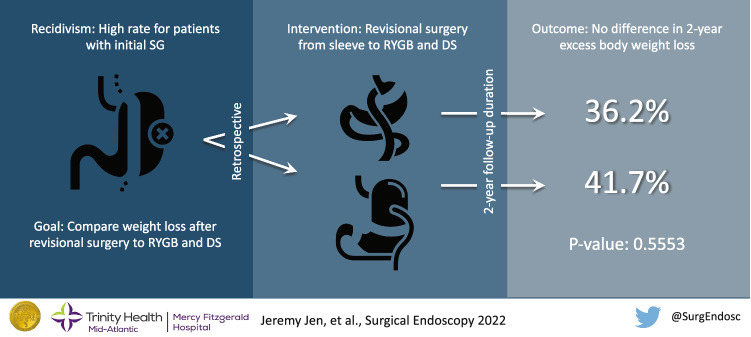
Abstract
Background
Recidivism after initial sleeve gastrectomy (SG) remains common. Revisional surgery to convert SG to Roux-en-Y gastric bypass (RYGB) or duodenal switch (DS) for additional weight loss is increasing. This study aims to compare the outcomes after conversion of SG to RYGB or DS.
Methods
A retrospective single-institution review was conducted from 2015 to 2021, identifying 75 patients who underwent conversion from prior SG to either RYGB (40) or DS (35). Mean excess body weight loss (EBWL) at 3, 6, 12, and 24 months was assessed and compared. Secondary measures of length of stay (LOS), procedure length, and 30-day readmission rate were also reviewed.
Results
Percentage EBWL for RYGB vs DS was 24.0% vs 18.8% at 3 months (N = 36 vs 26; P < 0.0491), 34.8% vs 29.0% at 6 months (N = 29 vs 17; P < 0.2192), 43.0% vs 40.1% at 12 months (N = 28 vs 12; P < 0.6828), and 36.2% vs 41.7% at 24 months (N = 27 vs 7; P < 0.5553). Average LOS was 2.6 days ± 1.4 for RYGB and 2.8 days ± 1.3 for DS (P < 0.6032). Average procedure length was 134.4 min for RYGB and 189.8 min for DS (P < 0.0001). 30-day readmission rate was 27.5% (N = 11) for RYGB and 14.3% (N = 5) for DS (P < 0.1645). Significant weight loss was observed in both subgroups up to 12 months, with no significant weight loss between 12 and 24 months (RYGB N = 21, P < 0.2961; DS N = 5, P < 0.7233).
Conclusion
Both revisional RYGB and revisional DS procedures had significant and sustained weight loss in the first 12 months. There was no significant excess body weight loss difference between revisional RYGB and revisional DS patients at 6, 12, and 24 months, with only significant greater weight loss for RYGB patients at 3 months. Additionally, procedure length was significantly longer for DS compared to RYGB, with no significant differences in LOS and 30-day readmission rates.
Graphical abstract
Keywords: Bariatric surgery, Gastric bypass, Duodenal switch, Laparoscopic, Morbid obesity, Revisional surgery
Morbid obesity in America has been described as an epidemic with worrisome, increasing prevalence and multiple associated comorbidities. The Centers for Disease Control and Prevention reports an increase of obesity rates of 30.5% to 42.4% between 1999–2000 and 2017–2018, whereas severe obesity increased from 4.7% to 9.2% over the same period. The estimated annual medical cost of obesity was estimated to be $147 billion in 2018 [1]. Bariatric surgery has been proven to provide significant reduction in mean percent excess weight loss as well as risk reduction for developing cardiovascular, cancer, endocrine, infectious, psychiatric, and mental health disorders. Additionally, relative risk of death was also reduced with weight loss surgery [2].
Currently, laparoscopic sleeve gastrectomy (SG) is the most commonly performed bariatric procedure, comprising greater than 59% of all bariatric procedures [3], and patients are expected to lose 50–60% of their excess body weight, compared to 60–70% for laparoscopic Roux-en-Y gastric bypass (RYGB) and 70–80 + % for laparoscopic biliopancreatic diversion with duodenal switch (BPD-DS) [4, 5]. However, long-term studies have demonstrated that 20–30% of sleeve gastrectomy patients will require revisional surgery, mostly due to failure in weight loss and recidivism [6, 7]. Because of this, revisional bariatric surgery has increased in recent years from 6.0% in 2013 to 16.7% in 2019 [3]. Revisional RYGB, BPD-DS, and the newer single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) have all demonstrated to be effective techniques for further weight reduction [8–10], with revisional bariatric procedures on average showing lower excess body weight loss compared to initial bariatric procedures [9, 10]. However, there have been few studies directly comparing revisional bariatric surgeries regarding excess body weight loss, specifically SG converted to RYGB and SG converted to DS, and thus the optimal revisional procedure is still under investigation. This study aims to compare the outcomes after conversion of SG to RYGB versus SG to DS.
