Abstract
The potential use of smallpox as an agent of bioterrorism has renewed interest in the development of a modern vaccine capable of replacing the standard Dryvax® vaccine. Vaccinia virus (ACAM2000), clonally isolated from Dryvax® and manufactured in cell culture, was tested for immunogenicity and protective activity in a non-human primate model. Cynomolgus monkeys vaccinated with ACAM2000, Dryvax®, or ACAM2000 diluent (control) were challenged 2 months post-vaccination with a lethal, intravenous dose of monkeypox virus. ACAM2000 proved immunogenic and efficacious in protecting against lethal monkeypox challenge, as evident from a lack of post-challenge viral replication, and the absence of any significant clinical signs attributable to monkeypox infection. This protection correlated (with) neutralizing antibody titers equivalent to those generated in the Dryvax® group post-vaccination, as well as a similar significant increase in the presence of neutralizing antibodies post-challenge. Control animals showed no signs of vaccine-induced seroconversion, displayed post-challenge tissue-associated viral replication and viremia, and developed severe monkeypox-specific clinical symptoms. The protective efficacy of ACAM2000 was found to be equivalent to the currently approved vaccine, Dryvax®.
Keywords: Smallpox vaccine, Monkeypox virus, Cynomolgus macaques
Introduction
Since the formal declaration of smallpox eradication by the World Health Organization in 1980 there has been a heightened concern over the use of variola virus as a potential bioterrorism agent. Vaccination of the general US population ceased in 1972 amid concerns over the adverse effects of vaccination in light of the dwindling threat that smallpox posed. With the termination of vaccination programs worldwide, commercial incentives for vaccine production waned and Dryvax® vaccine production in calf skin ceased altogether in 1982. Only military personnel and a limited population of healthcare, laboratory workers, and first responders currently receive the vaccine [1].
Due to cessation of routine childhood vaccination the general public has become increasingly susceptible to the disease. By the mid 1990s the US government had identified smallpox as a potential bioterrorist threat [2] primarily due to the possible persistence of unknown frozen stocks of virulent variola virus in ‘rogue’ states [3], [4], [5]. Although studies have shown that up to a 1:10 dilution of the Dryvax® vaccine can still elicit an effective cutaneous reaction or dermal “take” [6], [7], current emergency vaccination plans which call for use of the Dryvax® vaccine at a 1:5 dilution would only be sufficient to dose an estimated 75 million people [8]. The shortage of stockpiled vaccine and concerns over the quality of vaccine, formerly produced in the skin of calves, required a shift in focus toward the establishment of a next generation smallpox vaccine. In response, the US Government contracted for the large-scale production and stockpile of a vaccine at least equivalent to the Dryvax® vaccine in protective efficacy, and made in accordance with modern in vitro manufacturing standards.
Previous research demonstrated that smallpox vaccines could be produced successfully using cell culture [10], [11], [12]. An extension of this approach involves selection of a clonal virus population that would likely have a more consistent safety and immunogenicity profile compared to a lymph-derived vaccine (Dryvax®), consisting of a heterogeneous collection of viral subpopulations with discrepant properties of virulence [13], [14], [15].
Vaccine candidate identification began by isolating clones from the Dryvax® vaccine by plaque purification in MRC-5 human diploid cells. One clone (ACAM1000) was chosen based on similarity in “take” response in rabbit skin and an attenuated neurotropic phenotype in mice compared to Dryvax®. Initial phase I trials using the ACAM1000 candidate confirmed that the clinical response profile was similar to that of Dryvax® and resulted in a “take” response in 100% of test subjects [13]. In conjunction with Baxter Bioscience, the ACAM1000 master virus seed was used to prepare vaccine (ACAM2000) on a large scale using Vero cells propagated under serum-free conditions. In phase I clinical trials, ACAM2000 produced major cutaneous reactions and elicited significant neutralizing antibody production and cell-mediated immune responses with a similar reactogenicity profile to that of Dryvax® in the vast majority of test subjects. ACAM2000 has also demonstrated protective efficacy in rodents [9]. This type of clonal vaccine is advantageous as it promotes a more stable viral phenotype, is more consistent in manufacture, and it eliminates the possibility of the accumulation of adventitious bovine contaminants due to passage in livestock [13].
