are not specific to HeLa cells. Interestingly, CPXV gene expression seems to be necessary for induction of these cytokines, as no increase in IL-6, IL-8 or CXCL1 secretion could be observed following infection of HeLa cells with UV-inactivated CPXV or VACV (data not shown). Therefore, it may be assumed that the induction of these cytokines is specifically mediated by replicating CPXV. Furthermore, the induction of IL-6 and IL-8 cannot be explained to be mediated indirectly by enhanced IFN-γ production by infected cells, as no increase in IFN-γ secretion was shown following CPXV infection compared to non-infected or VACV-infected cells (Fig. 2). Future work is necessary to investigate if the induction of IL-6, IL-8 and CXCL1 by CPXV infection is due to the specific action of a CPXV-encoded protein or if the induction of these cytokines is triggered by OPV replication in general and is specifically prevented by VACV.
Interestingly, in the case of CPXV we could observe similar induction of cytokine secretion following infection with two phylogenetically distantly related CPXV. As recent progress in CPXV phylogeny showed that CPXV comprises several distinct species (Carroll et al., 2011) a more detailed comparison of immune modulation by different CPXV species, and especially a comparison to VACV-like CPXV species, might be an interesting issue for future research.
In the case of VACV virus infection, our results are supported by previous reports on cytokine regulation by the Western Reserve strain of VACV which induces neither IL-6 nor IL-8 following infection, in contrast to infection with the highly attenuated Modified Vaccinia Ankara virus (MVA) (Guerra et al., 2004, Ludwig et al., 2005, Ramirez et al., 2000). Thus, CPXV-mediated induction of IL-6 and IL-8 closely resembles the consequences of infection with a highly attenuated virus and therefore may reflect an inability of CPXV to counteract host defences in human cells. However, induction of IL-6 and IL-8 mRNA expression was also observed following infection with MPXV which is even more pathogenic in humans than VACV or CPXV.
Regarding MPXV, the observation of increased IL8 gene expression following MPXV infection is consistent with an earlier report by Alkhalil et al. (2010) which profiled gene expression of MPXV-infected Macaca mulatta kidney epithelial (MK2) cells. Therefore, we suggest that MPXV-triggered induction of IL8 gene expression may not be specific to infection of human cells. Similarly, a strong induction of IL-6, IL-8, and G-CSF secretion following intrabronchial infection of cynomolgus macaques (Macaca fascicularis) with MPXV was reported (Johnson et al., 2011a).
Concerning CPXV infection, an increased secretion of IL-6 and CCL-2 (MCP-1) in vivo following intrabronchial infection of cynomolgus macaques was also described (Smith et al., 2012). Furthermore, a previous study by Johnson et al. (2011b) utilizing intravenous inoculation of CPXV in cynomolgus macaques reported elevated concentrations of CCL-2, IL-6, IL-8, and IFN-γ in the course of infection. This is in agreement with our results obtained for in vitro infection of human HeLa cells.
However, the importance of increased IL-6, IL-8 and CCL-2 secretion during CPXV infection, e.g. as triggers of leucocyte recruitment to the site of infection, is yet unclear. Lehmann et al. (2009) suggested CCL-2 to be an important trigger of leucocyte immigration into the lung following intranasal infection of mice with MVA. Consistent with our results, they could show that CCL-2 expression was only induced by MVA and not by any other non-attenuated strain of VACV. Therefore, it would be interesting to analyse leucocyte recruitment following CPXV infection. In our study we could show that enhanced secretion of cytokines and chemokines following CPXV infection in vitro is associated with an increased chemotactic activity of cell culture supernatants of CPXV-infected cells towards U937 cells and PMA-treated U937 cells, respectively, which were used as a model for monocytes and macrophages, respectively. This was in contrast to supernatants of VACV-infected cells.
We therefore conclude that the induction of chemotactic factors by CPXV infection is responsible for leucocyte recruitment in vitro. If also true in vivo, this may be seen as a part of the immune response of the host, leading to immigration of leukocytes to the site of infection. But then, early recruitment of circulating macrophages as one of the initial target cells may also facilitate systemic spread of OPV (Rubins et al., 2011). However, in the case of human CPXV infections, this has not yet been observed in immunocompetent individuals.
Acknowledgements
The authors would like to thank Jule Hinzmann and Jung-Won Sim-Brandenburg for excellent technical assistance, Ursula Erikli for copy-editing and Daniel Stern for statistics advice.
