A 37-year-old man attended our hospital with a 1-week history of fever, chills, headaches, sore throat, generalised malaise, and a rash on his arms, legs, trunk, and in his groin. The patient also reported significant pain and discomfort in his rectum when defecating. He had a history of HIV and metastatic Kaposi sarcoma; secondary syphilis, which had been treated; and hypertension. He was prescribed the following medications: emtricitabine-tenofovir, doravarine, darunavir-cobicistat, and hydrochlorothiazide.
The patient reported no recent travel or contact with human or animal with a similar rash or illnesses. He reported no history of varicella zoster virus infection as a child and said he had received all childhood vaccinations. The patient reported no sexual activity for the past 6 months.
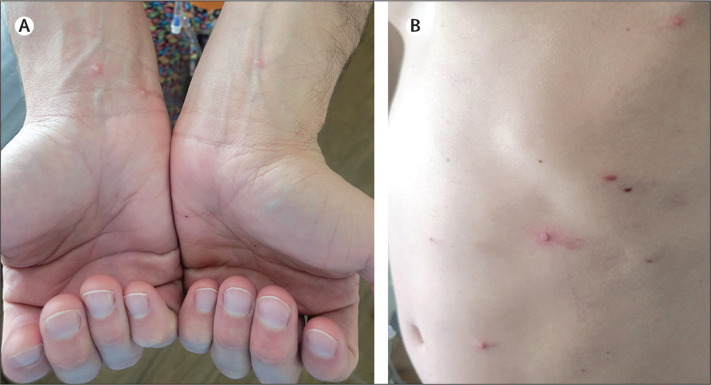
On examination, the patient was afebrile: temperature 37·3°C. He appeared uncomfortable and had several scattered, pink, umbilicated papules, vesicles, and pustules on the trunk, upper and lower extremities, groin, and peri-anal region (figure ).
Figure.
Monkeypox infection in HIV-positive person
(A) Photograph shows pustules on volar wrists. (B) Photograph shows pustules at left hypochondriac and lumbar regions.
Laboratory investigations showed a haemoglobin concentration of 12·7 g/dL (normal 13·3–16·3), CD4+ T-helper cell count of 262 cells per mcL (normal 490–1740), HIV-1 RNA PCR less than 20 copies per mL, and an absolute lymphocyte count of 3·01 per uL (normal 1·1–2·7). Serology was negative for Bartonella henselae, Aspergillus spp, hepatitis A, B, and C, cryptococcal antigen, herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), and (1→3)-β-D-glucan.
Immunohistochemical analysis of a biopsy sample of one of the truncal papules was negative for HSV-1, HSV-2, and Treponema pallidum. Warthin-Starry staining was negative for spirochetes and periodic-acid-Schiff staining was negative for fungal organisms. Histopathological analysis of the biopsy sample showed focal epidermal necrosis with underlying acute inflammation in the superficial dermis and individually necrotic keratinocytes at the periphery. A Dacron swab of one of the truncal lesions was positive for human monkeypox virus.
Considering our patient's history of immunosuppression, we prescribed doxycycline, ceftriaxone, and valacyclovir; we subsequently gave him a 2-week course of 600 mg of tecovirimat twice a day. He reported no significant adverse effects and the skin lesions healed rapidly. At follow-up one month later, the patient reported areas of hyperpigmentation at the sites of the healed lesions and said he was doing well.
Monkeypox is caused by the monkeypox virus, a member of the Poxviridae family and Orthopoxvirus genus. The virus is transmitted via large respiratory droplets, direct or indirect contact with bodily fluids or lesion material, or contact with fomites—including bedding or towels. Malaise, lymphadenopathy, headache, myalgia, and cutaneous symptoms can occur within 1–3 days of the onset of fever. Lesions may initially appear as macules progressing to papules, vesicles, and pustules that dry up and fall off. The development of skin lesions, in addition to viral prodromal symptoms, in immunocompromised patients should raise the possibility of other diagnosis, such as primary or secondary infection with varicella zoster virus or other organisms such as Cryptococcus neoformans, Histoplasma capsulatum, or Bartonella henselae; we considered this unlikely in our patient given his presentation. Molluscum contagiosum also presents with umbilicated papules, but usually without systemic symptoms. Notably, proctitis has recently been recognised as being associated with a human monkeypox infection. Tecovirimat is approved for treating human smallpox disease. Treatment in cases of human monkeypox should be considered in severe cases, immunocompromised patients, paediatric populations, pregnant or breastfeeding women, and people with concurrent disease or other comorbidities.
Declaration of interests
We declare no competing interests.
Contributors
We were all involved in the patient's care. AJ and LEH wrote the manuscript under RSK's supervision. Written consent for publication was obtained from the patient.