Abstract
Studies indicate that West African and Congo basin isolates of monkeypox virus (MPXV) are genetically distinct. Here, we show Congo basin MPXV-ZAI-V79 is more virulent for cynomolgus monkeys as compared to presumed West African MPXV-COP-58. This finding may explain the lack of case-fatalities in the U.S. 2003 monkeypox outbreak, which was caused by a West African virus. Virulence differences between West African and Congo basin MPXV are further supported by epidemiological analyses that observed a similar prevalence of antibodies in non-vaccinated humans in both regions, while >90% of reported cases occurred in the Congo basin, and no fatal cases were observed outside of this region. To determine the basis for this difference in virulence, we sequenced the genomes of one human West African isolate, and two presumed West African isolates and compared the sequences to Congo basin MPXV-ZAI-96-I-16. The analysis identified D10L, D14L, B10R, B14R, and B19R as possible virulence genes, with D14L (ortholog of vaccinia complement protein) as a leading candidate.
Keywords: Monkeypox, Genomic sequences, Genetic diversity, Virulence genes, Non-human primates
Introduction
MPXV causes a smallpox-like disease in humans and may threaten the population either as a zoonotic infection or through a criminal event. The case-fatality rate of human monkeypox in a 1980s prospective study in the Democratic Republic of the Congo (DRC former Zaire) was approximately 10% (Jezek and Fenner, 1988), compared to variola virus (VARV) smallpox, which ranged from >1% to about 15% in Africa and to approximately 30% in Asia (Fenner et al., 1988). Unlike smallpox, which had secondary attack rates ranging to 60%, human monkeypox during the 1980s prospective study was about 10%, with interhuman transmission rarely over two or three generations (Jezek et al., 1986). Recent retrospective studies suggest that disease incidence is increasing due to encroachment of humans into habitats of animal reservoirs for MPXV (Mukinda et al., 1997, Hutin et al., 2001). Also, the first outbreak in the Western hemisphere occurred in the U.S. Midwest from April to June of 2003 (Reed et al., 2004). MPXV entered the U.S. in a shipment of African rodents from Ghana (West Africa) destined for the pet trade. At a pet distribution center, prairie dogs became infected and in turn were responsible for 72 confirmed or suspected cases of human monkeypox. Unlike African outbreaks, the U.S. outbreak resulted in no fatalities and there was no documented human-to-human transmission (Reed et al., 2004). This less severe epizootic could be due to higher natural resistance of the U.S. population, a healthier patient population lacking background infections, and/or better supportive care for patients. There is, however, a significant possibility that this variability in pathogenicity is secondary to strain-specific differences in the virulence of the infecting virus.
The U.S. Midwest isolates belong to the West African MPXV group which is genetically distinct from Congo basin isolates as determined by restriction fragment length polymorphisms (RFLP) and DNA sequencing analysis of the hemagglutinin and TNFR genes (Mukinda et al., 1997, Esposito and Knight, 1985, Esposito and Fenner, 2001). Virulence differences between West African and Congo basin MPXV are supported by epidemiological analyses that observed a similar prevalence of antibodies in non-vaccinated humans in both regions (Jezek and Fenner, 1988), while >90% of reported cases occurred in the Congo basin, and no fatal cases were observed outside of this region (Esposito and Fenner, 2001). In this study, we formally compare the virulence of West African and Congo basin isolates of MPXV in cynomolgus monkeys, and use comparative genomics to identify and characterize predicted genes that may be responsible for virulence differences.
Results and discussion
Virulence of West African and Congo basin isolates of MPXV in non-human primates
We examined the virulence of MPXV-COP-58 (COP-58) and a Congo basin isolate MPXV-ZAI-V79 (ZAI-V79) by aerosol infection of cynomolgus monkeys with high and low doses of virus. MPXV-COP-58, although derived from a primate shipped from Singapore to Copenhagen which developed disease 62 days after arriving in Copenhagen, is presumed to be of West African origin by virtue of restriction enzyme profiles (Esposito and Knight, 1985), geographically-restricted primate anti-orthopoxvirus seroprevalences (Arita et al., 1972), and historic inferences (Fenner et al., 1989). This supposition is strengthened by the sequence analysis provided here. MPXV-COP-58 caused no deaths and little morbidity following aerosol challenge, whereas infections with the Congo basin isolate resulted in severe morbidity at the low dose and uniform mortality at the high dose (Table 1 ). These data further support the contention that the Congo basin isolates are more virulent for humans than those from West Africa.
