Abstract
The first-generation smallpox vaccine was based on live vaccinia virus (VV) and it successfully eradicated the disease worldwide. Therefore, it was not administered any more after 1980, as smallpox no longer existed as a natural infection. However, emerging threats by terrorist organisations has prompted new programmes for second-generation vaccine development based on attenuated VV strains, which have been shown to cause rare but serious adverse events in immunocompromised patients. Considering the closely related animal poxviruses that might also be used as bioweapons, and the increasing number of unvaccinated young people and AIDS-affected immunocompromised subjects, a safer and more effective smallpox vaccine is still required. New avipoxvirus-based vectors should improve the safety of conventional vaccines, and protect from newly emerging zoonotic orthopoxvirus diseases and from the threat of deliberate release of variola or monkeypox virus in a bioterrorist attack. In this study, DNA and fowlpox recombinants expressing the L1R, A27L, A33R and B5R genes were constructed and evaluated in a pre-clinical trial in mouse, following six prime/boost immunisation regimens, to compare their immunogenicity and protective efficacy against a challenge with the lethal VV IHD-J strain. Although higher numbers of VV-specific IFNγ-producing T lymphocytes were observed in the protected mice, the cytotoxic T-lymphocyte response and the presence of neutralising antibodies did not always correlate with protection. In spite of previous successful results in mice, rabbits and monkeys, where SIV/HIV transgenes were expressed by the fowlpox vector, the immune response elicited by these recombinants was low, and most of the mice were not protected.
Keywords: Recombinant vaccine; Fowlpox virus; Prime/boost; OPXV vaccine; L1R, A27L, A33R and B5R VV genes
1. Introduction
The original vaccinia virus (VV) smallpox vaccine was administered by scarification. Due to the successful eradication of smallpox worldwide, the use of this vaccine was discontinued from 1980, later to be replaced by VV-derived, second-generation, cell-cultured vaccines, such as ACAM2000® (Weltzin et al., 2003). These vaccines showed similar immune responses as Dryvax®, but were associated with health risks and contraindications (Nalca and Zumbrun, 2010, Wiser et al., 2007), as they could spread to immunocompromised, non-vaccinated subjects (Bray, 2003, Centers for Disease Control and Prevention, 2007, Jacobson et al., 2008, Lane and Goldstein, 2003). In the attempt to develop strains with lower reactogenicity and fewer side effects, and to face the potential re-emergence or accidental/deliberate release of orthopoxviruses (OPXV) in the human population (Cardeti et al., 2011, Megid et al., 2012, Vogel et al., 2012, Whitley, 2003), a third generation of attenuated vaccines was developed. Although not as virulent as variola, the monkeypox (MPXV) and cowpox (CPXV) viruses are also a threat to public health, as they cause mortality in underdeveloped countries (Reed et al., 2004) and can become a potential bioweapon if adapted to grow and spread in humans (Lewis-Jones, 2004).
The new attenuated vaccines, which include the VV-derived Lister clone LC16m8 (Kenner et al., 2006) and the most advanced modified vaccinia Ankara (MVA; e.g., the IMVAMUNE® (Kennedy and Greenberg, 2009)), have an improved safety profile and induce more rapid responses (Earl et al., 2004). However, their ability to induce long-term immunity is controversial (Earl et al., 2007, Ferrier-Rembert et al., 2008), and they also fail to induce protective immunity in immunocompromised animals (Edghill-Smith et al., 2005a). MVA also induces lower immunogenicity than the traditional smallpox vaccine (Ferrier-Rembert et al., 2008) and shows a limited replication in mammals (Blanchard et al., 1998). A further caveat may be represented by possible recombination events that may rescue endogenous OPXV genes and generate a fully-replicative genotype (Okeke et al., 2009, Verheust et al., 2012). MVA also failed to protect animals with CD4/CD8 combined immunodeficiency (Wiser et al., 2007) and Rhesus macaques infected with simian immunodeficiency virus showing a very low cell count of the immune repertoire (Edghill-Smith et al., 2005a).
Although the VV antigens that protect against smallpox are not completely defined, neutralising antibodies have mainly been raised against the surface proteins of the two OPXV infectious particles: the mature virions (MVs) released after cell lysis and the extracellular virions (EVs), which are wrapped by an additional envelope (Moss, 2011, Pacchioni et al., 2013, Roberts and Smith, 2008, Smith et al., 2002). In particular, the combined use of the L1 and A27 proteins of MV, and the A33 and B5 proteins of EV has conferred better protection than the individual use of MV or EV proteins (Fogg et al., 2004, Hooper et al., 2002). These subunit vaccines are protective against VV intranasal challenge in mice or MPXV intravenous challenge in monkeys (Buchman et al., 2010, Fogg et al., 2007, Hirao et al., 2011, Hooper et al., 2010).
In the present study, four DNA recombinants that express the VV L1R, A27L, A33R and B5R genes (called the 4DNAmix) were used, followed by four novel fowlpox (FP) recombinants that express the same genes (Pacchioni et al., 2013) (called the 4FPmix). MVA, which also contains the same genes, was used as a control, either alone or in a prime/boost regimen, followed by the 4FPmix. A direct comparison was performed between humoral and cell-mediated responses and the ability to induce protection in the immunised mice. Attenuated avipox viruses have been developed as novel vectors for the construction of recombinant vaccines against several human infectious diseases (Radaelli et al., 1994, Zanotto et al., 2010). These vectors are restricted for replication to avian species, but permissive for entry and transgene expression in most mammalian cells. They are also immunologically non-cross-reactive with vaccinia (Baxby and Paoletti, 1992), and they can escape neutralisation by vector-generated antibodies in smallpox-vaccine-experienced humans.
2. Materials and methods
2.1. Cells
Specific-pathogen-free primary chick embryo fibroblasts (CEF) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% heat-inactivated calf serum (Gibco Life Technologies, Grand Island, NY, USA), 5% Tryptose Phosphate Broth (Difco Laboratories, Detroit, MI, USA), 100 U/ml penicillin and 100 mg/ml streptomycin. Green monkey kidney (Vero) cells, and Balb/C mouse fibroblasts (B77 cells) were grown in DMEM supplemented with 10% heat-inactivated calf serum, 100 U/ml penicillin and 100 mg/ml streptomycin.