References
- Alcami A., Lira S.A. Modulation of chemokine activity by viruses. Current Opinion in Immunology. 2010;22(4):482–487. doi: 10.1016/j.coi.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalil A., Hammamieh R., Hardick J., Ichou M.A., Jett M., Ibrahim S. Gene expression profiling of monkeypox virus-infected cells reveals novel interfaces for host–virus interactions. Virology Journal. 2010;7:173. doi: 10.1186/1743-422X-7-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzhanova D., Fruh K. Modulation of the host immune response by cowpox virus. Microbes and Infection. 2010;12(12–13):900–909. doi: 10.1016/j.micinf.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna I.J., Slifka M.K., Crotty S. Immunity and immunological memory following smallpox vaccination. Immunological Reviews. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Letters. 1992;307(1):97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- Baxby D., Bennett M., Getty B. Human cowpox 1969–93: a review based on 54 cases. British Journal of Dermatology. 1994;131(5):598–607. doi: 10.1111/j.1365-2133.1994.tb04969.x. [DOI] [PubMed] [Google Scholar]
- Bourquain, D., Dabrowski, P.W., Nitsche, A., 2012. Comparison of host cell gene expression in cowpox, monkeypox or vaccinia virus-infected cells reveals virus-specific regulation of immune response genes. Virology Journal, in press. [DOI] [PMC free article] [PubMed]
- Buatois V., Fagete S., Magistrelli G., Chatel L., Fischer N., Kosco-Vilbois M.H., Ferlin W.G. Pan-CC chemokine neutralization restricts splenocyte egress and reduces inflammation in a model of arthritis. Journal of Immunology. 2010;185(4):2544–2554. doi: 10.4049/jimmunol.1000182. [DOI] [PubMed] [Google Scholar]
- Carroll D.S., Emerson G.L., Li Y., Sammons S., Olson V., Frace M., Nakazawa Y., Czerny C.P., Tryland M., Kolodziejek J., Nowotny N., Olsen-Rasmussen M., Khristova M., Govil D., Karem K., Damon I.K., Meyer H. Chasing Jenner's vaccine: revisiting cowpox virus classification. PLoS One. 2011;6(8):e23086. doi: 10.1371/journal.pone.0023086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri G., Panchanathan V., Bluethmann H., Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. Journal of Virology. 2006;80(13):6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clahsen T., Schaper F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. Journal of Leukocyte Biology. 2008;84(6):1521–1529. doi: 10.1189/jlb.0308178. [DOI] [PubMed] [Google Scholar]
- Czerny C.P., Eis-Hubinger A.M., Mayr A., Schneweis K.E., Pfeiff B. Animal poxviruses transmitted from cat to man: current event with lethal end. Zentralblatt fuer Veterinaermedizin. Reihe B. 1991;38(6):421–431. doi: 10.1111/j.1439-0450.1991.tb00891.x. [DOI] [PubMed] [Google Scholar]
- de Souza Trindade G., Drumond B.P., Guedes M.I., Leite J.A., Mota B.E., Campos M.A., da Fonseca F.G., Nogueira M.L., Lobato Z.I., Bonjardim C.A., Ferreira P.C., Kroon E.G. Zoonotic vaccinia virus infection in Brazil: clinical description and implications for health professionals. Journal of Clinical Microbiology. 2007;45(4):1370–1372. doi: 10.1128/JCM.00920-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., Reimann K.A., Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nature Medicine. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Essbauer S., Pfeffer M., Meyer H. Zoonotic poxviruses. Veterinary Microbiology. 2010;140(3–4):229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Sigal L.J. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. Journal of Immunology. 2005;175(10):6829–6836. doi: 10.4049/jimmunol.175.10.6829. [DOI] [PubMed] [Google Scholar]
- Fenner F., Anderson D.A., Arita I., Jezek Z., Ladnyi I.D. World Health Organization; Geneva: 1988. Smallpox and Its Eradication. [Google Scholar]
- Guerra S., Lopez-Fernandez L.A., Conde R., Pascual-Montano A., Harshman K., Esteban M. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. Journal of Virology. 2004;78(11):5820–5834. doi: 10.1128/JVI.78.11.5820-5834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra S., Lopez-Fernandez L.A., Pascual-Montano A., Munoz M., Harshman K., Esteban M. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. Journal of Virology. 2003;77(11):6493–6506. doi: 10.1128/JVI.77.11.6493-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra S., Najera J.L., Gonzalez J.M., Lopez-Fernandez L.A., Climent N., Gatell J.M., Gallart T., Esteban M. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. Journal of Virology. 