Table 1.
Aerosol infection of cynomolgus monkeys with West and Congo basin isolates monkeypox virus
| MPXV isolate | Aerosol dose (PFU/monkey)a | Morbidity (Day 7)b | Mortality | Mean day of death |
|---|---|---|---|---|
| COP-58 | 110 | 0/3 | 0/3 | – |
| 20,000 | 0/3c | 0/3 | – | |
| ZAI-V79 | 90 | 2/3 | 0/3 | – |
| 50,000 | 3/3 | 3/3 | 10 ± 1 |
An aerosol route of challenge was used, as it is possibly the major route for a person-to-person transmission of MPXV. The aerosol challenge particle size was 1–3 μm.
Exanthem, enanthem, cough, depression.
Late enanthem ∼10 days.
Genome sequencing of West African isolates SL-V70, WRAIR-61, and COP-58
To identify the putative genetic basis of this difference in virulence, we needed to acquire the genomic sequences of West African isolates and compare them to those from MPXV-ZAI-96-I-16 (ZAI-96), the sole Congo basin group member whose genomic sequence is available in GenBank (Shchelkunov et al., 2001, Shchelkunov et al., 2002). Therefore, we sequenced the genomes of West African isolates SL-V70, WRAIR-61, and COP-58 and obtained contiguous consensus sequences of 198,756, 199,195, and 199,469 nucleotides, respectively. The genomes were resolved to an average 4.8-fold or greater redundancy (lowest coverage 2-fold); we did not sequence the palindromic hairpin terminal loop (∼80 bp) of each end of the genome. The first nucleotide of each genomic sequence of SL-V70, WRAIR-61, and COP-58 was equivalent to nucleotide 160, 155, and 149 of the genomic sequence of VACV-COP, respectively. This position was 25, 30, and 36 nucleotides, respectively, beyond the end of the first primer used to amplify the sequencing templates.
Genetic diversity between West African and Congo basin isolates
Based upon multiple nucleic acid sequence alignments of the core conserved genomic region of each orthopoxvirus (OPV) species, we determined the evolutionary relationships between these viruses which are shown in Fig. 1A. To further examine the relatedness of the MPXV isolates, we compared the genome of West African isolate SL-V70 with the genomes of the other MPXV isolates. We chose SL-V70 as the prototypic West African isolate because it was isolated from a case of human monkeypox in Sierra Leone (Foster et al., 1972). Fig. 1B is a graphic representation of the substitutions, insertions, and deletions (InDel) observed between the aligned genomes. SL-V70 is more closely related to isolates WRAIR-61 and COP-58 than to ZAI-96. There are no differences in the set of genes predicted for SL-V70, COP-58, and WRAIR-61, and examination of the minor sequence differences among them does not support any biological consequence for these changes (Table S1 in Appendix A). A similar comparative analysis between COP-58 and WRAIR-61 shows that they are more closely related to each other than to SL-V70, but are distinguished from each other by the number and position of minor repeat elements and a series of polyA/T tracts distributed along the genome; these differences may be useful for distinguishing closely related isolates in molecular epidemiological studies (Supporting online data in Appendix A). Fig. 1C summarizes percent difference values for individual paired comparisons. The SL-V70, COP-58, and WRAIR-61 isolates show 0.55–0.56% difference with ZAI-96 as compared to 0.01–0.07% nucleotide difference among themselves. For comparison, we observe the following OPV intraspecies nucleotide difference values: 0.2% VARV (major strains Bangladesh-1975 [BSH-75] and India-1967); 0.4% VARV (BSH-75, major strain and Garcia-1966, minor strain); 1.1% VACV (strains WR and COP); 0.4% ECTV (NAV and MOS isolates), and a 0.1% camelpox virus (M-96 and CMS isolates). An interspecies comparison of SL-V70 with ECTV-MOS, VACV-COP, VARV-BSH-75, and CPXV-BR yields <4.9% nucleotide difference. These intraspecies nucleotide difference values are reflected in the branch length differences of the evolutionary tree shown in Fig. 1A. This high level of identity among OPVs is consistent with the genes having a similar function in all OPV. In summary, there is significantly greater sequence diversity between the West African SL-V70 and Congo basin ZAI-96 isolates than between SL-V70 and the two other presumptive West African isolates (COP-58 and WRAIR-61) indicating that analyzed West and Congo basin isolates belong to separate clades; this confirms and extends the MPXV RFLP studies of others (Mukinda et al., 1997, Douglass et al., 1994, Esposito and Knight, 1985).
