Abstract
Background
Since the eradication of smallpox in 1980, monkeypox has been the most prevalent zoonosis caused by orthopoxviruses. The virus has been largely endemic to the rainforests of Central and West Africa with occasional exportation to other countries. The disease typically runs a self-limited course with case fatality rates ranging from 4 to 11%. Currently, the world is faced with a multi-country outbreak of monkeypox, whose extent and impact remains to be seen.
Objective
The objective of this article is to discuss the changing epidemiology of the monkeypox virus, with special reference to the current outbreak.
Content
Since the beginning of this outbreak which started on May 7th, till 14th of June 2022, a total of 1879 cases of monkeypox have been reported worldwide, spanning across 35 non-endemic, and commonly involving men who have sex with men. The magnitude of this unprecedented outbreak has highlighted the lacunae in our understanding of the viral epidemiology and ecology. What was earlier a rare sporadic zoonotic disease is now an emergent pathogen with documented human-to-human transmission potential, both in household as well as nosocomial settings. Waning immunity due to cessation of smallpox vaccination, wide host range of the virus, undetected circulation in wildlife in pan-geographic areas, emergence of better adapted strains of the virus due to unchecked replication in the HIV positive immunocompromised population along with deforestation and human encroachment of reservoir areas are some of the plausible reasons for an increased incidence of the disease. Unflinching government commitment, healthcare worker training, education of the masses, stockpiling of the available vaccine and drug, intersectoral co-ordination in lines of the One Health approach are simultaneously needed to avert the current spread of monkeypox. There is also a compelling need to strengthen surveillance systems to curb future outbreaks.
Keywords: Monkeypox, Zoonosis, Outbreak, Epidemiology
Abbreviation: MPXV, Monkeypox virus
1. Introduction
Monkeypox was considered a rare sporadic viral zoonotic disease with a limited capacity to spread between humans in the past [1]. Historically, the virus has been endemic in the rainforests of Central and West Africa with occasional exportation to other countries. Till May 2022, the largest outbreak in a non-endemic country was reported form United States of America in 2003. However, starting in May 2022, several countries non-endemic for the virus have reported outbreaks of monkeypox bringing forth this disease that has been lurking for decades.
2. Virus biology
The monkeypox virus (MPXV) is one of the four human pathogens belonging to the genus Orthopoxvirus within the Poxviridae family; the other three being the cowpox virus, vaccinia virus and the variola virus, the causative agent of smallpox, now eradicated. MPXV is a brick-shaped enveloped virus measuring 200–250 nm, which replicates in the cytoplasm, despite being a DNA virus [2]. The double stranded DNA genome is approximately 197 kbp in size, which is among the largest of all viral genomes, consisting of about 190 non-overlapping open reading frames. There is a high degree of homology in the genes located in the central part of the genome, with the terminal regions on either end demonstrating high variability, as is the norm with orthopoxviruses. The conserved genes are mostly involved in essential viral functions like replication and virion assembly, and the variable regions of the genome most likely contribute to the virulence of different orthopoxviruses [3].
Genomic sequencing has identified two distinct phylogenetic clades of MPVXes, with a distinct geographical distribution. They are the West African (WA) clade and the Congo Basin (CB) clade (also called Central Africa clade) [4]. WA and CB MPXV also differ in virulence and transmissibility, as discussed ahead.
3. Historical perspectives
MPVX got its name in 1958 when it was first isolated during an outbreak of pustular disease in imported macaques in an animal facility in Denmark, and was placed under the genus Orthopoxvirus [5]. The following years witnessed a number of outbreaks in animals, mostly non-human primates. It was in 1970 that the first human case of infection with the virus was reported in an infant in the Democratic Republic of Congo (DRC), then Zaire, along with other countries spanning through the rain forests of the Central and West Africa [6]. The number of confirmed and probable cases of infection with the virus has increased from 48 in 1970s to 343 in 1980s to a further 511 in 1990s [7]. DRC is the sole country to have reported monkeypox cases continually for the last five decades. The first outbreak outside the African continent occurred in the United States of America (USA) in 2003 when native prairie dogs served as vectors and amplifying hosts after they were cohabited with imported small African mammals, leading to 47 cases across five states [8]. The second outbreak which led to the expansion of the traditional ecology in the Congo basin and West Africa region was described in the dry savannah region in south Sudan in 2005 [9].
