Abstract
Genes of the Schlafen family, first discovered in mouse, are expressed in hematopoietic cells and are involved in immune processes. Previous results showed that they are candidate genes for two major phenomena: meiotic drive and embryonic lethality (DDK syndrome). However, these genes remain poorly understood, mostly due to the limitations imposed by their similarity, close location and the potential functional redundancy of the gene family members.
Here we use genomic and phylogenetic studies to investigate the evolution and role of this family of genes. Our results show that the Schlafen family is widely distributed in mammals, where we recognize four major clades that experienced lineage-specific expansions or contractions in various orders, including primates and rodents. In addition, we identified members of the Schlafen family in Chondrichthyes and Amphibia, indicating an ancient origin of these genes. We find evidence that positive selection has acted on many Schlafen genes. Moreover, our analyses indicate that a member of the Schlafen family was horizontally transferred from murine rodents to orthopoxviruses, where it is hypothesized to play a role in allowing the virus to survive host immune defense mechanisms. The functional relevance of the viral Schlafen sequences is further underscored by our finding that they are evolving under purifying selection. This is of particular importance, since orthopoxviruses infect mammals and include variola, the causative agent of smallpox, and monkeypox, an emerging virus of great concern for human health.
Abbreviations: OPV, orthopoxvirus; ORF, open reading frame; EST, expression sequence tags
Keywords: Schlafen, Poxvirus, Horizontal transfer, Immune system, Gene duplication, Om
1. Introduction
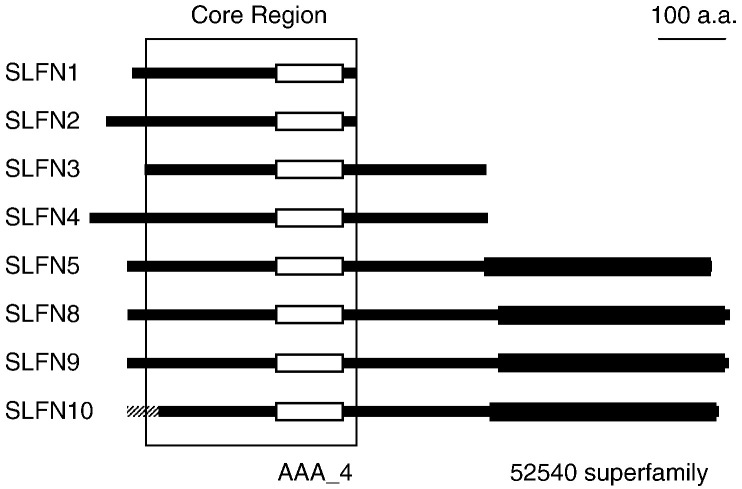
The Schlafen (Slfn) genes were first described in mouse as a family of genes that are transcribed during thymocyte maturation (Schwarz et al., 1998). Slfn1, 2, 3, and 4 share a common core region that contains a divergent AAA domain (AAA_4) that, presumably, has ATP binding activity. This region is also present in Slfn5, 8, 9, and 10, which code for longer proteins that contain motifs similar to the superfamily I helicases (Geserick et al., 2004) (Fig. 1 ).
Fig. 1.
SLFN proteins in mouse. The location of the common core region analyzed in our phylogenetic study is shown, as well as the AAA_4 (Pfam04326) and 52540 superfamily domains (P-loop containing nucleoside triphosphate hydrolases superfamily) (www.ensembl.org; Appendix A). The latter domain is present in DNA and RNA helicases and some other proteins. Slfn14 and LOC435271 are predicted genes without sufficient expression support (Table 1 and Appendix C) and, therefore, their products have not been represented. An alternative Slfn10 translation initiation site is indicated with a dashed line.
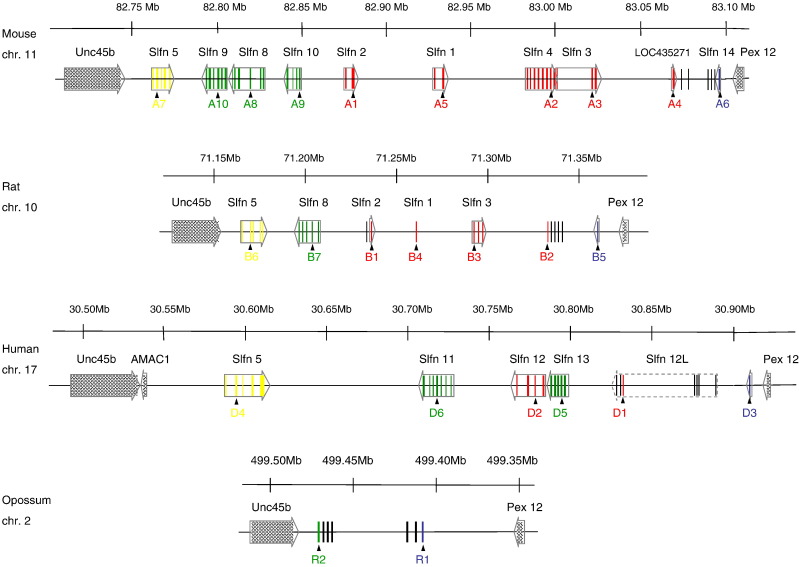
In mouse, the Slfn genes are clustered in a 350 kb interval of chromosome 11 (Fig. 2 ) within the Om (Ovum mutant) candidate region. We and others have mapped two interesting phenomena to this region: DDK syndrome of embryonic lethality and meiotic drive. In crosses involving the DDK inbred strain, early embryonic lethality occurs when a maternal cytoplasmic factor present in DDK oocytes interacts with a paternal gene provided by the sperm of other mouse strains (Wakasugi, 1974, Mann, 1986, Renard and Babinet, 1986, Gao et al., 2005). Both the maternal and paternal loci responsible for this DDK syndrome map to the Om region (Baldacci et al., 1992, Sapienza et al., 1992, Pardo-Manuel de Villena et al., 1997, Pardo-Manuel de Villena et al., 1999, Bell, 2006). Meiotic drive in the Om region occurs due to unequal segregation of chromatids during the second meiotic division in mouse eggs (Pardo-Manuel de Villena et al., 1997, Pardo-Manuel de Villena et al., 2000a, Pardo-Manuel De Villena et al., 2000b, De la Casa-Esperon, 2002).
Fig. 2.
Orthologous regions in mouse, rat, human and opossum that contain the Slfn genes. Indicated are the Ensembl annotated Slfn genes and the related sequences found in our search, their exons, and direction of transcription when expression support was found (Appendix A). The location of the sequences identified and used in our phylogenetic analyses (Fig. 4, Fig. 5) is indicated below with arrows. Colors indicate their relationships according to our phylogenetic results (red = Group 1; yellow = Group 2; blue = Group 3; green = “Group 4”; black = unidentified). The position of the human Slfn12L predicted transcript is indicated, although the dashed arrow box also encompasses several additional sequences that are not included in the prediction but that we subsequently identified as Slfns (Appendix A). Opossum R2 sequence was not included in the phylogenetic analysis (see Methods), but our analyses indicate that it is more similar to Slfn8, 9 and 10 (Group 4) sequences than to any other mouse sequences (data not shown).
In addition, several studies support a role of Slfn members in immune response. Slfn genes are expressed in tissues of the immune system and their expression levels vary during T-cell and macrophage development, as well as in response to infections (Schwarz et al., 1998, Geserick et al., 2004, Lund, 2006, Sohn, 2007). The Slfn genes are located in a region that has been associated with several autoimmune disorders in human and mouse (Fujikado et al., 2006, Griffiths et al., 1999, Wandstrat and Wakeland, 2001). Both Slfn1 and Slfn8 transgenic mice show a reduction in thymus size and thymocyte proliferation (Schwarz et al., 1998, Geserick et al., 2004). These and other results, especially the ectopic expression of Slfn1 in mouse fibroblasts, suggest a role in inhibition of cell growth (Schwarz et al., 1998, Brady et al., 2005, Zhang, 2008, Patel, 2009), although this antiproliferative activity is neither shared by all Slfn genes (Geserick et al., 2004, Lee et al., 2008) nor confirmed by other studies (Zhao et al., 2008). The only knock-out studied (that of Slfn1 [Schwarz et al. 1998]) showed no apparent phenotype, suggesting functional redundancy among Slfn family members.