Materials and methods
Our community hospital began performing revisional SG to RYGB surgery in May 2015 and revisional SG to DS surgery in April 2018. We performed a retrospective chart review of our bariatric procedure database as well as our electronic medical records from May 1, 2015 through August 31, 2021 based on ICD-10 codes and found 75 patients who underwent conversion from prior SG to either RYGB or DS. Of the 75 patients, 40 were SG to RYGB revisional surgery patients and 35 were SG to DS revisional surgery patients. No distinction was made between BPD-DS patients (32) and SADI-S patients (3) for this study, with both grouped under the DS category. For these 75 patients, we collected their pre-operative weight before their revisional surgery, as well as their post-operative weights from their 3-month, 6-month, 12-month, and 24-month office visits. Chart review was performed to evaluate indications for surgery. Additional data were also collected on all patients for secondary outcomes, including length of stay, length of operation, emergency department (ED) visits within 30 days, readmission rates within 30 days, and any operative intervention within the 24-month follow-up period.
Pre-operative workup included a thorough bariatric program with monthly extensive nutritional counseling with on-site registered dieticians for 3–6 months, psychiatric evaluation and clearance, appropriate cardiac and medical clearances, and counseling on dietary changes. All patients in the study had their revisional surgery performed by one of two bariatric surgeons using the same surgical technique. All procedures were performed in a laparoscopic fashion, with no patients converted to open. For the RYGB patients, the Roux limbs were 150 cm in length and the biliopancreatic limbs were 80 cm in length. The gastric pouch was 30 mL in volume and created by inflating a 30-mL balloon within the stomach. For the DS patients, the common channels were 150 cm in length, and the Roux limbs were 200 cm in length. The patients from both subgroups were given the same perioperative care. All patients except for one single patient had their surgeries performed at the same community hospital. The outlier patient was included because the surgery was performed by the same two surgeons at a nearby community hospital in the same hospital system, and the patient underwent the same perioperative care at that other hospital. Post-procedure upper endoscopies were not routinely performed within our practice for all revisional bariatric patients, and they were only performed when patients complained of symptoms concerning for peptic ulcer disease.
The weights of 36 RYGB and 26 DS patients at the 3-month interval, 29 RYGB and 17 DS patients at the 6-month interval, 28 RYGB and 12 DS patients at the 12-month interval, and 27 RYGB and 7 DS patients at the 24-month interval were collected. Their age at the time of surgery, sex, and height were used to calculate ideal body weight. The patient’s weight loss compared to the weight at the time of surgery was then calculated, as well as their percent excess body weight loss (%EBWL) and percent total body weight loss (%TWL). Of note, EBWL was calculated from the time of revisional surgery and not from the time of the initial sleeve gastrectomy, as many patients did not receive their initial sleeve at our community hospital with such data incomplete. The average %EBWL and %TWL were then calculated for each group at each time interval, and the EBWL and TWL for each subgroup of RYGB and DS were compared at each time interval using unpaired T tests with a 95% confidence interval (CI). To investigate whether significant weight loss occurred in each subgroup between each time interval, average %EBWL was also compared for individuals within each subgroup between each time interval, from 3–6 months, 6–12 months, and 12–24 months, using paired T tests with a 95% CI. Subgroup patient characteristics were compared using unpaired T tests and population proportion Z tests. Statistical analysis was performed using SPSS version 26.0.
Results
Out of 75 total patients, 65 were female and 10 were male (Table 1). Among the 40 RYGB patients, 35 were female and 5 were male, with a mean age of 45.9 ± 10.3 (range 27–67). Among the 35 DS patients, 30 were female and 5 were male, with a mean age of 45.3 ± 9.4 (range 26–65). For the RYGB group, 90% (36/40) were African American patients and 10% (4/40) were Caucasian patients. For the DS group, 77.1% (27/35) were African American patients, 20% (7/35) were Caucasian patients, and 2.9% (1/35) were other. Average BMI at time of surgery was 46.9 ± 7.6 (range 36.0–71.4) for RYGB and 49.8 ± 8.7 (range 33.8–72.0) with no statistically significant subgroup difference (P = 0.3589). At time of surgery, prevalence of comorbidities was compared between RYGB and DS, with 57.5% (23/40) vs 60% (21/35) for hypertension, 20% (8/40) vs 20% (7/35) for diabetes mellitus, and 30% (12/40) vs 42.9% (15/35) for obstructive sleep apnea. Indications for conversion to RYGB included inadequate weight loss/weight regain (33), gastroesophageal reflux disease (GERD) (1), and both (6), while indications for conversion to DS were all due to inadequate weight loss/weight regain (35). Of note, a single patient with BMI < 35 (BMI 33.8) underwent revisional DS due to multiple comorbidities, patient preference, and previous plan for DS as the second of a two-step procedure if initial sleeve gastrectomy did not create sufficient weight loss.
Table 1.