4. Changing epidemiology of the virus
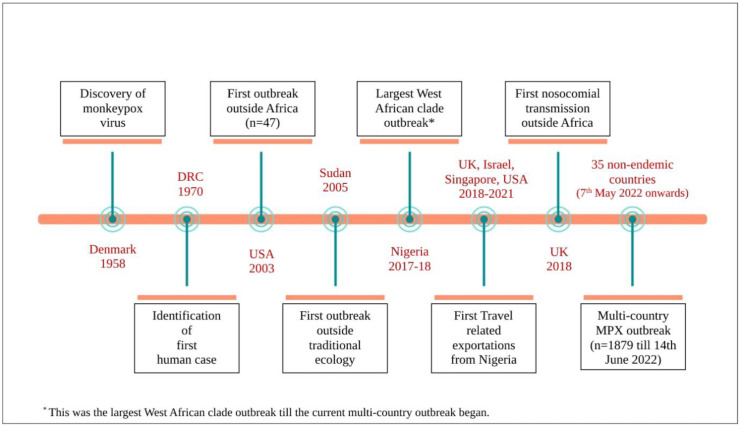
With the advent of the 21st century, the virus had not only crossed borders of the African continent, but it was also being increasingly reported as larger outbreaks than as singular cases. The median age increased from 4 to 5 years in 1970–1989 to 10 years in 2000–2009 and 21 years in 2010–2019 [7]. The Nigerian outbreak of 2017–2018 befits a special mention as it was till then the largest West African clade outbreak to occur, that too after about four decades of no reported cases in the country [10]. Both primary zoonotic and secondary human-to-human transmission was noted [11]. The outbreak also triggered off a series of travel related exportations to the United Kingdom (three cases), Israel (one case), and Singapore (one case), USA (one case) through to 2018 to 2021 [12,13]. A report of nosocomial transmission to a healthcare worker also surfaced from the United Kingdom [14](Fig. 1 ).
Fig. 1.
Timeline of major events in the history of monkeypox (DRC is Democratic Republic of Congo).
The dramatic surge of confirmed and probable cases reported from DRC, re-emergence of the virus in Nigeria and notable geographic spread brought about considerable concern over the changing epidemiology and further resurgence of monkeypox. It has also been proved that the increase in reported cases in DRC is a true rise in disease occurrence and cannot merely be attributed to improvised surveillance systems [15]. A number of reasons for the resurgence of monkeypox cases have been proposed, the primary ones being waning immunity after the cessation of smallpox vaccination in 1980, deforestation, climatic change, armed conflict, mass population displacement, increased consumption of bush meat and greater global travel [15,16]. The Nigerian outbreak was preceded by heavy rainfall and flooding that is hypothesised to have increased animal-human contact as both reached out for dryer, high lands [16].
Half a century since the first reported case, the natural host for the monkeypox virus remains elusive. The Gambian pouched rat and the rope squirrel are the most probable candidates, based on evidence from field and laboratory investigations [2]. The susceptibility of a large number of animal species, mainly rodents and non-human primates reflects upon the adaptability of the virus, with the latter believed to be incidental hosts. Experimental infections have been induced in animals through numerous inoculation routes; however the natural route of transmission is believed to be species-specific [17]. The primary zoonotic transmission of the virus can occur via contact with blood, body fluid and lesions of infected animals that most commonly happens while hunting. Similar routes have been described for human to human transmission, along with fomites and respiratory transmission which needs prolonged face to face contact. Nosocomial and sexual transmission from infected individuals with genital lesions have been reported [18]. The CB clade is more transmissible than the WA in terms of secondary attack rate and serial transmission events. Earlier studies found the human-to-human transmission reproductive rate (R0) to be 0.8, thus dismissing the self-sustained transmission and epidemic potential of the virus [19]. However, the same models may not hold true today considering the trans-continental spread of the virus, ecological changes and a large immunocompromised population of PLHA which may favour repeated circulation, viral evolution and emergence of better human adapted pathogens [20]. Moreover, it has also been shown that genomic destabilization and genetic polymorphism is leading to evolution of circulating MPXV strains in DRC, which may be better human adapted [21].