Therefore, selective forces related to embryonic lethality (DDK syndrome), meiotic drive at Om, and immune response might have shaped the evolution of the Slfn genes. However, the role of each of the Slfn genes in these processes is still unclear. The chromosomal proximity and high degree of sequence similarity of the Slfn genes, as well as their possible functional redundancy, are major obstacles to functional studies. In addition, several sequences within the Om region show similarities to the described Slfn genes, but have not been systematically annotated and analyzed. Our knowledge of the Slfn genes is quite limited, since only a few studies have been conducted in any vertebrate species other than mouse.
To address these issues, we investigated the diversity, origin and evolution of the Slfn gene family, incorporating data from Mus musculus and expanding our survey and comparative analyses to other organisms. We also tested for the possible role of selection in the evolution of these genes. Our results show that the Slfn family has a broad but patchy phylogenetic distribution in vertebrates, and has expanded (or contracted) independently several times in mammals within the same orthologous region. Moreover, our results suggest that one of these genes was horizontally transferred from rodents to orthopoxviruses (OPVs) and appears to be functional in some of them. These viruses infect mammals and include variola, the causative agent of smallpox, vaccinia (the vaccine virus used to eradicate smallpox), and monkeypox, which has been responsible for recent outbreaks of human illness (http://www.cdc.gov/ncidod/monkeypox/). We hypothesize that, in OPVs, Slfns might play an important role in eluding the host immune system during the infectious processes, as well as in the viral host specificity.
2. Methods
2.1. Identification of Schlafen sequences in diverse organisms
Mouse, human, opossum and rat Slfn sequences were obtained from Ensembl (Appendix A) and aligned using ClustalW2 (Larkin et al., 2007). Additional, non annotated Slfn related sequences were obtained from these mammalian genomes by blastn and tblastn alignment with annotated Slfn sequences (Appendix A). The 329 amino acid portion of Slfn2 corresponding to the region of greatest similarity (positions 82882846–82883832 on mouse chromosome 11 in the m37 genome assembly) was used as the query for searches with tblastn (Altschul et al. 1997) of the NR, WGS, HTGS, and EST NCBI databases. Sequences were retrieved with a threshold of e < 0.01 (Appendix B).
Slfn sequences were searched in orthopoxviruses by aligning all mouse Slfn sequences with representative strains of the nine sequenced OPV species (NC_003663.2 (cowpox), NC_004105.1 (ectromelia), NC_003310.1 (monkeypox), NC_003391.1 (camelpox), NC_008291.1 (taterapox), NC_006998.1 (vaccinia), NC_001611.1 (variola), AY484669.1 (rabbitpox), DQ792504.1 (horsepox)). Significant hits (e < 0.01) were almost identical to the viral sequences obtained by the above approach (Appendix B), except for a few extra nucleotides (Appendix D).
2.2. Mouse Schlafen expression analysis
Mouse EST and RNA-containing NCBI databases were searched by blastn (Altschul et al. 1997) with the identified mouse Slfn nucleotide sequences. We established a lower limit of 97% identity in order to account for the high error rate of EST sequences (Nishikawa and Nagai 1996) and hits were manually inspected in order to unequivocally ascribe each of the retrieved RNA sequences to a single Slfn gene (Appendix C).
2.3. Phylogenetic analyses
Slfn amino acid sequences (see section 2.1) were aligned with ClustalW2 (Larkin et al., 2007) and edited with GENEDOC (http://www.psc.edu/biomed/genedoc). Regions of ambiguous alignment and very short sequences were excluded from the analyses, and a few sequences were manually edited in order to reconstruct ORFs (Appendix B). We obtained an unrooted neighbor-joining phylogram using MEGA 3.1 (Kumar et al., 2004) and employing the equal input model for amino acid evolution, which corrects for variation in amino acid frequency. Node support was assessed by conducting 5000 nonparametric bootstrap pseudoreplicates.
Bayesian reconstruction of Slfn gene phylogeny was performed using MrBayes v. 3.1 (Ronquist and Huelsenbeck, 2003). We used flat priors and applied the “mixed” model for amino acid sequences, under which MrBayes examines trees generated based on a wide range of models of protein evolution. Two independent runs with four Markov chains each were conducted for three million generations, with sampling at every 100th generation. When the standard deviation of split frequencies was < 0.02, we considered the searches within a run to have converged, and we discarded the first 25% of trees as “burn-in”. The Bayesian tree shown here represents the majority rule consensus of the last 2.25 million generations from each of the two separate runs combined (4.5 million generations total).
2.4. Codon evolution analysis
For each Slfn group identified by phylogenetic analysis, amino acid sequences encoded by Slfn genes from human, chimp, macaque, dog, horse, cow, mouse, rat and opossum (or the subset of these taxa present in each group) were aligned using MAFFT version 6 (Katoh and Toh 2008), as were viral Slfn (v-slfn) sequences from phylogenetic Group 1. These multialignments were used to obtain the corresponding codon alignment with PAL2NAL (Suyama et al., 2006). The program CODEML in the suite PAML 4 (Yang 2007) was used to test Slfn genes and v-slfn sequences for adaptive evolution under three codon substitution models (see details in Appendix E).
The “branch” models were used to test whether v-slfn sequences are evolving neutrally or under purifying selection. Two sets of “site” models (M1a-M2a and M7-M8) were used to detect specific sites under positive selection. Finally, the “branch-site” models allowed inspection of specific lineages for sites showing evidence of adaptive evolution. A likelihood ratio test (LRT) was used to compare models in each test. Phylogenetic trees were edited using MEGA 3.1 (Kumar et al., 2004).
3. Results
3.1. Genomic clustering of the Schlafen genes in mouse and expression in immunity-related tissues
In mouse, eight Slfn genes have been annotated within a cluster in chromosome 11 (www.ensembl.org; Fig. 2; Appendix A). They are flanked by the Unc45/Unc45b and Pex12 genes, which are involved in myosin folding and peroxisome biogenesis, respectively (Chang et al., 1997, Srikakulam et al., 2008). The Slfn genes range in size from 992 to 23,806 nucleotides and encode putative proteins that range in size from 337 to 910 amino acids and display 31–92% identity and 51–96% similarity over 312–331 amino acids. These proteins share an AAA_4 domain that is 101 amino acids in size and is located within the most conserved region (Fig. 1).
To identify new mouse Slfn family members, the m37 genome assembly was searched using blastn and tblastn with sequences of the eight known mouse Slfns. We found four additional sequences (42–88% identity, 62–92% similarity to previously described Slfn sequences over 334–499 amino acids), all clustered within the Om region and flanked by the Unc45/Unc45b and Pex12 genes (Appendices A and B, and Fig. 2). One overlaps with sequences encoding the SLFN14 predicted protein (XP_899217 in Gubser et al., 2007). The other three are most likely fragments of a single duplication of a gene related to Slfn4/Slfn3 (see section 3.2) and two overlap with the predicted gene LOC435271 (Bell et al., 2006). We excluded a sequence annotated as Slfn-like 1 on chromosome 4, which contains a partial AAA_4 domain, because of the extremely low similarity to and phylogenetic distance from Slfn genes (data not shown). Therefore, we conclude that a total of 10 “bona fide” Slfn members are present in the m37 mouse sequence.