Patient characteristics of revisional RYGB and revisional DS subgroups
| Characteristics | n (%), RYGB subgroup | n (%), DS subgroup | P value, unpaired T-test |
|---|---|---|---|
| Total patients | 40 | 35 | |
| Male | 5 (12.5) | 5 (14.3) | |
| Female | 35 (87.5) | 30 (85.7) | |
| Age (in years) | |||
| Mean | 45.9 ± 10.3 | 45.3 ± 9.4 | 0.8058 |
| Median | 42.5 | 46 | |
| Range | 27–67 | 26–65 | |
| Race | |||
| Caucasian | 4 (10.0) | 7 (20.0) | |
| African American | 36 (90.0) | 27 (77.1) | |
| Other | 0 (0.0) | 1 (2.9) | |
| BMI at time of revision | |||
| Mean | 46.9 ± 7.6 | 49.8 ± 8.7 | 0.3589 |
| Median | 45.8 | 50.3 | |
| Range | 36.0–71.4 | 33.8–72.0 | |
| Comorbidities at time of revision | |||
| Hypertension | 23 (57.5) | 21 (60.0) | |
| Diabetes mellitus | 8 (20.0) | 7 (20.0) | |
| Obstructive sleep apnea | 12 (30.0) | 15 (42.9) |
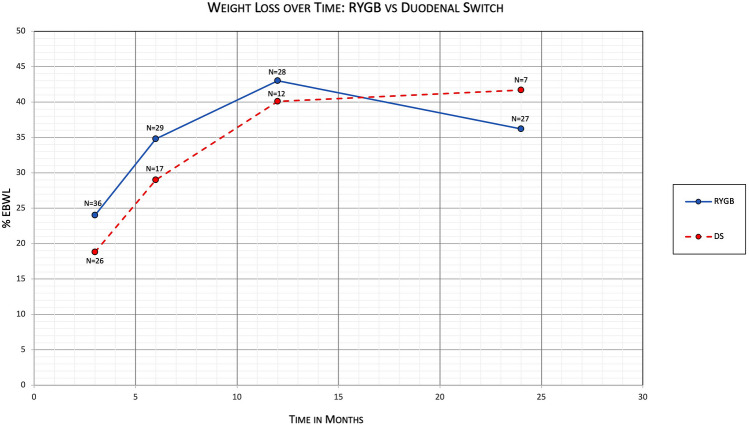
For the RYGB group, mean percentage of excess body weight loss (%EBWL) was 24.0% (95% CI 20.7–27.3) at 3 months (N = 36), 34.8% (95% CI 29.3–40.3) at 6 months (N = 29), 43.0% (95% CI 36.0–49.9) at 12 months (N = 28), and 36.2% (95% CI 27.9–44.5) at 24 months (N = 27) (Table 2). For the DS group, mean %EBWL was 18.8% (95% CI 14.5–23.0) at 3 months (N = 26), 29.0% (95% CI 20.5–37.5) at 6 months (N = 17), 40.1% (95% CI 24.7–55.5) at 12 months (N = 12), and 41.7% (95% CI 18.4–65.2) at 24 months (N = 7). Comparing %EBWL of patients with conversion to RYGB vs patients with conversion to DS, at 3 months there was a mean difference of 5.2 (95% CI 0.02 to 10.4, P = 0.0491); at 6 months there was a mean difference of 5.8 (95% CI − 3.6 to 15.2, P = 0.2192); at 12 months there was a mean difference of 2.8 (95% CI − 11.1 to 16.8, P = 0.6828); and at 24 months there was a mean difference of − 5.5 (95% CI − 24.4 to 13.3, P = 0.5553) (Fig. 1).
Table 2.
Primary outcome comparing %EBWL between RYGB and DS subgroups
| Time (months) | N (RYGB) | %EBWL (95% CI), RYGB subgroup | N (DS) | %EBWL (95% CI), DS subgroup | Mean diff (95% CI) | P value |
|---|---|---|---|---|---|---|
| 3 | 36 | 24.0 (20.7–27.3) | 26 | 18.8 (14.5–23.0) | 5.2 (0.02 to 10.4) | 0.0491 |
| 6 | 29 | 34.8 (29.3–40.3) | 17 | 29.0 (20.5–37.5) | 5.8 (− 3.6 to 15.2) | 0.2192 |
| 12 | 28 | 43.0 (36.0–49.9) | 12 | 40.1 (24.7–55.5) | 2.8 (− 11.1 to 16.8) | 0.6828 |
| 24 | 27 | 36.2 (27.9–44.5) | 7 | 41.7 (18.4–65.2) | − 5.5 (− 24.4 to 13.3) | 0.5553 |
Fig. 1.