5. Current outbreak
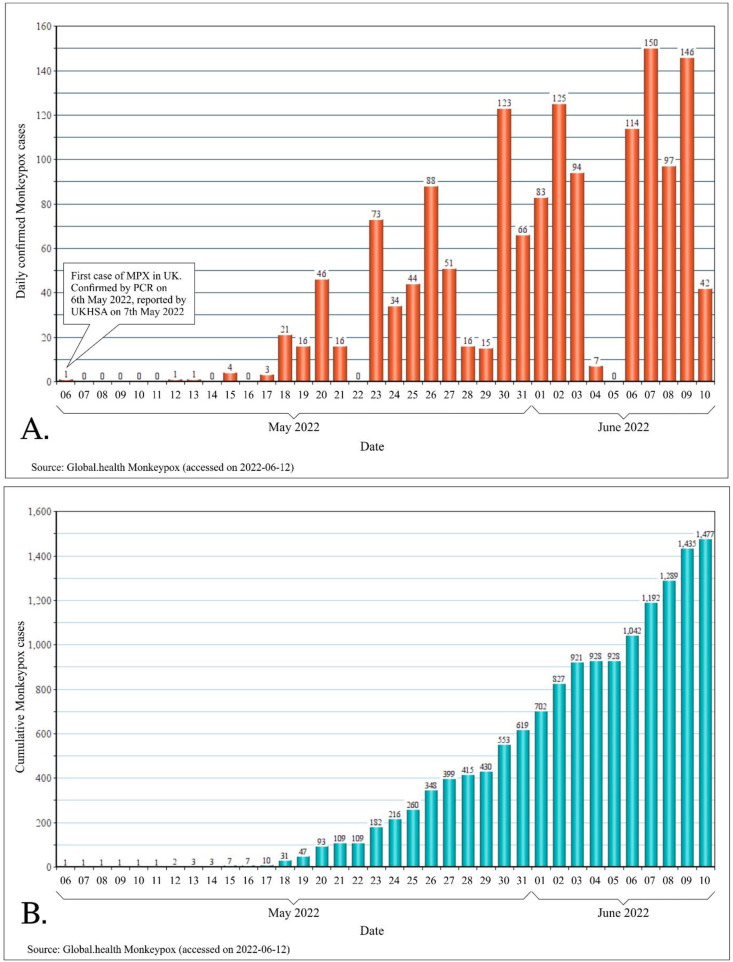
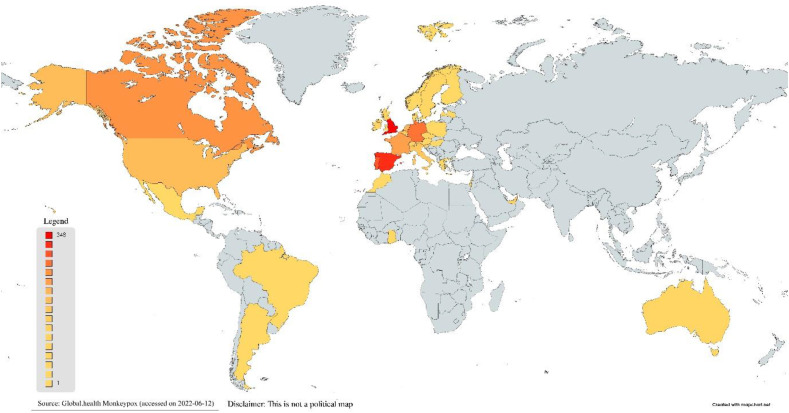
On 7th May 2022, the United Kingdom (UK) reported an imported case of monkeypox in a person travelling from Nigeria. On 13th May 2022, UK reported another two cases which did not have any epidemiological link to the first case. Following these developments, UK further reported cluster of cases on the 15th, 18th and 20th May. Starting from 18th May 2022, multiple countries in the European Union reported monkeypox cases (Fig. 2 ).Since the beginning of this outbreak, till 14th of June 2022, 1879 cases have been reported worldwide, spanning across 35 non-endemic countries [22] (Fig. 2, Fig. 3 ).
Fig. 2.