Our analysis of available expression data (ESTs) for the annotated mouse Slfn genes (Table 1 ; Appendix C) confirms previous reports showing that the Slfn genes are expressed in (but not necessarily restricted to) cells of the immune system (Schwarz et al., 1998, Geserick et al., 2004). This is also the case for the human Slfn genes (data not shown). Moreover, expression of some Slfn members in reproductive organs, oocytes and early embryos is suggestive of their possible role in DDK syndrome of embryonic lethality and/or meiotic drive at Om (Mann, 1986, Renard and Babinet, 1986, Pardo-Manuel de Villena et al., 2000a). We found a few EST sequences that support transcription of some, but not all, the newly identified Slfn sequences. As observed in mouse, expression of the MM_A6/Slfn14 rat and human orthologs (section 3.2) was represented by a few transcripts (data not shown).
Table 1.
Expression of Slfn sequences in mouse.
| Sequence | Expression in immunity-related tissues | Expression in other or mixed tissues |
|---|---|---|
| Slfn1 | Hematopoietic stem cells, thymus, spleen, T-cells, bone marrow, lymph nodes, macrophages | Adipose tissue, aorta and veins, spinal cord |
| Slfn2 | Thymus, spleen, CD11 +ve dendritic cells, hematopoietic stem cells, macrophages | Tumors, mammary gland, lung, 19.5 dpc fetus, joints, synovial fibroblasts, inner ear, taste buds |
| Slfn3 | Thymus, CD11 +ve dendritic cells, spleen | Testis, melanoma/melanocyte cells |
| Slfn4 | Spleen, bone, lymph nodes, hematopoietic stem cells | Vagina, embryo, mammary and lung tumors, taste buds unfertilized eggs, 8-cell embryos |
| Slfn5 | Thymus, spleen, activated macrophages, bone | Lung, kidney, embryonic Rathke's pouches, testis |
| Slfn8 | Macrophages, CD11 +ve dendritic cells, spleen | Kidney |
| Slfn9 | Hematopoietic stem cells, activated macrophage, bone | Embryonic nasal region, tumors, prostate, eye, 6.5-dpc to 14-dpc embryos, mammary gland, ES cells |
| Slfn10 | Activated macrophage, thymus, hematopoietic stem cells | Pancreatic islet, lung, mammary tumor |
| 83067775–83068818 (within LOC435271) | CD11 +ve dendritic cells, | |
| Slfn14, 1st exon | Spleen |
Summary of the expression results obtained by BLAST search of NCBI databases (Appendix C). No transcripts were found for the rest of the Slfn14 predicted exons as well as for Slfn sequences in mouse m37 chromosome 11 positions 83,073,547–83,074,000 and 83,078,898–83,079,059 (Fig. 1 and Appendix A). In the latter case, the reading frame was disrupted.
3.2. Phylogenetic distribution of the Schlafen family: expansion within a cluster in mammals
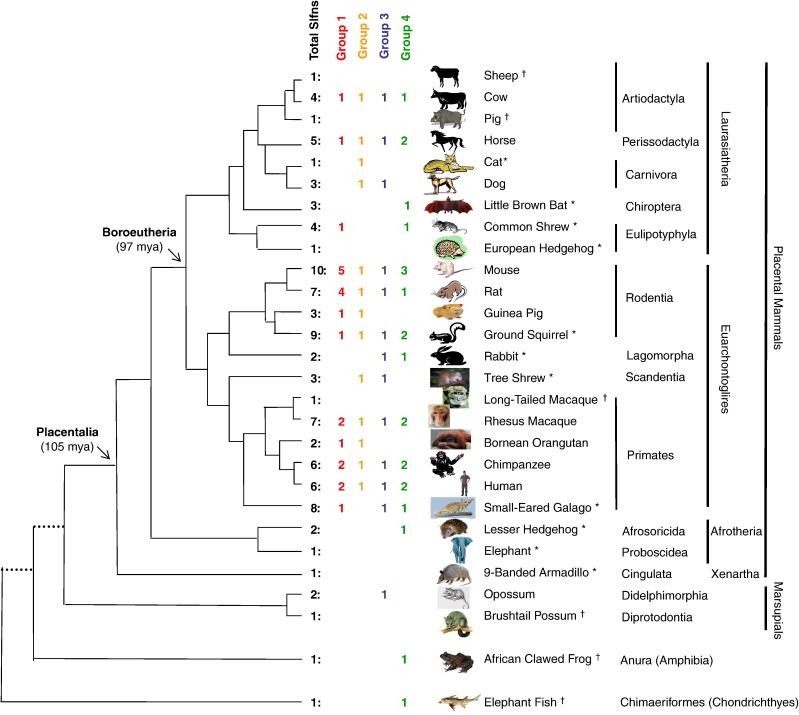
To explore the distribution and evolution of the Slfn family, we searched for orthologs/paralogs of mouse Slfn genes in other species, using the core region shared by all SLFN proteins (Fig. 1) as a query to search all databases. Putative genes related to Slfn were identified in 26 animal genomes in addition to mouse (Fig. 3 ; Appendix B). Pairwise comparisons to mouse Slfns and phylogenetic analyses show clearly that they are members of the Slfn family (Fig. 4, Fig. 5 ). Slfn genes were identified in every mammalian genome with at least 2× coverage (except platypus). In addition, single copies of Slfn genes were identified in the African clawed frog Xenopus laevis and the elephant “fish” Callorhinchus milii (a chondrichthyan, representing the deepest split in the phylogeny of living gnathostomes). No Slfn genes were identified in any other non-viral genome. However, Slfn-related sequences were found in the genomes of orthopoxviruses (Appendix B; see section 3.3).
Fig. 3.
Distribution of Schlafen sequences among taxa. The topology and divergence times of the mammalian tree are drawn according to www.tolweb.org/tree and Murphy et al. (2007). The total numbers of Slfn sequences for each taxon are indicated, as well as the number of Slfn sequences that fell within each of the four groups identified in our phylogenetic analyses (Fig. 4, Fig. 5). Only sequences that were suitable for our phylogenetic analyses (see Methods) could be classified into one of the 4 groups. Genome sequencing status at the time of this analysis: ⁎2× (or less) whole genome shotgun; †underway, sequencing projects at early stages.
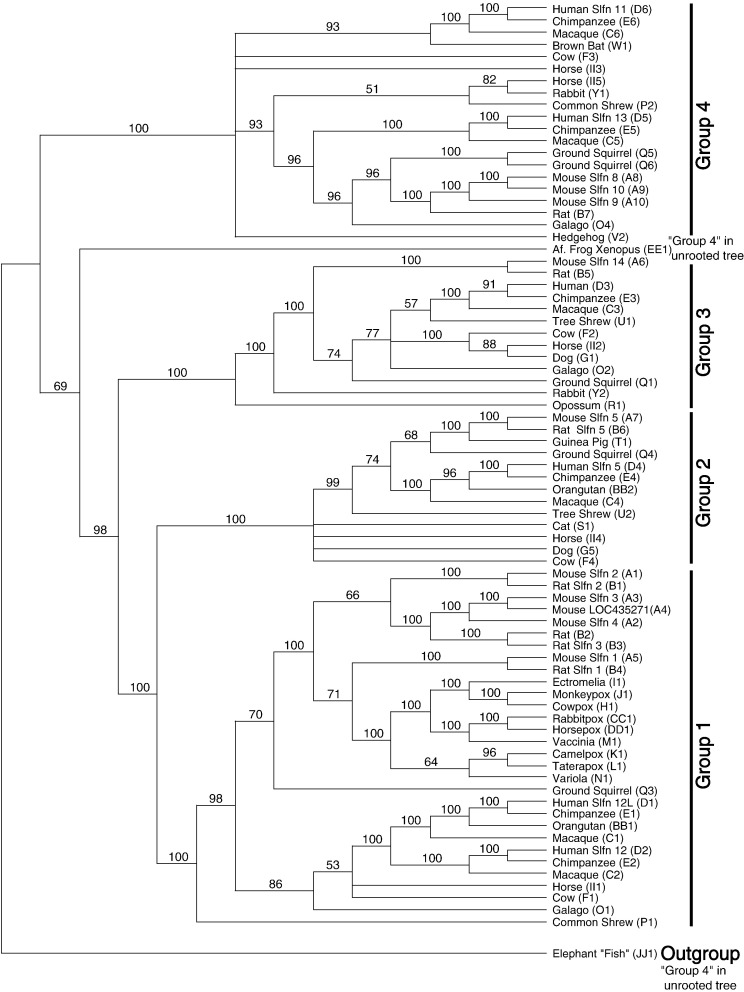
Fig. 4.