Primary outcome comparing %EBWL between RYGB and DS subgroups over time
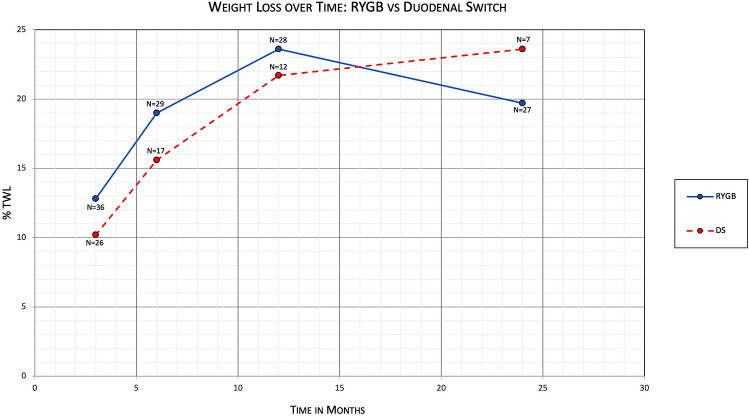
Mean percentage of total body weight loss (%TWL) was also calculated (Table 3). For the RYGB group, %TWL was 12.8% (95% CI 11.2–14.5) at 3 months (N = 36), 19.0% (95% CI 16.0–21.9) at 6 months (N = 29), 23.6% (95% CI 19.8–27.4) at 12 months (N = 28), and 19.7% (95% CI 15.1–24.4) at 24 months (N = 27). For the DS group, mean %TWL was 10.2% (95% CI 7.97–12.4) at 3 months (N = 26), 15.6% (95% CI 11.4–19.7) at 6 months (N = 17), 21.7% (95% CI 13.9–29.4) at 12 months (N = 12), and 23.6% (95% CI 11.9–35.3) at 24 months (N = 7). Comparing %TWL of patients with conversion to RYGB vs patients with conversion to DS, at 3 months there was a mean difference of 2.7 (95% CI 0.035 to 5.27, P = 0.0471); at 6 months there was a mean difference of 3.4 (95% CI − 1.47 to 8.23, P = 0.1673); at 12 months there was a mean difference of 1.9 (95% CI − 5.40 to 9.26, P = 0.5979); and at 24 months there was a mean difference of − 3.9 (95% CI − 14.2 to 6.46, P = 0.4521) (Fig. 2).
Table 3.
Primary outcome comparing %TWL between RYGB and DS subgroups
| Time (months) | N (RYGB) | %TWL (95% CI), RYGB subgroup | N (DS) | %TWL (95% CI), DS subgroup | Mean diff (95% CI) | P value |
|---|---|---|---|---|---|---|
| 3 | 36 | 12.8 (11.2–14.5) | 26 | 10.2 (7.97–12.4) | 2.7 (0.035 to 5.27) | 0.0471 |
| 6 | 29 | 19.0 (16.0–21.9) | 17 | 15.6 (11.4–19.7) | 3.4 (− 1.47 to 8.23) | 0.1673 |
| 12 | 28 | 23.6 (19.8–27.4) | 12 | 21.7 (13.9–29.4) | 1.9 (− 5.40 to 9.26) | 0.5979 |
| 24 | 27 | 19.7 (15.1–24.4) | 7 | 23.6 (11.9–35.3) | − 3.9 (− 14.2 to 6.46) | 0.4521 |
Fig. 2.
Primary outcome comparing %TWL between RYGB and DS subgroups over time
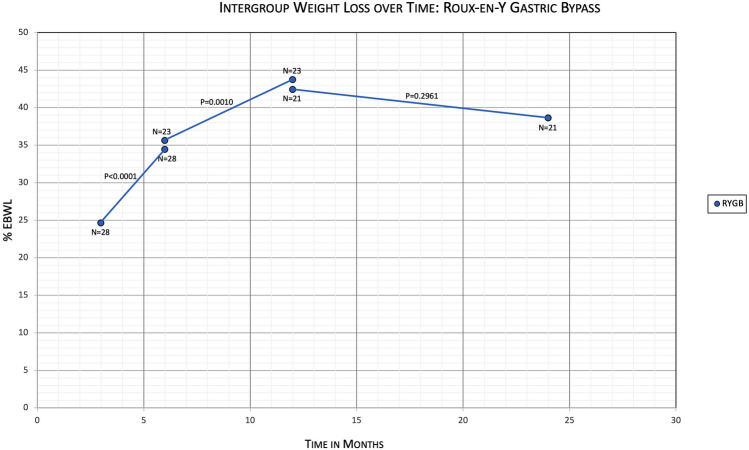
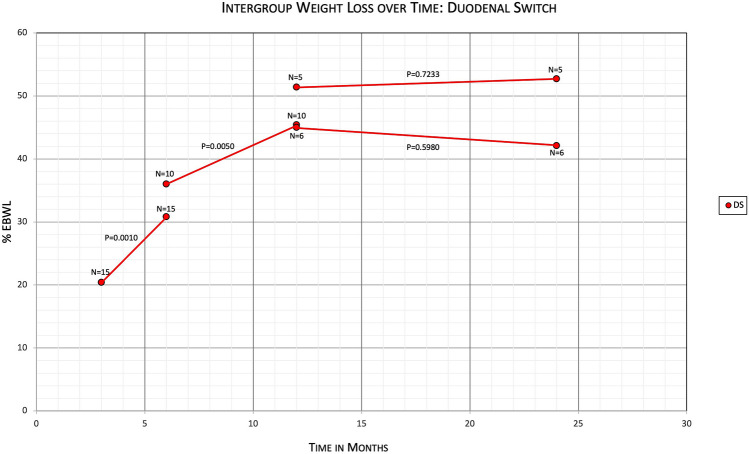
Within the RYGB group, when comparing %EBWL of patients at 3 months with the same patients at 6 months, the means were 24.6% and 34.4%, with a mean difference of − 9.8 (95% CI − 12.9 to − 6.7, N = 28, P < 0.0001) (Table 4). Comparing %EBWL of RYGB patients at 6 months with the same patients at 12 months, the means were 35.6% and 43.7%, with a mean difference of − 8.1 (95% CI − 12.5 to − 3.7, N = 23, P = 0.0010). And comparing %EBWL of RYGB patients at 12 months with the same patients at 24 months, the means were 42.4% and 38.6%, with a mean difference of 3.8 (95% CI − 3.6 to 11.3, N = 21, P = 0.2961) (Fig. 3). Within the DS group, when comparing %EBWL of patients at 3 months with the same patients at 6 months, the means were 20.4% and 30.8%, with a mean difference of − 10.4 (95% CI − 15.8 to −5.0, N = 15, P = 0.0010) (Table 5). Comparing %EBWL of DS patients at 6 months with the same patients at 