Abstract
γδ T cells are unconventional lymphocytes that could play a role in bridging the innate and adaptive immune system. Upon initial exposure to an antigen, some activated T cells become memory T cells that could be reactivated upon secondary immune challenge. Recently, subsets of γδ T cells with a restricted antigen repertoire and long-term persistence have been observed after clearance of viral and bacterial infections. These γδ T cells possess the hallmark ability of memory T cells to respond more strongly and proliferate to a higher extent upon secondary infection. Murine and primate models of Listeria monocytogenes and cytomegalovirus infection display these memory hallmarks and demonstrate γδ T cell memory responses. In addition, human and non-human primate infections with Mycobacterium tuberculosis, as well as non-human primate infection with monkeypox and studies on patients suffering from autoimmune disease (rheumatoid arthritis and multiple sclerosis) reveal memory-like responses corresponding with disease. Murine models of psoriatic disease (imiquimod) and parasite infections (malaria) exhibited shifts to memory phenotypes with repeated immune challenge. These studies provide strong support for the formation of immune memory in γδ T cells, and memory γδ T cells may have a widespread role in protective immunity and autoimmunity.
Abbreviations: APC, Antigen presenting cell; BCG, Bacille Calmette-Guerin; CCR7, C-C chemokine receptor type 7; CD, Cluster of differentiation; CMV, Cytomegalovirus; DC, Dendritic cell; DETC, Dendritic epidermal T cell; EYFP, Enhanced yellow fluorescent protein; HC, Healthy control; HLA, Human leukocyte antigen; HMBPP, Hydroxy-3-methyl-but-2-enyl pyrophosphate; HSP, Heat-shock protein; IEL, Intraepithelial lymphocyte; IFN, Interferon; IL, Interleukin; IMQ, Imiquimod; IPP, Isopentyl pyrophosphate; LM, Listeria Monocytogenes; MCMV, Murine cytomegalovirus; MHC, Major histocompatibility complex; MICA, MHC class I polypeptide-related sequence A; mLN, Mesenteric lymph node; MPV, Monkeypox virus; MS, Multiple sclerosis; NHP, Non-human primate; NKR, Natural killer receptor; PBMC, Peripheral blood mononuclear cell; PE, Phycoerythrin; pLN, Peripheral lymph node; RA, Rheumatoid arthritis; RRMS, Relapsing-remitting multiple sclerosis; SLO, Secondary lymphoid organ; TCM, Central memory T cell; TCR, T cell receptor; TEFF, Effector T cell; TEM, Effector memory T cell; TEMRA, T effector memory re-expressing CD45RA; TLR, Toll-like receptor; TNF, Tumor necrosis factor; TRM, Resident memory T cell
Keywords: Gamma delta T cell, Immune memory, Adaptive immunity, Infections, Autoimmunity
1. Introduction
Gamma delta (γδ) T cells are a relatively small subset of unconventional lymphocytes which possess both innate and adaptive characteristics, bridging these two arms of the immune system [1]. Compared to traditional CD4+ and CD8+ alpha beta (αβ) T cells, γδ T cells bear innate-like characteristics such as non-major histocompatibility complex (MHC) restricted antigen recognition similar to Natural Killer cells, and an ability to attack cells and microbes directly with their cytotoxic activity [2], [3]. γδ T cells can be found circulating in the blood and lymphoid organs, or as resident cells in peripheral tissues and barrier surfaces such as the gut (γδ intraepithelial lymphocytes, IELs) and skin (γδ dendritic epidermal T cells, DETCs) [4]. γδ T cells possess a unique ability to also act as antigen presenting cells (APCs) to αβ T cells in the context of tumour antigens and microbial peptides [5], [6]. Additionally, human γδ T cells are effective at cross presentation of antigens to CD8+ T cells; surprisingly, γδ T cells can be even more effective than monocyte-derived dendritic cells (DCs) at cross-presentation in vitro [6]. Mouse γδ T cells have been noted to express MHC II and co-stimulatory molecules after activation in vitro with anti-CD3 and anti-CD28 antibodies, showing further APC capabilities [7]. Murine and human γδ T cells can act as a first defense while also influencing the behavior of their αβ T cell counterparts in infection and disease.
While γδ T cells are considered innate-like and have several innate characteristics, they are adaptive immune lymphocytes which have T cell receptors (TCRs) that undergo V(D)J recombination similar to their αβ counterparts. However, only a small fraction of putative γδ TCR ligands have been discovered [8]. It is generally believed that conventional and superantigens which activate αβ TCRs do not activate γδ T cells [9]. Using a combination of the γδ TCR, toll-like receptors (TLRs), and natural killer receptors (NKRs), γδ T cells can recognize self-proteins such as classical and non-classical MHC molecules, like MHC class I polypeptide-related sequence A (MICA), and CD1c, as well as pyrophosphate-containing small molecules (termed phosphoantigens), heat-shock proteins (HSPs), and lipids (via CD1) [9], [10]. Some examples of these small non-peptide phosphoantigens include mevalonate-derived isopentyl pyrophosphate (IPP) associated with infected and transformed cells, as well as (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) produced by pathogenic bacteria and parasites [11]. IPP and HMBPP can stimulate the γδ TCR directly, leading to TCR-dependant activation and a rapid cytotoxic response [12]. Interestingly, enhanced diversity of γδ TCR V-J and V-D-J junctional regions has been observed in γδ T cells collected from the intestinal epithelium, as compared to γδ T cells collected from the thymus, lymph nodes, and epidermis [13]. This finding further supports the role for recognition of non-self-molecules by the γδ TCR, as the gut is continually exposed to luminal bacterial and fungal antigens, which could be a reason for this increased TCR junctional diversity. Consequently, γδ T cells can recognize a wide selection of both self (stress signals) and non-self (bacterial, viral, parasitic) antigens, and respond accordingly.
With such a diverse repertoire of recognized antigens and a lack of APC antigen processing required for recognition, it comes as no surprise that γδ T cells have been implicated in infection, immunity, and autoimmunity since their discovery. Knockout and adoptive transfer experiments with γδ T cells have revealed a substantial protective role in models of infection, including animal models of Mycobacterium tuberculosis, Listeria monocytogenes, and influenza viruses [11]. An important role for γδ T cells also occurs in autoimmunity, where they have been noted to contribute to the pathogenesis of Type 1 diabetes, rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus; although, their exact role is not yet clear in most conditions [14]. Keeping this in mind, another hallmark of the adaptive immune system is forming long-lasting immune memory T cells. Memory T cells are antigen experienced T cells residing in lymphoid organs, the circulation, and peripheral tissues, which possess a lower activation threshold and respond faster and more effectively upon re-exposure with their cognate antigen [15]. After T cells mount a response upon antigenic stimulation, some effector cells will further differentiate into long-lived memory T cells. Memory T cells can provide different signals than their naïve and effector counterparts and proliferate faster in response to antigen exposure. All of this leading to more effective host protection. Evidence also suggests that cytokines and chemokines play a crucial role in TCR activation along with cognate antigen recognition in a memory recall response [16].
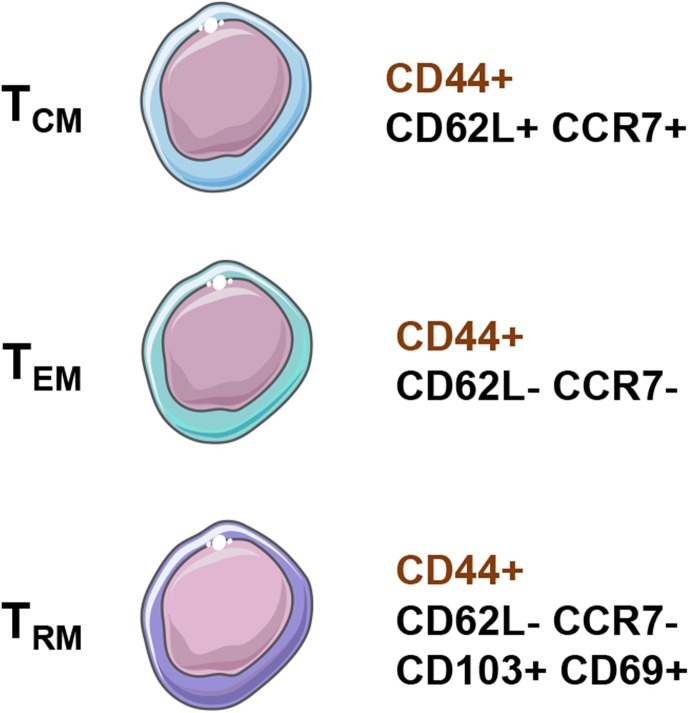
Naïve, effector, and memory T cells have distinct DNA methylation patterns which likely contribute to their unique properties, including increased longevity, potent effector function, and unique memory expression programs [17]. Generally, memory T cells can be subdivided into 3 classes: central memory T cells (TCM), effector memory T cells (TEM), and resident memory T cells (TRM) [18]. Long-lived TCM cells recirculate between the secondary lymphoid organs (SLOs) and blood, while TEM cells can recirculate through non-lymphoid tissues, SLOs, and/or blood. Finally, TRM are understood to reside permanently in peripheral non-lymphoid tissues, SLOs, and local vascular compartments without recirculating (Fig. 1 ).
Fig. 1.