Phylogeny of Slfn genes based on neighbor-joining analysis of amino acid sequences. Only sequences that aligned unambiguously to the mouse SLFN core region (Fig. 1) were analyzed. This is an unrooted phylogram obtained using the “equal input” model implemented in MEGA 3.1 (Kumar et al., 2004). We designate four major clades ”Groups 1–4”. The pink arrow indicates the hypothesized occurrence of horizontal transfer of a Slfn gene from a rodent to the OPV ancestor. Numbers at nodes represent bootstrap support values based on 5000 pseudoreplicates. ⁎ indicates 70% or above bootstrap support (Hillis and Bull 1993). Values below 50% were removed. Scale: 0.1 indicates 10% estimated number of substitutions per position.
Fig. 5.
Phylogeny of Slfn genes based on Bayesian analysis of amino acid sequences. The tree is rooted using the sequence from “elephant fish” (chondrichthyan). Numbers at nodes represent posterior probabilities. Groups 1–4 (designated based on the unrooted neighbor-joining tree, Fig. 4) are indicated. Results from 4.5 million generations run under the “mixed” (multiple model) criterion for evolution are shown.
?>In all mammalian genomes with high-quality coverage and assembly, we observed that Slfn genes form a cluster of paralogs within the same orthologous region flanked by the Unc45 and Pex12 genes, as we previously determined in mouse. The organization of this cluster has been further investigated in the genome of mouse, rat, human and opossum (Fig. 2; Appendix A). In these species, the Slfn cluster appears to have been shaped by multiple tandem duplication events and in some cases by lineage-specific inversions (Fig. 2).
Our neighbor-joining tree including all Slfn amino acid sequences indicates that Schlafen members cluster in four major lineages, with strong bootstrap support (80%–98%) for three of them (Fig. 4; Appendix B). The unrooted topology of the Bayesian tree (Fig. 5) is identical in nearly all major respects, with extremely strong posterior probability support for all key nodes. We arbitrarily designated the focal clades as Groups 1–4, with the caveat that the root of the tree is unknown (i.e., at least one of the groups is likely to be paraphyletic with respect to the true outgroup sequence). Given the generally accepted basal split between Chondrichthyes and all other living jawed vertebrates, we consider the most plausible root to lie on the branch between the Slfn from “elephant fish” and the others, and rooted the Bayesian tree accordingly. Under this rooting, elephant fish obviously is removed from “Group 4”, and the Slfn from the frog Xenopus appears as sister to those comprising groups 1+2+3 (rooting the N-J tree with elephant fish would place the Xenopus Slfn sister to Group 4), while in all other respects the groups that we recognize are monophyletic.
The major Slfn groups that we recognize appear to have originated by gene duplications (Fig. 3, Fig. 4, Fig. 5). At least two of these groups (1 and 4) have expanded recently in some mammalian lineages by additional gene duplications (based on the phylogenies shown in Fig. 4, Fig. 5 and copy number). Interestingly, group 1 also contains the viral copies (v-slfn).
3.3. Evolutionary origin of the Schlafen-related genes in orthopoxviruses: horizontal transfer from rodents
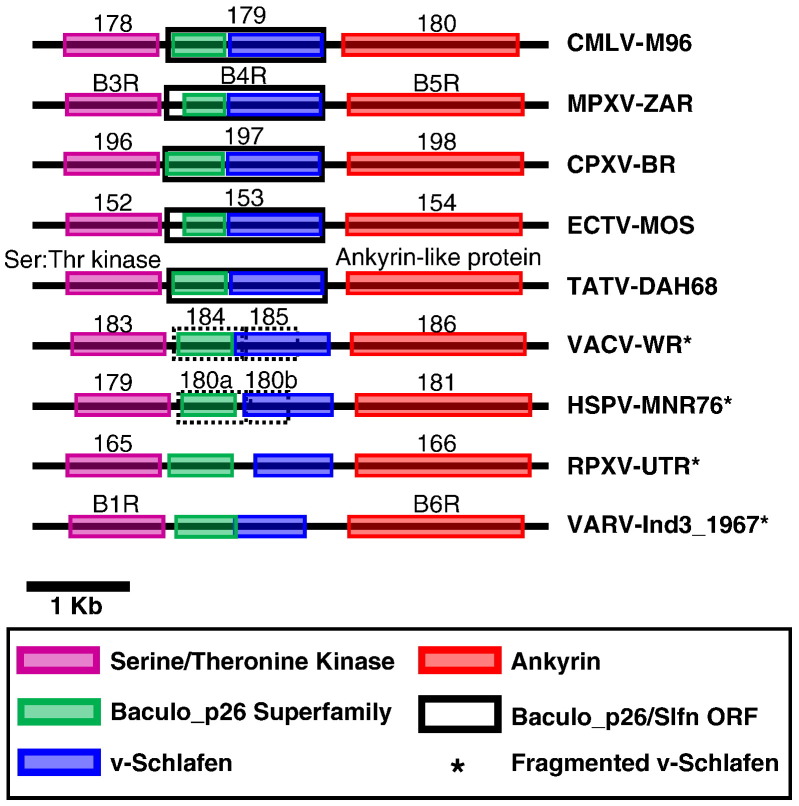
Although the presence of Schlafen sequences in OPVs (v-slfn) has previously been reported (Schwarz et al., 1998, McLysaght et al., 2003, Gubser, 2007), their evolutionary origin was unclear. We have identified v-slfn sequences in all OPVs for which data are available, but not in any other viruses, including other members of the poxvirus group. Only one v-slfn sequence is found in each OPV (Appendices B and D); v-slfn sequences are located in orthologous positions in all OPV genomes (Fig. 6 ) and share 84–99% sequence similarity over 299–308 amino acids. Some, but not all OPVs (such as variola), have retained an open reading frame (ORF). This ORF extends at the 5′ end to include a sequence that encodes for a baculovirus p26 domain of unknown function (Goenka and Weaver 2008) (Fig. 6).
Fig. 6.
v-slfn sequences in orthopoxviruses. A representative strain of each OPV species is depicted. v-slfn and surrounding genes are annotated according to www.poxvirus.org. v-slfn is flanked by sequences that code for a serine/threonine kinase and an ankyrin protein in all OPVs. The predicted v-slfn ORFs (www.poxvirus.com) often include non-Slfn sequences that code for a baculovirus p26 protein domain. The v-slfn sequences of all OPVs (Appendix D), as well as all the other sequences represented, were identified by BLAST search by using all mouse SLFN and the camelpox sequences as queries, respectively.
Our phylogenetic analyses show that the amino acid sequences of the viral Slfn genes are most closely related to the mouse and rat Slfn genes of our Group 1 (Fig. 4, Fig. 5). Although it is possible that Slfn genes are derived from a viral sequence, several lines of evidence argue against this scenario. First, v-slfn sequences have a very restricted distribution in viruses, whereas the Slfn family is widely present and diversified in multiple clades in mammals and other vertebrates, with v-slfn deeply nested within only one of these lineages (Fig. 4, Fig. 5). Furthermore, the Slfn group containing viral sequences (Group 1) is unlikely to be the most ancient Slfn clade, as it does not include genes from more “basal” vertebrates like the chondrichthyan elephant “fish” and the frog X. laevis, which are instead located deeper in the rooted tree (Fig. 4, Fig. 5). Finally, an independent phylogenetic analysis of sequences containing the AAA_4 domain from eukaryotes and prokaryotes supports that OPV sequences cluster within the Slfn Group 1 genes of mammals (http://www.ebi.ac.uk/goldman-srv/pandit/pandit.cgi?action=browse&fam=PF04326). Therefore, it is most likely that Slfn sequences were horizontally transferred from a rodent to an OPV and subsequently have diverged to give rise to the v-slfn genes.