2.2. Viruses and fowlpox recombinants
The highly pathogenic IHD-J strain of VV was supplied by S. Dales (University of Western Ontario, London, Canada) (Wilton et al., 1986), and was grown in Vero cells, to use as the challenging virus (1 × 106 PFU/mouse) through the airways. IHD-J was amplified in Vero cells, purified on discontinuous sucrose density gradient, and titrated as described previously (Pacchioni et al., 2013). The 4FP recombinants, FPL1R, FPA27L, FPA33R and FPB5R, that express the L1, A27, A33 and B5 proteins of VV, respectively, were generated in our laboratory by in-vivo homologous recombination (Pozzi et al., 2009). Gene insertion was performed downstream of the VV H6 early/late promoter (Rosel et al., 1986), inside the 3-β-hydroxysteroid dehydrogenase 5-delta 4 isomerase gene interrupted by a multiple cloning site. Recombinants were grown and amplified in CEF, and purified on discontinuous sucrose gradients. MVA was kindly obtained by A. Siccardi (Dept. of Biology, University of Milan, Italy), and amplified and purified on CEF, as already described (Soprana et al., 2011).
2.3. Plasmids
The expression plasmids pcDNA3.1A27L, pcDNA3L1R, pcDNA3A33R and pcDNA3B5R were constructed in our laboratory by insertion of the same genes used for the FP recombinants. In particular, pcDNA3.1 (Invitrogen Corp., San Diego, CA, USA) was used for the A27L gene, which was inserted into the HindIII/NotI restriction sites, whereas pcDNA3 (Invitrogen Corp.) was used for the L1R, A33R and B5R genes, which were separately inserted into the HindIII/XhoI restriction sites. Both pcDNA3.1 and pcDNA3 contain the human CMV promoter. The mixture of these four recombinants is called the 4DNAmix. PcDNA3gag/pol was used as an irrelevant negative control, and described previously (Zanotto et al., 2010).
2.4. Immunisation protocols
Six groups of eight female Balb/C mice (Charles River Laboratories, Wilmington, MA, USA) were immunised by multiple injections. All of the mice were inoculated three times at 3-week intervals, before challenge. After immunisation, all of the mice remained in good health, without loss of weight. Before each inoculation, the mice were anaesthetised with 300 μl 2.5% 2,2,2-tribromoethanol (Avertin®) (Sigma, St Louis, MO, USA) i.p., and bleeding was performed from the retro-orbital eye plexus. The plasma fraction was aliquoted and frozen at −80 °C. Six different prime/boost immunisation protocols were followed (Fig. 1 ), using: (i) plasmid 4DNAmix (100 μg of each recombinant/mouse; i.m.) in combination with recombinant FPgagpol (107 PFU/mouse; s.c.) (Group 1); (ii) 4DNAmix (100 μg of each recombinant/mouse; i.m.) in combination with recombinant 4FPmix (107 PFU of each recombinant/mouse; s.c.) (Group 2); (iii) recombinant 4FPmix (107 PFU of each recombinant/mouse; s.c.) used for the three inoculations (Group 3); (iv) MVA (107 PFU/mouse; s.c.) used for the three inoculations (Group 4); (v) MVA (107 PFU/mouse; s.c.) in combination with 4FPmix (107 PFU of each recombinant/mouse; s.c.) (Group 5); and (vi) pcDNAgagpol plasmid (100 μg/mouse; i.m.) in combination with FPgagpol recombinant (107 PFU/mouse; s.c.) (Group 6). All of the mice were maintained in accordance with the Italian national guidelines. Mice were observed for signs of disease and weighed daily, provided food and water ad libitum. Every effort was made to minimise suffering and, based on predetermined criteria (loss of more than 30% body weight) moribund animals were euthanized. The approval for this study was granted by the ethical Committee of the University of Milan.
Fig. 1.
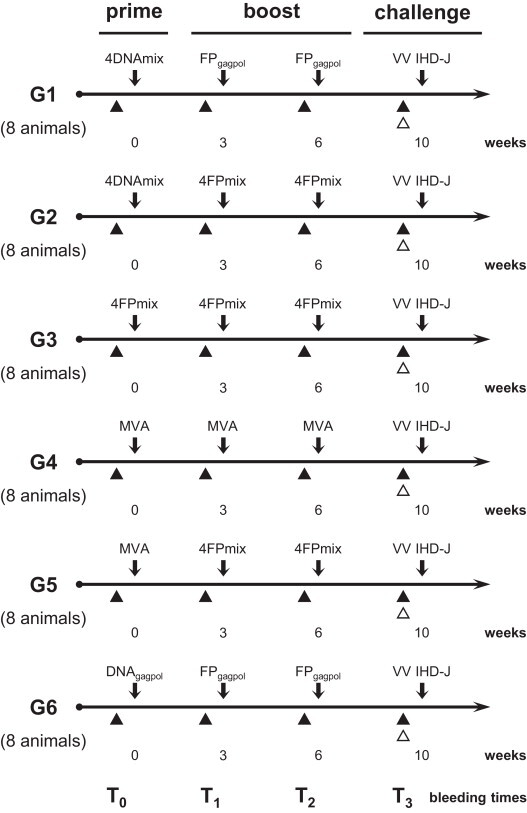
Immunisation protocols. Six different prime/boost immunisation protocols were followed using 8 mice per group. The L1R, A27L, A33R and B5R genes were expressed either by pcDNA3 or by FP recombinants, and administered together as the 4DNAmix or 4FPmix, respectively. Each plasmid was administered i.m. at 100 μg/mouse, and each virus was administered s.c. at 107 PFU/mouse. MVA was used as a positive control or for priming. Plasmid pcDNAgagpol and FPgagpol were used as negative controls. G1 to G6, immunisation group numbers.