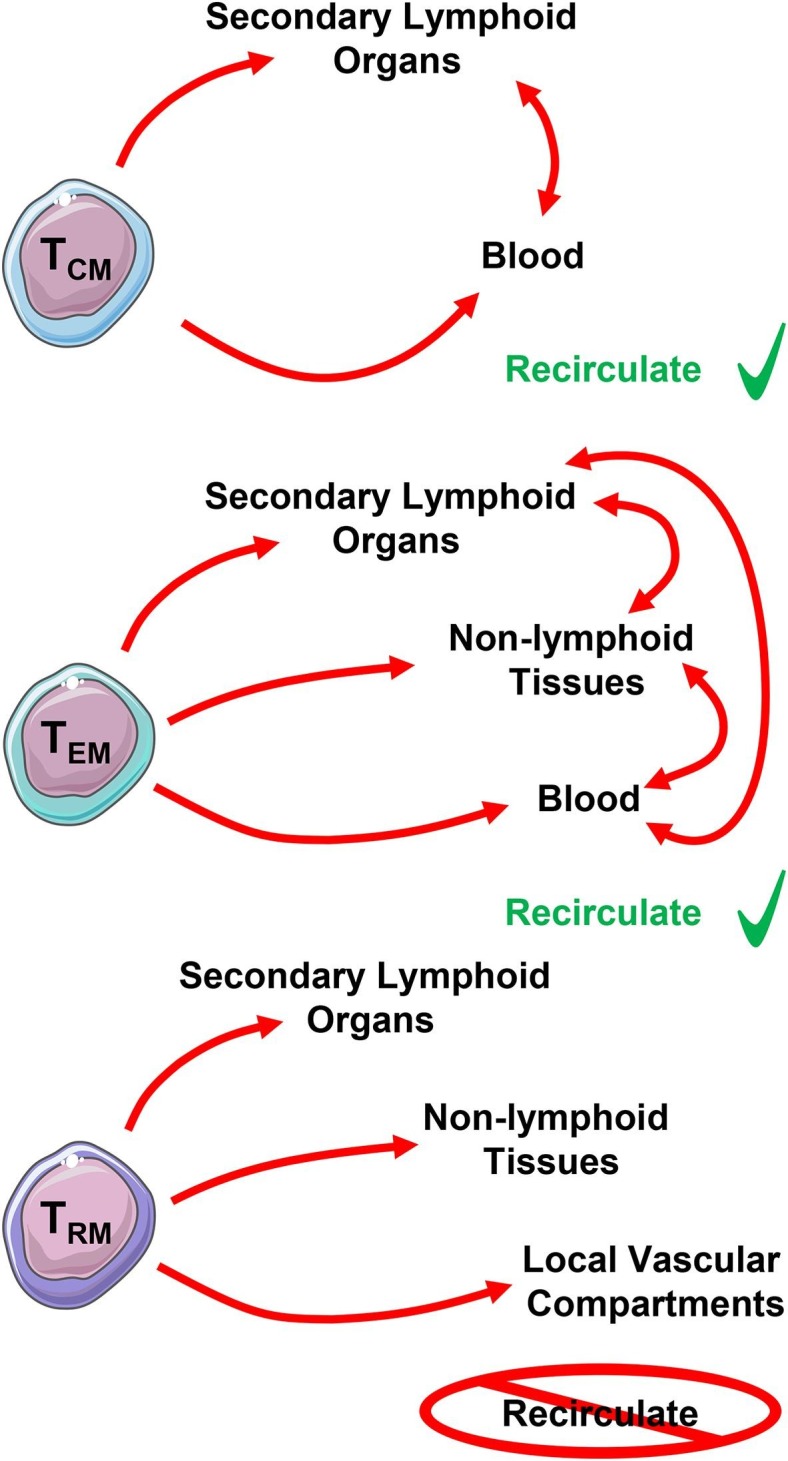
Dynamics of memory T cell populations and their putative localization. Central memory (TCM) and effector memory (TEM) T cells are able to recirculate, while resident memory (TRM) T cells reside permanently in their location.
In mice, TCM cells express the lymphoid tissue homing receptors C—C chemokine receptor type 7 (CCR7) and L-selectin (CD62L) (Fig. 2 ), while TRM cells express CD69 and integrin alpha E (CD103), known to be involved in tissue retention and recruitment, respectively [19], [20]. On the other hand, TEM cells fall somewhere in the middle usually not expressing any of these markers, notably not expressing the lymphoid homing receptors CCR7 and CD62L [21]. All of these murine memory T cell subsets express the hyaluronic acid receptor and activation marker, CD44, which is upregulated after antigen encounter and stays upregulated in memory T cells [22]. Naïve murine T cells, while expressing CD62L and CCR7, are distinctly CD44− [23].
Fig. 2.
Memory marker expression of murine central memory (TCM), effector memory (TEM), and resident memory (TRM) γδ T cells. The expression of CD44 in murine memory γδ T cells denotes previous encounter with antigen, and CD44 stays upregulated in memory T cells.
Human memory T cell populations can be subdivided in a similar fashion, albeit, with some different markers to the mouse. While humans share CCR7, CD62L, CD69, and CD103 with the mouse, human memory T cell subsets are further characterized using CD45RA and CD45RO, and do not express CD44 (Fig. 3 ) [24]. CD45RA is a marker of naïve T cells, while CD45RO denotes previous activation in a similar manner to CD44 in mice [25]. Naïve human T cells are CD45RA+ CD45RO− CD62L+ CCR7+, and after activation by TCR-mediated antigen recognition the naïve T cells become effector T cells (TEFF) with potent cytotoxic and proinflammatory capabilities [15]. Following the primary immune response and contraction of TEFF cell populations, some TEFF become long-lived CD45RA- CD45RO+ CD62L+ CCR7+ TCM cells [26]. These TCM cells can become TEM cells after re-exposure to their cognate antigen, yielding CD45RA− CD45RO+ CD62L− CCR7− effector memory T cells capable of a quick and robust proinflammatory response [27]. On the other hand, human TRM are CD45RA− CD45RO+ CD62L− CCR7− CD103+ CD69+, sharing similar markers to their murine counterparts [24]. TRM are thought to be derived from precursors that entered the tissues during the effector phase of the immune response, which remain positioned in the periphery [28]. Both mouse and human T cells are thought to follow a similar stepwise, progressive shift from naïve T cells, to TEFF cells, then TCM cells, and eventually TEM cells based on antigen recognition, activation, and the presence of cytokines [21], [26]. However, it is speculated that memory T cell development may not be entirely linear, as several hypothetical models exist with supporting evidence for the development pathway of memory T cells [29].
Fig. 3.
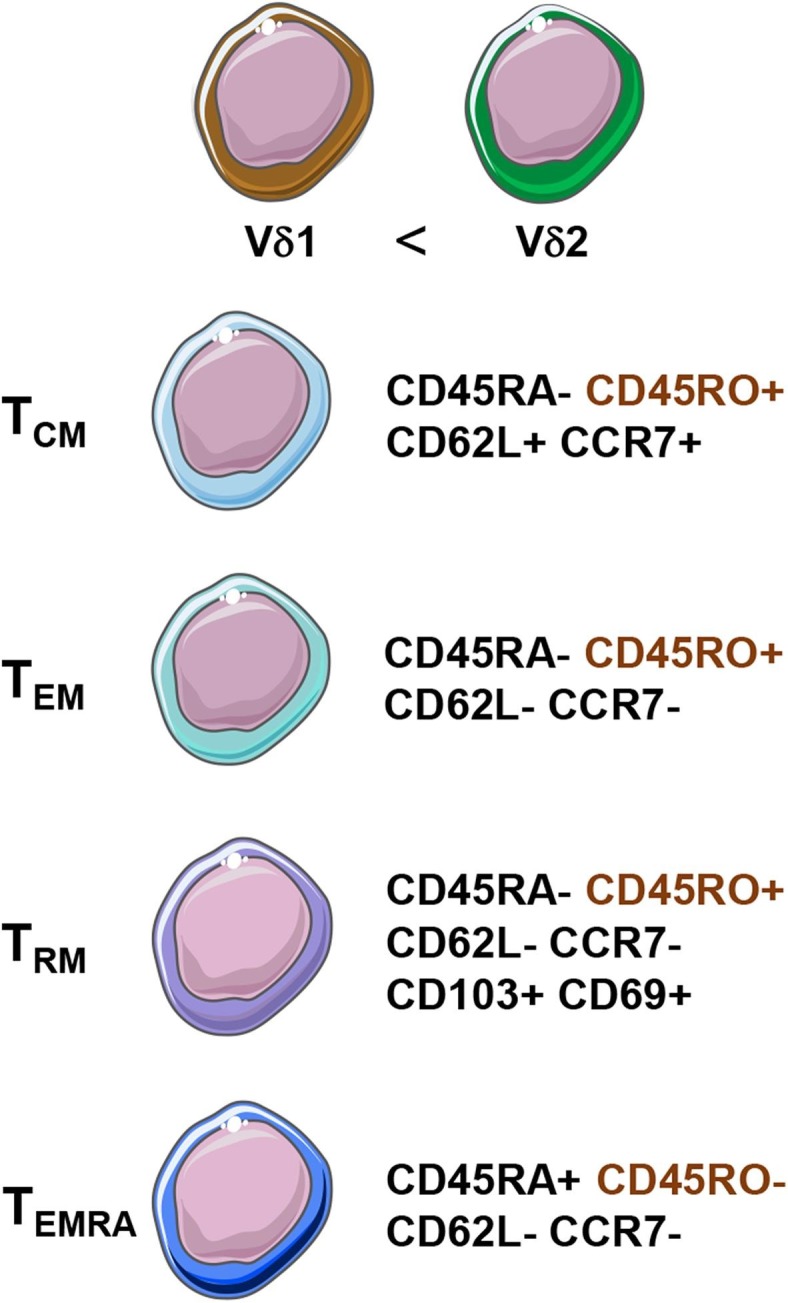
Memory marker expression of human central memory (TCM), effector memory (TEM), resident memory (TRM), and effector memory re-expressing CD45RA (TEMRA) γδ T cells. Vδ2 γδ T cells make up the vast majority of human γδ T cells. Human memory γδ T cells are defined by their expression of CD45RO and lack of expression of CD45RA, except in the case of γδ TEMRA cells, which re-express CD45RA.