Eradication of naturally occurring smallpox makes field tests for vaccine efficacy impossible so we set out to test vaccine immunogenicity and protective activity in a non-human primate model. Previous work has shown the pathology of monkeypox infection in cynomolgus monkeys (Macaca fascicularis) to be similar to that of smallpox infections in humans [16]. This is regarded as the best model of orthopoxvirus infection in a non-human primate [8] and has been consistently used to compare protective efficacies of candidate vaccines and therapeutics [17], [18], [19]. This study was designed to evaluate whether ACAM2000 is effective for prophylaxis, compared to Dryvax®, against virulent monkeypox virus administered intravenously to cynomolgus monkeys as a surrogate efficacy model for vaccination against smallpox infection in humans.
Materials and methods
Test system
Twenty-four male (n = 12) and female (n = 12) cynomolgus macaques aged 22 months or more were utilized for this study. Monkeys were screened by plaque reduction neutralization tests to certify the animals as seronegative to vaccinia virus and were quarantined and under observation for a minimum of 6 weeks prior to the study. Animals identified with chest tattoos and individual cage cards were individually housed while on study. Supplemental feeding, due to cessation of voluntary feeding, was administered via an oro-gastric tube when necessary. During the quarantine period animals were randomly assigned by gender and weight to three study groups such that each treatment group would contain equal numbers of males and females covering an equivalent weight range.
Vaccine inoculations
On study Day 0 all animals were anesthetized and administered either ACAM2000 (n = 8) (dose formulation: 4.4 × 108 pfu/mL), Dryvax® (n = 8) (Wyeth-Lederle; dose formulation: 1.5 × 108 pfu/mL), or a negative control (n = 8) [ACAM2000 glycerol–phenol diluent: 50% (w/w) glycerin, USP; 0.21% (w/w) phenol, USP in Water for Injection, USP] via the percutaneous route (scarification) with a sterile bifurcated needle delivering a nominal volume of 2.5 μL. Vaccines were delivered by administering a minimum of 15 jabs to the shaved skin in the subscapular region.
Challenge material inoculations
Sixty-one days after vaccination, all animals were administered a challenge dose of 0.5 mL containing 3.8 × 107 total pfu of monkeypox virus (Monkeypox strain Zaire 79; CDC V79-I-005) in HEPES buffer into the femoral vein in a single IV injection.
Clinical observations
All animals were observed twice daily (a.m. and p.m.) for the duration of the study. Observations included, but were not limited to, behavior, physical appearance, feces/urine output, eating behaviors, and movement/activity. Vaccination sites were visually inspected for measurement and characterization of appearance of erythema (i.e. vesicle, papule, pustule, ulcer, scab). Body weight measurements were taken during the vaccination and challenge periods and daily rectal body temperatures were taken post-challenge. Pox lesion development was observed by body region and characterized by degree of severity based on predetermined ranges of lesion counts per body part (mild: 5–25; moderate: 26–100; severe: 101–250; grave: >250).
Hematology
Blood was collected into EDTA tubes on Days 0, 60, 67, 73, 79 and on the day of death prior to necropsy (when possible) for hematology determinations. A Bayer Health Care ADVIA 120 was used to evaluate the following parameters: white blood cell count, relative leukocyte percentages, differential leukocyte count, hemoglobin, hematocrit, red blood cell count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red cell distribution width, platelet count, and mean platelet volume.
Clinical chemistry
Clinical chemistry evaluation was performed on blood collected into serum separator tubes (SST) on Days 0, 60, 67, 73, 79 and on the day of death prior to necropsy (when possible) using a Bayer Health Care ADVIA 1200. Serum chemistry evaluation was performed on the following parameters: albumin, albumin/globulin (A/G ratio), alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, urea nitrogen, BUN/creatinine ratio, calcium, chloride, creatinine, globulin, glucose, lactate dehydrogenase, sodium, potassium, phosphorus, and total protein.
