Highlights
► We evaluate whether monkeypox can fill the niche left vacant by smallpox eradication. ► We discuss ecologic and epidemiologic limits that could impede monkeypox's emergence. ► We assess genetic constrains that may hamper monkeypox from becoming a human-adapted virus.
Abstract
In 1980, the World Health Assembly announced that smallpox had been successfully eradicated as a disease of humans. The disease clinically and immunologically most similar to smallpox is monkeypox, a zoonosis endemic to moist forested regions in West and Central Africa. Smallpox vaccine provided protection against both infections. Monkeypox virus is a less efficient human pathogen than the agent of smallpox, but absent smallpox and the population-wide immunity engendered during eradication efforts, could monkeypox now gain a foothold in human communities? We discuss possible ecologic and epidemiologic limitations that could impede monkeypox's emergence as a significant pathogen of humans, and evaluate whether genetic constrains are sufficient to diminish monkeypox virus’ capacity for enhanced specificity as a parasite of humans.
Background
The history of vaccination begins with the use of an animal virus to immunize humans against smallpox [1]. It ends with this same practice. By the close of 1979, the concerted application of vaccinia virus-based vaccine in at-risk populations had effectively interrupted the spread of smallpox, resulting in the eradication of naturally occurring disease throughout the world. This was possible because of antigenic similarities between vaccinia and variola (the agent of smallpox) viruses, and the fact that Variola is human-specific, leaving no potential for zoonotic reservoirs.
Vaccinia and variola are Orthopoxviruses. Orthopoxviruses encompass an array of pathogens that elicit serologic cross-reactivity, among which only one, variola, is an exclusive parasite of humans. Several zoonotic Orthopoxviruses — including vaccinia virus, cowpox virus, and monkeypox virus — can infect humans opportunistically (in the event of an encounter between a virus-infected animal and a susceptible individual), but none manifest variola's capacity for relatively efficient inter-human spread, with the possible exception of monkeypox.
Initial observations of a ‘smallpox-like’ illness caused by infection with monkeypox virus rather than variola were made in 1970, during the final stages of smallpox eradication [2]. The discovery occurred during time of intensified effort to verify that smallpox had been eliminated from regions of West and Central Africa that had been deemed ‘smallpox-free’. The two diseases, smallpox and monkeypox, share a distinctive clinical presentation and almost certainly existed historically in sympatry across what is recognized now as the endemic range for monkeypox (Figure 1 ). But, in the absence of laboratory testing to specify the etiologic agent responsible for the condition, it is likely that most Orthopoxvirus-associated ‘smallpox-like’ illnesses were assumed to be smallpox; smallpox being broadly distributed and extremely well known.
Figure 1.
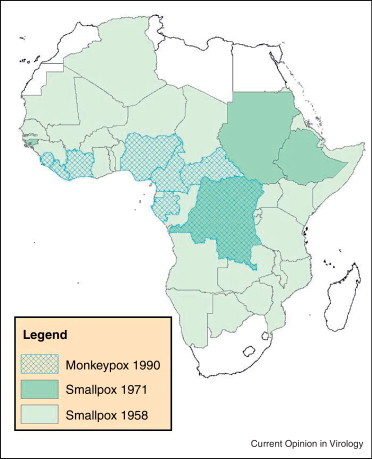
Map depicting the distribution of the smallpox in Africa 1954–1958, by country, before the inception of global eradication efforts (‘Smallpox 1958’, light green), and during the latter stages of eradication, at the time human monkeypox was discovered (‘Smallpox 1971’, dark green) [12]. Countries reporting at least one case of human monkeypox through 1990 are depicted with cross-hatching.
Image courtesy of Benjamin Monroe, CDC.
It is now more than 30 years since the WHO recommended cessation of routine smallpox vaccination. Very few individuals born since eradication have received smallpox vaccination, and among those over 30 years of age who did receive vaccination, immunity is waning. This increasing deficit of human immunity raises the specter of whether, under these conditions, monkeypox might emerge as a more significant human pathogen, perhaps even ‘replacing’ smallpox. Indeed recent reports of increasing monkeypox incidence in the Democratic Republic of the Congo (DRC) [3•], as well as sporadic occurrences in neighboring countries imply that this may be a possibility [4, 5, 6]. But before concluding that smallpox eradication and the cessation of vaccination have opened an ecologic, or immunologic, niche for monkeypox to exploit, it seems reasonable to address the following questions. First, during the era before smallpox eradication, was the level of immunity in human populations — engendered either by smallpox vaccination or by the circulation of smallpox — responsible in some way for suppressing the emergence and spread of monkeypox? Or, are there in fact particular characteristics intrinsic to monkeypox (ecologic requirements, genetic determinants, among others) that have served to establish fundamental limits on the virus’ capacity to emerge and spread beyond its current geographic confines? And if so, are there mechanisms or opportunities that could allow monkeypox to overcome these limitations?