12 months, the means were 36.0% and 45.4%, with a mean difference of − 9.5 (95% CI − 15.3 to − 3.7, N = 10, P = 0.0050). And comparing %EBWL of DS patients at 12 months with the same patients at 24 months, the means were 45.0% and 42.1%, with a mean difference of 2.9 (95% CI − 10.3 to 16.1, N = 6, P = 0.5980). There was an outlier patient when comparing DS patients at 12 months with 24 months who had %EBWL decrease from 13.2% to −10.9% within that time. If the outlier patient is removed, comparing %EBWL at 12 months with the same patients at 24 months, the means were 51.4% and 52.7%, with a mean difference of − 1.3 (95% CI − 11.2 to 8.5, N = 5, P = 0.7233) (Fig. 4).
Table 4.
Primary outcome comparing %EBWL of different time intervals within the RYGB subgroup
| Time interval (months) | N | %EBWL initial | %EBWL final | Mean diff (95% CI) | P value |
|---|---|---|---|---|---|
| 3 vs 6 | 28 | 24.6 | 34.4 | − 9.8 (− 12.9 to − 6.7) | < 0.0001 |
| 6 vs 12 | 23 | 35.6 | 43.7 | − 8.1 (− 12.5 to − 3.7) | 0.0010 |
| 12 vs 24 | 21 | 42.4 | 38.6 | 3.8 (− 3.6 to 11.3) | 0.2961 |
Fig. 3.
Primary outcomes evaluating %EBWL over time within the RYGB subgroup
Table 5.
Primary outcome comparing %EBWL of different time intervals within the DS subgroup
| Time interval (months) | N | %EBWL initial | %EBWL final | Mean diff (95% CI) | P-value |
|---|---|---|---|---|---|
| 3 vs 6 | 15 | 20.4 | 30.8 | − 10.4 (− 14.8 to − 5.0) | 0.0010 |
| 6 vs 12 | 10 | 36.0 | 45.4 | − 9.5 (− 15.3 to − 3.7) | 0.0050 |
| 12 vs 24 | 6 | 45.0 | 42.1 | 2.9 (− 10.3 to 16.1) | 0.5980 |
| 12 vs 24 (w/o outlier) | 5 | 51.4 | 52.7 | − 1.3 (− 11.2 to 8.5) | 0.7233 |
Fig. 4.
Primary outcomes evaluating %EBWL over time within the DS subgroup
Average length of procedure was 134.4 ± 35.2 (range 60–213) minutes for RYGB and 189.8 ± 48.9 (range 110–303) minutes for DS (Table 6). Comparing procedure length for RYGB to that of DS, there was a mean difference of − 55.4 min (P = 0.6032). Average length of stay (LOS) was 2.6 ± 1.4 (range 1–9) days for RYGB and 3.1 ± 2.2 (range 1–13) days for DS. Comparing LOS for RYGB to that of DS, there was a mean difference of − 0.46 days (P = 0.2731). There was an outlier patient for the DS subgroup that had a LOS of 13 days, more than twice the next highest LOS of 6 days, due to persistent abdominal pain and tachycardia. Patient eventually underwent diagnostic laparoscopy which was negative for pathology, and she subsequently improved and was discharged without further intervention. If the outlier patient is removed, average LOS would be 2.8 ± 1.3 (range 1–6) days for DS. Comparing LOS for RYGB to that of DS after outlier removal, there would a mean difference of − 0.16 days (P = 0.6032).
Table 6.