Fig. 1.
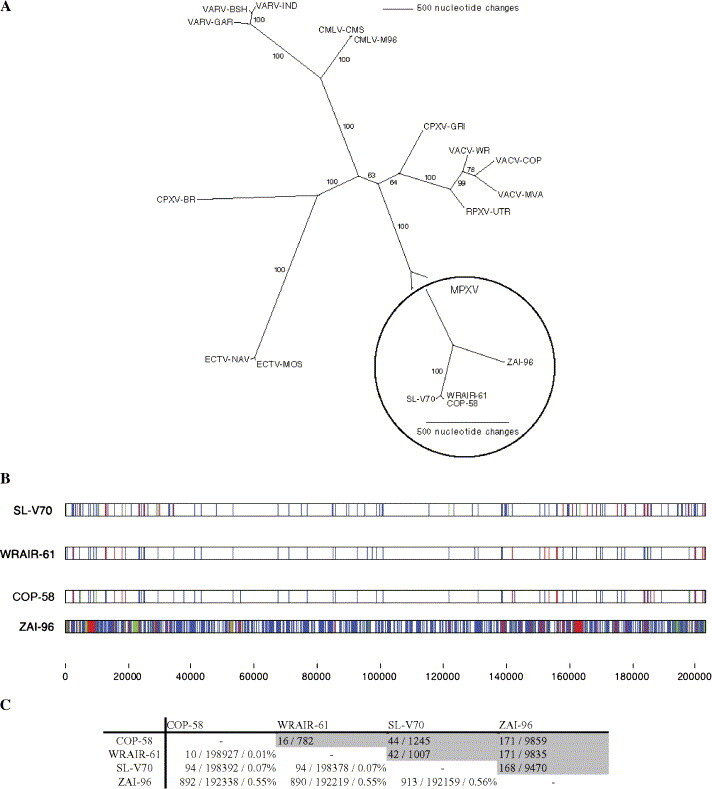
Genomic comparison of West African and Congo basin isolates of MPXV. (A) OPV phylogenetic predictions based upon the multiple nucleic acid sequence alignments of the core genomic region of each representative orthopoxvirus species, strain, or isolate. Bootstrap resampling confidence percentage based on 1000 replicates are displayed at each branch point. Branch lengths are proportional to the number of nucleotide changes. (B) CLUSTALW software was used to align the genomes of SL-V70, COP-58, WRAIR-61, and ZAI-96 and the alignment was manually optimized using Base-By-Base (Brodie et al., 2004). Each mismatched base was identified as a substitution (blue bar), deletion (red bar), or insertion (green bar) relative to a consensus; blue bars in all genomes indicate no consensus. InDels were counted as one mismatch regardless of size. The scale is such that several substitutions in close proximity may generate a single blue bar. (C) Summary of nucleotide difference comparisons: upper (grey) = gap number (segments) / total gap length; lower = number substitutions / number identical (non-gap) residues / percent difference (includes number of gaps).
Comparison of the West African SL-70 and Congo basin ZAI-96 genomes
The preceding data show that West African and Congo Basin MPXV isolates differ in virulence for cynomolgus monkeys, and that they are genetically distinct. To identify genes potentially responsible for the observed virulence difference, more extensive comparative analyses were carried out between West African SL-V70 and Congo basin ZAI-96. Within the SL-V70 genome, we predict 171 functional unique genes, 26 non-functional open reading frames (ORF) regions, and small vestiges of an additional 10 ORFs (Fig. 2 , Table 2, Table 3 ). The ZAI-96 genome is predicted to contain 173 unique genes (Table 2) and 16 truncated ORFs. SL-V70 and ZAI-96 share 170 unique orthologs that are on average more than 99.4% identical at the protein level. Comparisons of transcription regulatory sequences of SL-V70 and ZAI-96 ORFs revealed no major differences (Supporting online data in Appendix A).