Immunologic niche
Smallpox (variola major) is associated with higher fatality rates than monkeypox, but the clinical presentation of monkeypox is difficult to distinguish from discrete, ordinary smallpox (Figure 2 ), and smallpox vaccine is protective against both. A debate continues as to the duration of immunity provided by smallpox vaccine in the absence of periodic boosting [7, 8, 9], but it is inarguable that lifelong protection from re-infection was a lasting indemnity for having survived smallpox [10]. Presumably, a smallpox survivor would also possess life-long protection against infection with monkeypox virus (and vice versa), thus an individual infected with one virus would be permanently removed from the pool of susceptible hosts for the other.
Figure 2.
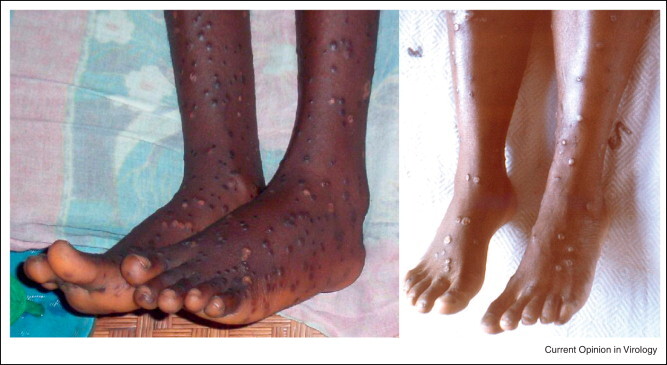
(a) Legs and feet of monkeypox patient (photo courtesy of J. Harvey). (b) Legs and feet of smallpox patient at similar stage of rash (pustular) (photo courtesy of J. Nobel, CDC).
In contrast, smallpox vaccination provides only limited-term protection from infection with either monkeypox or variola. Could smallpox circulation in monkeypox endemic regions of Africa then have been sufficient to impede the spread of human monkeypox? What level of vaccination would have been required to achieve the same effect?
During a 40-year period from 1919 to 1958 an estimated 122 600 cases of smallpox were reported by the Colonial authorities in DRC (Belgian Congo), on average ∼3065 cases per year [11]. During that same period, roughly 78 million vaccinations and re-vaccinations were administered, the vast majority after 1945. In the 7 years immediately before the inception of the concerted vaccination programs, 14 000 total cases of smallpox were reported (from a population of ∼7.7 million persons [11]). In time, vaccination had a clear impact on smallpox and ultimately led to eradication, and collaterally on potential human monkeypox infections. However, in the absence of data describing the incidence of monkeypox human infections before and during much of the eradication era, it is difficult to determine the role of smallpox population (herd) immunity for vaccine induced immunity in the incidence of monkeypox human disease. Despite the absence of quality control of vaccines before the 1960s, the combination of smallpox and vaccine-derived immunity would provide protection against monkeypox infection. Since vaccination rates exceeded smallpox case rates in central Africa during this period, it is easy to imagine a converse relationship between smallpox vaccination and human monkeypox incidence during this period and immediately following eradication of smallpox.
Between 1967 and 1971, at the height of the smallpox eradication efforts, an estimated 15 236 000 doses of vaccine were provided to 21 countries in West and Central Africa for purposes of vaccination or re-vaccination against smallpox [12]. The sheer numbers of immunizations doubtless had an impact on the incidence, and possibly the geographic distribution of not just smallpox, but of other Orthopoxvirus-associated human infections including monkeypox. No human monkeypox cases have been reported from West Africa since 1981 [13], though evidence points to the fact that monkeypox virus still circulates enzootically [14, 15]. And in the years immediately following smallpox eradication (1985–95), reports of monkeypox from the Congo Basin declined measurably [16].
It is difficult to assess what the ecologic impact of variola might have been on monkeypox virus over earlier time-periods, when the viruses were circulating independent of the influence of vaccine. As a solely human pathogen, variola's ability to persist in populations is vulnerable to immunologically driven interruptions in human-to-human transmission chains. By contrast, monkeypox is a sylvatic zoonosis and human infections are incidental and probably of little consequence to the overall persistence of the virus in nature. The current endemic distribution of monkeypox is in all likelihood governed by the distribution of its principal host(s). However, incidence of human infection is also dependent on cross protective immunity stemming from the vaccination campaign and previous exposure to variola or other Orthopoxviruses. Monkeypox virus is, however, capable of infecting a broad range of hosts, and spillover into a new permissive host with a more cosmopolitan distribution could — in theory — contribute to the virus’ emerging as a threat to humans.