γδ T cells share memory marker expression patterns with αβ T cells, and they can be subdivided into memory subsets based on the same marker expression criteria. However, how well do memory γδ T cells fill their memory roles in comparison to αβ memory T cells? In recent years, models of infection and autoimmune disease focusing on memory γδ T cells has yielded useful knowledge about the influence of these cells in infection and immunity. In this review we will explore the evidence for adaptive immune memory in γδ T cells across humans and mice in various models of disease and infection.
2. Human memory γδ T cells
Human γδ T cells can be divided into two general subsets following their usage of the TCRδ chain: Vδ1+ and Vδ2+ γδ T cells. Vδ1+ cells possess more diversity in their TCRγ chain expression (Vγ2/3/4/5/8) than their Vδ2+ counterparts (almost exclusively Vγ9+) [30], [31]. γδ T cells comprise roughly 5% of T cells in the peripheral blood of healthy adults [32]. The majority of γδ T cells in peripheral blood (roughly two-thirds) bear the Vγ9Vδ2 TCR, while the remainder are mostly Vδ1+ with even fewer Vδ3+ and Vδ5+ γδ T cells [4]. Most research into the ability of human γδ T cells to form long-lived memory cells has focused on the more frequent Vδ2+ subset. Human Vγ9Vδ2 T cells are clinically relevant as upon exposure to cells expressing non-peptidic antigens (ex. fibroblasts, monocytes, tumour cells), they respond with a cascade of immune reactions leading to αβ T cell activation, cytokine release, cytotoxicity, and DC and B cell activation [33]. These responses may aid in fighting pathogens, killing transformed cells, or alternatively, worsen autoimmune and inflammatory diseases. The ability of Vδ2+ and Vδ2− γδ T cells to form memory cell populations has been investigated in recent years, with evidence pointing towards the existence of these long-lived memory γδ T cell populations.
2.1. Mycobacterium tuberculosis and Listeria monocytogenes: Bacterial infections in primates
Early research into the γδ T cell response to M. tuberculosis infection led to speculation about the formation of adaptive immune memory in γδ T cells. In 1998, Hoft et al. showed that Bacille Calmette-Guerin (BCG) vaccination could induce memory-like characteristics and responses in γδ T cells [34]. Peripheral blood mononuclear cells (PBMCs) from BCG vaccinated and non-vaccinated healthy adults were exposed to M. tuberculosis antigens in vitro, and then immune cell populations characterized after 7 days of antigen exposure. γδ T cells had the most dramatic expansion in response to antigen exposure, and γδ T cells from BCG-vaccinated individuals expanded more than non-vaccinated controls. This expansion was observed with interleukin (IL)-2 alone, and in co-culture with CD4+ T cells, revealing that the expansion was not solely dependent on CD4+ T cell help. However, optimal expansion required CD4+ T cells in co-culture. Proliferation and expansion to a greater extent upon re-exposure to antigen is a hallmark of immune memory and previous antigen experience. This pioneering work revealed a potential role for memory formation in γδ T cells.
More recently, the memory-like responses of γδ T cells to re-exposure to M. tuberculosis antigens observed in vitro was confirmed in vivo using macaques [35]. Upon both intravenous and pulmonary reinfection with BCG, αβ and γδ T cells exhibited enhanced expansion versus the initial infection, eliciting this response for >4 weeks after the first infection. In vitro activation using BCG phosphoantigens and γδ T cells collected from infected macaques before the BCG infection and 4 weeks later confirmed that these γδ T cells were antigen specific and expanding in vivo in response to the BCG reinfection. Building on this concept, the authors wanted to elicit a Vδ2+ γδ T cell specific memory response in vivo [36]. Macaques were vaccinated with an attenuated strain of HMBPP producing Listeria monocytogenes (LM), and then infected with a moderate dose of M. tuberculosis 12 weeks after vaccination. Both LM and M. tuberculosis are known to produce HMBPP, a potent phosphoantigen activator of the γδ TCR [37]. Initial immunization caused an expansion of HMBPP-specific Vγ2Vδ2+ γδ T cells, and upon re-challenge with M. tuberculosis, the vaccinated macaques fared better losing less weight and with a lower bacterial burden in the lungs than the control groups (controls vaccinated with a non-HMBPP-producing attenuated LM strain or saline). The HMBPP-producing LM strain vaccinated macaques also had better control of the infection, with more CD4+ and CD8+ T cell recruitment and less M. tuberculosis dissemination outside of the lungs. Repeating the same protocol but reinfecting 35 days after vaccination with the attenuated HMBPP-producing LM strain instead of M. tuberculosis yielded similar results, with a much larger expansion of Vγ2Vδ2+ γδ T cells after the second infection versus the first [38]. These expanded Vγ2Vδ2+ γδ T cells were also found to be potent effector cells producing, or co-producing, proinflammatory cytokines, interferon (IFN)− γ, tumour necrosis factor (TNF)-α, IL-17, and/or perforin after LM infection.
These M. tuberculosis and LM experiments show that Vγ2Vδ2-specific immunization may lead to a potent adaptive memory response upon re-challenge. However, although these studies show a memory-like response in γδ T cells to M. tuberculosis and LM reinfection, they do not provide a profile of γδ T cells further than characterization of their Vδ and Vγ chain usage. Further characterization of memory subsets and memory marker expression would be helpful in determining the role and phenotype of these cells. Nonetheless, this provides a useful framework for further investigations of human and non-human primate (NHP) memory γδ T cell responses to bacterial infection.
2.2. Monkeypox and CMV: Viral infections in primates
γδ T cells have also shown memory-like responses to viral infections in NHP models. Cynomolgus monkeys vaccinated with vaccinia virus-derived Dryvax followed by infection with monkeypox (MPV) 2 months later led to potent memory-like expansion of γδ T cells upon reinfection [39]. Researchers co-vaccinated macaques with vaccinia virus and the antiviral drug cidofovir to produce sub-optimal anti-MPV immunity, and then challenged animals with MPV 2 months later. Despite the suboptimal immune priming due to cidofovir, 4 of 6 immunized macaques had an up to 10-fold increase in the frequency of Vγ2Vδ2+ T cells in the blood after MPV infection. This expansion was greater than what was seen in the mock-vaccinated macaques after MPV challenge, and greater than what was observed after vaccination alone without secondary infection. Vaccinia virus/cidofovir co-vaccination alone induced little to no expansion of Vγ2Vδ2+ T cells. The memory-like expansion of Vγ2Vδ2+ T cells after MPV infection in vaccinated macaques represents a hallmark of adaptive immune memory and trained immunity. Interestingly, orthopoxviruses do not produce phosphoantigen, so this response may be mediated by non-TCR mechanisms or reciprocal interactions with αβ T cells. On the other hand, there may be an unknown, non-phosphoantigen TCR antigen within the viral genome that activates the Vδ2+ γδ TCR. The latter seems more likely given the antigen/TCR driven memory responses observed in γδ T cells.
Human cytomegalovirus (CMV) also induces characteristics of immune memory in γδ T cells post-infection. Analyzing PBMCs from blood samples collected from CMV+ and CMV- patients by flow cytometry revealed changes in Vδ2− γδ T cell populations, but not Vδ2+ [40]. Within the Vδ2− compartment, CMV+ donors exhibited an increased frequency of terminally differentiated γδ TEM re-expressing CD45RA (termed TEMRA) γδ T cells versus CMV- donors (68.4% vs 13.7% respectively, P < 0.001). TEMRA cells are similar to TEM cells, however, they can be associated with a unique upregulation of cytotoxic molecules [41]. TEMRA cells re-express the CD45RA isoform after antigenic stimulation, while their TEM counterparts express CD45RO [42]. The researchers also studied the recall response of γδ T cells in renal allografts from CMV+ patients into CMV- patients and vice versa. They observed quicker expansions of Vδ2− γδ T cells in organ transplant patients experiencing CMV reactivation under immunosuppressive treatment (CMV+ patients transplanted with CMV- allografts, mean of 17 days for expansion) compared with patients undergoing primary infection (CMV- patient transplanted with CMV+ allograft, mean of 66 days for expansion, P < 0.005) [40]. An increase in TEMRA cells was also seen over the course of the primary infection in D+/R- patients, while the frequency remained stable and high in the D-/R+ patients following CMV reactivation from immunosuppression. The expansion of effector-memory Vδ2− γδ T cells in response to CMV infection, coupled with a faster expansion upon re-challenge with CMV is yet another example of memory-like responses within the γδ T cell subset. As Vδ2− T cells can kill CMV-infected cells in vitro, it is likely that Vδ2− T cells possessing a terminally differentiated TEMRA phenotype with an increased potential for cytotoxicity play an important role in the antiviral response [43]. Consequently, viral models induce a similar memory recall response upon reinfection to bacterial infection models in humans and NHPs, and together these data suggest a role for γδ T cell adaptive immune memory in protective immunity.