2.5. ELISA
The mice plasma was tested for the presence of antibodies against the L1, A27, A33 and B5 VV-specific proteins before the first immunisation and after each immunisation. Purified IHD-J virus was plated at a concentration of 2 PFU × 105/well in a 96-well MaxiSorp microtitre plate (Nunc, Naperville, IL, USA) in 0.05 M carbonate-bicarbonate buffer, pH 9.6, and incubated overnight at 4 °C. The ELISA was performed in triplicate, essentially as described previously (Radaelli et al., 2010). The sera were diluted 1:1000 and the reactions were revealed using a 1:1000 dilution of goat anti-mouse horseradish–peroxidase-conjugated sera (DakoCytomation, Glostrup, Denmark) and tetramethylbenzidine substrate (Sigma). Preimmune mouse sera were used as negative controls. The absorbance of each well was read at 490 nm using a 550 microplate reader (Bio-Rad, Hercules, CA, USA).
2.6. Virus neutralisation assays
The neutralising activity of the mice sera, that were obtained at different times post-immunisation and after the challenge, was tested in duplicate by measuring the extent of inhibition of IHD-J infectivity. The assays were performed by preincubation for 1 h at 37 °C in a 48-well plate of an equal volume of IHD-J with heat-inactivated mouse serum, used at different dilutions in DMEM. The viral inoculum was adjusted to give approximately 5 × 102 PFU of IHD-J/ml. Infection was performed in duplicate on Vero cells, and was allowed to proceed for 1 h at 37 °C. The same amount of virus incubated with DMEM was used as a negative control, whereas the virus incubated with hyperimmune mouse serum was used as a positive control. Three days later, 1.5% neutral red was added, and the plaques were counted the next day, as described previously (Pacchioni et al., 2013). Sera from each group at the different times post-infection (p.i.) were pooled before use. Sera from Group 5 were also tested individually. Neutralisation was expressed as the percentage of inhibition of the infection compared to the negative control, where the virus was incubated with DMEM only.
2.7. In-vivo cytotoxicity assays
B77 syngeneic cells were separately infected with each FP recombinant (6 PFU/cell) for 1 h at 37 °C. Five hours later, the cells were washed twice with Ca2+- and Mg2+-free phosphate-buffered saline (PBS−), trypsinised, and harvested in DMEM with 5% calf serum, and counted, to prepare a mixture with equivalent numbers of cells infected with each recombinant. The same number of cells infected with FPwt was also prepared. The two cell populations were loaded with two different concentrations of the vital fluorescent carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE) dye (Life Technologies) and mixed at a 1:1 ratio (6 × 106 cells infected with the recombinants, as cytotoxic T-lymphocyte (CTL) targets, and 6 × 106 cells infected with FPwt, as a control, for each mouse). Briefly, the cells were incubated separately with 0.75 μM or 0.075 μM CFDA-SE in DMEM plus 1% foetal bovine serum for 30 min, and washed twice with DMEM plus 10% foetal bovine serum before use. The staining and the cell ratio was further checked by flow cytometry and set as a control. Each mouse was injected with 1.2 × 107 cells, in the caudal vein. Two mice for each group of immunised animals were used for this assay, which was performed after the third immunisation, at 10 weeks p.i. Target and control cells were recovered from the spleen 24 h later.
2.8. Ex-vivo cytokine production
Popliteal lymph nodes from 2 mice per each group were dissected mechanically, until single-cell suspensions were obtained. The cells from draining and non-draining lymph nodes were cultured for 5 h at 37 °C with 10 μg/ml brefeldin A (Sigma), 50 ng/ml phorbol 12-myristate 13-acetate (Sigma) and 100 ng/ml ionomycin (Sigma) in complete DMEM medium, and processed for surface and intracellular cytokine staining as described previously (Radaelli et al., 2003). Briefly, after treatment with 2 mM EDTA in PBS and an incubation at 37 °C for 5 min, the cells were washed twice with PBS, surface-stained with a fluorescein-isothiocyanate-tagged anti-CD4 antibody (5 μg/ml), washed again, and fixed and permeabilised with Cytofix/Cytoperm (BD Immunocytometry Systems, San José, CA, USA) for 15 min on ice in the dark. Following two further washes, the cells were stained with anti-IFNγ-APC (14 μg/ml) (BioLegend, BD) and anti-IL4-PE (14 μg/ml) (BD Immunocytometry Systems), incubated for 30 min at 4 °C, washed extensively three times with PBS containing 5% foetal bovine serum, and analysed by four-colour flow cytometry (FACSCalibur, BD).
2.9. Mouse challenge
Preliminary tests were first performed by challenging naïve animals, to evaluate the minimum lethal dose to kill 50% of the mice (LD50). These mice were inoculated with high (5 × 105, 20 × 105, 30 × 105 PFU IHD-J), or low (1 × 105, 2 × 105 PFU IHD-J) doses of the VV IHD-J strain, with the LD50 defined as 2 × 105 PFU IHD-J. For the experimental assay, six mice out of the eight per group were challenged 6 weeks after the third immunisation, using intranasal administration of 1 × 106 PFU IHD-J in 30 μl PBS-, administered through a plastic pipette tip after anaesthetising the animals with 300 μl avertin i.p. All of the mice were followed daily, with measurements of their weight and monitoring for disease symptoms.
2.10. Statistical analyses
Statistical analyses were performed using one-way ANOVA parametric tests and Bonferroni/Newman–Keuls analysis of variance, using the GraphPad Prism software, version 2.0, as well as the Student's t-test. The statistical significance was set as p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
3. Results
3.1. Low humoral response in immunised mice
With the aim of developing a vaccine strategy that can protect from OPXV infections, six different immunisation protocols were compared for the raising of antibodies and for effector-T-cell-specific responses in the mice. Both DNA and FP virus recombinants were constructed to prime six groups of eight mice, and they were followed by two boosts with FP recombinants as shown in Fig. 1. All of the animals remained in good health after all of the rounds of immunisation. The specific humoral response against L1, A27, A33 and B5 proteins expressed by DNA and/or FP recombinants was measured in the plasma by ELISA, using the plate-bound purified IHD-J virus as the antigen (Fig. 2 ). The animals of Group 5 showed a significant increase at T2 (p < 0.01 and < 0.001 vs Group 4 and 6, respectively), which was observed in the mice of Group 3 both at T2 (p < 0.001 vs Groups 4 and 6), and at T3 (p < 0.001 vs Groups 4, 5, and 6). In Group 4, the increase was seen at T3 (p < 0.001 vs Groups 5 and 6). The results are shown for the 1:1000 serum dilutions.