Ecologic context of monkeypox
Broad host range zoonotic agents have been highlighted as being more likely to be emerging or re-emerging human pathogens. Over 50% of zoonotic viruses with 3 or more types of non-human hosts have been classified as emerging agents [17]. Monkeypox virus can infect an array of mammalian taxa including Sciurid, Glirid and Nesomyid rodents (Cynomys sp., Funisciurus sp., Graphiurus sp., Cricetomys sp.), marsupials (Monodelphis domestica, Delphius marsupialis), and primates (Callithrix jacchus, Homo sapiens) [18, 19•]. In each of the examples provided, infections occurred without experimental induction by humans, but for most, human intervention was responsible for bringing the species in question into proximity with the virus. Monkeypox virus has only been isolated once from an animal captured in its natural environment — in 1986 monkeypox virus was isolated from the carcass of a Funiscirus squirrel found in Equateur Province of DRC [20]. The host range of naturally occurring (sylvatic) monkeypox remains undefined, but given its capacity to infect many different types of animals, it is likely to exceed the 3-host threshold.
Large mammals, gazelles and primates, have been singled out as potentially important sources of human infection in Central Africa [21], but the consistency of associations between rodent hosts and viruses across the Orthopoxvirus clade suggest that a rodent reservoir (or reservoirs) would be more likely for monkeypox [1, 22] (Table 1 ). The perpetuation of acute viral infections in small populations is often theorized to necessitate either virus persistence or latency in the host (which is not characteristic of Orthopoxvirus infections) or high host turnover [23], which again points toward a rodent reservoir. Rodent fauna, such as squirrels (Funiscirus sp., Heliosciurus sp.) and Cricetomys, that are known to be susceptible to monkeypox virus infection, and that exploit food sources and refuges in areas adjacent to forest margins and human communities in DRC, are perhaps the most likely reservoirs and agents of virus transmission to humans [24, 25]. Virus spillover into a more widely distributed sister taxa could raise concerns about the spread of disease beyond Africa.
Table 1.
Examples of (non-primate) mammalian species that are susceptible to infection with monkeypox virus, and their suitability as vectors of infection to humans.
| Order | Family | Species | Circumstance of infection | Potential as significant monkeypox vector to humansa |
|---|---|---|---|---|
| Rodentia | Sciuridae | Sciurus vulgaris | Experimental infectionb | Not a significant vector of directly transmitted zoonotic pathogens |
| Funisciurus spp. | Natural infection (sylvatic); experimental infectionb | Range restricted | ||
| Heliosciurus spp. | Captive animal; experimental infectionb | Range restricted | ||
| Marmota monax | Captive animal | Not a significant vector of directly transmitted zoonotic pathogens; range restricted | ||
| Cynomys ludovicianus | Captive animal; experimental infectionb | Not a significant vector of directly transmitted zoonotic pathogens; range restricted | ||
| Gliridae | Graphiurus spp. | Captive animal; experimental infectionb | Range restricted | |
| Nesomyidae | Cricetomys spp. | Captive animal | Range restrictedd | |
| Dipodidae | Jaculus sp. | Captive animal | Range restricted | |
| Muridae | Rattus norvegicus | Experimental infectionb (1–3-day-old animals)c | Only very young animals are susceptible to infection | |
| Mus musculus | Experimental infectionb (8–15-day-old animals)c | Only very young animals are susceptible to infection | ||
| Langomorpha | Leporidae | Oryctolagus cuniculus | Experimental infectionb (10-day-old animals)c | Only very young animals are susceptible to infection |
| Didelphimorphia | Didelphidae | Monodelphis domestica | Captive animal | Range restricted |
| Didelphis marsupialis | Captive animal | Range restricted | ||
| Erinaceomorpha | Erinaceidae | Atlerix spp. | Captive animal | Range restricted |
Refers to the species’ potential to serve as significant monkeypox vector to humans outside of the current areas of endemic monkeypox disease in Africa.
Routes of experimental infection include abrasion, foot pad inoculation, intranasal introduction of virus [26, 27, 53, 54].
In these experiments, adult animals refractory to infection [27].
A colony of introduced Cricetomys sp. has been described in the State of Florida, USA [55].
In artificial settings, the common European squirrel Scirurus vulgaris has proven to be sensitive to infection with monkeypox virus [26] and the North American Sciurid rodent Cynomys ludovicianus has proven to be not only susceptible to infection but also capable of transmitting infection to humans [14, 19•]. The more common commensal rodents, Rattus spp. and Mus spp. are not considered to be especially susceptible to monkeypox virus infection, although monkeypox virus can be propagated in several inbred strains of mice and in immature animals [27, 28].
In the absence of virus spillover and perpetuation in a readily susceptible, broadly distributed animal host, the spread of monkeypox beyond its areas of current endemicity in Africa would be dependent on human-to-human transmission which prompts the question of whether the inter-human transmission of monkeypox is sufficiently robust for this to occur?