2.3. Multiple sclerosis and rheumatoid arthritis: Autoimmune disease in humans
Memory γδ T cells in human autoimmune disease has been investigated to a much lower extent. Shifts in memory T cell populations and recall-like expansions upon immune challenge have been observed in several human autoimmune disorders, including systemic lupus erythematosus, multiple sclerosis (MS), rheumatoid arthritis (RA), and Crohn’s disease [15]. The memory response of γδ T cells in autoimmunity is much less understood. Recently, circulating γδ T cells were characterized in relapsing-remitting multiple sclerosis (RRMS) patients (either in remission or relapse) versus healthy controls (HC) [44]. Researchers found no differences in frequency of total γδ T cells in peripheral blood among RRMS patients and HC, but increased circulating naïve γδ T cells in the RRMS group versus HC. Interestingly, a strong decrease in γδ TCM cells was observed in remission RRMS versus relapse RRMS and HC, potentially a result of decreased antigen stimulation needed to maintain the TCM pool over time. In addition, relapse RRMS patients had fewer circulating terminally differentiated TEMRA γδ T cells. The researchers hypothesized that this was the result of migration of these cells out of the circulation into inflamed tissues in relapse RRMS patients, where TEMRA γδ T cells are highly represented. TEMRA cells express receptors for migration into inflamed tissue compartments, and express intracellular perforin and granulysin showing potent cytotoxicity; TEMRA cells are not commonly found in SLOs [45], [46]. This RRMS example highlights the dynamics of memory cell populations in autoimmune disease, with increased disease severity linked to probable extravasation of TEMRA, and decreased disease severity associated with depletion of TCM γδ T cells (potentially leading to decreased circulating memory cells capable of a strong immune response). However, more research is required to support this hypothesis, as the migration of these cells and their effector functions have not been fully elucidated.
γδ TEM cells have also shown that they possess the potential to worsen RA in humans. Vγ9Vδ2+ γδ T cells were observed in high numbers in the peripheral blood and synovial fluid collected from RA patients, most bearing markers of TEM γδ T cells [47]. These Vγ9Vδ2+ TEM cells exhibited a strong APC capability (high expression of human leukocyte antigen (HLA)-DR and CD80/86, molecules associated with professional APC function), and the ability to simultaneously secrete both IFN-γ and IL-17 (two potent proinflammatory cytokines) upon in vitro stimulation with IPP. These results suggest that human Vγ9Vδ2+ γδ TEM cells possess the ability to both activate CD4+ T cells through APC function, and directly secrete proinflammatory cytokines in the context of RA, hinting to a role in the development of the disease. This example highlights the cytokine potential of an upregulated population of memory γδ T cells in RA, but it does not examine the memory response itself. While these autoimmune examples do not present direct evidence for a role of memory γδ T cells in human autoimmune disease progression, they show interesting evidence in favour of this hypothesis by characterizing memory marker expression and analyzing the responses of these cells in vitro. Further analysis of the recall response of human memory γδ T cells in autoimmune disease is needed to support this evidence.
3. Murine memory γδ T cells
Murine γδ T cells can be subdivided based on their Vγ and Vδ chain usage. However, their isoforms differ from what is observed in humans. Mouse γδ T cells are primarily grouped based on Vγ chain expression. γδ T cell Vγ chains are most often characterized using either the Heilig & Tonegawa or Garman naming systems. The Garman system proposes 7 Vγ chain subsets: Vγ1.1, Vγ1.2, Vγ1.3, Vγ2, Vγ3, Vγ4, and Vγ5, which coincide with Heilig & Tonegawa’s Vγ1, Vγ2, Vγ3, Vγ4, Vγ5, Vγ6, and Vγ7 subsets, respectively [48], [49]. Following Heilig & Tonegawa’s naming system, some important murine γδ T cell subsets are the Vγ5Vδ1+ DETCs in the epidermis, Vγ1+ and Vγ7+ IELs in the gastrointestinal tract, Vγ4+ γδ T cells in the dermis, Vγ6Vδ1+ γδ T cells in the female reproductive tract, tongue, and peritoneal cavity, and Vγ1+ and Vγ4+ γδ T cells in the SLOs and lung [50]. Whereas murine γδ T cells do show some diversity in their Vδ chain usage, they are usually classified based on their Vγ chain [51]. Murine γδ T cells represent roughly 3% of spleen and lymph node CD3+ T cells in rodents, and exhibit a similar capability to produce the cytokines IL-17 and/or IFN-γ to human γδ T cells [4], [52]. Knockout models of various Vγ γδ T cell subsets has revealed that the composition of murine γδ T cells influences population dynamics of memory CD4+ and CD8+ αβ T cells in non-immunized mice [53]. γδ T cells display characteristics of immune memory in murine bacterial, viral, and parasitic infections, and experimental models of autoimmune disease. In mice, phycoerythrin (PE) is a putative antigen of the γδ TCR. After immunizing mice with PE, PE specific γδ T cells were shown to transition from a naïve (CD44− CD62L+) to a TEM (CD44+ CD62L−) phenotype [54]. It was also reported that these cells have a rapid induction of effector capabilities, producing large amounts of IL-17A after PE immunization. These data suggest a TCR dependant shift from naïve to memory γδ T cells upon encounter with cognate TCR antigen, and further examples will build on this concept.
3.1. Listeria monocytogenes and Staphylococcus aureus: Murine bacterial infections
Similar to human γδ T cells, murine γδ T cells possess memory characteristics in models of bacterial infection and reinfection. In a murine model of oral LM infection, LM elicited a population of CD44+ Vγ4Vδ1+ (Garman notation) γδ T cells in the mesenteric lymph node (mLN), but not the peripheral lymph nodes (pLN) [55]. Mice were reinfected with LM 170 days after primary infection either orally or intravenously, and then rested for an additional 124 days prior to a tertiary oral LM infection. After secondary oral challenge with LM, the frequency of CD44+ γδ T cells increased to a higher extent than the primary infection within 5 days of the secondary infection (Fig. 4 ). The frequency of CD44+ γδ T cells was even higher after the tertiary infection, pointing to a stepwise acquisition of trained immune memory with each infection. This increase in CD44+ γδ T cells was not elicited when mice were administered LM intravenously, showing specificity to oral (mucosal) infection. Further, the researchers confirmed a role for γδ T cell mediated immunity in LM by using antibodies to deplete CD4+ and CD8+ T cells and/or internalize the γδ TCR before reinfection with LM. Antibody mediated γδ TCR internalization or CD4+/CD8+ depletion alone caused only minimal loss of protection, while both combined resulted in the least protection among LM-experienced mice. This suggests that γδ T cells exert an important protective role in oral LM infection in conjunction with CD4+ and CD8+ αβ T cells. Using antibodies against the Vγ1.1+ and Vγ2+ TCRs (the other γδ T cells commonly found in the mucosa) along with anti-CD4+/CD8+ antibodies conferred more protection than using an antibody not specific for certain γδ TCRs, showing that it is likely the memory Vγ4+ γδ T cells that play the most significant role in protection from LM among all γδ T cells. LM-experienced mice also had lower viral loads in the mLN and small intestine upon reinfection than naïve mice undergoing primary infection. These experiments demonstrate that LM-elicited CD44+ γδ T cells are maintained long term and can respond to secondary and tertiary infection with robust proliferation and effector functions (that increase host immunity). These are hallmarks of adaptive immune memory.
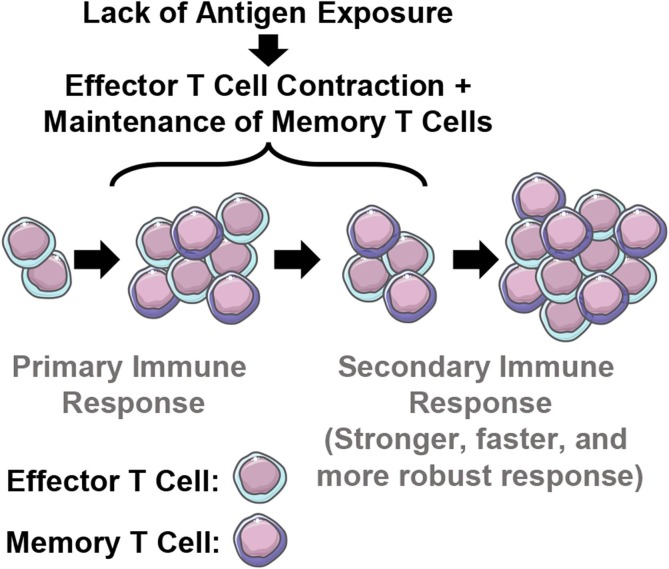
Fig. 4.
The primary immune response to pathogen infection elicits effector and some memory T cells. As demonstrated by Lefrancois et al. (2013), upon resolution of infection and contraction of immune effectors coinciding with reduced antigen exposure, a population of long-lived memory T cells develops which can react more strongly to reencounter with cognate antigen (secondary, tertiary, etc. immune response).
Further investigation into LM-elicited memory Vγ4+ γδ T cells in mice revealed that these cells possess characteristic markers of TRM in the mLN after infection [56]. RNA sequencing demonstrated that most (>70%) LM-elicited Vγ4+ CD44+ γδ T cells in the mLN expressed TRM tissue retention marker CD69, but not TCM markers CD62L and CCR7 30 days post primary infection. Furthermore, parabiosis of LM-experienced Thy1.2 mice and naïve Thy1.1 mice revealed that these Vγ4+ γδ T cells are indeed resident memory T cells, as frequencies did not equilibrate in the mLN between naïve and LM-experienced animals after 9–11 days. On the other hand, circulating and mLN frequencies of naïve CD4+ αβ T cells and Vγ1.1/2/3+ γδ T cells equilibrated fully between animals, which showed the tissue-resident properties of these Vγ4+ γδ T cells. γδ TRM cells were more IL-17A positive than any other cells in the mLN upon re-challenge with LM (analyzed via flow cytometry staining), and blockade of IL-17A with antibodies resulted in increased bacterial burden and delayed clearance of LM. Therefore, as these γδ TRM cells are a primary source of IL-17A in the mLN upon LM re-challenge, and IL-17A production correlates with bacterial clearance, it seems probable that TRM γδ T cells play an important role in controlling LM infection in mice. Together, these experiments demonstrate that memory Vγ4+ γδ T cells may help in resisting LM infection: LM-elicited Vγ4+ γδ T cells are primarily of the TRM subtype, Vγ4+ γδ T cells undergo progressively increased proliferation upon each re-challenge with LM (a hallmark of immune memory), and these γδ TRM are a crucial source of IL-17A upon reinfection with LM (which correlates with increased protection and decreased bacterial burden).