Antibody and viremia determinations
Blood drawn into SST tubes was separated and the serum was tested for neutralizing antibody determination on Days 0, 30, and 60 post-vaccination, 30 days post-challenge, as well as terminal draw dates when available. All pre-challenge serum samples were shipped to Acambis Inc. for assay in a 50% plaque reduction neutralization test (PRNT50) performed in Vero cells as previously described [13], using the ACAM1000 vaccinia virus in place of the referenced virus (vaccinia strain WR). Post-challenge samples were analyzed by Battelle in a similar assay to detect monkeypox-specific neutralizing antibodies.
Post-challenge blood was drawn into sodium citrate (CPT) tubes for viremia determination. Peripheral blood mononuclear cells (PBMCs) were separated, collected, and counted for cell-associated virus titer determination. Separated plasma from CPT processing (and serum from SST processing where available) was tested for cell-free virus. PBMCs were held on ice, sonicated for cell lysis and viral release, and stored at −80 °C until tested. Throat swabs were collected to determine virus shedding in the oral cavity. All throat swabs were collected and placed in a storage buffer containing sterile PBS with antibiotics and 1% FBS and stored at −80 °C until analysis. Virus titer determinations were performed by plaque assay. Briefly, serial dilutions of prepared samples were inoculated onto Vero cell monolayers and incubated 60 min at 37 °C/5% CO2. Cell culture medium containing methylcellulose was used to overlay the monolayers; these culture plates were incubated for 72 h at 37 °C/5% CO2. Monolayers were stained with a formalin/ethanol/crystal violet solution, plaques were manually enumerated, and virus titers were determined and expressed as pfu/106 PBMCs for cell-associated virus and pfu/mL for all other sample types. Limit of detection for samples inoculated undiluted is 10 pfu/mL.
Necropsy and histopathological examination
A full necropsy and associated routine histopathology supervised by a board certified veterinary pathologist was performed on any monkey found dead or euthanized and consisted of collection of the following tissues: skin, tonsil, spleen, lymph nodes, liver, brain, lungs, heart, kidneys, adrenal glands, ovaries or testicles, gross lesions. Histopathology consisted of collection of gross lesions on listed tissues. Samples were fixed in 10% neutral buffered formalin, processed to 5-μm sections for routine H & E staining, and examined microscopically.
Results
Post-vaccination clinical observations
During the 60-day vaccination period all animals displayed normal behavior with no observed morbidity or mortality. No significant abnormal clinical observations were recorded for any animal in either the ACAM2000 or Dryvax® group following vaccination.
Immunogenicity
The cutaneous reaction to vaccination was assessed by recording the scored progression and size of erythema and/or central lesion from the inoculation site. Measurements were taken at five time-points during the first 15 days post-immunization. All monkeys vaccinated with ACAM2000 or Dryvax® exhibited cutaneous “take” responses to the vaccine material. No significant differences were seen in the size or appearance (i.e. papule, pustule, etc.) of cutaneous reactions between vaccine groups. Cutaneous lesion size peaked on Days 7–10 for both vaccination groups (Fig. 1 ).
Figure 1.
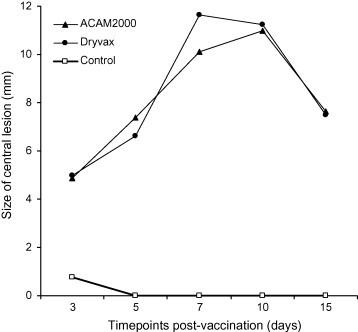
Vaccination site response. Statistical analysis showed no significant difference in the size of central lesion between the two vaccine groups on any of the 5 days on which measurements were taken (Day 3: p = 0.8539; Day 5: p = 0.2874; Day 7: p = 0.1515; Day 10: p = 0.7856; Day 15: p = 0.9070, ANOVA). Likewise, no difference in lesion score was exhibited (p = 0.5870, ordinal logistic model).