Inter-human transmission potential
Whether monkeypox virus can exploit humans as a viable maintenance host will inevitably depend on the virus’ capacity for sustained inter-human transmission. Epidemiologic modeling studies performed in the 1980s led to the conclusion that it would be highly improbable for monkeypox to become established in human populations owing to the virus’ intrinsic lack of transmissibility [29•, 30]. The stochastic models used in these studies incorporated numerical estimates for contact and transmission rate variables that were derived from directly observed data [31]. Observations collected from 1980 to 1984 in DRC showed that people living in communities at risk for monkeypox had on average 10.7 close contacts (with 50% being high-risk household contacts), that secondary attack rates were approximately 6.7 times higher for unvaccinated contacts than vaccinated contacts, and that approximately 70% of the population had been vaccinated. Assuming these conditions, only 2% of model simulations resulted in a 3rd-generation virus transmission event, and no iterations resulted perpetuation beyond the 6th generation of spread. And even assuming ‘worst case scenario’ conditions — whereby vaccine-derived immunity in the starting population was 0% — the resultant number of cases per simulation increased by approximately a factor of 4, but still no simulations resulted in indefinite, sustained virus transmission [30]; the R 0 never achieved ≥1.
The basic reproductive rate of an infection, R 0, describes the inherent transmissibility of an infection within a population which has no prior immunity [32], effectively however the value is subject to influence by population demographics, contact patterns, and heterogeneities of susceptibility among individuals. Employing a straightforward calculation of the number of new cases generated by a single monkeypox infection [29•], the R 0 of the modeled scenario above could pass the threshold of 1 by simply augmenting of the total number of close contacts from 10.7 to 13.7. Alternatively, increasing the proportion of contacts that are high-risk household contacts from 50% to 80% achieves the same outcome. Thus, within this framework (which assumes an absence of vaccine-derived immunity), fairly minor shifts in the epidemiologic context of monkeypox could tip the balance in favor of sustained spread even in the absence of other ecologic or evolutionary modifications.
Obtaining modern estimates of secondary contact rates and knowledge of human contact patterns in monkeypox endemic areas will be important for assessing the epidemiologic potential of monkeypox for sustained inter-human transmission in contemporary at-risk communities. Regardless, however, of the current reproductive rate of monkeypox in human populations, probabilistic arguments suggest that a zoonotic pathogen with an R 0 near to one (such as monkeypox) retains a greater potential to evolve to a state of higher transmissibility as transmission chains lengthen and as the number of primary introductions increases [33]. Under this scenario, evolutionary advancements could accrue in stepwise fashion through individual character state changes, provided each step were to confer an incremental advantage in transmissibility (fitness) [33]. For example, an initial (hypothetical) virus mutation that enhances seeding and proliferation of virus in the epithelium of the human throat, followed by a second mutation that potentiates irritation and coughing, could provide a theoretical fitness advantage at each step; whereas reversing the steps would likely not.
Zoonotic pathogens of intermediate transmissibility to humans such as monkeypox may be well positioned to derive selective advantage (for heightened transmissibility) from minor gains in host specialization. But, would increasing the inter-human transmission potential of monkeypox necessarily require increased specialization for humans and, if so, would that in turn necessarily lead toward recapitulation of a pathogen with the virulence and characteristics of variola?
Evolutionary constraints
Though monkeypox and (discrete ordinary) smallpox would be difficult to distinguish from one another in a clinical setting, there are subtle clues that point toward one illness as opposed to the other. Lymphadenopathy, for example, is a prominent feature of monkeypox [34, 35] yet was nearly absent in smallpox patients. Nodal swelling has been described with smallpox [36, 37], but the underlying process for this — localized edema — is distinct from the process of lymphoid hyperplasia (lymphocyte proliferation) observed in non-human primates infected with monkeypox virus [38, 39]. Other functional differences affecting immune evasion and manipulation of the host immune system are predicted based on genome-level comparisons between variola and monkeypox viruses.
A core set of 90 conserved genes has been proposed as the ‘minimum essential genome’ of all Chrodopoxviruses (the subfamily that encompasses those poxviruses that parasitize vertebrate animals) [40]. This set accounts for only ∼50% of the haploid gene content of variola virus [41•]. A typical Orthopoxvirus such as variola or monkeypox will have, in addition, genes associated with host specificity, immunomodulation and subcellular trafficking (for example), as well as a complement of open reading frames (orfs) with unknown function, regions of non-coding sequence, and long inverted terminal repeats (ITRs). Fluctuations in gene content — gene gain, gene loss — can provide opportunities for Orthopoxvirus adaptation to alternative hosts [42]. In fact, broad-scale evaluation of Orthopoxviruses genomes suggests that it is not uncommon for genes that have been acquired or lost to be those associated with host-specific properties [40, 42].