Research on memory γδ T cells in bacterial infections using a model of Staphylococcus aureus revealed a similar role for Vγ4+ γδ T cells in vivo in mice [57]. γδ T cells were the predominant source of IL-17 during infection, and prior immune challenge with Staphylococcus aureus expanded a population of Vγ4+ T cells capable of upregulated IL-17 production during reinfection 21 days after initial exposure. Mice previously exposed to S. aureus infection had lower bacterial loads, increased bacterial clearance, and increased IL-17 production post-infection than mice with no prior exposure, demonstrating a memory response. The frequency of CD44+ and IL-17+ γδ T cells was also higher in mice with prior exposure versus mice not previously exposed 21 days after infection, showing the formation and longevity of memory γδ T cells. This increased cytokine response is a characteristic of TEM cells. However, the authors did not phenotype the cells for TEM memory marker expression further than CD27 and CD44. Consequently, it seems murine and human Vγ4+ and Vδ2+ γδ T cells share many common aspects in their memory response to LM infection. The murine immune response to Staphylococcus aureus infection has many similarities to the memory Vγ4+ γδ T cell response to LM, notably in cytokine production.
3.2. Murine Cytomegalovirus: Viral infection model in mice
Memory γδ T cell responses have also been observed in viral infections of the mouse. Murine CMV (MCMV) reproduces a similar T cell and natural killer cell response as human CMV, and therefore serves as a useful model to study immune responses to CMV [58]. Recently, γδ T cells have been shown to have an important influence in resistance to MCMV. Mice lacking either TCRα or TCRδ were able to survive MCMV infection, while mice lacking CD3ε (which have no T cells) died at low doses of MCMV [59]. These authors then determined the frequency and marker expression patterns of γδ T cells in response to MCMV infection. They found increased numbers of Vγ1+, and to a lesser extent, Vγ4+ γδ T cells in the liver and lungs 3 days after infection, and an increase in only Vγ1+ γδ T cells in the spleen (Heilig & Tonegawa notation). Notably, the proportion of TEM (CD44+ CD62L−) cells among Vγ1+ and Vγ4+ γδ T cells increased after 3 days post infection in the liver, lungs, and spleen, eventually representing up to ~80% of each Vγ γδ T cell subset by day 56. This increase in TEM was concomitant with a decrease in TCM (CD44+ CD62L+) Vγ1+ and Vγ4+ γδ T cells, potentially showing the dynamics of memory T cell differentiation in infection. To reveal the capability of γδ T cells to mount a memory response upon re-challenge with MCMV, the researchers transferred γδ T cells from MCMV-experienced and MCMV-negative TCRα knockout mouse donors into CD3ε knockout mice and infected the recipient mice with MCMV after transfer. The CD3ε knockout mice that received γδ T cells from MCMV positive donors had a much higher survival (>60% survival) than the mice that received γδ T cells from MCMV negative donors (0% survival) after 40 days post-infection. The marked increase in TEM cells after both human and mouse CMV infection, which is associated with increased pathogen control and clearance, presents strong evidence for a role of γδ T cell memory in this context. The ability of MCMV-induced γδ T cells, but not MCMV-inexperienced γδ T cells, to confer increased protection from MCMV in mice lacking all T cells further supports this hypothesis. However, it is not yet known if murine γδ T cells recognize stressed-induced antigens on CMV-infected host cells like their human counterparts [60].
3.3. Malaria: Murine and human similarities
γδ T cells also possess distinctive anti-parasitic properties like the αβ T cells. Recently, studies using mouse models of malaria (Plasmodium chabaudi) have detailed the memory response of γδ T cells to parasitic reinfection. C57BL/6 mice were infected with P. chabaudi and then treated on day 14 post-infection [61]. Twelve weeks after drug treatment, splenocytes from P. chabaudi-experienced and naïve mice were harvested and stimulated in vitro with P. chabaudi-infected red blood cells. The splenocytes from previously infected mice had a significantly higher amount of CD107a+ (a marker for cytotoxicity) expressing and IFN-γ producing γδ T cells after re-exposure than splenocytes from naïve mice [61], [62]. Repeating this experiment followed by flow cytometric characterization of memory markers revealed that after P. chabaudi infected red blood cell re-exposure, γδ T cells from previously infected mice had a significantly higher proportion of cytotoxic TEM (CD44+ CD62L- CD107a+ IFN-γ+) γδ T cells than naïve mice. This shows that the γδ T cell memory response to malaria is largely confined to the cytotoxic TEM compartment. RNA-sequencing revealed that the TEM γδ T cells from malaria-experienced mice had enhanced IFN-γ production, cytotoxicity, and chemokine expression. They also observed an increase in genes related to antigen presentation, and generally, P. chabaudi-experienced γδ T cells displayed more activation characteristics than naïve γδ T cells.
Interestingly, experiments in humans have demonstrated a similar role for TEM γδ T cells in the primate immune response to Plasmodium falciparum [63]. These CD45RO+ CD62L- γδ TEM cells showed a comparable ability to their murine counterparts to produce IFN-γ in response to in vitro infection by Plasmodium falciparum, and the majority of cytokine producing T lymphocytes had a TEM phenotype (including γδ T cells). The memory response was observed for up to 140 days post-infection in vitro by collecting PBMCs from immunized patients and challenging them with Plasmodium falciparum infection at various time points. The percentage of IFN-γ producing TEM lymphocytes in previously immunized individuals increased more dramatically after infection of PBMCs compared to malaria-inexperienced individuals, demonstrating a trained response. In addition, adding γδ T cells isolated from PBMCs pre-exposed to Plasmodium falciparum to PBMCs that had not previously encountered Plasmodium falciparum resulted in a higher proportion of IFN-γ producing lymphocytes after in vitro rechallenge than adding naïve PBMCs to naïve γδ T cells. The proportion of IFN-γ producing cells also increased when pre-exposed PBMCs were added to pre-exposed γδ T cells and stimulated in vitro with Plasmodium falciparum, however, IFN-γ producing cells reached a similar level to that when only the γδ T cells had been pre-exposed. This suggests that the memory response is heavily dependent on the γδ T cell subset. Additionally, the combined IFN-γ recall response by all immune subsets remained undiminished for at least 14 months after a single infection. Together, these experiments demonstrate a role for both human and murine memory γδ T cells in protective immunity from malaria. However, further research is required to unravel the role and specific γδ T cell subset responsible for the memory response and protective effects observed in malaria and other parasitic infections. It would be useful to perform adoptive transfer experiments with malaria exposed and not exposed γδ T cells into TCRδ knockout animals to see the level of protection conferred by the malaria-experienced γδ T cells upon reinfection in vivo.
3.4. Imiquimod: A model of murine psoriatic autoimmune disease
Similar to their human counterparts, memory γδ T cells may play a role in mouse models of autoimmunity. Treatment with the topical TLR-7/8 ligand and immune activator, imiquimod (IMQ), can induce an inflammatory psoriasis-like skin condition in humans and mice [64]. IMQ treatment is considered a valid mouse model of psoriatic disease [65]. Researchers found that mice exposed to IMQ had elevated numbers of Vγ4+ (Heilig & Tonegawa notation) γδ T cells in noninflamed skin and pLNs after treatment, and they stayed elevated post-treatment [66]. After treating the skin of the back of animals with IMQ, researchers took biopsies from unaffected ear skin and analyzed γδ T cell frequencies. Surprisingly, they observed a 20-fold increase in Vγ4+ γδ T cells in the skin of the ear 30 days after IMQ treatment, which persisted for 7 months. This may explain the ability of IMQ to induce skin inflammation at sites distant from application. Interestingly, Vγ4+ γδ T cells from the ear tissue of animals sensitized to IMQ, which was initially applied to the skin of the back, had greater proliferation and IL-17 production (quantified via intracellular flow cytometry staining) upon re-exposure to IMQ (applied to the ear for secondary exposure) than naïve animals. This coincided with a more rapid increase in inflammation of the ear skin in IMQ-experienced animals, showing a marked memory-like response upon secondary exposure to IMQ. IMQ-sensitized mice had a more rapid increase in the thickness of the ear (quantifying inflammation) and in neutrophil accumulation than naïve mice upon secondary exposure to IMQ. The accelerated worsening of inflammation in sensitized wild-type mice was not observed in Sox13-mutant mice (which lack Vγ4+ γδ T cells), revealing that it is indeed the Vγ4+ γδ T cell subset responsible for this memory response.