3.4. Selection on the vertebrate and viral Schlafen coding sequences
Many genes involved in the immune response are known to evolve under positive selection (Waterston, 2002, Ellegren, 2008). To determine whether the Slfn family has been evolving adaptively, we focused on the high coverage genomes of mouse, rat, human, chimp, macaque, dog, cow, horse and opossum. The ratio between non-synonymous and synonymous substitutions per site, or dN/dS, was used as a proxy to infer how selection shaped the coding region of Slfn genes; values of dN/dS higher than one are interpreted as a signature of positive selection (Hill and Hastie, 1987, Hughes and Nei, 1988). The CODEML program of the PAML package (Yang 2007) was used to estimate dN/dS in our datasets (see Methods and Appendix E). A strong signature of positive selection was identified in all Slfn groups by at least one set of CODEML models (Table 2, Table 3 ; Appendix E). Genes in Slfn group 4 seem to have been particularly affected by episodes of adaptive evolution, with 39 different codons estimated by the various CODEML models as having dN/dS significantly > 1 (p < 0.05; Table 2, Table 3; Appendices E–F).
Table 2.
Positive selection detected using CODEML “site” models on Slfn lineages.
| Model | ω | Sites | |
|---|---|---|---|
| Group 1 | M7 | 0.568 | 1⁎ |
| M8 | 0.665 | ||
| Group 2 | M1a | 0.588 | – |
| M2a | 0.725 | ||
| Group 2 | M7 | 0.589 | 1⁎ |
| M8 | 0.712 | ||
| Group 3 | M7 | 0.334 | – |
| M8 | 0.357 | ||
| Group 4 | M1a | 0.513 | 13⁎+4⁎⁎ |
| M2a | 0.793 | ||
| Group 4 | M7 | 0.512 | 29⁎+4⁎⁎ |
| M8 | 0.771 |
Major Slfn lineages showing significant (p value < 1%) evidence of positive selection. Complete results are shown in Appendix E, Table 1, Table 2. M1a–M2a and M7–M8 are “site” models. ω: estimated average dN/dS value of the tree. Sites: Positively selected sites according to the Bayes Empirical Bayes analysis. ⁎: Significant test (p value < 5%). ⁎⁎: extremely significant test (p value < 1%).
Table 3.
Positive selection detected using CODEML “branch-site” models on Slfn subtrees.
| Model | ω | Sites | |
|---|---|---|---|
| Group 2 | Null | 1 | 1⁎+1⁎⁎ |
| WG | Alternative | 1.5 | |
| Group 4 | Null | 1 | 14⁎+2⁎⁎ |
| WG | Alternative | 1.5 | |
| Group 4 | Null | 1 | 3⁎ |
| R | Alternative | 1.5 | |
| Group 4 | Null | 1 | 2⁎+2⁎⁎ |
| P+L | Alternative | 1.5 | |
| Group 4 | Null | 1 | – |
| P+L Slfn11 | Alternative | 1.5 | |
| Group 4 | Null | 1 | – |
| P+L Slfn13 | Alternative | 1.5 |
Major Slfn lineages showing significant (p value < 1%) evidence of positive selection. Complete results are shown in Appendix E, Table 1, Table 2, Table 3. Single-branch analyses are reported only in Appendix E. Null and Alternative refer to “branch-site” models also known as “test 2”. ω: initial dN/dS value. Sites: Positively selected sites according to the Bayes Empirical Bayes analysis. ⁎: Significant test (p value < 5%). ⁎⁎: extremely significant test (p value < 1%). WG: whole-group; R: rodents; P: primates; L: laurasiatheria (dog, horse and cow).
Using “branch-site” codon substitution models (Zhang et al., 2005, Yang, 2007; see also Methods), we found that different Slfn gene groups in several mammalian lineages (called subtrees in Table 3 and Appendices E–F) have been evolving under positive selection (Appendix E, Table 3; Appendix G). To determine if paralogous lineages have experienced different selective regimes after gene duplication, we applied “branch-site” models to test single paralogous branches in Slfn groups 1 and 4 (Appendices E and G). This analysis identified six branches with dN/dS significantly > 1, although in five of these cases only one of the two paralogous lineages experienced positive selection (Appendix E, Table 4 ; Appendix G, Fig. 1, Fig. 2, Fig. 3, Fig. 4).
Table 4.
Evolutionary hypotheses tested on v-slfn sequences.
| Hypotheses | Model | ω0 | ω1 | ω2 | 2⁎(ΔlnL) |
|---|---|---|---|---|---|
| Positive selection | M1a | 0.514 | – | – | 0.86 |
| “site” models | M2a | 0.564 | – | – | (rejected) |
| Positive selection | M7 | 0.507 | – | – | 0.88 |
| “site” models | M8 | 0.563 | – | – | (rejected) |
| Positive selection on the v-slfn clade | Null | 1 | – | – | 0 |
| “branch-site” models | Alternative | 1.5 | – | – | (rejected) |
| v-slfn clade is evolving neutrally | Two ratios | 0.609 | 0.3524 | – | 70.1⁎ |
| One ratio | 0.586 | 1 | – | (rejected) | |
| v-slfn pseudogenes are NOT evolving neutrally | Two ratios | 0.43 | 1.0354 | – | 0 |
| One ratio | 0.43 | 1 | – | (rejected) | |
| v-slfn genes are NOT evolving neutrally | Three ratios | 0.613 | 1.1558 (P) | 0.2964 (G) | 80.36⁎ |
| Two ratios | 0.586 | 0.8987 (P) | 1 (G) | (accepted) | |
Parameters and models associated with some CODEML tests performed on v-slfn sequences. Tests for positive selection involved only the nine v-slfn sequences. The last three hypotheses were tested comparing a model of neutrality (ω0 or ω1 = 1) with an alternative model where ω is estimated, and were performed on a tree also including the three mouse genes Slfn1–3 as outgroups. Such models were applied to the v-slfn whole clade, v-slfn pseudogenes and v-slfn genes. Tested hypotheses are showed in the first column. ω0: estimated or initial dN/dS in positive selection tests, background dN/dS in the other tests; ω1: estimated or fixed second value dN/dS; ω2: estimated or fixed third value dN/dS; 2⁎(ΔlnL): twice the log likelihood difference between null and alternative models; P: dN/dS of v-slfn pseudogenes; G: dN/dS of v-slfn genes; the asterisk in the last column marks tests with p value < 1%. Further details and other tests are described in the text and Appendices E and H.
We also tested for selection during the evolution of OPV v-slfn sequences. McLysaght et al. (2003) inferred positive selection in v-slfn genes by comparing CODEML models M7 and M8. However, we found no evidence of positive selection among nine OPV v-slfn sequences (Appendix E, Table 1, Table 2, Table 3), or on a smaller data set without the four v-slfn sequences that lack ORFs and likely represent pseudogenes (data not shown).
Analyses using the “branch” CODEML models (see Methods; Table 4 and Appendix H) showed that the four v-slfn pseudogenes are evolving neutrally, whereas the five v-slfn sequences with an intact ORF have an average dN/dS = 0.2964, suggesting that the latter are evolving under purifying selection (Table 4 and Appendix H).
4. Discussion
4.1. Distribution and phylogeny of the Schlafen genes
Since the Slfn genes were discovered in M. musculus, most studies in animals have been performed in this species in spite of the poor annotation of the members of this gene family. Our comprehensive analysis has confirmed the existence of the eight annotated genes in mouse, plus two additional Slfn copies that include some sequences of two annotated gene predictions (Fig. 2). However, given our observation of rapid evolution of this gene family in rodents, future studies in mice and rats must be performed with caution, because the number of Slfn copies might not be fixed (Fig. 4, Fig. 5; Bell et al., 2006).