Fig. 2.

Analysis of the specific humoral responses. The anti-L1, A27, A33 and B5 antibody levels were determined by ELISA using purified IHD-J VV as the plate-bound antigen. The bars indicate the average of the values of the animals of each group. The responses were compared among the groups, and the mice of G3, G4, and G5 showed significant increases in specific antibody titres at T2 and T3. Statistical differences using one-way ANOVA parametric tests and Bonferroni/Newman-Keuls analysis of variance are shown: (**) p < 0.01; (***) p < 0.001.
3.2. Neutralising activity against IDH-J does not always correlate with mouse survival
To determine whether the humoral responses seen after these regimens of immunisation prevented IDH-J infectivity, neutralisation tests were performed (Fig. 3A). As a whole, a low neutralising activity was raised in Group 1 (p < 0.01 vs Group 2 and Group 6, Student t-test) and in Group 3 (p < 0.05 vs Group 5, and p < 0.001 vs Group 2 and Group 6), whereas no neutralising activity was detected in Group 2 or 6, with low neutralising activity in Group 5 (p < 0.01 vs Group 2 and Group 6). In contrast, the mice of Group 4, which were used as a positive control, raised high levels of neutralising antibodies against IDH-J (p < 0.001 vs Groups 1, 2, 3, 5, 6). As 50% of the mice of Group 5 (mice numbers 4, 5, 7) were protected from IDH-J infection (see Fig. 6C), the sera from this group were also separately analysed at post-immunisation times, to try to evaluate the correlation between viral neutralisation and survival and the contribution of the specific immunogen (Fig. 3B). The analysis was not performed for animals 3 and 6, that were used for the in-vivo cytotoxicity assays. The results were expressed as the average of two determinations, and they showed that mouse number 7 had a neutralising activity >40% (p < 0.001 vs mice 1, 2, 4, 5, 8), whereas three mice had neutralising activities >20%: mouse 1 (p < 0.001 vs mouse 5), mouse 4 (p < 0.01 vs mouse 1 and p < 0.001 vs mice 2, 5, 8), and mouse 8 (p < 0.05 vs mouse 5). Conversely, after each immunisation, mice 2 and 5 showed low neutralising activities ≤15%.
Fig. 3.
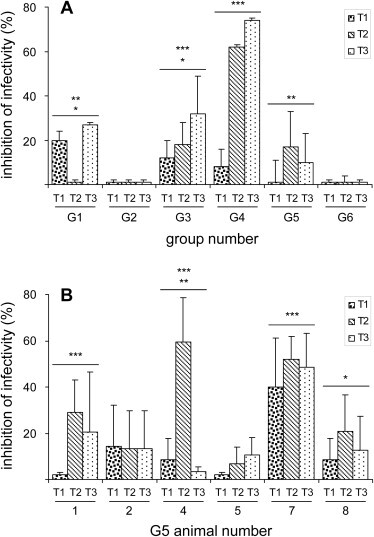
Neutralising antibodies elicited by the different mouse immunisation protocols. Virus neutralisation assays were performed on Vero cells after each immunisation. Plaque reduction was quantified as compared to the control, in which the viral inoculum was incubated with the preimmune serum, and expressed as percentages of inhibition of infectivity. (A) Except for Group 2 (G2), all of the groups of mice raised neutralising antibodies against IHD-J, and inhibition of infectivity generally increased after the second immunisation. (B) Sera from mice 1, 4, 7 and 8 of Group 5 showed significantly higher inhibition of infectivity, although not all of these mice were protected. Conversely, mouse 5 of Group 5 was protected in the absence of significant levels of neutralising antibodies. Statistical significances using the Student t-test are shown: (**) p < 0.01; (***) p < 0.001.
Fig. 6.
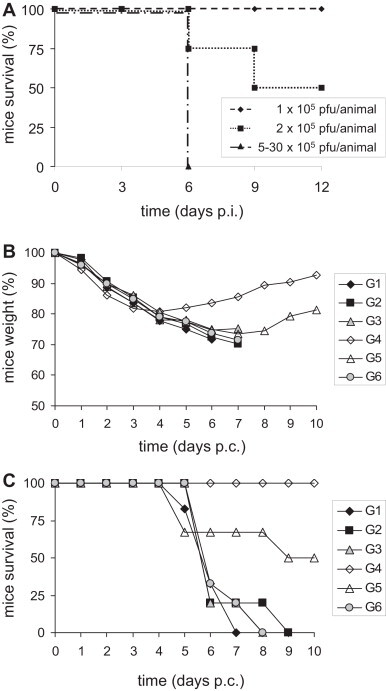
Efficacy of the vaccine-induced responses by the immunisation regimens. Different viral doses of IHD-J were tested on naïve mice to define the LD50 (A). Six weeks after the third immunisation, six animals per group were challenged by intranasal administration with 1 × 106 PFU IHD-J, and were monitored for weight (B) and survival (C). All of the animals progressively lost weight to 20% at day 4 p.c., with no relevant differences among the groups of vaccinated mice (B). From day 5 p.c., all of the animals of Group 4 (G4) started regaining weight and survived. 50% of the mice of Group 5 (G5) recovered and were protected after day 7 p.c. All of the animals of the other groups died between days 7 and 8 p.c. (C).
3.3. Comparable cell-mediated cytotoxicity is elicited by MVA vs MVA/FP-recombinant vaccines
The antigen-specific CTL activity was analysed in vivo on splenocytes. B77 syngeneic cells were infected with each FP recombinant or with FPwt, stained with two different CFDA-SE concentrations, and injected i.v. into the immunised mice. These were evaluated the next day using flow cytometry. Antigen-specific CTL-mediated killing was calculated by the decrease in the ratio between the percentage of B77 cells infected with FP recombinants and B77 cells infected with FPwt, after infusion in the immunised mice. The results are expressed as the percentages of specific killing (Fig. 4 ), and they show that there were more dead target cells in Groups 2, 4 and 5 than in the negative control, although no statistical differences were found between Groups 2, 4 and 5.