Evolution of the current multi-country outbreak of monkeypox (07.05.2022 through 10.06.2022). A. Daily confirmed cases of monkeypox since the outbreak began. B. Cumulative cases of monkeypox in the current outbreak.
Fig. 3.
Geographical distribution of confirmed cases of monkeypox in non-endemic countries during the current outbreak (as of 10 June 2022).
All the isolates from the current outbreak have been reported to belong to the WA Clade. No deaths have been reported so far due to monkeypox in any of the non-endemic countries [23]. Although sexual transmission has not been established for the virus previously, early evidence from the ongoing outbreak has suggested transmission within sexual networks, particularly in men having sex with men [24]. There is a possibility that chains of transmission are being missed because some countries are reporting new cluster of cases among individuals who give no contact history with previously confirmed cases, suggesting undetected circulation of the virus [23]. Despite the risk to human health being low currently, what is worrisome is the fact that the public health risk may skyrocket if the virus creates a niche for itself in the immune naïve populations.
6. The Indian scenario
As of June 14th, 2022, India is yet to report a case of monkeypox. However, with the ongoing global spread, it is inevitable that India, home to one-sixth of mankind, would register its first case sooner or later. A serological survey in healthy Rhesus monkeys done in 1974 in outskirts of Delhi did not reveal the presence of antibodies against monkeypox, thus negating the possibility of existence of reservoirs for the virus in the country [25]. It is not improbable that the virus may be present in the Indian wildlife undetected. It is highly plausible that once introduced into the country, the virus may behave in the similar fashion as the buffalopox virus, another member of the genus Orthopoxvirus and a close variant of Vaccinia virus, which has established itself in water buffaloes of India, causing periodic outbreaks in buffaloes, cows and humans handling them [26].
7. Clinical features, diagnosis and management
The incubation period of the monkeypox ranges from 5 to 17 days, with the disease occurring in two phases. The prodrome lasts for 1–5 days and is marked by a distinctive lymphadenopathy along with constitutional symptoms. This is followed by the eruptive phase wherein a centrifugal rash persists for 2–3 weeks till drying and desquamating. The number of lesions may range from a few to several thousands, and the current outbreak has been marked by lesions beginning from or spreading to the genitals [27]. Severe complications like secondary bacterial infections of skin and soft tissue, pneumonia, severe dehydration, encephalitis and sepsis are rare but present. The pooled case fatality rate has been found to be 8.7% (range 4–11%), with most deaths seen in young children and immunocompromised individuals [7].
With the advent of the current outbreak, CDC and WHO have laid down guidelines for screening and triaging of the viral infection. Polymerase chain reaction (conventional/real time) with or without sequencing is the mainstay of laboratory diagnosis, with swab from skin lesions being the recommended sample [28]. While isolation, symptomatic treatment and rehydration form the mainstay of management of mild cases, Tecovirimat (TPOXX, ST-246), an envelope protein synthesis inhibitor, is the first antiviral to have been licensed by the European Medicines Agency against MPVX based on experimental animal data and pre-clinical trials [29]. The drug has a safe pharmacokinetic and pharmacodynamic profile and has been found to be effective in non-human primates. WHO recommends the rashes to be managed conservatively and antibiotics be used to treat secondary bacterial infections [30]. Cidofovir and brincidofovir, despite affirmative in-vitro and animal studies, have suboptimal safety profiles and have not yet been licensed for the treatment of monkeypox infections. Similarly, data on the efficacy of Vaccinia Immune Globulin (VIG) is not available. However, CDC recommends the prophylactic use of VIG in immunocompromised individuals who cannot be offered smallpox vaccination following exposure to MPVX [31].
8. Vaccination
The year 1980 marked the cessation of routine smallpox vaccination after WHO coordinated and certified elimination of the disease. Smallpox vaccination with the vaccinia virus has been found to provide 85% protection against monkeypox [19]. Patients vaccinated against smallpox with the vaccinia vaccine have significantly lesser morbidity than the non-vaccinated [32].
In September 2019 FDA approved the first vaccine JYNNEOS™ (also known as Imvamune or Imvanex) for prevention of monkeypox as well as smallpox in USA [33]. This is a replication deficient modified vaccinia Ankara virus vaccine (MVA-BN). The vaccine is administered subcutaneously as two doses, 4 weeks apart. Another vaccine called ACAM2000®, which uses a live vaccinia virus is recommended by CDC for active immunisation against smallpox in high risk individuals [34].