Secondary outcomes: Length of procedure, length of stay, 30-day readmission rate, 2-year reoperations, and complications
| n (%), RYGB subgroup | n (%), DS subgroup | P value | |
|---|---|---|---|
| Length of procedure (min) | |||
| Mean | 134.4 ± 35.2 | 189.8 ± 48.9 | < 0.0001 |
| Median | |||
| Range | 60–213 | 110–303 | |
| Length of stay (days) | |||
| Mean | 2.6 ± 1.4 | 3.1 ± 2.2 | 0.2731 |
| Median | 2 | 2 | |
| Range | 1–9 | 1–13 | |
| Length of stay (days) with DS outlier (13) removed | |||
| Mean | 2.6 ± 1.4 | 2.8 ± 1.3 | 0.6032 |
| Median | 2 | 2 | |
| Range | 1–9 | 1–6 | |
| Thirty-day readmission | 11 (27.5) | 5 (14.3) | 0.1645 |
| Nausea and vomiting | 6 | 2 | |
| Abdominal pain | 2 | 0 | |
| Other | 3 | 3 | |
| 2-year reoperations | 11 (27.5) | 3 (8.57) | 0.0357 |
| Cholecystectomy | 3 | 1 | |
| Gastrojejunal revision | 3 | 0 | |
| Diagnostic laparoscopy | 2 | 1 | |
| Internal hernia | 3 | 0 | |
| Perforated gastric ulcer | 1 | 1 | |
| Small bowel obstruction | 2 | 0 | |
| Other | 2 | 1 | |
| 2-year complications | |||
| Ulcers | 5 | 1 | |
| Strictures | 2 | 0 | |
| 2-year unrelated surgeries | 7 | 3 | |
| Elective hernia repair | 2 | 0 | |
| Orthopedic surgery | 3 | 2 | |
| Hidradenitis excision | 1 | 0 | |
| Lipoma excision | 0 | 1 | |
| Ureteral lithotripsy | 1 | 0 | |
Thirty-day readmission was 27.5% (11/40) for RYGB and 14.3% (5/35) for DS, with difference of 13.2% (P = 0.1645). Reasons for readmission included intractable nausea and vomiting (8), abdominal pain (2), and one each of fever, constipation, bowel obstruction (RYGB subgroup), perforated gastric ulcer, pulmonary embolism, and uncontrolled atrial fibrillation (DS subgroup). Of note, three readmissions for the RYGB subgroup were due to a single patient.
Patients requiring relevant reoperation within the 24 months of follow-up were 11/40 (27.5%) for RYGB and 3/35 (8.57%), with several patients requiring multiple operative interventions. Reasons for relevant reoperation included cholecystectomy (4), gastrojejunal (GJ) anastomotic revision (3), diagnostic laparoscopy (3), internal hernia (3, all single patient), gastric ulcer perforation with Graham patch (2), small bowel obstruction (2), and one each of GJ stricture requiring multiple endoscopic dilations, intra-abdominal abscess drainage (RYGB subgroup), and small bowel resection secondary to strangulated incisional hernia (DS subgroup). The RYGB patients had statistically significant higher numbers of patients requiring reoperation compared to DS patients (P = 0.0357). There were additional unrelated surgical procedures performed on the patients (Table 6), as well as one death in the RYGB subgroup due to cardiac arrest after septic shock from pneumonia.
One patient from each subgroup had a 30-day readmission requiring surgery, with the RYGB having SBO requiring J–J anastomotic revision, and DS having a perforated gastric ulcer with Graham patch, multiple takebacks for washout, and strangulated incisional hernia requiring small bowel resection within the same readmission hospital visit. All other readmissions were treated nonsurgically and discharged home. Ulcer formation occurred much more frequently in the RYGB subgroup and required more surgical intervention for ulcers, with eventually 3 patients requiring GJ revision. Ulcer formation occurred in 5 (12.5%) RYGB patients and 1 (2.9%) DS patient. Two (5.0%) RYGB patients developed anastomotic strictures, with only 1 requiring further intervention with serial dilations. No confirmed leaks were noted, although one RYGB patient had intra-abdominal collection adjacent to the J–J anastomosis requiring two drainage procedures with no leakage seen on multiple CTs with oral contrast nor intraoperatively.
Electronic medical records (EMR) indicate clinical visit follow-up for the RYGB subgroup was 35/40 (87.5%) at 3 months, 24/37 (64.9%) at 6 months, 26/37 (70.3%) at 12 months, and 17/34 (50%) at 24 months, while clinical visit follow-up for the DS subgroup was 29/33 (87.9%) at 3 months, 15/26 (57.7%) at 6 months, 12/16 (75.0%) at 12 months, and 5/9 (55.6%) at 24 months. Of note, EMR was incomplete before 2017, potentially affecting RYGB but not DS data. The denominator (patient pool) varied depending on the number of patients who could potentially have the clinical visit follow-up, as certain patients were unable to due to recent surgery without the full 24-month follow-up.
Discussion
Both groups had significant weight loss from 3 to 6 months and from 6 to 12 months, with no significant weight change thereafter, plateauing at approximately 40% EBWL and 22% TWL for both groups. This demonstrates significant and effective weight loss for revisional bariatric procedures during the first year after surgery, with sustained weight loss during the second year after surgery. Certain studies show 35–55% EBWL after revisional surgery [9], and other studies showed %TWL ranging from 6.9 to 41.25% [11]. Care must be taken when comparing between studies, as certain studies seem to calculate %EBWL from the patient’s weight prior to the sleeve gastrectomy rather than prior to the revisional surgery [12], resulting in 65–80% EBWL. Furthermore, many of these studies with high %EBWL have lower patient numbers and are more highly selective. However, our weight loss at two years is still equivalent to multiple studies for revisional surgery, showing that revisional surgeries performed at a community hospital are efficacious.