To further investigate this model, a reporter mouse was generated using Cre-lox technology in which IL-17A/F co-producing cells express the reporter enhanced yellow fluorescent protein (EYFP) [67]. Sixty days after initial treatment with IMQ, macro and microscopic inflammation was totally resolved. However, a higher proportion of EYFP+ cells was observed in the skin of the ear than in unexposed mice, and most of these EYFP+ cells were Vγ4+ γδ T cells. In the skin draining lymph nodes, most EYFP+ γδ T cells were CD44+, CD62L-, and CD103+ after primary IMQ exposure, revealing a resident memory phenotype. Secondary challenge with IMQ after 60 days of recovery induced stronger and more robust inflammation and resulted in a significant increase in the number of EYFP+ Vγ4+ γδ T cells in the skin of the ear compared to primary exposure. To ensure that it was the γδ T cells that were responsible for the memory response to secondary IMQ exposure, TCRα knockout mice and wild-type mice were challenged and re-challenged with IMQ. There was a similar increase in ear skin inflammation in the TCRα knockout mice to that observed in the wild-type mice after repeated IMQ exposure. The inflammation observed in response to the secondary exposure to IMQ was the same when IMQ was applied to the either the same ear as the primary exposure, or the other ear, in both wild-type and TCRα knockout mice. Taking into account the prolonged persistence of Vγ4+ γδ T cells observed in the skin after primary IMQ treatment, these γδ T cells display hallmarks of TRM cells. Administration of a TCR internalizing anti-Vγ4+ antibody before and during IMQ treatment resulted in loss of exacerbated disease and resulted in identical inflammation to both the primary and secondary IMQ exposure, which was lower than in animals not treated with the anti-Vγ4+ antibody. Together, these data suggest that: the Vγ4+ γδ T cell subset is sensitive to IMQ treatment, Vγ4+ γδ T cells respond more strongly to secondary IMQ challenge than to primary challenge by enhanced production of proinflammatory cytokines (IL-17), Vγ4+ γδ T cells have the characteristics of long-lived TRM in the skin, and depletion of Vγ4+ γδ T cells blunts the inflammatory response to primary and secondary exposure to IMQ. However, the putative TCR antigen that the γδ TCR recognizes in response to IMQ-induced inflammation remains unknown. These experiments suggest that memory γδ T cells may play a role in murine autoimmune disease progression and sterile inflammation, specifically in the context of models of IMQ-induced psoriatic disease.
4. Conclusion
The ability to knockout, deplete, and transfer γδ T cell subsets and control infection parameters in mice has been extremely useful in showing a role for memory γδ T cells in infection and immunity. In addition, models using NHPs provide another animal model which can be manipulated experimentally, while more closely resembling humans. At the moment, experiments in humans investigating γδ T cell memory have only been able to characterize the memory γδ T cells rather than manipulate them directly, except in the context of in vitro experiments. Nonetheless, human and NHP experiments using bacterial and viral infections/immunizations have revealed characteristics distinct to adaptive immune memory in γδ T cells. Shifts in memory γδ T cell populations have been seen in human models of relapsing autoimmune disease, and γδ T cells bearing memory markers have been shown to increase in human autoimmunity. The evidence for memory γδ T cells in murine autoimmunity is slightly stronger (as in the case of psoriasis), but more work needs to be done using other animal models of autoimmune disease.
While countless experiments have shown a prominent role for γδ T cells in infection and immunity, only recently has there been an interest in adaptive immune memory development in these innate-like cells [68]. Memory γδ T cells share many common aspects between mice and primates, and most importantly, they share the hallmark ability of memory cells to respond more strongly to secondary and tertiary exposure to the pathogenic agent. Given that γδ T cells are potent sources of proinflammatory mediators across species (notably IL-17 and IFN-γ), it makes sense that γδ T cells have been found to have a significant role in inflammation and the response to infection and cellular stress [69]. In addition, their ability to be activated in a non-MHC restricted manner and act as professional APCs allows them to also act as first-line innate-like sensors able to manipulate the immune response [1]. γδ T cells perform important reciprocal interactions with αβ T cells, and can influence the heterogeneity of αβ T cell populations in response to inflammation and stress [53]. These characteristics along with the ability of γδ T cells to express NKRs and TLRs yields a very potent, yet small population of immune cells capable of mounting a rapid proinflammatory response to immune challenge [12]. The ability of γδ T cells to respond quickly to immune challenge and orchestrate the subsequent immune response uniquely bridges innate and adaptive immunity.
On the other hand, the ability of γδ T cells to undergo TCR-mediated activation and V(D)J TCR recombination is a distinct feature of the adaptive immune system [70]. Hallmarks of adaptive immunity include specificity, immunological memory, and self/non-self-recognition, and γδ T cells display all of these characteristics in various contexts of infection and disease [71]. Further research will be required to determine the antigen repertoire of the γδ TCR, as putative γδ TCR antigens remain mostly unknown in mice and primates. Even with this limited knowledge of γδ TCR diversity, it is almost undoubtable that through TCR-dependent mechanisms γδ T cells have a capacity to become long-lived memory cells capable of a more robust secondary response. Prominent roles for TEM and TRM γδ T cell subsets have been shown in experimental models of disease, but the role of γδ TCM cells is less well-developed. Further investigations using immune challenge/re-challenge models of infections and autoimmunity will help shed light on the role of the γδ TCM subset in comparison to the better characterized γδ TEM and TRM cells.
In many ways memory γδ T cells resemble their memory αβ T cell counterparts, notably in both phenotype and recall-like response [72]. It is understood that antigen-specific naive T cells can become immune memory cells after cognate antigen recognition [73]. Where γδ T cells differ from traditional αβ T cells is that γδ T cells do not require MHC presentation for antigen recognition; therefore, the initial mechanics of memory γδ T cell generation post antigen-recognition may be slightly different than in αβ T cells [3]. It is probable that γδ T cell immune memory generation is primarily antigen driven, and enhanced TCR signal strength combined with local cytokine milieu likely drives the differentiation from effector γδ T cells to memory γδ T cells in a similar manner to αβ T cell memory generation [74]. As many of the pathogens mentioned above produce potent activators of the γδ TCR (for example: HMBPP and IPP [11]), it is likely that TCR antigens are directly responsible for the initial activation and subsequent immune memory response observed in γδ T cells. This response may be tissue localization-dependent as the γδ TCR has varying degrees of TCR junctional diversity across tissue compartments, which could coincide with the level of diversity of local pathogens [13]. The effector functions and cytokine-producing capabilities of γδ T cells also differ depending on tissue localization. Accordingly, tissue compartment localization and frequency of local pathogens in number and recurrence may have an effect on the ability of γδ T cells to form long-lived memory cell populations [73].
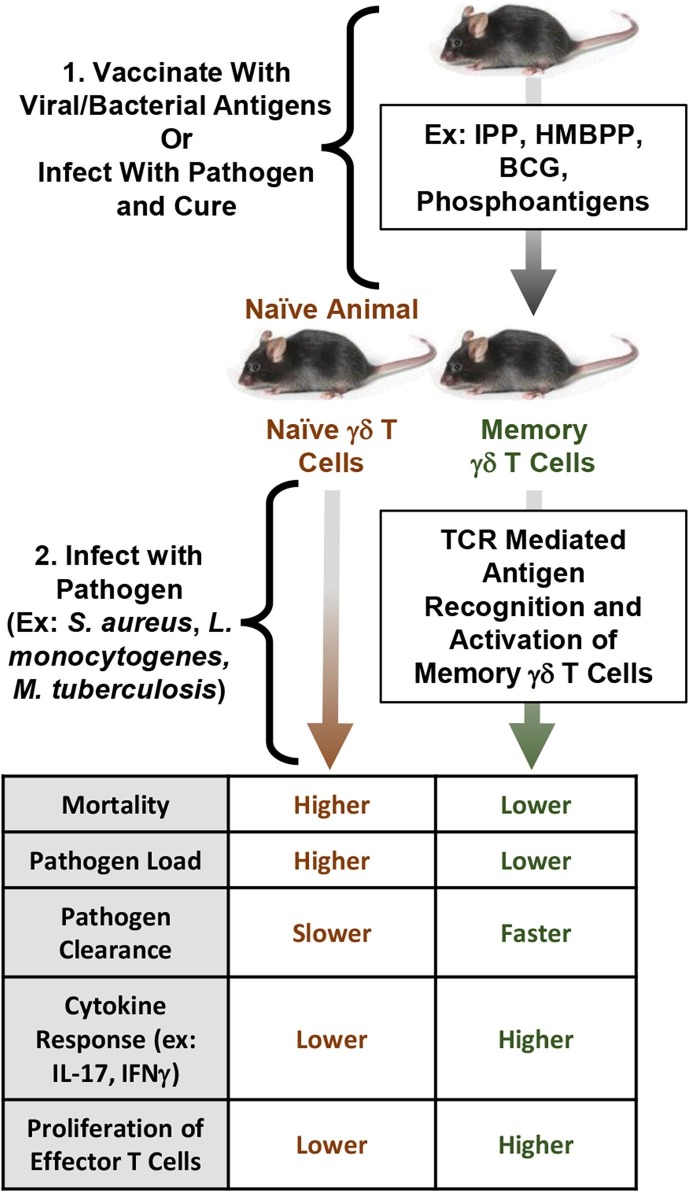
Clonal selection and expansion of various functionally useful γδ TCRs accompanied by phenotypic differentiation and acquisition of distinct functional biology has been observed in γδ T cells, which suggests acquisition of trained immune memory based on effectiveness of TCR recognition [75]. As increases in certain antigen-specific Vγ and Vδ subsets of memory γδ T cells were observed across several models of infection, it seems that memory γδ T cells exhibit restricted clonality and a strong ability to clonally expand antigen-specific cells post-reinfection. Evidence from human CMV infection supports this restricted clonality hypothesis, as most CMV+ allogenic stem cell donors had a primarily oligoclonal Vδ1, polyclonal Vδ2, and oligoclonal Vδ3 profile, showing a primarily oligoclonal Vδ2− γδ T cell population in CMV+ individuals [76]. Targeting various antigen-specific γδ T cell clonotypes for vaccination may be a useful strategy to induce protective immunity, as these experiments point to an important role for antigen-specific memory γδ T cells in resisting bacterial and viral infections. In general, development of γδ T cell memory after exposure to a pathogen yields more resilient immunity, allowing for faster pathogen clearance, a higher frequency of cytokine producing cells, and lower individual mortality (Fig. 5 ). The longevity of γδ T cells with immune memory post infection remains unknown, but the experiments mentioned above reveal that they can persist for months to years. There is no current evidence in the literature to suggest that memory γδ T cells are any more long-lived or, on the contrary, more short-lived, than their αβ T cell counterparts. Consequently, memory cell longevity may be primarily influenced by the inflammatory environment of the T cell itself [77].