Fig. 4.
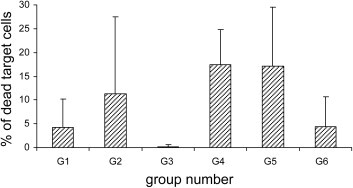
In-vivo cell-mediated cytotoxicity. The mice were injected with B77 syngeneic cells infected with the 4FPmix or FPwt and loaded with two concentrations of CFDA-SE dye. The CTL responses were evaluated using flow cytometry, after recovering target and control cells ex-vivo from the spleen 24 h after the inoculation. The percentages of dead cells were higher than the negative control in Groups 2, 4 and 5 (G2, G4, G5), with Group 4 and Group 5 being almost similar, although no statistical differences were found.
3.4. IFNγ- and IL4-mediated immune responses after vaccination
The secretion of effector cytokines by CD4+ T cells in the mice immunised following the different immunisation regimens was assessed by intracellular cytokine staining. Single-cell suspensions from draining lymph nodes near the site of injection were cultured in vitro in the presence of phorbol 12-myristate 13-acetate and ionomycin, and the percentages of CD4+ cells producing IFNγ or IL4, as parameters of Th1 and Th2 responses, were determined by cytofluorimetric analysis (Fig. 5 ).
Fig. 5.

Functional virus-specific T-cell responses induced by the vaccinations. IFNγ and IL4 production were measured after in-vitro stimulation with phorbol 12-myristate 13-acetate and ionomycin in an intracellular staining assay. IFNγ (A) and IL4 (B) were evaluated as parameters representative of Th1 and Th2 responses, respectively. There was a higher number of CD4+ T cells producing IFNγ in Group 4 mice (A, G4), as compared to the other groups of mice, which gave a significantly greater IFNγ/IL4 ratio vs the negative control (C). IL4 production was low in all of the groups. Statistical significance using one-way ANOVA parametric tests and Bonferroni/Newman-Keuls analysis of variance is shown: (***) p < 0.001.
No increases in the percentages of cells that produced IFNγ or IL4 effector cytokines were observed in the different mouse groups (Fig. 5A and B), except for Group 4, which showed a significant increase (p < 0.001) in IFNγ production vs the Group 6 negative control.
3.5. Protection of immunised mice
To evaluate the efficacy of the vaccine-induced responses according to the different immunisation regimens, the mice were monitored for weight and survival after the challenge with the VV IHD-J strain. Preliminary assays showed that mice injected with 5–30 × 105 PFU IHD-J died at day 6 post-challenge (p.c.), whereas all of the mice infected with 1 × 105 PFU IHD-J remained alive and developed strong immunity and protection against re-infection with high viral doses. When 2 × 105 PFU IHD-J were used, 50% of the mice survived (i.e., the LD50, Fig. 6A) and were protected from high viral doses up to 5 × 106. The dose of 1 × 106 PFU IHD-J (5-fold the LD50) was then chosen for the challenge. In the experimental challenge, soon after the first day p.c., all of the mice progressively lost weight, by 20% at day 4 p.c., with no relevant differences between the groups of vaccinated mice (Fig. 6B). At day 5 p.c., all of the mice of Group 4 started regaining weight and survived, whereas only 50% of the mice of Group 5 recovered after day 7 and survived (Fig. 6B and C). All of the mice of Groups 1, 2, 3, 6, 8 died between days 7 and 8 p.c.
4. Discussion
The development of safer vaccines against OPXV infection of humans is still an emerging concern that might arise either through accidental exposure to CPXV, MPXV or other OPXV (Reed et al., 2004), or following deliberate bioterrorist release of variola virus. The discontinuation of the smallpox vaccination campaign resulted in declined herd immunity, with increased MPXV zoonotic infections (Hutin et al., 2012), which has encouraged the development of safer smallpox vaccines (Artenstein, 2008, Poland, 2005, Wiser et al., 2007). Although with low frequency, serious side effects can result from live VV-based vaccines, especially in immunocompromised subjects and patients with skin diseases (Schulze et al., 2007). Highly attenuated MVA is thought to be safe (Kennedy and Greenberg, 2009), although it requires 1000-fold the dose of the current live VV vaccine.
It is already well established that combined immunisation regimens where DNA priming is followed by protein or viral vaccine boosts elicit higher immune responses compared with the non-combined use of the immmunogens (Lu, 2009, Radaelli et al., 2003, Radaelli et al., 2007, Wang et al., 2008). In particular, heterologous DNA prime/protein boost strategies have been effective in eliciting improved quality of antigen-specific antibody responses (Vaine et al., 2010), and thus this approach might offer new possibilities to develop safe and improved vaccines.
In the present study, four new FP recombinants, FPL1R, FPA27L, FPA33R and FPB5R, were combined with DNA recombinants, all of them expressing the VV L1R, A27L, A33R and B5R genes, and were administered as novel homologous/heterologous prime/boost immunisation regimens. Our aim was to evaluate the humoral and cell-mediated responses as well as the protection in mice, through the comparison of different vaccination protocols. We have demonstrated that: (i) the specific antibody responses that were elicited by the different protocols do not correlate with protection; (ii) the presence of neutralising antibodies in the serum does not always allow mouse survival; (iii) the putative protective role of the cellular responses does not always occur, as shown by IFN-γ/IL4 cytokine determination as a surrogate for the Th1/Th2 responses.
Although still controversial, the critical role of the antigen-specific humoral response for vaccine-induced protection against OPXV has already been described (Edghill-Smith et al., 2005b, Panchanathan et al., 2006, Sarkar et al., 1975). In particular, while CD8+ T cells appear not to be necessary for protection from a lethal MPXV challenge (Edghill-Smith et al., 2005b), passive transfer of VV-specific sera was shown to confer protection both in mice and monkeys (Golden et al., 2011). Vaccination with live VV appears to be effective also in subjects with dysfunctions in their humoral response, although not in subjects with T-cell related immunodeficiencies (Golden and Hooper, 2013).