Statistical significance was also observed between both groups at 3 months, with increased RYGB %EBWL compared to DS %EBWL. However, no statistically significant difference was observed at follow-up times of 6 months, 12 months, and 24 months. This is contradictory to some other studies that have demonstrated higher percentage of total weight loss for patients undergoing the DS procedure when compared directly to the RYGB. However, this may be due to prior studies having higher pre-revisional procedure BMI for DS patients compared to RYGB patients, as well as longer follow-up times [11, 12]. Our patients had statistically equivalent BMIs prior to the revisional procedure. Additionally, this difference might also be attributed to the combined restrictive and malabsorptive effects of the RYGB procedure causing greater initial weight loss, compared with the solely malabsorptive effect of the DS procedure. Although both groups of patients are given the same post-operative bariatric diet regimen, the stomach is not re-sleeved or revised for DS patients, allowing them to continue eating similar quantities of food before and after surgery. Because RYGB patients are given a 30-mL gastric pouch, they initially have greater feelings of satiety after surgery, likely causing a greater initial effect on weight loss compared to DS patients. Eventually, the larger malabsorptive effect of the DS procedure, which has a much shorter common channel and alimentary tract compared to the RYGB procedure, likely compensates for the lack of restrictive effect, causing no statistically significant difference in %EBWL afterward between the two groups.
Mean %TWL was also calculated for comparison between revisional RYGB and revisional DS. However, there is no significant difference between %EBWL and %TWL for this comparison, validating no statistical significance between the two groups in terms of BMI and confirming that %EBWL is an accurate measurement of the two subgroups’ weight losses.
For secondary outcomes, length of procedure was statistically significant between the two groups, with DS procedure length greater than RYGB procedure length. The DS procedure is a newer procedure, and our bariatric surgeons first began performing this procedure in 2018, creating a greater learning curve for DS compared to the RYGB and likely contributing to increased operating time. Further research can be performed to see if the length of operation decreases as surgeon experience increases. Other secondary outcomes showed no statistical significance between the two groups. Despite greater operative length with DS procedures, there was no difference between length of stay for the two groups. There was also no statistical significance between the groups for 30-day readmission rate, although there was a trend toward greater readmission rate for the RYGB patients. This likely reflects the greater complication rate and reoperation rate for RYGB revisions, which is seen and is statistically significant, as well as possibly more initial symptomatic complaints compared to the DS procedure, a possible reflection of the restrictive nature of the RYGB procedure. Overall, our readmission and adverse event rates seem similar to other studies, which report 31–33% for revisional RYGB and 28% for revisional DS [11, 13], showing that revisional bariatric surgery can be safely performed at community hospitals.
The advantage of this study is that it encompassed a single institution, with two bariatric surgeons who performed procedures with the same surgical technique and gave patients the same perioperative care and follow-up. This decreased the variability that would often be observed across different institutions, especially regarding Roux limb, BP limb, and common channel length as well as perioperative care. Because patients had the same nursing care, operating room staff, and decision-making regarding discharge disposition, there was decreased variability with regards to LOS and procedure length.
Limitations of this study include being a retrospective study rather than a prospective study, with randomization not able to be performed due to multiple factors. Patients with severe gastroesophageal reflux disease would preferentially undergo revisional RYGB to better relieve reflux disease and symptoms [14], while those with a strong tobacco history would preferentially undergo revisional DS, as relapse rates are high [15] and patients who smoke after undergoing RYGB procedure have a high rate of marginal ulcers and related complications [16, 17]. Certain patient insurances also do not cover the DS procedure. Additional limitations include short duration and low power. Revisional RYGB procedures were started in 2015, while revisional DS procedures were started in 2018. Accordingly, 26/35 (74.3%) of DS procedures were performed after August 31, 2019, not allowing the full 24-month duration of follow-up due to our study cutoff date of August 31, 2021. After adjusting for this, clinical visit follow-ups were above 50% for both groups at 24 months. Furthermore, there were multiple disruptions in bariatric surgical procedures and follow-up since March 2020 due to the COVID-19 pandemic. As the pandemic becomes better controlled with fewer OR schedule disruptions and better follow-up, future studies will be able to have larger patient populations to observe with longer duration, resulting in higher powered data.
In conclusion, performing revisional laparoscopic Roux-en-Y gastric bypass and revisional laparoscopic duodenal switch after laparoscopic sleeve gastrectomy at a community hospital is safe and effective, achieving statistically significant and sustained weight loss up to 12 months. Revisional RYGB has a statistically significant increase in weight loss compared to revisional DS over the first three months, but no statistically significant difference is observed afterward, showing equivalent excess body weight loss between patients undergoing the two procedures. Statistically significant difference was also noted for length of procedure, with revisional DS having longer operating times compared to revisional RYGB. However, no statistically significant difference was observed with length of stay or 30-day readmission rate, and complication rates were consistent with prior studies. Future research is needed to increase power and duration of this study, to better delineate any differences between procedures, to evaluate long-term trends, and to assess resolution of pre-procedural comorbidities. Other potential studies include comparing revisional BPD-DS and revisional SADI-S, measuring and changing Roux limb, BP limb, and alimentary limb lengths for RYGB, or observing the effect of metabolic agents such as phentermine or GLP-1 agonists on augmenting or decreasing weight loss after revisional bariatric procedures.