In general, monkeypox virus genomes have, or have retained, considerably more DNA content than variola. A comparison of the Zaire-96 strain of monkeypox [41•, 43] and the Kuwait-1967 strain of variola captures trends present across a broader sampling of each species: here, the monkeypox genome includes 4 additional genes and is ∼11 000 nucleotides longer than the variola genome; it has ∼10.5× longer ITRs, and extra coding sequences within the ITRs (whereas variola has none) [41•]. Variola unquestionably has one of the most significantly size-restricted genomes of all the Orthopoxviruses, yet it is not a trimmed-down version of monkeypox. Variola has (depending on the analysis) up to 9 defined coding sequences that monkeypox viruses do not have, or of which monkeypox viruses have only retained fragments [44•]. In contrast, monkeypox has ∼16 defined orfs not present in variola [44•, 45] (Table 2 ).
Table 2.
| Locus | Function | Variola | Monkeypox (Central African) |
|---|---|---|---|
| C3L | Inhibitor of complement enzymes | Co-factor in cleavage of C3b and C4b; C3 and C5 decay-accelerating activity | Truncated version of protein; C3b and C4b cleavage activity (lower efficiency that variola protein); no decay-accelerating activity |
| C10L | IL-β antagonist protein | C-terminal domain of protein binds host IL-β receptor blocking IL-β-mediated cellular activation pathways | Truncated version of protein lacking C-terminal domain |
| K3L | elF-2α protein | Mimic of host cell translation initiation factor, mimic protein binds to the host's INF-induced inhibitor of translation, thereby allowing translation to continue in infected cells | Gene absent or fragmented |
| E3L | INF-resistance protein | N-terminal domain of protein binds Z-DNA and may influence the expression of immune response genes; C-terminal domain of protein binds dsRNA and inhibits Type I interferon-mediated host cell activation | Truncated version of protein with only the C-terminal, dsRNA-binding domain |
| A49R | Phosphotransferase | No predicted role in virulence | Gene absent |
Several of the loci found in variola that are missing or truncated in monkeypox are hypothesized to play a role in immune evasion and virulence. For example, the variola genome harbors a virulence-associated gene (C3L) that expresses an inhibitor of complement enzymes. The ortholog of this gene (D14L) is either missing or expressed as a truncated (but functional) protein in monkeypox viruses [45, 46•]. The question of how pivotal the protein is to establishing robust Orthopoxvirus infections in humans is still the subject of investigation, but the smallpox protein is presumed to modulate a critical feature of the host innate immune system early during infection [45, 46•, 47, 48]. (Experimental attempts to demonstrate the functional importance of this locus to other orthopoxirus virulence phenotypes — either by adding the locus to deficient genetic backgrounds or by ablating the function from virulent background — have generated inconsistent results [49, 50].)
If the gene complement of monkeypox is lacking certain essential coding sequences related to host specialization, monkeypox virus’ larger genome size and unique orfs could theoretically provide enough genetic plasticity to overcome the limitation. For instance, deficiencies in certain variola-specific functions could be met through alternative pathways — that is, functional pathways for immune evasion or inhibition that differ from variola's, yet ultimately impact the same target within the host.
A scan of the genome indicates that monkeypox viruses are deficient with respect to full-length orthologs for the two prominent loci in variola that influence interferon-resistance (E3L, K3L) [44•, 45]. Yet, host-expression microarrays generated following infection of primary human monocytes with monkeypox virus unambiguously demonstrated diminution of interferon-associated host gene expression [51]. Thus, although monkeypox virus lacks full-length orthologs for these variola genes associated with interferon resistance, suppression of host interferon-induced gene expression is still achieved. This particular phenomenon, though not fully characterized, provides one example of a virus phenotype, common to both variola and monkeypox, that is manifest through non-equivalent processes. The inhibition of host interleukin-1 beta (IL1-β) may be another.
Variola expresses a full-length IL1-β antagonist protein (C10L ortholog) that binds at its C-terminal end to host IL-1β receptors effectively preventing or diminishing host cell activation by the cytokine [52•]. Only the N-terminal portion of the protein is expressed by monkeypox virus [45] suggesting that the monkeypox protein would not demonstrate IL1-β receptor binding capacity. However, some Central African strains of monkeypox appear to possess the capacity to interfere with host cell activation by IL1-β. These variants of monkeypox virus putatively express a protein (B15R) that binds directly to IL-1β, rather than to its host cell receptor [44•, 46•, 52•]. If borne out by functional studies, this could constitute and alternative means — not found in variola — of achieving the same host immune-modulatory effect.
Conceivably, further adaptation of monkeypox virus to humans, if it happens at all, could arrive through gene gain, or through nucleotide changes and optimization of these non-equivalent, redundant pathways (convergent evolution).