Fig. 5.
Memory γδ T cells primed via previous exposure to pathogens expedite the immune response to infection. Memory T cells proliferate faster and respond more strongly via increased cytokine production and enhanced effector functions in the memory response. Vaccination using pathogen-specific γδ TCR antigens (ex: HMBPP, IPP, phosphoantigens) may result in higher survival and enhanced pathogen clearance upon infection.
In conclusion, γδ T cells play a part in both innate and adaptive immunity, and recent research has revealed characteristics of adaptive immune memory in γδ T cells in both mice and primates. Research on infection and immunity that has found a prominent role for γδ T cells would benefit from further exploring the possibility of long-lived memory γδ T cells that could worsen disease or promote immunity.
Source of funding
The work of the authors was supported by Canadian Institutes of Health Research (CIHR) First Pilot Foundation Grant 143348, a Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, and by the Canada Fund for Innovation, all to ELS.
CRediT authorship contribution statement
Kevin Comeau: Conceptualization, Investigation, Writing - original draft, Visualization. Pierre Paradis: Writing - review & editing, Visualization. Ernesto L. Schiffrin: Writing - review & editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lawand M., Déchanet-Merville J., Dieu-Nosjean M.-C. Key features of gamma-delta t-cell subsets in human diseases and their immunotherapeutic implications. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Y., Niu C., Cui J. Gamma-delta (γδ) T cells: friend or foe in cancer development? J. Transl. Med. 2018;16:3. doi: 10.1186/s12967-017-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh R.M., Lin M.Y., Lohman B.L., Varga S.M., Zarozinski C.C., Selin L.K. Alpha beta and gamma delta T-cell networks and their roles in natural resistance to viral infections. Immunol. Rev. 1997;159:79–93. doi: 10.1111/j.1600-065x.1997.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 4.Khairallah C., Chu T.H., Sheridan B.S. Tissue adaptations of memory and tissue-resident gamma delta t cells. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02636. https://www.frontiersin.org/article/10.3389/fimmu.2018.02636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandes M., Willimann K., Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 6.Brandes M., Willimann K., Bioley G., Lévy N., Eberl M., Luo M., Tampé R., Lévy F., Romero P., Moser B. Cross-presenting human γδ T cells induce robust CD8+ αβ T cell responses. Proc. Natl. Acad. Sci. 2009;106:2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng L., Cui Y., Shao H., Han G., Zhu L., Huang Y., O’Brien R.L., Born W.K., Kaplan H.J., Sun D. Mouse gammadelta T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J. Neuroimmunol. 2008;203:3–11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi X., Guo Y., Chen H., Xu C., Zhang H., Hu H., Cui L., Ba D., He W. Antigen specificity of gammadelta T cells depends primarily on the flanking sequences of CDR3delta. J. Biol. Chem. 2009;284:27449–27455. doi: 10.1074/jbc.M109.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.M.K. Heng, M.C.Y. Heng, Antigen Recognition by γδ T-Cells, Landes Bioscience, 2013. https://www.ncbi.nlm.nih.gov/books/NBK6165/ (accessed May 12, 2020).
- 10.Kazen A.R., Adams E.J. Evolution of the V, D, and J gene segments used in the primate γδ T-cell receptor reveals a dichotomy of conservation and diversity. Proc. Natl. Acad. Sci. 2011;108:E332–E340. doi: 10.1073/pnas.1105105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y., Lin L., Xiao Z., Li M., Wu X., Li W., Li X., Zhao Q., Wu Y., Zhang H., Yin J., Zhang L., Cho C.H., Shen J. Protective role of γδ T cells in different pathogen infections and its potential clinical application. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/5081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu D., Wu P., Qiu F., Wei Q., Huang J. Human γδT-cell subsets and their involvement in tumor immunity. Cell. Mol. Immunol. 2017;14:245–253. doi: 10.1038/cmi.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosley R.L., Whetsell M., Stickney D., Whetsell L., Schaefer F.V., Miller K.S., Klein J.R. Phenotype and TCR gamma delta variable gene repertoire of intestinal intraepithelial lymphocytes in wild mice (Mus musculus domesticus): abundance of V gamma 1 transcripts and extensive delta gene diversity. Int. Immunol. 1994;6:231–238. doi: 10.1093/intimm/6.2.231. [DOI] [PubMed] [Google Scholar]
- 14.Paul S., Shilpi G. Lal, Role of gamma-delta (γδ) T cells in autoimmunity. J. Leukoc. Biol. 2015;97:259–271. doi: 10.1189/jlb.3RU0914-443R. [DOI] [PubMed] [Google Scholar]
- 15.Devarajan P., Chen Z. Autoimmune effector memory T cells: the bad and the good. Immunol Res. 2013;57:12–22. doi: 10.1007/s12026-013-8448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauvau G., Soudja S.M. Mechanisms of memory T cell activation and effective immunity. Adv. Exp. Med. Biol. 2015;850:73–80. doi: 10.1007/978-3-319-15774-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youngblood B., Hale J.S., Kissick H.T., Ahn E., Xu X., Wieland A., Araki K., West E.E., Ghoneim H.E., Fan Y., Dogra P., Davis C.W., Konieczny B.T., Antia R., Cheng X., Ahmed R. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552:404–409. doi: 10.1038/nature25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jameson S.C., Masopust D. Understanding subset diversity in T cell memory. Immunity. 2018;48:214–226. doi: 10.1016/j.immuni.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cibrián Danay D. CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017;47:946–953. doi: 10.1002/eji.201646837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fear V.S., Lai S.P., Zosky G.R., Perks K.L., Gorman S., Blank F., von Garnier C., Stumbles P.A., Strickland D.H. A pathogenic role for the integrin CD103 in experimental allergic airways disease. Physiol. Rep. 2016;4 doi: 10.14814/phy2.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golubovskaya V., Wu L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers. 2016;8 doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budd R.C., Cerottini J.C., Horvath C., Bron C., Pedrazzini T., Howe R.C., MacDonald H.R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. Baltim. Md. 1987;1950(138):3120–3129. [PubMed] [Google Scholar]
- 23.Jenkins M.K., Moon J.J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. Baltim. Md. 2012;1950(188):4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benichou G., Gonzalez B., Marino J., Ayasoufi K., Valujskikh A. Role of memory T cells in allograft rejection and tolerance. Front Immunol. 2017;8:170. doi: 10.3389/fimmu.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth N.J., McQuaid A.J., Sobande T., Kissane S., Agius E., Jackson S.E., Salmon M., Falciani F., Yong K., Rustin M.H., Akbar A.N., Vukmanovic-Stejic M. Different proliferative potential and migratory characteristics of human CD4 + regulatory T cells that express either CD45RA or CD45RO. J. Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 26.Mahnke Y.D., Brodie T.M., Sallusto F., Roederer M., Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur. J. Immunol. 2013;43:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 27.Opata M.M., Stephens R. Early decision: effector and effector memory T cell differentiation in chronic infection. Curr. Immunol. Rev. 2013;9:190–206. doi: 10.2174/1573395509666131126231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenkel J.M., Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasper D.J., Tejera M.M., Suresh M. CD4 T-cell memory generation and maintenance. Crit. Rev. Immunol. 2014;34:121–146. doi: 10.1615/critrevimmunol.2014010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesch D., Hinz T., Kabelitz D. Analysis of the TCR Vgamma repertoire in healthy donors and HIV-1-infected individuals. Int. Immunol. 1998;10:1067–1075. doi: 10.1093/intimm/10.8.1067. [DOI] [PubMed] [Google Scholar]
- 31.Bottino C., Tambussi G., Ferrini S., Ciccone E., Varese P., Mingari M.C., Moretta L., Moretta A. Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J. Exp. Med. 1988;168:491–505. doi: 10.1084/jem.168.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.S. Fonseca, V. Pereira, C. Lau, M. dos A. Teixeira, M. Bini-Antunes, M. Lima, Human Peripheral Blood Gamma Delta T Cells: Report on a Series of Healthy Caucasian Portuguese Adults and Comprehensive Review of the Literature, Cells. 9 (2020). https://doi.org/10.3390/cells9030729. [DOI] [PMC free article] [PubMed]
- 33.Nedellec S., Bonneville M., Scotet E. Human Vγ9Vδ2 T cells: from signals to functions. Semin. Immunol. 2010;22:199–206. doi: 10.1016/j.smim.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Hoft D.F., Brown R.M., Roodman S.T. Bacille calmette-guérin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J. Immunol. Baltim. Md. 1998;1950(161):1045–1054. [PubMed] [Google Scholar]