In the present study, although high variability was found among the animals which might weaken the results, the highest level of humoral response was obtained both after the second and third immunisation for the mice of Group 3, which received the 4FPmix repeatedly. However, none of these animals survived the challenge, although antibodies showed virus neutralising activity. Conversely, the mice of Group 4 showed highly significant humoral responses and were protected. In Group 5, only 50% of the mice survived, in spite of their VV-specific antibody response. The neutralisation of infectivity, which generally correlates with the level of antibodies against virus surface antigens, was significantly higher in the mice of Group 4 that were all protected, but remained low in the mice of Group 5. Neutralisation was thus performed separately on the sera from each mice of Group 5 to determine whether the neutralising activity correlated with the protection. Surprisingly, the significantly higher level of neutralising antibodies of the protected mice 4 and 7 was not confirmed by mouse 5, which was also protected, but which showed lower neutralising antibody levels than the unprotected mice (mice 1, 2, 8). This suggests that protection cannot be ascribed only to the level of neutralising antibodies.
Although the protective role of CTL after OPXV vaccination remains to be defined (Buchman et al., 2010), immunogens that target T-cell epitopes also appear to be effective (Goulding et al., 2013, Hirao et al., 2011, Moise et al., 2011, Snyder et al., 2004). As specific cytokine release is one of the main characteristics of the Th1/Th2 responses, both IFNγ and IL4 production were assessed. IL4 production varied only slightly among the groups, whereas the number of IFNγ-producing cells was higher in the mice of Group 4, which resulted in a significantly higher IFNγ/IL4 ratio in protected mice. When testing the effector function of specific CD8+ T-cells, similar higher cytotoxic activity was found in the spleen of the mice of Groups 4 and 5. This suggests a pivotal role of IFNγ-producing T cells and cytotoxic cells in the mice of Group 4, which is also in agreement with other recent studies that have underlined the need for T-cell responses for survival after VV challenge.
Although the weight decrease soon after the challenge was similar in all of the immunised groups, it is surprising that this loss was consistently almost 5% higher in the mice of Group 4, which then progressively regained weight at day 5 p.c. and were protected. As three out of six mice of Group 5 also regained weight at day 7 p.c. and were protected, these results suggest a transient negative effect on animals vaccinated with MVA, and confirms that MVA can provide 100% protection only after repeated administrations (Handley et al., 2009, Kennedy and Greenberg, 2009, Kenner et al., 2006). Although the use of a different vector might avoid the neutralisation elicited by MVA priming, the boosting with FP-based recombinants did not elicit protection in all of the animals of Group 5.
In spite of the evidence from other studies, DNA recombinants alone did not provide any protection. This might be ascribed to the absence of repeated DNA immunisation or to a different way of DNA administration. It is well known that a more effective immune response can be obtained by heterologous prime/boost, although the route and dose of vaccine administration might also be determinants (Bansal et al., 2008). This is further supported by a recent study in which an optimal dose of the VV Tiantan attenuated strain was used, with higher levels of long-lasting neutralising antibody responses induced in protected mice through the intranasal/oral than the i.m. and s.c. routes (Lu et al., 2011). If FP evasion mechanisms can be hypothesised that can inactivate cytokines, these mechanisms were not active in previous immunisation experiments in which other FP recombinants were used, and it prompts us to improve immunogenicity by different routes of immunisation.
Acknowledgements
The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: the NR-417 monoclonal antibody against VV (WR) L1 protein, residues 1 to 185; the NR-631 polyclonal antibody against VV (WR) L1 protein; the NR-567 monoclonal antibody against VV (WR) A27 protein, residues 1 to 110; the NR-627 polyclonal antibody against VV (WR) A27 protein; the NR-565 monoclonal antibody against VV (WR) A33 protein, residues 58 to 185; the NR-628 polyclonal antibody against VV (WR) A33 protein; the NR-551 monoclonal antibody against VV (WR) B5 protein, residues 20 to 275; and the NR-629 polyclonal antibody against VV (WR) B5 protein. We also thank Dr. Christopher Berrie for editorial assistance with the manuscript.
References
- Artenstein A.W. New generation smallpox vaccines: a review of preclinical and clinical data. Rev. Med. Virol. 2008;18:217–231. doi: 10.1002/rmv.571. [DOI] [PubMed] [Google Scholar]
- Bansal A., Jackson B., West K., Wang S., Lu S., Kennedy J.S., Goepfert P.A. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J. Virol. 2008;82:6458–6469. doi: 10.1128/JVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxby D., Paoletti E. Potential use of nonreplicating vectors as recombinant vaccines. Vaccine. 1992;10:8–9. doi: 10.1016/0264-410x(92)90411-c. [DOI] [PubMed] [Google Scholar]
- Blanchard T.J., Alcami A., Andrea P., Smith G.L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101–114. doi: 10.1016/s0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Buchman G.W., Cohen M.E., Xiao Y., Richardson-Harman N., Silvera P., DeTolla L.J., Davis H.L., Eisenberg R.J., Cohen G.H., Isaacs S.N. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine. 2010;28:6627–6636. doi: 10.1016/j.vaccine.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardeti G., Brozzi A., Eleni C., Polici N., D’Alterio G., Carletti F., Scicluna M.T., Castilletti C., Capobianchi M., Di Caro A., Autorino G.L., Amaddeo D. Cowpox virus in llama, Italy. Emerg. Infect. Dis. 2011;17:1513–1515. doi: 10.3201/eid1708.101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Household transmission of vaccinia virus from contact with a military smallpox vaccinee—Illinois and Indiana, 2007. MMWR Morb. Mortal. Wkly. Rep. 2007;56:478–481. [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Montefiori D.C., Byrum R., Piatak M., Lifson J.D., Amara R.R., Robinson H.L., Huggins J.W., Moss B. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;366:84–97. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D., Kulesh D.A., Martinez M.J., Miller D.M., Mucker E.M., Shamblin J.D., Zwiers S.H., Huggins J.W., Jahrling P.B., Moss B. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y., Bray M., Whitehouse C.A., Miller D., Mucker E., Manischewitz J., King L.R., Robert-Guroff M., Hryniewicz A., Venzon D., Meseda C., Weir J., Nalca A., Livingston V., Wells J., Lewis M.G., Huggins J., Zwiers S.H., Golding H., Franchini G. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 2005;191:372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Ferrier-Rembert A., Drillien R., Tournier J.N., Garin D., Crance J.M. Short- and long-term immunogenicity and protection induced by non-replicating smallpox vaccine candidates in mice and comparison with the traditional 1st generation vaccine. Vaccine. 2008;26:1794–1804. doi: 10.1016/j.vaccine.2007.12.059. [DOI] [PubMed] [Google Scholar]
- Fogg C., Americo J.L., Lustig S., Huggins J.W., Smith S.K., Damon I.K., Resch W., Earl P.L., Klinman D.M., Moss B. Adjuvant enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007;25:2787–2799. doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg C., Lustig S., Whitbeck J.C., Eisenberg R.J., Cohen G.H., Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 2004;78:10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J.W., Hooper J.W. The strategic use of novel smallpox vaccines in the post-eradication world. Expert Rev. Vaccines. 2013;10:1021–1035. doi: 10.1586/erv.11.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J.W., Zaitseva M., Kapnick S., Fisher R.W., Mikolajczyk M.G., Ballantyne J., Golding H., Hooper J.W. Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease. Virol. J. 2011;8:441. doi: 10.1186/1743-422X-8-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding J., Bogue R., Tahiliani V., Croft M., Salek-Ardakani S. CD8T cells are essential for recovery from a respiratory vaccinia virus infection. J. Immunol. 2013;189:2432–2440. doi: 10.4049/jimmunol.1200799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley L., Buller R.M.L., Frey S.E., Bellone C., Parker S. The new ACAM2000 vaccine and other therapies to control orthopoxvirus outbreaks and bioterror attacks. Expert Rev. Vaccines. 2009;8:841–850. doi: 10.1586/erv.09.55. [DOI] [PubMed] [Google Scholar]
- Hirao L.A., Draghia-Akli R., Prigge J.T., Yang M., Satishchandran A., Wu L., Hammarlund E., Khan A.S., Babas T., Rhodes L., Silvera P., Slifka M., Sardesai N.Y., Weiner D.B. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2011;203:95–102. doi: 10.1093/infdis/jiq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper J.W., Custer D.M., Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicitis appropriate antibody responses in nonhuman primates. Virology. 2002;306:181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper J.W., Ferro A.M., Golden J.W., Silvera P., Dudek J., Alterson K., Custer D.M., Rivers B., Morris J., Owens G., Smith J.F., Kamrud K.I. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine. 2010;28:494–511. doi: 10.1016/j.vaccine.2009.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin Y.J., Williams R.J., Malfait P., Pebody R., Loparev V.N., Ropp S.L., Rodriguez M., Knight J.C., Tshioko F.K., Khan A.S., Szczeniowski M.V., Esposito J.J. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2012;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson I.G., Smith T.C., Smith B., Wells T.S., Ryan M.A. US military service members vaccinated against smallpox in 2003 and 2004 experience a slightly higher risk of hospitalization postvaccination. Vaccine. 2008;26:4048–4056. doi: 10.1016/j.vaccine.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Kennedy J.S., Greenberg R.N. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev. Vaccines. 2009;8:13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner J., Cameron F., Empig C., Jobes D.V., Gurwith M. LC16m8: an attenuated smallpox vaccine. Vaccine. 2006;24:7009–7022. doi: 10.1016/j.vaccine.2006.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J.M., Goldstein J. Adverse events occurring after smallpox vaccination. Semin. Pediatr. Infect. Dis. 2003;14:189–195. doi: 10.1016/s1045-1870(03)00032-3. [DOI] [PubMed] [Google Scholar]
- Lewis-Jones S. Zoonotic poxvirus infections in humans. Curr. Opin. Infect. Dis. 2004;17:81–89. doi: 10.1097/00001432-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Lu B., Yu W., Huang X., Wang H., Liu L., Chen Z. Mucosal immunization induces a higher level of lasting neutralizing antibody response in mice by a replication-competent smallpox vaccine: vaccinia Tiantan strain. J. Biomed. Biotechnol. 2011;2011:970424. doi: 10.1155/2011/970424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megid J., Borges I.A., Trindade G.S., Appolinário C.M., Ribeiro M.G., Allendorf S.D., Antunes J.M., Silva-Fernandes A.T., Kroon E.G. Vaccinia virus zoonotic infection, São Paulo State, Brazil. Emerg. Infect. Dis. 2012;18:189–191. doi: 10.3201/eid1801.110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise L., Buller R.M.L., Schriewer J., Lee J., Frey S.E., Martin W., De Groot A.S. VennVax, a DNA-prime, peptide-boost multi-T-cell epitope poxvirus vaccine, induces protective immunity against vaccinia infection by T cell response alone. Vaccine. 2011;29:501–511. doi: 10.1016/j.vaccine.2010.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 2011;239:8–26. doi: 10.1111/j.1600-065X.2010.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalca A., Zumbrun E.E. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Devel. Ther. 2010;4:71–79. doi: 10.2147/dddt.s3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke M.I., Nilssen O., Moens U., Tryland M., Traavik T. In vitro host range, multiplication and virion forms of recombinant viruses obtained from co-infection in vitro with a vaccinia-vectored influenza vaccine and a naturally occurring cowpox virus isolate. Virol. J. 2009;6:55. doi: 10.1186/1743-422X-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchioni S., Bissa M., Zanotto C., De Giuli Morghen C., Illiano E., Radaelli A. L1R, A27L, A33R and B5R vaccinia virus genes expressed by fowlpox recombinants as putative novel orthopoxvirus vaccines. J. Transl. Med. 2013;11:95. doi: 10.1186/1479-5876-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan V., Chaudhri G., Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 2006;80:6333–6338. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland G.A. Smallpox vaccines: from first to second to third generation. Vaccine. 2005;365:362–363. doi: 10.1016/S0140-6736(05)17840-4. [DOI] [PubMed] [Google Scholar]