Conclusion
If the question initially posed was ‘What is the intrinsic potential of monkeypox to fill the void left by the eradication of smallpox?’, we conclude here with a mixed assessment. The scope of human immunity generated by eradication-era vaccinations unquestionably had an impact on the prevalence and distribution of both monkeypox and smallpox. But only smallpox was eradicable through the human vaccination program. The immunologic picture appears favorable for the resurgence of monkeypox in disease endemic areas — owing to increasing population-level vulnerability — but several factors inherent to the genetic makeup and ecology of monkeypox virus would seem to diminish the probability that this disease will spread to a significant degree outside the moist tropical forests of West and Central Africa.
The 2003 outbreak of monkeypox in the United States, which began with importation of infected animals from West Africa, provided a stark example of how spillover and propagation in a permissive animal could, at least temporarily, expand the range of monkeypox. Yet the most plausible animal taxa for monkeypox virus propagation and spread (Sciurid rodents, for example) are likely to be inefficient transmitters of infection to humans. Conversely, taxa more frequently implicated in transmission of zoonotic diseases to humans (Mus and Rattus) are not particularly susceptible to infection with monkeypox virus. It is arguable that for emergence to occur, gains in transmission efficiency and in the capability of monkeypox virus to exploit humans as hosts would be required. The path to achieving these gains (and an R 0 >1 in human populations) could involve relatively minor changes to the epidemiology of the disease (e.g. increasing the number of high-risk contacts by ∼20%) or evolutionary modifications that enhance infection success and specificity in humans hosts. But, in the immediate future, neither path is likely to lead to the recapitulation of a pathogen with the same virulence properties as smallpox.
In the meantime, monkeypox will continue to be a significant public health concern for people living in endemic areas. Waning immunity, inadequate housing and health infrastructure, and the lack of alternatives to bush meat consumption all likely contribute to increasing the concern that monkeypox may re-emerge in Central Africa. This in turn contributes to fears about export of the virus to neighboring countries. Appropriate and effective interventions are urgently needed to prevent ongoing human infections. By focusing on disease prevention efforts in areas already affected by monkeypox, we may ultimately diminish the probability that monkeypox will be a future threat in other environments.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
Acknowledgement
The authors would like to thank Benjamin Monroe for helping to produce figures for this article.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the funding agency.
References
- 1.Carroll D.S., Emerson G.L., Li Y., Sammons S., Olson V., Frace M., Nakazawa Y., Czerny C.P., Tryland M., Kolodziejek J., et al. Chasing Jenner's vaccine: revisiting cowpox virus classification. PLoS One. 2011;6:e23086. doi: 10.1371/journal.pone.0023086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Org. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 3•.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., Blumberg S., Thomassen H.A., Pike B.L., Fair J.N., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides an empirical description of a 20-fold increase in the incidence of human monkeypox in the Democratic Republic of the Congo since the eradication of smallpox and cessation of programs for routine smallpox vaccination.
- 4.Berthet N., Nakoune E., Whist E., Selekon B., Burguiere A.M., Manuguerra J.C., Gessain A., Kazanji M. Maculopapular lesions in the Central African Republic. Lancet. 2011;378:1354. doi: 10.1016/S0140-6736(11)61142-2. [DOI] [PubMed] [Google Scholar]
- 5.Damon I.K., Roth C.E., Chowdhary V. Discovery of monkeypox in Sudan. N Engl J Med. 2006;355:962–963. doi: 10.1056/NEJMc060792. [DOI] [PubMed] [Google Scholar]
- 6.Learned L.A., Reynolds M.G., Wassa D.W., Li Y., Olson V.A., Karem K., Stempora L.L., Braden Z.H., Kline R., Likos A., et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–434. [PubMed] [Google Scholar]
- 7.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 8.Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I., Wong S.W., Yoshihara P., Hanifin J.M., Slifka M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 9.Karem K.L., Reynolds M., Hughes C., Braden Z., Nigam P., Crotty S., Glidewell J., Ahmed R., Amara R., Damon I.K. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vacc Immunol. 2007;14:1318–1327. doi: 10.1128/CVI.00148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karem K.L., Reynolds M.G. Protection from smallpox: beyond immune biomarkers. Future Virol. 2011;6:709–719. [Google Scholar]
- 11.Schneider W.H. Smallpox in Africa during colonial rule. Med Hist. 2009;53:193–227. doi: 10.1017/s002572730000363x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. World Health Organization; Geneva: 1988. Smallpox and its Eradication. [Google Scholar]
- 13.Breman J.G., Nakano J.H., Coffi E., Godfrey H., Gautun J.C. Human poxvirus disease after smallpox eradication. Am J Trop Med Hyg. 1977;26:273–281. doi: 10.4269/ajtmh.1977.26.273. [DOI] [PubMed] [Google Scholar]
- 14.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds M.G., Carroll D.S., Olson V.A., Hughes C., Galley J., Likos A., Montgomery J.M., Suu-Ire R., Kwasi M.O., Jeffrey R.J., et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg. 2010;82:746–754. doi: 10.4269/ajtmh.2010.09-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Technical Advisory Group on Human Monkeypox . World Health Organization; Geneva: 1999. Report of a WHO Meeting. WHO/CDS/CSR/APH/99.5. pp. 1–11. [Google Scholar]
- 17.Woolhouse M.E., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gough A.W., Barsoum N.J., Gracon S.I., Mitchell L., Sturgess J.M. Poxvirus infection in a colony of common marmosets (Callithrix jacchus) Lab Anim Sci. 1982;32:87–90. [PubMed] [Google Scholar]
- 19•.Hutson C.L., Lee K.N., Abel J., Carroll D.S., Montgomery J.M., Olson V.A., Li Y., Davidson W., Hughes C., Dillon M., et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. [PubMed] [Google Scholar]; This article illustrates the broad range of animal taxa that can become infected with monkeypox virus in the absence of specific experimental induction of infection by humans. Rather these infections were the consequence of the animals having been in proximity to other infected animals or to their having had contact with virus-contaminated environments (cages, bedding).