- 35.Lai X., Shen Y., Zhou D., Sehgal P., Shen L., Simon M., Qiu L., Letvin N.L., Chen Z.W. Immune biology of macaque lymphocyte populations during mycobacterial infection. Clin. Exp. Immunol. 2003;133:182–192. doi: 10.1046/j.1365-2249.2003.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen L., Frencher J., Huang D., Wang W., Yang E., Chen C.Y., Zhang Z., Wang R., Qaqish A., Larsen M.H., Shen H., Porcelli S.A., Jacobs W.R., Chen Z.W. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl. Acad. Sci. U.S.A. 2019;116:6371–6378. doi: 10.1073/pnas.1811380116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belmant C., Espinosa E., Poupot R., Peyrat M.-A., Guiraud M., Poquet Y., Bonneville M., Fournié J.-J. 3-Formyl-1-butyl pyrophosphate A novel mycobacterial metabolite-activating human γδ T Cells. J. Biol. Chem. 1999;274:32079–32084. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- 38.Ryan-Payseur B., Frencher J., Shen L., Chen C.Y., Huang D., Chen Z.W. Multieffector-functional immune responses of HMBPP-specific Vγ2Vδ2 T cells in nonhuman primates inoculated with Listeria monocytogenes ΔactA prfA*. J. Immunol. Baltim. Md. 2012;1950(189):1285–1293. doi: 10.4049/jimmunol.1200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao L., Huang D., Wei H., Wang R.C., Chen C.Y., Shen L., Zhang W., Jin J., Chen Z.W. Expansion, reexpansion, and recall-like expansion of Vγ2Vδ2 T cells in smallpox vaccination and monkeypox virus infection. J. Virol. 2009;83:11959–11965. doi: 10.1128/JVI.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitard V., Roumanes D., Lafarge X., Couzi L., Garrigue I., Lafon M.-E., Merville P., Moreau J.-F., Déchanet-Merville J. Long-term expansion of effector/memory Vδ2− γδ T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–1324. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Y., Babor M., Lane J., Schulten V., Patil V.S., Seumois G., Rosales S.L., Fu Z., Picarda G., Burel J., Zapardiel-Gonzalo J., Tennekoon R.N., De Silva A.D., Premawansa S., Premawansa G., Wijewickrama A., Greenbaum J.A., Vijayanand P., Weiskopf D., Sette A., Peters B. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 2017;8:1473. doi: 10.1038/s41467-017-01728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 43.Halary F., Pitard V., Dlubek D., Krzysiek R., de la Salle H., Merville P., Dromer C., Emilie D., Moreau J.-F., Déchanet-Merville J. Shared reactivity of V{delta}2(neg) gamma}{delta T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteiro A., Cruto C., Rosado P., Martinho A., Rosado L., Fonseca M., Paiva A. Characterization of circulating gamma-delta T cells in relapsing vs remission multiple sclerosis. J. Neuroimmunol. 2018;318:65–71. doi: 10.1016/j.jneuroim.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Dieli F., Poccia F., Lipp M., Sireci G., Caccamo N., Di Sano C., Salerno A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J. Exp. Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caccamo N., Meraviglia S., Ferlazzo V., Angelini D., Borsellino G., Poccia F., Battistini L., Dieli F., Salerno A. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vγ9Vδ2 naive, memory and effector T cell subsets. Eur. J. Immunol. 2005;35:1764–1772. doi: 10.1002/eji.200525983. [DOI] [PubMed] [Google Scholar]
- 47.Hu C., Qian L., Miao Y., Huang Q., Miao P., Wang P., Yu Q., Nie H., Zhang J., He D., Xu R., Chen X., Liu B., Zhang D. Antigen-presenting effects of effector memory Vγ9Vδ2 T cells in rheumatoid arthritis. Cell. Mol. Immunol. 2012;9:245–254. doi: 10.1038/cmi.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garman R.D., Doherty P.J., Raulet D.H. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45:733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 49.Heilig J.S., Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 50.Pang D.J., Neves J.F., Sumaria N., Pennington D.J. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136:283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ezquerra A., Cron R.Q., McConnell T.J., Valas R.B., Bluestone J.A., Coligan J.E. T cell receptor delta-gene expression and diversity in the mouse spleen. J. Immunol. Baltim. Md. 1990;1950(145):1311–1317. [PubMed] [Google Scholar]
- 52.J. Gertner, E. Scotet, M. Poupot, M. Bonneville, J.-J. Fournié, Lymphocytes: Gamma Delta, in: John Wiley & Sons, Ltd (Ed.), Encycl. Life Sci., John Wiley & Sons, Ltd, Chichester, UK, 2007: p. a0001195.pub2. https://doi.org/10.1002/9780470015902.a0001195.pub2.
- 53.Phalke S.P., Huang Y., Rubtsova K., Getahun A., Sun D., Reinhardt R.L., O’Brien R.L., Born W.K. γδ T cells shape memory-phenotype αβ T cell populations in non-immunized mice. PloS ONE. 2019;14 doi: 10.1371/journal.pone.0218827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng X., Wei Y., Huang J., Newell E.W., Yu H., Kidd B.A., Kuhns M.S., Waters R.W., Davis M.M., Weaver C.T., Chien Y. γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen specific Interleukin 17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheridan B.S., Romagnoli P.A., Pham Q.M., Fu H.H., Alonzo F., Schubert W.D., Freitag N.E., Lefrancois L. Gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romagnoli P.A., Sheridan B.S., Pham Q.M., Lefrancois L., Khanna K.M. IL-17A-producing resident memory gammadelta T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc Natl Acad Sci USA. 2016;113:8502–8507. doi: 10.1073/pnas.1600713113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy A.G., O’Keeffe K.M., Lalor S.J., Maher B.M., Mills K.H., McLoughlin R.M. Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J. Immunol. 2014;192:3697–3708. doi: 10.4049/jimmunol.1303420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krmpotic A., Bubic I., Polic B., Lucin P., Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5:1263–1277. doi: 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Khairallah C., Netzer S., Villacreces A., Juzan M., Rousseau B., Dulanto S., Giese A., Costet P., Praloran V., Moreau J.-F., Dubus P., Vermijlen D., Déchanet-Merville J., Capone M. γδ T cells confer protection against murine cytomegalovirus (MCMV) PLOS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willcox C.R., Pitard V., Netzer S., Couzi L., Salim M., Silberzahn T., Moreau J.-F., Hayday A.C., Willcox B.E., Déchanet-Merville J. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 2012;13:872–879. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 61.Kumarasingha R., Ioannidis L.J., Abeysekera W., Studniberg S., Wijesurendra D., Mazhari R., Poole D.P., Mueller I., Schofield L., Hansen D.S., Eriksson E.M. Transcriptional memory-like imprints and enhanced functional activity in γδ T cells following resolution of malaria infection. Immunology. 2020 doi: 10.1101/2020.05.05.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aktas E., Kucuksezer U.C., Bilgic S., Erten G., Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009;254:149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Teirlinck A.C., McCall M.B.B., Roestenberg M., Scholzen A., Woestenenk R., de Mast Q., van der Ven A.J.A.M., Hermsen C.C., Luty A.J.F., Sauerwein R.W. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Fits L., Mourits S., Voerman J.S.A., Kant M., Boon L., Laman J.D., Cornelissen F., Mus A.-M., Florencia E., Prens E.P., Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. Baltim. Md. 2009;1950(182):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 65.Sakai K., Sanders K.M., Youssef M.R., Yanushefski K.M., Jensen L., Yosipovitch G., Akiyama T. Mouse model of imiquimod-induced psoriatic itch. Pain. 2016;157:2536–2543. doi: 10.1097/j.pain.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramirez-Valle F., Gray E.E., Cyster J.G. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci USA. 2015;112:8046–8051. doi: 10.1073/pnas.1508990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hartwig T., Pantelyushin S., Croxford A.L., Kulig P., Becher B. Dermal IL-17-producing γδ T cells establish long-lived memory in the skin. Eur. J. Immunol. 2015;45:3022–3033. doi: 10.1002/eji.201545883. [DOI] [PubMed] [Google Scholar]
- 68.Lalor S.J., McLoughlin R.M. Memory gammadelta T cells-newly appreciated protagonists in infection and immunity. Trends Immunol. 2016;37:690–702. doi: 10.1016/j.it.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Haas J.D., González F.H.M., Schmitz S., Chennupati V., Föhse L., Kremmer E., Förster R., Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur. J. Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 70.Legut M., Cole D.K., Sewell A.K. The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy. Cell. Mol. Immunol. 2015;12:656–668. doi: 10.1038/cmi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.J. Charles A Janeway, P. Travers, M. Walport, M.J. Shlomchik, Principles of innate and adaptive immunity, Immunobiol. Immune Syst. Health Dis. 5th Ed. (2001). https://www.ncbi.nlm.nih.gov/books/NBK27090/ (accessed May 19, 2020).
- 72.Lombes Amélie A. Adaptive immune-like g/ T lymphocytes share many common features with their a/b T cell counterparts. J. Immunol. 2015;195:1449–1458. doi: 10.4049/jimmunol.1500375. [DOI] [PubMed] [Google Scholar]
- 73.Vantourout P., Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams M.A., Ravkov E.V., Bevan M.J. Rapid culling of the CD4+ T Cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davey M.S., Willcox C.R., Joyce S.P., Ladell K., Kasatskaya S.A., McLaren J.E., Hunter S., Salim M., Mohammed F., Price D.A., Chudakov D.M., Willcox B.E. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 2017;8:14760. doi: 10.1038/ncomms14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knight A., Madrigal A.J., Grace S., Sivakumaran J., Kottaridis P., Mackinnon S., Travers P.J., Lowdell M.W. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116:2164–2172. doi: 10.1182/blood-2010-01-255166. [DOI] [PubMed] [Google Scholar]
- 77.Pennock N.D., White J.T., Cross E.W., Cheney E.E., Tamburini B.A., Kedl R.M. T cell responses: naïve to memory and everything in between. Adv. Physiol. Educ. 2013;37:273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]