Declarations
Disclosures
Drs. Jeremy Jen, Hau Phan, Brett Johnson, and Piotr Krecioch have no conflicts of interest or financial ties to disclose. Medical students Corliann Blyn, Janet Lavrich, Krishna Mallem, and Priya Kalsank Pai have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention (nd) Adult Obesity Facts [Fact sheet]. https://www.cdc.gov/obesity/data/adult.html. Accessed 9 Mar 2022
- 2.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–424. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Society for Metabolic and Bariatric Surgery (2021) Estimate of Bariatric Surgery Numbers, 2011–2019 [Fact sheet]. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed 19 Mar 2022.
- 4.Azagury D, Papasavas P, Hamdallah I, Gagner M, Kim J. ASMBS position statement on medium- and long-term durability of weight loss and diabetic outcomes after conventional stapled bariatric procedures. Surg Obes Relat Dis. 2018;14:1425–1441. doi: 10.1016/j.soard.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Anderson B, Gill RS, de Gara CJ, Karmali S, Gagner M. Biliopancreatic diversion: the effectiveness of duodenal switch and its limitations. Gastroenterol Res Pract. 2013 doi: 10.1155/2013/974762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan B, Chong TH, Peng J, Chen Y, Wang C, Yang J. Mid-long-term revisional surgery after sleeve gastrectomy: a systematic review and meta-analysis. Obes Surg. 2019;29:1965–1975. doi: 10.1007/s11695-019-03842-3. [DOI] [PubMed] [Google Scholar]
- 7.El Ansari W, Elhag W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps—a scoping review. Obes Surg. 2021;31:1755–1766. doi: 10.1007/s11695-020-05160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andalib A, Alamri H, Almuhanna Y, Bouchard P, Demyttenaere S, Court O. Short-term outcomes of revisional surgery after sleeve gastrectomy: a comparative analysis of re-sleeve, Roux en-Y gastric bypass, duodenal switch (Roux en-Y and single-anastomosis) Surg Endosc. 2021;35:4644–4652. doi: 10.1007/s00464-020-07891-z. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu H, Annaberdyev S, Motamarry I, Kroh M, Schauer PR, Brethauer SA. Revisional bariatric surgery for unsuccessful weight loss and complications. Obes Surg. 2013;23:1766–1773. doi: 10.1007/s11695-013-1012-1. [DOI] [PubMed] [Google Scholar]
- 10.Hakan S, Halil A. Is revisional bariatric surgery effective as primary surgery? Obes Surg. 2020;30:1219–1229. doi: 10.1007/s11695-019-04280-x. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Ellenbogen Y, Doumouras AG, Gmora S, Anvari M, Hong D. Single- or double-anastomosis duodenal switch versus Roux-en-Y gastric bypass as a revisional procedure for sleeve gastrectomy: a systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15:556–566. doi: 10.1016/j.soard.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Carmeli I, Golomb I, Sadot E, Kashtan H, Keidar A. Laparoscopic conversion of sleeve gastrectomy to a biliopancreatic diversion with duodenal switch or a Roux-en-Y gastric bypass due to weight loss failure: our algorithm. Surg Obes Relat Dis. 2015;11:79–85. doi: 10.1016/j.soard.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Casillas RA, Um SS, Getty JLZ, Sachs S, Kim BB. Revision of primary sleeve gastrectomy to Roux-en-Y gastric bypass: indications and outcomes from a high-volume center. Surg Obes Relat Dis. 2016;12:1817–1825. doi: 10.1016/j.soard.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 14.El-Hadi M, Birch DW, Gill RS, Karmali S. The effect of bariatric surgery on gastroesophageal reflux disease. Can J Surg. 2014;57:139–144. doi: 10.1503/cjs.030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Rodríguez O, Secades-Villa R, Flórez-Salamanca L, Okuda M, Liu SM, Blanco C. Probability and predictors of relapse to smoking: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend Rep. 2013;132:479–485. doi: 10.1016/j.drugalcdep.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spaniolas K, Yang J, Crowley S, Yin D, Docimo S, Bates AT, Pryor AD. Association of long-term anastomotic ulceration after Roux-en-Y gastric bypass with tobacco smoking. JAMA Surg. 2018;153:862–864. doi: 10.1001/jamasurg.2018.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittrich L, Schwenninger MV, Dittrich K, Pratschke J, Aigner F, Raakow J. Marginal ulcers after laparoscopic Roux-en-Y gastric bypass: analysis of the amount of daily and lifetime smoking on postoperative risk. Surg Obes Relat Dis. 2020;16:389–396. doi: 10.1016/j.soard.2019.11.022. [DOI] [PubMed] [Google Scholar]