- 20.Khodakevich L., Jezek Z., Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arita I. The current status of monkeypox: memorandum from a WHO meeting. Bull World Health Org. 1984;62:703–713. [PMC free article] [PubMed] [Google Scholar]
- 22.Emerson G.L., Li Y., Frace M.A., Olsen-Rasmussen M.A., Khristova M.L., Govil D., Sammons S.A., Regnery R.L., Karem K.L., Damon I.K., Carroll D.S. The phylogenetics and ecology of the orthopoxviruses endemic to North America. PLoS One. 2009;4:e7666. doi: 10.1371/journal.pone.0007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathanson N. Virus perpetuation in populations: biological variables that determine persistence or eradication. Arch Virol Suppl. 2005:3–15. doi: 10.1007/3-211-29981-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khodakevich L., Szczeniowski M., Nambu M.D., Jezek Z., Marennikova S., Nakano J., Meier F. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop Geogr Med. 1987;39:56–63. [PubMed] [Google Scholar]
- 25.Khodakevich L., Jezek Z., Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Org. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- 26.Marennikova S.S., Shelukhina E.M., Zhukova O.A. Experimental infection of squirrels Sciurus vulgaris by monkey pox virus. Acta Virol. 1989;33:399. [PubMed] [Google Scholar]
- 27.Marennikova S.S., Seluhina E.M. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull World Health Org. 1976;53:13–20. [PMC free article] [PubMed] [Google Scholar]
- 28.Americo J.L., Moss B., Earl P.L. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J Virol. 2010;84:8172–8180. doi: 10.1128/JVI.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]; This influential paper utilized period virus-transmission data to construct epidemiologic models that suggested monkeypox virus transmission was unlikely to be sustainable in human society in the absence of repeated introductions from a zoonotic source.
- 30.Jezek Z., Grab B., Dixon H. Stochastic model for interhuman spread of monkeypox. Am J Epidemiol. 1987;126:1082–1092. doi: 10.1093/oxfordjournals.aje.a114747. [DOI] [PubMed] [Google Scholar]
- 31.Arita I., Jezek Z., Khodakevich L., Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am J Trop Med Hyg. 1985;34:781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- 32.Anderson R., May R. Oxford University Press; Oxford: 1992. Infectious Diseases of Humans. [Google Scholar]
- 33.Antia R., Regoes R.R., Koella J.C., Bergstrom C.T. The role of evolution in the emergence of infectious diseases. Nature. 2003;426:658–661. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., Damon I.K., Reynolds M.G., Kuehnert M.J. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 35.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 36.Councilman W.T. Some general considerations on the pathology of smallpox. Public Health Pap Rep. 1905;31:218–229. [PMC free article] [PubMed] [Google Scholar]
- 37.Wahl-Jensen V., Cann J.A., Rubins K.H., Huggins J.W., Fisher R.W., Johnson A.J., de Kok-Mercado F., Larsen T., Raymond J.L., Hensley L.E., Jahrling P.B. Progression of pathogenic events in cynomolgus macaques infected with variola virus. PLoS One. 2011;6:e24832. doi: 10.1371/journal.pone.0024832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyall J., Johnson R.F., Chen D.Y., Huzella L., Ragland D.R., Mollura D.J., Byrum R., Reba R.C., Jennings G., Jahrling P.B., et al. Evaluation of Monkeypox disease progression by molecular imaging. J Infect Dis. 2011 doi: 10.1093/infdis/jir663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goff A.J., Chapman J., Foster C., Wlazlowski C., Shamblin J., Lin K., Kreiselmeier N., Mucker E., Paragas J., Lawler J., Hensley L. A novel respiratory model of infection with monkeypox virus in cynomolgus macaques. J Virol. 2011;85:4898–4909. doi: 10.1128/JVI.02525-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upton C., Slack S., Hunter A.L., Ehlers A., Roper R.L. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J Virol. 2003;77:7590–7600. doi: 10.1128/JVI.77.13.7590-7600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Hendrickson R.C., Wang C., Hatcher E.L., Lefkowitz E.J. Orthopoxvirus genome evolution: the role of gene loss. Viruses. 2010;2:1933–1967. doi: 10.3390/v2091933. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a newly developed computational tool, the authors of this article analyzed a set of 17 fully sequenced Orthopoxvirus genomes and identified a series of putative gene gain/loss events that have occurred over the course of Orthopoxvirus evolution and diversification.