By Day 30 post-vaccination all monkeys in both the ACAM2000 and Dryvax® groups seroconverted (≥fourfold increase in vaccinia neutralizing antibody titer compared to baseline) and displayed vaccinia-specific antibody titers ranging from 40 to 320 (GMT = 160) and from 40 to 640, respectively (GMT = 174). Antibody titers remained relatively unchanged at Day 60 post-vaccination and ranged from 80 to 640 (GMT = 174) and from 80 to 320 (GMT = 190), respectively (Fig. 2 ). No statistical differences were found between the post-vaccination vaccinia-specific GMT values of the ACAM2000 and Dryvax® treatment groups at either Day 30 (p > 0.8473) or Day 60 (p > 0.6505). Sham vaccination resulted in 0% seroconversion.
Figure 2.
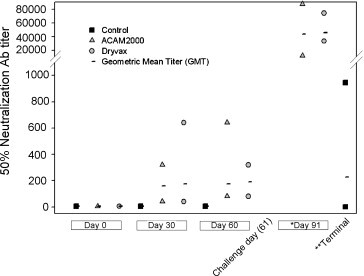
Antibody response. Graph shows the maximum, minimum, and geometric mean titer (GMT) values for each treatment group and time point. Note: reported prescreen and post-vaccination (time points: 0, 30, and 60) antibody titer results generated from vaccinia plaque reduction neutralization assays. Post-challenge (time point: terminal, 91) antibody titer results generated from monkeypox plaque reduction neutralization assays. *No statistical difference in post-challenge monkeypox-specific GMT values between ACAM2000 (Day 91, GMT = 43 782) and Dryvax® (Day 91, GMT = 46 072) vaccination groups were observed (p > 0.8456). **Post-challenge monkeypox-specific antibody titers in control group determined at time of death or sacrifice (varied).
Protective efficacy
All treatment groups were challenged with a virulent intravenous dose of monkeypox virus (3.8 × 107 total pfu) on Day 61 post-vaccination. All ACAM2000 vaccinated monkeys survived the challenge with few or no apparent clinical signs or symptoms of poxvirus infection. Protection afforded by the Dryvax® treatment was similar. Three animals in the Dryvax® group and one ACAM2000 vaccinee demonstrated minor rash-like skin eruptions in the immediate area of the femoral vein challenge site. These reactions were observed from Day 5 to 7 post-challenge and quickly resolved within a 2-day period. Monkeys in both vaccine groups displayed high levels of monkeypox-specific neutralizing antibodies after challenge. These antibody titer values ranged from 12 047 to 88 037 (GMT = 43782) and from 33 483 to 74 688 (GMT = 46072) for the ACAM2000 and Dryvax® groups, respectively (Fig. 2). No statistical differences were found between the post-challenge monkeypox-specific GMT values of the ACAM2000 and Dryvax® immunization groups at 30 days post-challenge (p = 0.8456). Rectal temperatures were similar between vaccine groups and showed little evidence any of these animals became febrile (Fig. 3 ). Neither vaccination nor challenge had any significant effect on body weight in either the ACAM2000 or Dryvax® group aside from generally negligible weight gain over the course of the 91-day study (Table 1 ).
Figure 3.
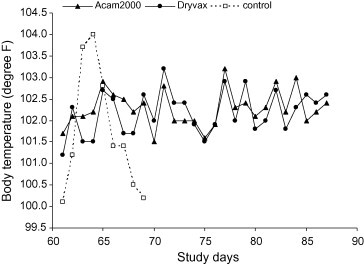
Post-challenge body temperatures. Data shown reflect mean values of all animals for each group. No significant differences in body temperatures were found to exist between ACAM2000 and Dryvax® treatment groups in the average, maximum, and incidence of elevated temperatures.
Table 1.