Fig. 2.

Physical map of SL-V70. Predicted genes are numbered and shown as straight arrows; regions containing fragments of larger genes in other OPVs are shown with staggered arrows to represent frame changes and are labeled A–Z. Open arrowheads indicate an ORF split over 2 lines of the diagram. Scale is shown in kilobases. The thick line represents the ITR.
Table 2.
Predicted ORFs in SL-V70
| SL-70a |
ZAI-96 orthologb |
Predicted motif/function | ||||||
|---|---|---|---|---|---|---|---|---|
| Name | Lengthc | Startd | Stope | Name | Length | Identical | Identity (%) | |
| 1 | 246 | 1510 | 770 | J1L | 246 | 246 | 100.0 | SecP/CC-Chemokine BP (C23L/B29R)f |
| 2 | 349 | 2686 | 1637 | J2L | 348 | 345 | 98.9 | SecP/TNF BP (crmB) (BR005/226) |
| 3 | 590 | 4548 | 2776 | J3L | 587 | 581 | 99.2 | Ankyrin/unknown (BR-006/225) |
| 4 | 437 | 6008 | 4695 | D1L | 437 | 432 | 98.9 | Ankyrin/unknown (BR-017) |
| 5g | 176 | 6653 | 6123 | – | – | – | – | Unknown (BR-018) |
| 6g | 153 | 7863 | 7402 | – | – | – | – | Unknown (C16L/B22R) |
| 7 | 142 | 9076 | 9504 | D3R | 142 | 142 | 100.0 | Growth factor (C11R) |
| 8 | 242 | 11,081 | 11,809 | D5R | 242 | 240 | 99.2 | RING finger/apoptosis (MOS-012) |
| 9 | 126 | 12,392 | 12,012 | D6L | 126 | 126 | 100.0 | SecP/IL-18 BP (BSH-D7L) |
| 10 | 660 | 14,434 | 12,452 | D7L | 660 | 650 | 98.5 | CHO Host range (BSH-D8L) |
| 11 | 64 | 14,764 | 14,570 | D8L | 64 | 63 | 98.4 | Retroviral pseudoprotease (BR-026) |
| 12 | 630 | 16,803 | 14,911 | D9L | 630 | 626 | 99.4 | Ankyrin (C9L) |
| 13 | 167 | 17,964 | 17,461 | D10L | 150 | 147 | 98.0 | Host range (C7L) |
| 14 | 159 | 18,617 | 18,138 | D11L | 153 | 151 | 99.3 | Unknown (C6L) |
| 15 | 206 | 19,381 | 18,761 | D12L | 206 | 204 | 99.0 | Unknown (C5L) |
| 16 | 316 | 20,376 | 19,426 | D13L | 315 | 312 | 98.7 | IL-1 receptor antagonist (C4L) |
| – | – | – | – | D14Lh | 216 | – | – | Inhibitor of complement enzymes (C3L) |
| 17 | 214 | 21,561 | 20,917 | D19L | 214 | 213 | 99.5 | Unknown (C1L) |
| 18 | 117 | 21,960 | 21,607 | P1L | 117 | 117 | 100.0 | Cytoplasmic P/virulence (N1L) |
| 19 | 177 | 22,620 | 22,087 | P2L | 177 | 176 | 99.4 | α-Amanitin sensitivity (N2L) |
| 20 | 446 | 23,988 | 22,648 | O1L | 442 | 439 | 99.3 | Ankyrin/unknown (M1L) |
| 21 | 220 | 24,720 | 24,058 | O2L | 220 | 218 | 99.1 | Unknown (M2L) |
| 22 | 284 | 25,679 | 24,825 | C1L | 284 | 283 | 99.7 | Ankyrin/host range (K1L) |
| 23 | 374 | 27,422 | 26,298 | C2L | 375 | 373 | 99.5 | Serpin (SPI-3)/unknown (K2L) |