Across a much broader phylogenetic spectrum, the number of Slfn sequences within species is highly variable. The Slfn genes are widespread in placental mammals, as well as present in opossum, a few other vertebrates including the deeply diverged Chondricthyes and Amphibia (Fig. 3), and OPVs. In mammals for which genome sequences with high coverage are available, they are most abundant in primates and rodents, and in all cases studied they are clustered in tandem within an orthologous region. No Slfn has been identified by our approach in monotremes and reptiles (including birds); in amphibians we found Slfn in Xenopus laevis but not in X. tropicalis. The only other non-mammalian vertebrate for which genome data are available that shows the presence of a Slfn is the chondrichthyan Callorhinchus milii (“elephant fish”). Since few complete genomes of non-mammalian vertebrates are currently available, we focus our analyses on mammals, where the Slfn genes clearly have retained and/or acquired important, yet mostly unknown, functions.
Phylogenetic analysis of Slfn genes reveals four distinct lineages, which appear to have experienced multiple duplications (and potentially losses) in mammals (Fig. 4, Fig. 5). Our “Group 4” (sensu lato) shows the widest phylogenetic distribution, with members occurring not only in placental mammals but also in two other vertebrates, and therefore it could represent the sister lineage to other Slfn groups. Our “most plausible” rooting (with the chondrichthyan Slfn) removes non-mammalian Slfns from our grouping scheme but still shows a basal split between mammalian Group 4 Slfns and those of amphibians and mammalian Groups 1–3, with the ancestor of Groups 1–3 having originated most recently. Given our evidence of recent duplications in mouse and rat, the Slfn genes probably are still rapidly evolving in some rodents. This agrees with previous evidence that in mouse the Slfn cluster is located in an unstable and rapidly changing region (Bell et al., 2006). Rapid evolution is also often observed in other gene families involved in immune function, both in terms of nucleotide substitution and gene gain/loss rates (Demuth et al., 2006, Ellegren, 2008).
Analyses of mouse SLFN proteins' length, domains, function, and cellular location have shown that members of the Group 1 identified in our phylogenetic studies (Fig. 4, Fig. 5) have some distinct features respect to the other groups. Mouse Slfn1–4 (Group 1) code for short proteins with reported antiproliferative activity (Schwarz et al., 1998), which has not been observed in larger members of the family that contain helicase-related domains (Slfn5 8, 9 and 10, located in our phylogenetic Groups 2 and 4) (Geserick et al., 2004). In addition, expression of FLAG-tagged Slfn genes showed that the SLFN1, 2, and 4 proteins (Group 1) are mainly or exclusively localized in the cytoplasm, whereas SLFN5, 8 and 9 (Groups 2 and 4) products are only observed in the nucleus (Neumann et al., 2008).
4.2. Horizontal transfer of a Schlafen sequence to orthopoxviruses and its implications
Our data provide important clues about the origin of the v-slfn genes in OPVs. We observed a single copy in all sequenced OPVs, but none in any other virus, including other poxviruses. OPVs are genetically, morphologically, and antigenically similar viruses that form a monophyletic group and infect mammals. They include variola (the causative agent of smallpox), vaccinia (used in vaccination against smallpox), and monkeypox (which is of major concern as an emerging, rodent-borne pathogen). Our phylogenetic analysis strongly supports horizontal transfer of Slfn sequences from a mammal (probably a rodent) to an OPV. Although the possibility of recombination among OPVs cannot be eliminated, the location of v-slfns at orthologous positions, the high number of substitutions along the ancestral branch of the viral sequences (Fig. 4; Appendix E), their high degree of sequence similarity, and the very strong support for their monophyly indicates that Slfn sequences were probably acquired only once by the progenitor of OPVs (see also Gubser et al., 2004, Hughes and Friedman, 2005, Lefkowitz et al., 2006, Bratke and McLysaght, 2008). Once acquired, v-slfn sequences may have been retained due to selective advantages and/or to the relative flexibility in genome size of the poxviruses, which have fewer packaging restrictions than many other viruses and possess other horizontally transferred genes (Smith and Moss, 1983, Moss and Shisler, 2001). Five of the nine OPV v-slfns that we examined have conserved an ORF and appear to be undergoing purifying selection. The other four seem to have lost function. Gubser et al. (2004) postulated that the presence of genes that conserve an ORF in some OPV genomes, but are fragmented in others, might reflect a recent radiation of OPVs from a common ancestor (i.e., the pseudogenes have not yet been completely lost). Regardless of the age of the OPV group, our observation of purifying selection on v-slfns in several members suggests a functional role.
What is the function of v-slfn sequences in orthopoxviruses? Horizontal transfer of host immune-related genes to viruses and selection on such genes to allow viruses to escape the host immune system has been reported repeatedly (Hughes, 2002, Shchelkunov, 2003, Hughes and Friedman, 2005, Bratke and McLysaght, 2008). Diverse poxviruses have different immune evasion genes, some of which may have been acquired during host adaptation of the virus (Moss and Shisler 2001). Like many of the genes involved in virulence and modulation of immune response, v-slfns are located in the terminal regions of OPV genomes (Seet et al. 2003), further suggesting the role of OPV v-slfn sequences in eluding the host immune system.
While the ancestral OPV probably acquired Slfn sequences from a rodent, only modern OPVs that conserve an intact v-slfn ORF infect rodents (OPVs with fragmented v-slfns have other natural hosts). This further suggests that v-slfns participate in the OPVs' ability to infect rodents. This has important implications, since the spread of smallpox by the variola virus was eradicated by human vaccination because no other animal was infected. Variola v-slfn is fragmented, but the monkeypox v-slfn has an ORF. Although human-to-human transmission efficiency of monkeypox is low, any major outbreak that could result from changes in this virus would be very difficult to control, because many rodents are natural reservoirs (Shchelkunov 2003).
4.3. Selection regime operating during the evolution of the Schlafen genes
We tested whether there is any sign of positive selection of the Slfn sequence captured by OPVs, as found by McLysaght et al. (2003). We found no evidence of positive selection in any of our analyses (Appendices E and H). This discrepancy could be the result of the differences between the two studies: the dataset analyzed by McLysaght et al. (2003) included less viral species and different variants of v-slfn pseudogenes from several variola and vaccinia strains, compared to ours. In addition, their use of predicted ORFs of v-slfn pseudogenes (www.poxvirus.org) resulted in shorter alignments. Moreover, the analysis of McLysaght et al. (2003) was focused on poxviruses and ignored the rodent origin of v-slfns.
Our data suggest that the v-slfn genes with an intact ORF are mostly evolving under purifying selection, although we cannot exclude the possibility that some sites in these sequences have been evolving under positive selection. Regardless of the nature of selection (for favorable mutations vs. against deleterious ones), our findings and those of McLysaght et al. support an active and important role of these genes in OPVs. The first data regarding the function of v-slfns were provided by a recent study of a vaccinia virus engineered to incorporate a v-slfn ORF from camelpox (Gubser et al. 2007). They showed that, after intranasal infection of mice, vaccinia virus titer decays more rapidly when camelpox v-slfn is present than when it is not. However, this v-slfn does not appear to prevent either infection or viral replication in mouse cells. Gubser et al. (2007) hypothesized that expression of v-slfn might actually accelerate OPV elimination by the immune system, preventing the virus from overwhelming its host. Further studies will be necessary to elucidate the role of the products of the v-slfn genes in virulence and their possible interactions with the host immune response and/or the host SLFN proteins.
