Human monkeypox is a systemic exanthem, resembling smallpox, that occurs as a sporadic zoonosis in rural rainforest villages of western and central Africa. The disease is caused by an orthopoxvirus, which is transmitted to human beings by handling infected animals; serosurveys have implicated squirrels [Funisciurus and Heliosciurus spp] as the probable reservoir. Secondary human-to-human spread by aerosol or direct contact accounts for about 28% of cases; tertiary and quaternary chains of transmission are rare.1
Between Feb 15, and Aug 31, 1996, 71 cases of human monkeypox, including six deaths, were reported in 13 villages in the Katako-Kombe health zone among a population of 15 698, in Sankuru subregion, Kasai Oriental, Zaire.2 The outbreak had gone largely unrecognised until the end of July, when an abrupt increase in the number of reported cases led to a preliminary investigation, which found that 42 cases of human monkeypox, including three deaths, had occurred in a small village (population 346) where squirrels were often hunted by men and boys. Most cases were in people under 25 years of age. Among those examined during a preliminary investigation, none had a scar of smallpox vaccination. From February to July, one person in the village appeared to be the primary case-patient who may have been the source of a cascade of human-to-human transmission through eight members of his clan. During this time, monkeypox infections also occurred in other families living together and in a few clans in nearby villages, raising the possibility of other introductions of human monkeypox into the population.
Monkeypox was confirmed in 11 clinically suspect cases from crusted scabs, vesicular fluid, or serum collected from July to September, including three pairs of samples representing secondary contact cases in separate households. Virus-specific polymerase chain reaction (PCR) amplifications of genes for the monkeypox virus haemagglutinin (HA)3 and tumour necrosis factor receptor (TNFR; unpublished data) were positive for three of four available scab samples, and monkeypox virus was isolated in culture from two of the PCR-positive specimens. In addition, western blot assay showed orthopoxvirus genusspecific IgG in ten different patient sera, and an experimental enzyme-linked-indicator serum assay that used orthopoxvirus antigen peptides showed monkeypox-specific IgM in five of six sera tested.
The present cluster of cases constitutes a reemergence of human monkeypox on a scale greater in magnitude than the approximate 65 annual cases previously indicated for Kasai Oriental, Bandundu, and Equateur regions from 1981 to 1986, and it contains a more extensive occurrence of person-to-person transmission than previously recognised.1 The extent of the outbreak in Katako-Kombe, which reported no cases during the previous surveillance, and the incidence of disease among household contacts, challenge previous modelling studies1 that suggested prolonged episodes or sustained cascades of transmission of human monkeypox would be unlikely even after smallpox vaccination, which is protective, ceased. Alternately, the events may represent multiple introductions into the same population because of increasing encroachment of larger populations into the primary habitat of animals in this and other areas of Africa. Because sequence analyses have indicated that Zairian monkeypox strains have not diverged greatly from the first isolate from the area in 1970 (figure ) and monkeypox and smallpox variola viruses are independently evolved species,5 notions of monkeypox virus mutating into variola virus are unfounded.
Figure.
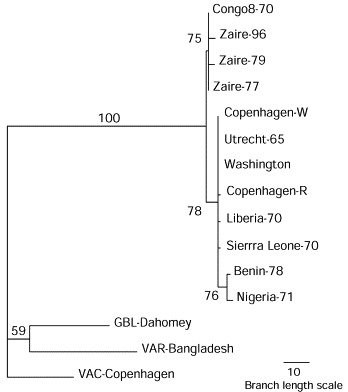
Phylogenetic analysis of orthopoxvirus TNFR open reading sequences of the current Zairian isolate compared with cognate sequences of monkeypox virus strains from Zaire in previous years and selected non-Zairian monkeypox strains, variola (VAR), gerbilpox (GBL), and vaccinia (VAC) viruses
Nucleotide sequences of genome PCR-generated amplicons were determined using dye-terminated, primer-walking, fluorescence-based Sanger-type reactions4. Maximum parsimony analysis of aligned sequences provided bootstrap confidence intervals (values in bold) after 1000 heuristic search replicates weighted for a transition to transversion ratio of 2 (PAUP software version 3·1·1).
In light of the 1996 episode, an international team coordinated by WHO, Centers for Disease Control and Prevention, and the Zairian Ministry of Health began an investigation in February, 1997, to evaluate the outbreak and determine current risk factors for infection. More specific rapid diagnostic assays should enable more precise monitoring of fluctuations in the virus and epidemiological pattern of this zoonosis as changes occur in human demographics, sanitary practices, and reservoir animal distributions.
Acknowledgments
Member at Médecins sans Frontières Belgique, Zaire: H Koen.
Members at Institut National de Recherche Biomédicale, Kinshasa, Zaire: M Delfi, J J Muyembe-Tamfum. Members at World Health Organization: T F Kweteminga, A Moudi. Members at Centers for Disease Control and Prevention, National Center for Infectious Diseases: L Mangindula, V N Loparev, J M Parsons, D L Jue, T W Crews, J C Knight.
References
- 1.Ježek Z, Fenner F. In: Melnick JL, editor. Vol 17. Karger; Basel, Switzerland: 1988. Human monkeypox. (Monographs in Virology). [Google Scholar]
- 2.WHO Monkeypox, Zaire. Wkly Epidemiol Rec. 1996;71:326. [Google Scholar]
- 3.Ropp SL, Jin Q, Knight JC, Massung RF, Esposito JJ. PCR strategy for identification and differentiation of smallpox and other orthopoxviruses. J Clin Microbiol. 1995;33:2069–2076. doi: 10.1128/jcm.33.8.2069-2076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massung RF, Esposito JJ, Liu LI, et al. Potential virulence determinants in terminal regions of variola smallpox virus genome. Nature. 1993;366:748–751. doi: 10.1038/366748a0. [DOI] [PubMed] [Google Scholar]
- 5.Douglass N, Dumbell KR. Independent evolution of monkeypox and variola viruses. J Virol. 1992;66:7565–7567. doi: 10.1128/jvi.66.12.7565-7567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