2007;81(16):8707–8721. doi: 10.1128/JVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E., Dasgupta A., Pinilla C., Norori P., Fruh K., Slifka M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(38):14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nature Medicine. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Johnson R.F., Dyall J., Ragland D.R., Huzella L., Byrum R., Jett C., St Claire M., Smith A.L., Paragas J., Blaney J.E., Jahrling P.B. Comparative analysis of monkeypox virus infection of cynomolgus macaques by the intravenous or intrabronchial inoculation route. Journal of Virology. 2011;85(5):2112–2125. doi: 10.1128/JVI.01931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.F., Yellayi S., Cann J.A., Johnson A., Smith A.L., Paragas J., Jahrling P.B., Blaney J.E. Cowpox virus infection of cynomolgus macaques as a model of hemorrhagic smallpox. Virology. 2011;418(2):102–112. doi: 10.1016/j.virol.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R.B., Ovsyannikova I.G., Jacobson R.M., Poland G.A. The immunology of smallpox vaccines. Current Opinion in Immunology. 2009;21(3):314–320. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramski M., Matz-Rensing K., Stahl-Hennig C., Kaup F.J., Nitsche A., Pauli G., Ellerbrok H. A novel highly reproducible and lethal nonhuman primate model for orthopox virus infection. PLoS One. 2010;5(4):e10412. doi: 10.1371/journal.pone.0010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach H., Kassub R., Patzold J., Korner J., Bruckel M., Verschoor A., Chaplin P., Suter M., Hochrein H. Immune requirements of post-exposure immunization with modified vaccinia Ankara of lethally infected mice. PLoS One. 2010;5(3):e9659. doi: 10.1371/journal.pone.0009659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M.H., Kastenmuller W., Kandemir J.D., Brandt F., Suezer Y., Sutter G. Modified vaccinia virus ankara triggers chemotaxis of monocytes and early respiratory immigration of leukocytes by induction of CCL2 expression. Journal of Virology. 2009;83(6):2540–2552. doi: 10.1128/JVI.01884-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., Davidson W., Galloway R., Khristova M.L., Reynolds M.G., Zhao H., Carroll D.S., Curns A., Formenty P., Esposito J.J., Regnery R.L., Damon I.K. A tale of two clades: monkeypox viruses. Journal of General Virology. 2005;86(Pt 10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- Ludwig H., Mages J., Staib C., Lehmann M.H., Lang R., Sutter G. Role of viral factor E3L in modified vaccinia virus ankara infection of human HeLa Cells: regulation of the virus life cycle and identification of differentially expressed host genes. Journal of Virology. 2005;79(4):2584–2596. doi: 10.1128/JVI.79.4.2584-2596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Huang X., Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathogens. 2010;6(3):e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Perrichot M., Stemmler M., Emmerich P., Schmitz H., Varaine F., Shungu R., Tshioko F., Formenty P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. Journal of Clinical Microbiology. 2002;40(8):2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. In: Fields Virology. 5th ed. Knipe D.M., editor. Lippincott Williams & Wilkins; Philadelphia: 2007. Poxviridae: the viruses and their replication; pp. 2905–2946. [Google Scholar]
- Moulton E.A., Atkinson J.P., Buller R.M. Surviving mousepox infection requires the complement system. PLoS Pathogens. 2008;4(12):e1000249. doi: 10.1371/journal.ppat.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan V., Chaudhri G., Karupiah G. Antiviral protection following immunization correlates with humoral but not cell-mediated immunity. Immunology and Cell Biology. 2010;88(4):461–467. doi: 10.1038/icb.2009.110. [DOI] [PubMed] [Google Scholar]
- Parker A.K., Parker S., Yokoyama W.M., Corbett J.A., Buller R.M. Induction of natural killer cell responses by ectromelia virus controls infection. Journal of Virology. 2007;81(8):4070–4079. doi: 10.1128/JVI.02061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen P.M., Tarvainen K., Hynninen A., Kallio E.R., Henttonen K., Palva A., Vaheri A., Vapalahti O. Cowpox with severe generalized eruption, Finland. Emerging Infectious Diseases. 2003;9(11):1458–1461. doi: 10.3201/eid0911.020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precopio M.L., Betts M.R., Parrino J., Price D.A., Gostick E., Ambrozak D.R., Asher T.E., Douek D.C., Harari A., Pantaleo G., Bailer R., Graham B.S., Roederer M., Koup R.A. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. Journal of Experimental Medicine. 2007;204(6):1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz M.M., Midgley C.M., Law M., Smith G.L. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nature Medicine. 2006;12(11):1310–1315. doi: 10.1038/nm1457. [DOI] [PubMed] [Google Scholar]