Clinical observations following monkeypox challenge
| Clinical signs | ACAM 2000 | Dryvax | Controls |
|---|---|---|---|
| Pox lesions | None | None | >250 per region |
| Body temperature | Normal | Normal | 104 °C on Day 3 (mean) |
| Body weight | Mean 1% gain | Mean 1% gain | 1–10% loss (mean 5% loss) |
| Hematology | Elevated lymphocytes | Elevated lymphocytes | Baso, lymph, WBC, HGB, and HCT values outside normal ranges |
| Clinical chemistry | All parameters within normal ranges | All parameters within normal ranges | Serum albumin, phosphorus, AST and LDH values outside normal ranges |
| Death | None | None | 100% by Day 9 |
| Pathology | None | None | Gross lesions consistent with monkeypox infection |
All control monkeys developed severe illness following challenge, manifested by cessation of voluntary feeding, lethargy, diarrhea, non-responsiveness, and progressive cutaneous lesion development. All control animals presented with “classical” severe pox-lesion development concentrated around the face, including the mucous membranes of the nose and mouth. Each monkey in the control group developed an elevated body temperature (considered ≥ 104 °F) during at least 1 day of observation following challenge. The majority of temperatures peaked 2–3 days post-challenge before declining just prior to death or euthanasia (Fig. 3). Body weight of control monkeys, which had remained steady or increased slightly during the post-vaccination period, declined an average of 5% from their pre-challenge body weight during the course of the post-challenge observation period. The weight loss observed ranged from 10% to 1% (Table 1). Six of eight monkeys in the control group developed a detectable antibody response to the monkeypox challenge by the final day of serum collection (Fig. 2). These positive antibody titers ranged from 70 to 946 with a GMT value of 227. This response did not prove protective from lethal monkeypox challenge as within 9 days post-challenge each of these monkeys succumbed to the infection or were humanely euthanized when deemed moribund.
Viremia and virus shedding
No detectable intracellular or cell-free virus was observed in any blood sample tested from either the ACAM2000 or Dryvax® group at any time post-challenge. Throat swabs taken every 2 days post-challenge were free from detectable virus by plaque assay in all ACAM2000 animals at all time points; however, three of eight Dryvax® vaccinated animals showed evidence of virus shedding in the oral cavity. Of these three positive Dryvax® animals, a very low level of virus was detected at Day 4 post-challenge for all three animals and for one 2 days later; this monkey (#19 738) displayed the most significant virus shedding (333 pfu/mL on Day 65 and 167 pfu/mL on Day 67). In contrast, viral replication was clearly apparent in all control animals. Virus shedding was confirmed from throat swabs in all control monkeys and appeared first on Day 2 post-challenge in three monkeys and in all by Day 6 post-challenge. Viremia in serum or plasma as well as PBMC-associated virus was detected in six of eight control animals (two of eight without reported positive values had incomplete sample sets) (Table 2 ).
Table 2.
Viremia and virus shedding in control animals
| Animal ID | PBMC (pfu/106 cells) |
Plasma/serum* (pfu/mL) |
Throat swab (pfu/mL inoculum) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 63 | Day 65 | Day 67 | Day 63 | Day 65 | Day 67 | Day 63 | Day 65 | Day 67 | |
| CO9557 | 0 | 6.69 × 100 | NS | 0 | 6.67 × 100 | 2.73 × 102* | 0 | 4.65 × 103 | NS |
| CO9568 | 0 | 3.81 × 101 | NS | 0 | 6.67 × 100 | 6.33 × 101* | 6.67 × 100 | 3.25 × 103 | NS |
| 19742 | 0 | 1.26 × 100 | NS | 0 | 3.33 × 100 | NS | 0 | 0 | 4.62 × 103 |
| 19781 | 0 | 0 | NS | 0 | 0 | NS | 0 | 3.58 × 103 | 5.97 × 104 |
| CO9472 | 0 | 0 | NS | 0 | 0 | NS | 8.33 × 101 | 1.32 × 104 | 1.05 × 104 |
| CO8579 | 1.14 × 101 | 2.08 × 103 | 3.61 × 105 | 6.67 × 100 | 4.00 × 102 | 1.61 × 107 | 0 | 4.15 × 102 | NS |
| 19679 | 0 | 1.84 × 101 | NS | 0 | 6.67 × 100 | NS | 0 | 5.78 × 102 | 3.83 × 104 |
| 19727 | 9.47 × 101 | 5.95 × 104 | NS | 0 | 4.94 × 103 | NS | 6.67 × 100 | 3.27 × 102 | NS |
NS: no sample for analysis.
The marked data was derived from analyzing serum rather than plasma.