In contrast with viruses, we find evidence of positive selection in mammalian Slfns of all four groups that we designated based on our phylogenetic analyses. This is particularly evident in Slfn Groups 1 and 4, in which a high proportion of codons show signs of positive selection (1 of every 14 codons in Group 1 and 1 of every 23 codons in Group 4; Appendix F). In Slfn Groups 2 and 3, it is possible that more sites evolving adaptively were missed by CODEML because of the reduced power of this program when small trees are analyzed (Anisimova et al., 2001, Anisimova et al., 2002). In addition, we showed that, after most episodes of gene duplication, paralogous lineages in Slfn Groups 1 and 4 experienced different selection regime, with one of the sister Slfn genes evolving adaptively and possibly diverging functionally (Appendices E and G).
Given the probabilistic nature of CODEML analyses, we caution that the role and function of specific sites that appear to be of adaptive significance should be experimentally validated. However, the influence of positive selection during the evolution of many Slfn lineages is supported by several lines of evidence. For instance, adaptive evolution has been detected by three different tests in Slfn groups 2 and 4 and by two tests in Slfn group 1 (Table 2, Table 3, Appendix E). In addition, the two “site” models M2a and M8 predicted a largely overlapping sets of sites with dN/dS > 1 in Slfn group 4 (Appendix F), and all the positively selected sites predicted by the CODEML Bayesian method show a posterior dN/dS mean > 2.4 (except one site in Slfn group 1 with dN/dS mean = 1.49; Appendix F). The consistency and robustness of our findings indicate that most likely several mammalian Slfn lineages experienced adaptive evolution.
4.4. Schlafen genes: open questions
Our study leaves open a number of important questions: has the evolution of the vertebrate and/or OPV Slfn genes been driven by the coevolution between hosts and viruses? Is there any functional overlap between the products of these genes in mammals? According to the “molecular mimicry” hypothesis of Murphy (1993), diversification and divergence of host immune-related genes from their horizontally transferred viral orthologs might prevent the interference of the viral products with the immune response. It is possible that such phenomena have underlain the evolution of some Slfn members: most mammalian Slfn groups have experienced positive selection and Slfn genes are highly diversified, especially in rodents. However, this hypothesis does not provide a comprehensive explanation for the evolution of the entire gene family since, as discussed above, the function of the Slfn genes is probably not restricted to defense against viral infections.
On the virus side, we observe purifying selection of the v-slfn sequences, but it is unclear if they have retained any of the functions of their mammalian ancestor (immunity-related or other), as well as if they have the ability of interacting or interfering with the host Slfn products. In addition, the contribution of the p26 baculovirus protein domain to the activity of the v-slfn product is unknown. Although the function of the p26 protein has not yet been identified, it recently has been shown to form dimers in vitro (Goenka et al. 2008). Oligomerization is also observed in AAA ATPases and v-slfn genes conserve an AAA-related domain (Hanson and Whiteheart 2005). Therefore, direct interaction between the v-slfn products and with the host Slfn copies is a strong possibility.
In conclusion, our studies show that the Slfn gene family is evolving rapidly, possibly in response to selective pressure related to their function in the immune response. The acquisition of Slfn copies by OPVs may have provided additional avenues for the evolution of this gene family. Further studies of the contribution of the Slfn genes to host-viral interactions have potential implications, not only for understanding the function of these genes in the immune response, but also in the battle against orthopoxviruses infections.
Acknowledgments
We thank Aaly Meherali, Pallavi Sattiraju and Vasha Huaman for their technical support and Dr. Esther Betrán for her invaluable comments and suggestions.
Received by I. King Jordan
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.gene.2009.07.006.
Appendix A.
Slfn sequences in mouse, human, rat and opossum.
Slfn core amino acid sequences identified in diverse organisms.
Expression analysis of mouse Slfn sequences.
Complete v-slfn nucleotide sequences identified by blast alignment of 9 representative OPV genomes with all mouse SLFN sequences.
Codon substitution analyses performed with CODEML on the four Slfn Groups.
List of sites with dN/dS significantly > 1 detected by “site” and “branch-site” models in Slfn genes.
Lineages that experienced positive selection in Slfn groups' phylogenetic trees.
Codon substitution analyses performed with CODEML on the poxviruses v-slfn genes and pseudogenes.
References
- Altschul S.F., et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova M., Bielawski J.P., Yang Z. Accuracy and power of the likelihood ratio test in detecting adaptive molecular evolution. Mol. Biol. Evol. 2001;18:1585–1592. doi: 10.1093/oxfordjournals.molbev.a003945. [DOI] [PubMed] [Google Scholar]
- Anisimova M., Bielawski J.P., Yang Z. Accuracy and power of Bayes prediction of amino acid sites under positive selection. Mol. Biol. Evol. 2002;19:950–958. doi: 10.1093/oxfordjournals.molbev.a004152. [DOI] [PubMed] [Google Scholar]
- Baldacci P.A., Richoux V., Renard J.P., Guénet J.L., Babinet C. The locus Om, responsible for the DDK syndrome, maps close to Sigje on mouse chromosome 11. Mamm. Genome. 1992;2:100–105. doi: 10.1007/BF00353857. [DOI] [PubMed] [Google Scholar]
- Bell T.A., et al. The paternal gene of the DDK syndrome maps to the Schlafen gene cluster on mouse chromosome 11. Genetics. 2006;172:411–423. doi: 10.1534/genetics.105.047118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady G., Boggan L., Bowie A., O'Neill L.A. Schlafen-1 causes a cell cycle arrest by inhibiting induction of cyclin D1. J. Biol. Chem. 2005;280:30723–30734. doi: 10.1074/jbc.M500435200. [DOI] [PubMed] [Google Scholar]
- Bratke K.A., McLysaght A. Identification of multiple independent horizontal gene transfers into poxviruses using a comparative genomics approach. BMC Evol. Biol. 2008;8:67. doi: 10.1186/1471-2148-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Lee W.H., Moser H., Valle D., Gould S.J. Isolation of the human PEX1 gene, mutated in group 3 of the peroxisome biogenesis disorders. Nat. Genet. 1997;15:385–388. doi: 10.1038/ng0497-385. [DOI] [PubMed] [Google Scholar]
- De la Casa-Esperon E., et al. X chromosome inactivation effect on maternal recombination and meiotic drive in the mouse. Genetics. 2002;161:1651–1659. doi: 10.1093/genetics/161.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth J.P., De Bie T., Stajich J.E., Cristianini N., Hahn M.W. The evolution mammalian gene families. PLoS ONE. 2006:e85. doi: 10.1371/journal.pone.0000085. Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H. Comparative genomics and the study of evolution by natural selection. Mol. Ecol. 2008;17:4586–4596. doi: 10.1111/j.1365-294X.2008.03954.x. [DOI] [PubMed] [Google Scholar]
- Fujikado N., Saijo S., Iwakura Y. Identification of arthritis-related gene clusters by microarray analysis of two independent mouse models for rheumatoid arthritis. Arthritis Res. Ther. 2006;8:R100. doi: 10.1186/ar1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Wu G., Han Z., de la Casa-Esperon E., Sapienza C., Latham K.E. Recapitulation of the Ovum Mutant (Om) phenotype and loss of Om locus polarity in cloned mouse embryos. Biol. Reprod. 2005;72:487–491. doi: 10.1095/biolreprod.104.035030. [DOI] [PubMed] [Google Scholar]
- Geserick P., Kaiser F., Klemm U., Kaufmann S.H., Zerrahn J. Modulation of T cell development and activation by novel members of the Schlafen (Slfn) gene family harbouring an RNA helicase-like motif. Int. Immunol. 2004;16:1535–1548. doi: 10.1093/intimm/dxh155. [DOI] [PubMed] [Google Scholar]
- Goenka S., Weaver R.F. The p26 gene of the Autographa californica nucleopolyhedrovirus: timing of transcription, and cellular localization and dimerization of product. Virus Res. 2008;131:136–144. doi: 10.1016/j.virusres.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Griffiths M.M., Encinas J.A., Remmers E.F., Kuchroo V.K., Wilder R.L. Mapping autoimmunity genes. Curr. Opin. Immunol. 1999;11:689–700. doi: 10.1016/s0952-7915(99)00038-2. [DOI] [PubMed] [Google Scholar]