| 24 | 424 | 29,041 | 27,767 | C4L | 424 | 423 | 99.8 | Phospholipase D-like/unknown (K4L) |
| 25 | 276 | 29,899 | 29,069 | C5L | 276 | 274 | 99.3 | MG lipase-like/unknown (BR045) |
| 26 | 149 | 30,035 | 30,484 | C6R | 149 | 146 | 98.0 | Unknown (K7R) |
| 27 | 219 | 31,206 | 30,547 | C7L | 219 | 216 | 98.6 | Apoptosis inhibitor (F1L) |
| 28 | 151 | 31,673 | 31,218 | C8L | 151 | 151 | 100.0 | dUTPase (F2L) |
| 29 | 492 | 33,148 | 31,670 | C9L | 487 | 477 | 98.4 | Kelch-like/unknown (F3L) |
| 30 | 319 | 34,118 | 33,159 | C10L | 319 | 319 | 100.0 | R. Reductase-small (F4L) |
| 31 | 343 | 35,180 | 34,149 | C11L | 343 | 339 | 98.8 | Major membrane protein (F5L) |
| 32 | 73 | 35,358 | 35,137 | C12L | 73 | 73 | 100.0 | Unknown (F6L) |
| 33 | 74 | 35,598 | 35,374 | C13L | 74 | 74 | 100.0 | Unknown (F7L) |
| 34 | 64 | 35,944 | 35,750 | C14L | 64 | 64 | 100.0 | Proline rich P/unknown (F8L) |
| 35 | 212 | 36,639 | 36,001 | C15L | 212 | 212 | 100.0 | Putative MP/unknown (F9L) |
| 36 | 439 | 37,945 | 36,626 | C16L | 439 | 438 | 99.8 | Ser/Thr kinase/morphogen (F10L) |
| 37 | 354 | 39,032 | 37,968 | C17L | 354 | 353 | 99.7 | Unknown (F11L) |
| 38 | 635 | 40,983 | 39,076 | C18L | 635 | 630 | 99.2 | IEV, actin tail, microtubule inter. (F12L) |
| 39 | 372 | 42,144 | 41,026 | C19L | 372 | 370 | 99.5 | Phospholipase/EEV (F13L) |
| 40 | 73 | 42,383 | 42,162 | C20L | 73 | 73 | 100.0 | Unknown (F14L) |
| 41 | 158 | 43,131 | 42,655 | C21L | 158 | 158 | 100.0 | Unknown (F15L) |
| 42 | 231 | 43,833 | 43,138 | C22L | 231 | 231 | 100.0 | MP/unknown (F16L) |
| 43 | 101 | 43,896 | 44,201 | C23R | 101 | 101 | 100.0 | IMV, DNA bound PP (F17R) |
| 44 | 479 | 45,637 | 44,198 | F1L | 479 | 477 | 99.6 | Poly(A) pol large (E1L) |
| 45 | 737 | 47,847 | 45,634 | F2L | 737 | 736 | 99.9 | Unknown (E2L) |
| 46 | 153 | 48,432 | 47,971 | F3L | 153 | 153 | 100.0 | PKR/OAS inhibitor (E3L) |
| 47 | 259 | 49,377 | 48,598 | F4L | 259 | 257 | 99.2 | RNA pol (RPO30) VITF-01 (E4L) |
| 48 | 567 | 50,150 | 51,853 | F5R | 567 | 565 | 99.7 | Unknown (E6R) |
| 49 | 166 | 51,935 | 52,435 | F6R | 166 | 166 | 100.0 | Myristyl MP/EEV (E7R) |
| 50 | 273 | 52,539 | 53,360 | F7R | 273 | 272 | 99.6 | ER-localized MP/unknown (E8R) |
| 51 | 1006 | 56,387 | 53,367 | F8L | 1006 | 1002 | 99.6 | DNA pol (E9L) |
| 52 | 95 | 56,419 | 56,706 | F9R | 95 | 93 | 97.9 | IMV, -S-S-bond PW (E10R) |
| 53 | 129 | 57,090 | 56,701 | F10L | 129 | 129 | 100.0 | IMV, core (E11L) |
| 54 | 665 | 59,074 | 57,077 | Q1L | 665 | 663 | 99.7 | MP/unknown (O1L) |