9. The lurking threat of monkeypox: vis-à-vis COVID-19
Over the last few weeks the world has woken up to monkeypox, an otherwise neglected disease of the African rainforests. Monkeypox is another example of zoonotic spillover that threatens mankind with greater than ever geographic spread and thus irrefutable epidemic potential. The capability of poxviruses to multiply rapidly despite low mutation rates defeating host defences [35], possible undetected circulation in wildlife [36] and emergence of viral strains better adapted for human to human transmission are a few factors that may lead to MPVX emerging as a major pathogen.
The news of this unprecedented outbreak has hit the world which was jolted by the COVID-19 pandemic not too long ago. But then, the current scenario may not be as worrisome for a number of reasons. The etiological agent is neither a novel virus nor an RNA virus like SARS-CoV2, there are vaccines and drugs available to combat it and most importantly, the agent isn't highly transmissible with a poor R0. Last but not the least, with the priming of the health sector by the pandemic, we may be better prepared to deal with the virus when the need arises.
10. The way forward
Monkeypox has been affecting the remotest and most marginalised part of the world for half a century, causing regular outbreaks. The numbers and the morbidity reported may possibly be the tip of the huge iceberg that lies underneath. Till the virus assumed outbreak proportions in the developed non-endemic world, there were no guidelines for clinical management of the infection. WHO, CDC, ECDC and the central government have been prompt in formulating guidelines for screening, triaging, diagnosing and managing monkeypox infections. It is also a good opportunity to bank on the advancement in molecular diagnostic capabilities that happened due to the COVID-19 pandemic. Educating the masses about the virus, guiding healthcare workers on dealing with the infection, rapid identification of new cases and stockpiling vaccines and drugs are urgently needed. It is also crucial that effective and affordable interventions be made available in the endemic countries, where the virus is a significant health concern, and not just hoarded by the high income countries. Monkeypox being a viral zoonosis mandates intersectoral coordination between human and animal health interventions. Stringent systematic surveillance programs must be put in place to study the burden of asymptomatic infections and seroprevalence of the disease.
11. Conclusion
Less than 30% of the population today is immunized against smallpox, and cross-protected against other orthopox viruses, thus leading to the speculation that MPVX may serve to occupy the epidemiological niche created by smallpox eradication. Although MPXV has been plaguing the African rain forest for decades, there exist huge lacunae in our understanding of its epidemiology, transmission and natural reservoirs. Being a zoonotic agent maintained in wild animals, the virus is not amenable to eradication measures. This outbreak should serve as a wakeup call for the scientific community to engage more into One Health approach and targeted surveillance programs to detect evolved viral strains in order to curb future outbreaks of monkeypox and other similar zoonoses. It also highlights the compelling need for better epidemic preparedness and global health, reiterating the lessons learnt from the COVID-19 pandemic.
CRediT author statement
NK: Data curation, writing: original draft.
PA: Conceptualization, writing: original draft, review and editing.
Declaration of competing interest
There is no conflict of interest.