- 42.McLysaght A., Baldi P.F., Gaut B.S. Extensive gene gain associated with adaptive evolution of poxviruses. Proc Natl Acad Sci U S A. 2003;100:15655–15660. doi: 10.1073/pnas.2136653100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shchelkunov S.N., Totmenin A.V., Babkin I.V., Safronov P.F., Ryazankina O.I., Petrov N.A., Gutorov V.V., Uvarova E.A., Mikheev M.V., Sisler J.R., et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides a comprehensive overview of key virus loci that are known to play a role in modulating the host immune response. The authors describe the roles of various proteins and the distribution of genes (presence, absence) across different Orthopoxvirus species (variola, vaccinia, monkeypox viruses).
- 45.Chen N., Li G., Liszewski M.K., Atkinson J.P., Jahrling P.B., Feng Z., Schriewer J., Buck C., Wang C., Lefkowitz E.J., et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., Davidson W., Galloway R., Khristova M.L., Reynolds M.G., et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]; The authors of this paper describe genome-level differences among viruses in two different genetic clades of monkeypox and suggest correlations linking specific gene profile to virulence phenotypes.
- 47.Liszewski M.K., Leung M.K., Hauhart R., Buller R.M., Bertram P., Wang X., Rosengard A.M., Kotwal G.J., Atkinson J.P. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J Immunol. 2006;176:3725–3734. doi: 10.4049/jimmunol.176.6.3725. [DOI] [PubMed] [Google Scholar]
- 48.Liszewski M.K., Bertram P., Leung M.K., Hauhart R., Zhang L., Atkinson J.P. Smallpox inhibitor of complement enzymes (SPICE): regulation of complement activation on cells and mechanism of its cellular attachment. J Immunol. 2008;181:4199–4207. doi: 10.4049/jimmunol.181.6.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estep R.D., Messaoudi I., O’Connor M.A., Li H., Sprague J., Barron A., Engelmann F., Yen B., Powers M.F., Jones J.M., et al. Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J Virol. 2011;85:9527–9542. doi: 10.1128/JVI.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girgis N.M., Dehaven B.C., Xiao Y., Alexander E., Viner K.M., Isaacs S.N. The Vaccinia virus complement control protein modulates adaptive immune responses during infection. J Virol. 2011;85:2547–2556. doi: 10.1128/JVI.01474-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubins K.H., Hensley L.E., Relman D.A., Brown P.O. Stunned silence: gene expression programs in human cells infected with monkeypox or vaccinia virus. PLoS One. 2011;6:e15615. doi: 10.1371/journal.pone.0015615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Alcami A., Smith G.L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]; The authors of the paper demonstrate novel functional activity for vaccinia virus mediated by the B15R protein. This protein is shown to bind directly to host IL-1 beta rather than to the IL-1 beta receptor. Variola viruses (which express a C10L homolog) demonstrate the latter, but not the former IL-1 beta inhibitory activity.
- 53.Hutson C.L., Olson V.A., Carroll D.S., Abel J.A., Hughes C.M., Braden Z.H., Weiss S., Self J., Osorio J.E., Hudson P.N., et al. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J Gen Virol. 2009;90:323–333. doi: 10.1099/vir.0.005108-0. [DOI] [PubMed] [Google Scholar]
- 54.Schultz D.A., Sagartz J.E., Huso D.L., Buller R.M. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology. 2009;383:86–92. doi: 10.1016/j.virol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson A.T., Papes M., Reynolds M.G., Perry N., Hanson B., Regnery R., Hutson C., Muizniek B., Damon I., Carroll D.S. Native-range ecology and invasive potential of Cricetomys in North America. J Mammal. 2006;87:427–432. [Google Scholar]
- 56.Shchelkunov S.N., Totmenin A.V., Safronov P.F., Gutorov V.V., Ryazankina O.I., Petrov N.A., Babkin I.V., Uvarova E.A., Mikheev M.V., Sisler J.R., et al. Multiple genetic differences between the monkeypox and variola viruses. Dokl Biochem Biophys. 2002;384:143–147. doi: 10.1023/a:1016016013042. [DOI] [PubMed] [Google Scholar]