Clinical pathology
Minor changes in hematology and clinical chemistry values were observed from post-vaccination to post-challenge for ACAM2000 immunized animals and were similar to changes observed among the Dryvax® group; however, few of these changes were statistically significant and were attributed to repeated manipulation versus indication of systemic viral infection. Post-challenge lymphocyte levels did rise above normal ranges in both vaccine groups but returned to normal by the end of study period (Table 1).
Notable findings in clinical pathology were limited to the non-vaccinated control group post-challenge. These findings include a substantial increase in total white cell counts; specifically, in lymphocytes and basophils 7–9 days post-challenge that, on average, reached two to four times normal baseline values. A modest increase in platelets was also observed. Clinical chemistry analysis showed decreases in mean serum albumin, total protein, and phosphorus concentrations (Table 1).
Pathology
All animals that succumbed to infection or were euthanized were members of the control group. All these animals had pathological findings consistent with death due to monkeypox infection. Gross monkeypox-related lesions were observed primarily in the skin, tongue, esophagus, tonsils, and stomach. Lesions considered related to monkeypox infection also include necrosis of the liver, lymph nodes, spleen, testes and ovaries, and incidence of focal to multi-focal fibrinohemorrhagic bronchitis and alveolitis. Other pathological findings include inflammation and neutrophilic infiltrates in the heart, kidney, and lymph nodes as well as myocardial degeneration, enlargement of the liver, various lymph nodes, and the spleen (Table 1). No surviving, healthy primates from either vaccine group were sacrificed at the study terminus as per IACUC guidelines governing this study.
Discussion
ACAM2000 is a clonal, cell culture-derived smallpox vaccine that has a similar biological profile to Dryvax®, including the ability to elicit major cutaneous reactions, systemic immune responses in animals and humans, and exhibits a similar protective efficacy in various animal challenge models [9], [13]. The present study was devised to further test the protective efficacy of the ACAM2000 vaccine in comparison with the established Dryvax® vaccine in a model more consistent with smallpox infection in humans. The intravenous challenge route was chosen in the absence of a developed model utilizing a more natural route of transmission (i.e. intranasal, intratracheal, or aerosol) involving the respiratory tract [20]. However, intravenous inoculation results in immediate viremia and systemic spread of the virus, is thus a severe route of infection and a rigorous test of efficacy for any therapeutic, and has consistently been used as such [17], [21], [22]. Previous comparative data from preclinical tests for safety, immunogenicity, and protective efficacy, and subsequent clinical observations [9], [13], [23] are also reinforced in light of this challenge study.
Successful vaccination occurred in all cynomolgus macaques vaccinated with ACAM2000 or with Dryvax®. The time course observed for development of major cutaneous reaction was 7–10 days for both vaccine groups and no significant differences were found in size or appearance of erythema following immunization. Seroconversion was consistent with development of a major cutaneous reaction and occurred in all subjects receiving a test vaccine with no differences in neutralizing antibody GMT seen across treatment groups at Days 30 and 60 post-vaccination.
A phase II clinical trial of ACAM2000 reported no clinically significant shifts in hematology or clinical chemistry parameters from baseline to Day 15 post-immunization [23]. This is consistent with the clinical pathology observed in the cynomolgus model as the minor shifts in hematology and clinical chemistry values observed over the course of the study were similar between vaccine groups and revealed no biologically or statistically significant differences. In contrast, significant changes in clinical pathology were observed in the control group values post-challenge, consistent with acute illness and inflammation due to viral infection.
A previous report on the pathology of aerosolized monkeypox in cynomolgus monkeys outlined the onset of exanthema (primarily inguinal, ventral abdominal, ventral thoracic, perineal, and facial), enanthema, fever, leukocytosis, and cell-associated virus 6–9 days post-exposure prior to death (mean 11.7 days). No cell-free virus or significant trends in clinical chemistry were reported [16]. Intravenous challenge of the non-vaccinated control animals in our study presented a much accelerated and systemic progression of disease. The onset of fever and detection of virus in buffy coat cells, serum/plasma, and from swabs taken from the oral mucosa was observed as early as Day 2 post-challenge. By Day 5 exanthema (primarily concentrated around the face, with generally equal distribution among the arms, legs, thorax and abdomen) was reported in all animals progressing until death (mean 7.5 days). Similar to the reported progression in the aerosol model, leukocytosis was reported by Day 6, but shifts in blood chemistry (decrease in mean serum albumin, total protein, and phosphorus concentrations) are also reported in this study.