- Pozzi E., Basavecchia V., Zanotto C., Pacchioni S., De Giuli Morghen C., Radaelli A. Construction and characterization of recombinant fowlpox viruses expressing human papilloma virus E6 and E7 oncoproteins. J. Virol. Methods. 2009;158:184–189. doi: 10.1016/j.jviromet.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Radaelli A., Bonduelle O., Beggio P., Mahe B., Pozzi E., Elli V., Paganini M., Zanotto C., De Giuli Morghen C., Combadière B. Prime-boost immunization with DNA, recombinant fowlpox virus and VLP(SHIV) elicit both neutralizing antibodies and IFNgamma-producing T cells against the HIV-envelope protein in mice that control env-bearing tumour cells. Vaccine. 2007;25:2128–2138. doi: 10.1016/j.vaccine.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Radaelli A., Gimelli M., Cremonesi C., Scarpini C., De Giuli Morghen C. Humoral and cell mediated immunity in rabbits immunized with live non replicating avipox recombinants expressing the HIV-1sf2 env gene. Vaccine. 1994;12:1110–1117. doi: 10.1016/0264-410x(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Radaelli A., Nacsa J., Tsai W.P., Edghill-Smith Y., Zanotto C., Elli V., Venzon D., Tryniszewska E., Markham P., Mazzara G.P., Panicali D.L., De Giuli Morghen C., Franchini G. Prior DNA immunization enhances immune response to dominant and subdominant viral epitopes induced by a fowlpox-based SIVmac vaccine in long-term slow-progressor macaques infected with SIVmac251. Virology. 2003;312:181–195. doi: 10.1016/s0042-6822(03)00184-3. [DOI] [PubMed] [Google Scholar]
- Radaelli A., Pozzi E., Pacchioni S., Zanotto C., De Giuli Morghen C. Fowlpox virus recombinants expressing HPV-16 E6 and E7 oncogenes for the therapy of cervical carcinoma elicit humoral and cell-mediated responses in rabbits. J. Transl. Med. 2010;8:40. doi: 10.1186/1479-5876-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., Swain G.R., Olson V.A., Sargent E.K., Kehl S.C., Frace M.A., Kline R., Foldy S.L., Davis J.P., Damon I.K. The detection of monkeypox in humans in the Western Hemisphere. New Engl. J. Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Roberts K.L., Smith G.L. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008;16:472–479. doi: 10.1016/j.tim.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Rosel J.L., Earl P.L., Weir J., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the Hindlll H genome fragment. J. Virol. 1986;60:436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J.K., Mitra A.C., Mukherjee M.K. The minimum protective level of antibodies in smallpox. Bull. World Health Organ. 1975;52:307–311. [PMC free article] [PubMed] [Google Scholar]
- Schulze C., Alex M., Schirrmeier H., Hlinak A., Engelhardt A., Koschinski B., Beyreiss B., Hoffmann M., Czerny C.P. Generalized fatal Cowpox virus infection in a cat with transmission to a human contact case. Zoonoses Public Health. 2007;54:31–37. doi: 10.1111/j.1863-2378.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Smith G.L., Vanderplasschen A., Law M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Snyder J.T., Belyakov I.M., Dzutsev A., Lemonnier F., Berzofsky J.A. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J. Virol. 2004;78:7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprana E., Panigada M., Knauf M., Radaelli A., Vigevani L., Palini A., Villa C., Malnati M., Cassina G., Kurth R., Norley S., Siccardi A.G. Joint production of prime/boost pairs of Fowlpox virus and modified vaccinia Ankara recombinants carrying the same transgene. J. Virol. Methods. 2011;174:22–28. doi: 10.1016/j.jviromet.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Vaine M., Wang S., Hackett A., Arthos J., Lu S. Antibody responses elicited through homologous or heterologous prime-boost DNA and protein vaccinations differ in functional activity and avidity. Vaccine. 2010;28:2999–3007. doi: 10.1016/j.vaccine.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheust C., Goossens M., Pauwels K., Breyer D. Biosafety aspects of modified vaccinia virus Ankara (MVA)-based vectors used for gene therapy or vaccination. Vaccine. 2012;30:2623–2632. doi: 10.1016/j.vaccine.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Vogel S., Sardy M., Glos K.K.H.C., Ruzicka T., Wollenberg A. The Munich outbreak of cutaneous cowpox infection: transmission by infected pet rats. Acta Derm. Venereol. 2012;92:126–131. doi: 10.2340/00015555-1227. [DOI] [PubMed] [Google Scholar]
- Wang S., Parker C., Taaffe J., Solorzano A., Garcia-Sastre A., Lu S. Heterologous HA DNA vaccine prime–inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–3633. doi: 10.1016/j.vaccine.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin R., Liu J., Pugachev K.V., Myers G.A., Coughlin B., Blum P.S., Nichols R., Johnson C., Cruz J., Kennedy J.S., Ennis F.A., Monath T.P. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 2003;9:1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- Whitley R.J. Smallpox: a potential agent of bioterrorism. Antiviral Res. 2003;57:7–12. doi: 10.1016/s0166-3542(02)00195-x. [DOI] [PubMed] [Google Scholar]
- Wilton S., Gordon J., Dale S. Identification of antigenic determinants by polyclonal and hybridoma antibodies induced during the course of infection by vaccinia virus. Virology. 1986;148:84–96. doi: 10.1016/0042-6822(86)90405-8. [DOI] [PubMed] [Google Scholar]
- Wiser I., Balicer R.D., Cohen D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine. 2007;25:976–984. doi: 10.1016/j.vaccine.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Zanotto C., Pozzi E., Pacchioni S., Volonté L., De Giuli Morghen C., Radaelli A. Canarypox and fowlpox viruses as recombinant vaccine vectors: a biological and immunological comparison. Antiviral Res. 2010;88:53–63. doi: 10.1016/j.antiviral.2010.07.005. [DOI] [PubMed] [Google Scholar]