- Gubser C., Hué S., Kellam P., Smith G.L. Poxvirus genomes: a phylogenetic analysis. J. Gen. Virol. 2004;85(Pt 1):105–117. doi: 10.1099/vir.0.19565-0. [DOI] [PubMed] [Google Scholar]
- Gubser C., et al. Camelpox virus encodes a Schlafen-like protein that affects orthopoxvirus virulence. J. Gen. Virol. 2007;88(Pt 6):1667–1676. doi: 10.1099/vir.0.82748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P.I., Whiteheart S.W. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- Hill R.E., Hastie N.D. Accelerated evolution in the reactive centre regions of serine protease inhibitors. Nature. 1987;326:96–99. doi: 10.1038/326096a0. [DOI] [PubMed] [Google Scholar]
- Hillis D.M., Bull J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993;42:182–192. [Google Scholar]
- Hughes A.L. Origin and evolution of viral interleukin-10 and other DNA virus genes with vertebrate homologues. J. Mol. Evol. 2002;54:90–101. doi: 10.1007/s00239-001-0021-1. [DOI] [PubMed] [Google Scholar]
- Hughes A.L., Friedman R. Poxvirus genome evolution by gene gain and loss. Mol. Phylogenet. Evol. 2005;35:186–195. doi: 10.1016/j.ympev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Hughes A.L., Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988;335:167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- Katoh K., Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Larkin M.A., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lee N.K., Choi H.K., Yoo H.J., Shin J., Lee S.Y. RANKL-induced schlafen2 is a positive regulator of osteoclastogenesis. Cell Signal. 2008;20:2302–2308. doi: 10.1016/j.cellsig.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Lefkowitz E.J., Wang C., Upton C. Poxviruses: past, present and future. Virus Res. 2006;117:105–118. doi: 10.1016/j.virusres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Lund S., et al. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J. Neuroimmunol. 2006;180:71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Mann J.R. DDK egg-foreign sperm incompatibility in mice is not between the pronuclei. J. Reprod. Fertil. 1986;76:779–781. doi: 10.1530/jrf.0.0760779. [DOI] [PubMed] [Google Scholar]
- McLysaght A., Baldi P.F., Gaut B.S. Extensive gene gain associated with adaptive evolution of poxviruses. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15655–15660. doi: 10.1073/pnas.2136653100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Shisler J.L. Immunology 101 at poxvirus U: immune evasion genes. Immunology. 2001;13:59–66. doi: 10.1006/smim.2000.0296. [DOI] [PubMed] [Google Scholar]
- Murphy P.M. Molecular mimicry and the generation of host defense protein diversity. Cell. 1993;72:823–826. doi: 10.1016/0092-8674(93)90571-7. [DOI] [PubMed] [Google Scholar]
- Murphy W.J., Pringle T.H., Crider T.A., Springer M.S., Miller W. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res. 2007;17:413–421. doi: 10.1101/gr.5918807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B., Zhao L., Murphy K., Gonda T.J. Subcellular localization of the Schlafen protein family. Biochem. Biophys. Res. Commun. 2008;370:62–66. doi: 10.1016/j.bbrc.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Nishikawa, T., Nagai, K., 1996. EST error analysis in a large-scale GenBank search of ESTs using rapid-identity-searching program for DNA sequences. In Genome mapping and sequencing, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Pardo-Manuel de Villena F., Naumova A.K., Verner A.E., Jin W.H., Sapienza C. Confirmation of maternal transmission ratio distortion at Om and direct evidence that the maternal and paternal "DDK syndrome" genes are linked. Mamm. Genome. 1997;8:64264–64266. doi: 10.1007/s003359900529. [DOI] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena F., de la Casa-Esperón E., Verner A., Morgan K., Sapienza C. The maternal DDK syndrome phenotype is determined by modifier genes that are not linked to Om. Mamm. Genome. 1999;10:492–497. doi: 10.1007/s003359901029. [DOI] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena F., de la Casa-Esperon E., Briscoe T.L., Sapienza C. A genetic test to determine the origin of maternal transmission ratio distortion. Meiotic drive at the mouse Om. locus. Genetics. 2000;154:333–342. doi: 10.1093/genetics/154.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Manuel De Villena F., de la Casa-Esperón E., Williams J.W., Malette J.M., Rosa M., Sapienza C. Heritability of the maternal meiotic drive system linked to Om and high-resolution mapping of the Responder locus in mouse. Genetics. 2000;155:283–289. doi: 10.1093/genetics/155.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B.B., et al. Schlafen 3, a novel gene, regulates colonic mucosal growth during aging. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G955–G962. doi: 10.1152/ajpgi.90726.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J.P., Babinet C. Identification of a paternal developmental effect on the cytoplasm of one-cell-stage mouse embryos. Proc. Natl. Acad. Sci. U. S. A. 1986;83:6883–6886. doi: 10.1073/pnas.83.18.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Paquette J., Pannunzio P., Albrechtson S., Morgan K. The polar-lethal Ovum mutant gene maps to the distal portion of mouse chromosome 11. Genetics. 1992;132:241–246. doi: 10.1093/genetics/132.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz D.A., Katayama C.D., Hedrick S.M. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9:657–668. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- Seet BT, et al. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- Shchelkunov S.N. Immunomodulatory proteins of orthopoxviruses. Mol. Biol. 2003;37:37–48. [PubMed] [Google Scholar]
- Smith G.L., Moss B. Infectious poxvirus vectors have capacity for at least 25 000 basepairs of foreign DNA. Gene. 1983;25:21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- Sohn W.J., et al. Novel transcriptional regulation of the Schlafen-2 gene in macrophages in response to TLR-triggered stimulation. Mol. Immunol. 2007;44:3273–3782. doi: 10.1016/j.molimm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Srikakulam R., Liu L., Winkelmann D.A. Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain. PLoS ONE. 2008;3:e2137. doi: 10.1371/journal.pone.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama M., Torrents D., Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609–W612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi N. A genetically determined incompatibility system between spermatozoa and eggs leading to embryonic death in mice. J. Reprod. Fertil. 1974;41:85–96. doi: 10.1530/jrf.0.0410085. [DOI] [PubMed] [Google Scholar]
- Wandstrat A., Wakeland E. The genetics of complex autoimmune diseases: non-MHC susceptibility genes. Nat. Immunol. 2001;2:802–809. doi: 10.1038/ni0901-802. [DOI] [PubMed] [Google Scholar]
- Waterston R.H., et al. (Mouse Genome Sequencing Consortium) nitial sequencing anc comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Zhang J., Nielsen R., Yang Z. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 2005;22:2472–2479. doi: 10.1093/molbev/msi237. [DOI] [PubMed] [Google Scholar]
- Zhang Y., et al. The Hsp40 family chaperone protein DnaJB6 enhances Schlafen1 nuclear localization which is critical for promotion of cell-cycle arrest in T-cells. Biochem. J. 2008;413:239–250. doi: 10.1042/BJ20071510. [DOI] [PubMed] [Google Scholar]
- Zhao L., Neumann B., Murphy K., Silke J., Gonda T.J. Lack of reproducible growth inhibition by Schlafen1 and Schlafen2 in vitro. Blood Cells Mol. Dis. 2008;41:188–193. doi: 10.1016/j.bcmd.2008.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Slfn sequences in mouse, human, rat and opossum.
Slfn core amino acid sequences identified in diverse organisms.
Expression analysis of mouse Slfn sequences.
Complete v-slfn nucleotide sequences identified by blast alignment of 9 representative OPV genomes with all mouse SLFN sequences.
Codon substitution analyses performed with CODEML on the four Slfn Groups.
List of sites with dN/dS significantly > 1 detected by “site” and “branch-site” models in Slfn genes.
Lineages that experienced positive selection in Slfn groups' phylogenetic trees.
Codon substitution analyses performed with CODEML on the poxviruses v-slfn genes and pseudogenes.