Published comparisons of ACAM1000, ACAM2000, and Dryvax® in mice challenged with vaccinia WR and cowpox demonstrated similar protective activities. Survival times of BALB/c mice challenged with vaccinia WR strain following immunization with graded doses of ACAM1000, ACAM2000, and Dryvax® did not differ significantly between vaccine groups. Required vaccine doses for survival of 50% of mice were also similar between groups [9].
Immunization of cynomolgus macaques with either ACAM2000 or Dryvax® proved equally efficacious against challenge with a lethal dose of monkeypox virus. Vaccinated animals did not develop fever, adverse clinical signs, laboratory abnormalities, or viremia. ACAM2000 and Dryvax® immunized animals exhibited greater than a 200-fold increase in levels of neutralizing antibodies following challenge, indicating that a limited monkeypox infection had occurred sufficient to boost immunity but insufficient to cause illness. The immunity afforded by ACAM2000 and Dryvax® is sufficient to inhibit systemic infection and development of advanced and dispersed dermal pocks; however, these vaccines do not provide a sterilizing immunity for such a severe dose and route of infection. Evidence of possible breakthrough lesion development observed in the immediate area surrounding the challenge site in one ACAM2000 vaccinee and three Dryvax® immunized animals, as well as breakthrough oral shedding in three Dryvax® vaccinated monkeys also suggests the presence of a limited infection. Indeed, a low level shedding of monkeypox virus in the oral cavity of three of eight Dryvax® vaccinated animals was observed, two of which also exhibited evidence of breakthrough lesion development local to the challenge site. This may indicate an enhanced protective profile of ACAM2000 over that of the standard Dryvax® vaccine. A comparative pathologic investigation of vaccinated animals beyond the scope of this study would be necessary to further elucidate this finding.
Protection was associated with high titers of neutralizing antibodies in response to vaccination with ACAM2000 or Dryvax®. Previous studies in monkeys, employing immune depletion methods, have shown that B-cell responses (antibodies) rather than CD4+ or CD8+ T-cell responses are sufficient to protect against intravenous challenge with a similar dose of monkeypox virus [24].
This study supports the conclusion that the ACAM2000 vaccine fully protects cynomolgus monkeys from developing a severe monkeypox infection following a harsh and uniformly lethal challenge, and that this protection is comparable to what is observed in monkeys vaccinated with Dryvax®. The eradication of naturally occurring human smallpox precludes pivotal efficacy trials in humans. Efficacy data presented here from the monkeypox-cynomolgus macaque challenge model, in concert with safety and immunogenicity data from human trials [23], is being used to demonstrate possible ACAM2000 vaccine efficacy against smallpox infection in humans.
Acknowledgements
All animal procedures were approved by the Battelle Institutional Animal Care and Use Committee and all research activities adhered to the “Guide for the Care and use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (US Department of Health and Human Services, Public Health Service, National Institutes of Health [NIH], revised 1996). We thank Peter Jahrling, US Army Medical Research Institute of Infectious Diseases (USAMRIID), Ft. Detrick, MD, for providing the monkeypox virus. We also thank (alphabetically) Jody Edwards, Tess Moir-Savitz, Don Moss, John Pyles, Josh Schmidt, Jen Tejada, Jean Truxall, and Daphne Vasconcelos for their excellent technical assistance, as well as, Gwendolyn Myers, Robert Schrader, and Paul Giannasca for their review of this manuscript.
Funds for this work were provided by grant #200-2002-00004 from the Centers for Disease Control and Prevention (CDC).
References
- 1.LeDuc J.W., Becher R. Current status of smallpox vaccine. Emerg Infect Dis. 1999;5(4):593–594. doi: 10.3201/eid0504.990429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson D.A., Inglesby T.V., Bartlett J.G., Ascher M.S., Eitzen E., Jahrling P.B., et al. Smallpox as a biological weapon: medical and public health management. J Am Med Assoc. 1999;281(22):2127–2137. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 3.Alibek K Biohazard. Handelman S, editor. New York, NY: Delta; 1999.