- Ramirez J.C., Gherardi M.M., Esteban M. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. Journal of Virology. 2000;74(2):923–933. doi: 10.1128/jvi.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., Blumberg S., Thomassen H.A., Pike B.L., Fair J.N., Wolfe N.D., Shongo R.L., Graham B.S., Formenty P., Okitolonda E., Hensley L.E., Meyer H., Wright L.L., Muyembe J.J. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonić, A., Dabrowski, P.W., Kurth, A., Nitsche, A., 2013. Genome analysis of Cowpox viruses reveals a clade closely related to Variola virus. Manuscript in preparation. [DOI] [PMC free article] [PubMed]
- Rubins K.H., Hensley L.E., Relman D.A., Brown P.O. Stunned silence: gene expression programs in human cells infected with monkeypox or vaccinia virus. PLoS One. 2011;6(1):e15615. doi: 10.1371/journal.pone.0015615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher C., Clark-Lewis I., Baggiolini M., Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(21):10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet B.T., Johnston J.B., Brunetti C.R., Barrett J.W., Everett H., Cameron C., Sypula J., Nazarian S.H., Lucas A., McFadden G. Poxviruses and immune evasion. Annual Review of Immunology. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- Shchelkunov S.N., Safronov P.F., Totmenin A.V., Petrov N.A., Ryazankina O.I., Gutorov V.V., Kotwal G.J. The genomic sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology. 1998;243(2):432–460. doi: 10.1006/viro.1998.9039. [DOI] [PubMed] [Google Scholar]
- Smith A.L., St Claire M., Yellayi S., Bollinger L., Jahrling P.B., Paragas J., Blaney J.E., Johnson R.F. Intrabronchial inoculation of cynomolgus macaques with cowpox virus. Journal of General Virology. 2012;93(Pt 1):159–164. doi: 10.1099/vir.0.036905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) International Journal of Cancer. 1976;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Trindade G.S., Emerson G.L., Carroll D.S., Kroon E.G., Damon I.K. Brazilian vaccinia viruses and their origins. Emerging Infectious Diseases. 2007;13(7):965–972. doi: 10.3201/eid1307.061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharke D.C., Karupiah G., Zhou J., Palmore T., Irvine K.R., Haeryfar S.M., Williams S., Sidney J., Sette A., Bennink J.R., Yewdell J.W. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. Journal of Experimental Medicine. 2005;201(1):95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y., Tagaya I. Plaque assay of variola virus in a cynomolgus monkey kidney cell line. Archiv fuer die gesamte Virusforschung. 1970;32(1):73–81. doi: 10.1007/BF01241522. [DOI] [PubMed] [Google Scholar]
- Vogel S., Sardy M., Glos K., Korting H.C., Ruzicka T., Wollenberg A. The Munich outbreak of cutaneous cowpox infection: transmission by infected pet rats. Acta Dermato-Venereologica. 2012;92(2):126–131. doi: 10.2340/00015555-1227. [DOI] [PubMed] [Google Scholar]
- Vorou R.M., Papavassiliou V.G., Pierroutsakos I.N. Cowpox virus infection: an emerging health threat. Current Opinion in Infectious Diseases. 2008;21(2):153–156. doi: 10.1097/QCO.0b013e3282f44c74. [DOI] [PubMed] [Google Scholar]
- Witkowski P.T., Schuenadel L., Wiethaus J., Bourquain D.R., Kurth A., Nitsche A. Cellular impedance measurement as a new tool for poxvirus titration, antibody neutralization testing and evaluation of antiviral substances. Biochemical and Biophysical Research Communications. 2010;401(1):37–41. doi: 10.1016/j.bbrc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Xu R., Johnson A.J., Liggitt D., Bevan M.J. Cellular and humoral immunity against vaccinia virus infection of mice. Journal of Immunology. 2004;172(10):6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- Zhang L., Derider M., McCornack M.A., Jao S.C., Isern N., Ness T., Moyer R., LiWang P.J. Solution structure of the complex between poxvirus-encoded CC chemokine inhibitor vCCI and human MIP-1beta. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):13985–13990. doi: 10.1073/pnas.0602142103. [DOI] [PMC free article] [PubMed] [Google Scholar]