References
- 1.Sklenovská N., Van Ranst M. Emergence of monkeypox as the most important Orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown K., Leggat P.A. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:E8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sklenovská N. In: Anim.-Orig. Viral Zoonoses. Malik Y.S., Singh R.K., Dhama K., editors. Springer; Singapore: 2020. Monkeypox virus; pp. 39–68. [DOI] [Google Scholar]
- 4.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnus P von, Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. doi: 10.1111/j.1699-0463.1959.tb00328.x. [DOI] [Google Scholar]
- 6.Breman J.G., Kalisa-Ruti, Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 7.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Neglected Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutson C.L., Lee K.N., Abel J., Carroll D.S., Montgomery J.M., Olson V.A., et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. [PubMed] [Google Scholar]
- 9.Formenty P., Muntasir M.O., Damon I., Chowdhary V., Opoka M.L., Monimart C., et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin Clade, Sudan, 2005. Emerg Infect Dis. 2010;16:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yinka-Ogunleye A., Aruna O., Ogoina D., Aworabhi N., Eteng W., Badaru S., et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCollum A., Disu Y., et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauldin M.R., McCollum A.M., Nakazawa Y.J., Mandra A., Whitehouse E.R., Davidson W., et al. Exportation of monkeypox virus from the African continent. J Infect Dis. 2020;225:1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Past U.S. Cases and outbreaks | monkeypox | poxvirus | CDC. 2022. https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html [accessed June 15, 2022]
- 14.Vaughan A., Aarons E., Astbury J., Brooks T., Chand M., Flegg P., et al. Human-to-Human transmission of monkeypox virus, United Kingdom, 2018. Emerg Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoff N.A., Doshi R.H., Colwell B., Kebela-Illunga B., Mukadi P., Mossoko M., et al. Evolution of a disease surveillance system: an increase in reporting of human monkeypox disease in the democratic republic of the Congo, 2001-2013. Int J Trop Dis Health. 2017;25 doi: 10.9734/IJTDH/2017/35885. IJTDH.35885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson K., Heymann D., Brown C.S., Edmunds W.J., Elsgaard J., Fine P., et al. Human monkeypox – after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker S., Buller R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:E1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 20.Grant R., Nguyen L.-B.L., Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98:638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kugelman J.R., Johnston S.C., Mulembakani P.M., Kisalu N., Lee M.S., Koroleva G., et al. Genomic variability of monkeypox virus among humans, democratic Republic of the Congo. Emerg Infect Dis. 2014;20:232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.2022 monkeypox and Orthopoxvirus outbreak global map | monkeypox | poxvirus | CDC. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html [accessed June 15, 2022]
- 23.Multi-country monkeypox outbreak: situation update. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390 n.d.
- 24.Mahase E. Monkeypox: what do we know about the outbreaks in Europe and North America? BMJ. 2022;377:o1274. doi: 10.1136/bmj.o1274. [DOI] [PubMed] [Google Scholar]
- 25.Sehgal C.L., Ray S.N. Survey of rhesus monkeys (Macaca mulatta) for haemagglutination inhibition antibody against vaccinia/variola and monkey pox viruses. J Commun Disord. 1974;6:233–235. [Google Scholar]
- 26.Venkatesan G., Balamurugan V., Prabhu M., Yogisharadhya R., Bora D.P., Gandhale P.N., et al. Emerging and re-emerging zoonotic buffalopox infection: a severe outbreak in Kolhapur (Maharashtra), India. Vet Ital. 2010;46:439–448. [PubMed] [Google Scholar]
- 27.Monkeypox cases confirmed in England – latest updates. GOVUK. https://www.gov.uk/government/news/monkeypox-cases-confirmed-in-england-latest-updates n.d.
- 28.Laboratory testing for the monkeypox virus: interim guidance. https://www.who.int/publications-detail-redirect/WHO-MPX-laboratory-2022.1 n.d.
- 29.Monkeypox. https://www.who.int/news-room/fact-sheets/detail/monkeypox n.d.
- 30.Clinical management and infection prevention and control for monkeypox: interim rapid response guidance. 2022. https://www.who.int/publications-detail-redirect/WHO-MPX-Clinical-and-IPC-2022.1 n.d. [accessed June 15, 2022]
- 31.Treatment | monkeypox | poxvirus | CDC. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html [accessed June 14, 2022]
- 32.Fenner F., Jezek Z. Karger Book; 1988. Human monkeypox. [n.d.] [Google Scholar]
- 33.Commissioner O of the. FDA approves first live, non-replicating vaccine to prevent smallpox and monkeypox. FDA. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-live-non-replicating-vaccine-prevent-smallpox-and-monkeypox [accessed June 14, 2022]
- 34.Vaccines | smallpox | CDC. 2019. https://www.cdc.gov/smallpox/clinicians/vaccines.html [accessed June 14, 2022]
- 35.Elde N.C., Child S.J., Eickbush M.T., Kitzman J.O., Rogers K.S., Shendure J., et al. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell. 2012;150:831–841. doi: 10.1016/j.cell.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakazawa Y., Mauldin M.R., Emerson G.L., Reynolds M.G., Lash R.R., Gao J., et al. A phylogeographic investigation of African monkeypox. Viruses. 2015;7:2168–2184. doi: 10.3390/v7042168. [DOI] [PMC free article] [PubMed] [Google Scholar]