- 4.Preston R. Random House; New York, NY: 2002. The demon in the freezer. [Google Scholar]
- 5.Henderson D.A. The looming threat of bioterrorism. Science. 1999;283(5406):1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- 6.Frey S.E., Couch R.B., Tackett C.O., Treanor J.J., Wolff M., Newman F.K., et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346(17):1265–1274. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 7.Frey S.E., Newman F.K., Cruz J., Shelton W.B., Tennant J.M., Polach T., et al. Dose-related effects of smallpox vaccine. N Engl J Med. 2002;346(17):1275–1280. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- 8.LeDuc J.W., Damon I., Relman D.A., Huggins J., Jahrling P.B. Smallpox research activities: U.S. Interagency Collaboration, 2001. Emerg Infect Dis. 2001;8(7):743–745. doi: 10.3201/eid0807.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monath T.P., Caldwell J.R., Mundt W., Fusco J., Johnson C.S., Buller M., et al. ACAM2000 clonal vero cell culture vaccinia virus (New York City Board of Health strain) a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8(Suppl. 2):S31–S44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimoto M., Yasuda A., Miki K., Morita M., Suzuki K., Uchida N., et al. Gene structure of low-neurovirulent vaccinia virus LC16m0, LCm16m8, and their lister original (LO) strains. Microbiol Immunol. 1985;29(5):421–428. doi: 10.1111/j.1348-0421.1985.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 11.Hekker A.C., Bos J.M., Rai N.K., Keja J., Cuboni G., Emmet B., et al. Large-scale use of freeze-dried smallpox vaccine prepared in primary cultures of rabbit kidney cells. Bull WHO. 1976;54(3):279–284. [PMC free article] [PubMed] [Google Scholar]
- 12.McCLain D.J., Harrison S., Yeager C.L., Cruz J., Ennis F.A., Gibbs P., et al. Immunologic responses to vaccinia vaccines administered by different peripheral routes. J Infect Dis. 1995;175(4):756–763. doi: 10.1086/513968. [DOI] [PubMed] [Google Scholar]
- 13.Weltzin R., Liu J., Pugachev K.V., Myers G.A., Coughlin B., Blum P.S., et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat Med. 2003;9(9):1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 14.Kutinova L., Ludvikova V., Simonova V., Otavova M., Krystofova J., Hainz P., et al. Search for optimal parent for recombinant vaccinia virus vaccines: study of three vaccinia virus vaccinal strains and several virus lines derived from them. Vaccine. 1995;13(5):487–493. doi: 10.1016/0264-410x(94)00019-j. [DOI] [PubMed] [Google Scholar]
- 15.Kutinova L., Ludvikova V., Krystofova J., Otavova M., Simonova V., Nemeckova S., et al. Influence of the parental virus strain on the virulence and immunogenicity of recombinant vaccinia viruses expressing HBV preS2-S protein or VZV glycoprotein I. Vaccine. 1996;14(11):1045–1052. doi: 10.1016/0264-410x(96)00008-4. [DOI] [PubMed] [Google Scholar]
- 16.Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensky L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81(12):1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 18.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., et al. Modified vaccinia virus Anakara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stittelaar K.J., Neyts J., Naesens L., van Amerongen G., van Lavieren R.F., Holy A., et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439(7077):745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 20.Breman J.G., Henderson D.A. Diagnosis and management of smallpox. N Engl J Med. 2002;346(17):1300–1308. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- 21.Hooper J.W., Thompson E., Wilhelmsen C., Zimmerman M., Ait Ichou M., Steffen S.E., et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heraud J.M., Edghill-Smith Y., Ayala V., Kalisz I., Parrino J., Kalyanaraman V., et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006;177(4):2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 23.Artenstein A.W., Johnson C., Marbury T., Morrison D., Blum P.S., Kemp T., et al. A novel, cell culture-derived smallpox vaccine in vaccinia-naïve adults. Vaccine. 2005;23(25):3301–3309. doi: 10.1016/j.vaccine.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 24.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]