| 55 | 108 | 59,447 | 59,121 | Q2L | 108 | 108 | 100.0 | Glutaredoxin/unknown (O2L) |
| 56 | 312 | 60,532 | 59,594 | I1L | 312 | 312 | 100.0 | IMV, core, morphogen (I1L) |
| 57 | 73 | 60,760 | 60,539 | I2L | 73 | 73 | 100.0 | MP/unknown (I2L) |
| 58 | 269 | 61,570 | 60,761 | I3L | 269 | 268 | 99.6 | DNA-binding PP (I3L) |
| 59 | 771 | 63,967 | 61,652 | I4L | 771 | 764 | 99.1 | R. Reductase-large (I4L) |
| 60 | 79 | 64,235 | 63,996 | I5L | 79 | 79 | 100.0 | MP/IMV (I5L) |
| 61 | 382 | 65,402 | 64,254 | I6L | 382 | 381 | 99.7 | Telomere BP (I6L) |
| 62 | 423 | 66,666 | 65,395 | I7L | 423 | 423 | 100.0 | IMV, core, CP (I7L) |
| 63 | 676 | 66,672 | 68,702 | I8R | 676 | 675 | 99.9 | RNA helicase, NPH-II (I8R) |
| 64 | 591 | 70,481 | 68,706 | G1L | 591 | 587 | 99.5 | Metalloprotease (G1L) |
| 65 | 220 | 70,807 | 71,469 | G3R | 220 | 220 | 100.0 | VLTF (G2R) |
| 66 | 111 | 70,813 | 70,478 | G2L | 111 | 111 | 100.0 | SecP/unknown (G3L) |
| 67 | 124 | 71,813 | 71,439 | G4L | 124 | 124 | 100.0 | IMV, -S-S-bond PW (G4L) |
| 68 | 434 | 71,816 | 73,120 | G5R | 434 | 431 | 99.3 | Unknown (G5R) |
| 69 | 63 | 73,129 | 73,320 | G6R | 63 | 63 | 100.0 | RNA pol (RPO7) (G5.5R) |
| 70 | 165 | 73,320 | 73,817 | G7R | 165 | 162 | 98.2 | Unknown (G6R) |
| 71 | 371 | 74,897 | 73,782 | G8L | 371 | 370 | 99.7 | IMV, core, matrix (G7L) |
| 72 | 260 | 74,928 | 75,710 | G9R | 260 | 260 | 100.0 | VLTF-1 (G8R) |
| 73 | 340 | 75,730 | 76,752 | G10R | 340 | 340 | 100.0 | Myristyl MP/unknown (G9R) |
| 74 | 250 | 76,753 | 77,505 | M1R | 250 | 250 | 100.0 | Myristyl MP/IMV (L1R) |
| 75 | 92 | 77,537 | 77,815 | M2R | 92 | 91 | 98.9 | MP/unknown (L2R) |
| 76 | 344 | 78,825 | 77,791 | M3L | 344 | 343 | 99.7 | Unknown (L3L) |
| 77 | 251 | 78,850 | 79,605 | M4R | 251 | 251 | 100.0 | IMV, core, ssDNA binding (L4R) |
| 78 | 128 | 79,615 | 80,001 | M5R | 128 | 128 | 100.0 | MP/unknown (L5R) |
| 79 | 152 | 79,958 | 80,416 | L1R | 152 | 151 | 99.3 | MP/IMV, morphogen (J1R) |
| 80 | 177 | 80,436 | 80,969 | L2R | 177 | 175 | 98.9 | Thymidine kinase (J2R) |
| 81 | 333 | 81,035 | 82,036 | L3R | 333 | 331 | 99.4 | Poly(A) poly-small (VP39) (J3R) |
| 82 | 185 | 81,951 | 82,508 | L4R | 185 | 185 | 100.0 | RNA pol (RPO22) (J4R) |
| 83 | 133 | 82,971 | 82,570 | L5L | 133 | 133 | 100.0 | MP/unknown (J5L) |
| 84 | 1286 | 83,078 | 86,938 | L6R | 1286 | 1278 | 99.4 | RNA pol (RPO147) (J6R) |
| 85 | 171 | 87,450 | 86,935 | H1L | 171 | 171 | 100.0 | Tyr/Ser phosphatase/unknown (H1L) |
| 86 | 189 | 87,464 | 88,033 | H2R | 189 | 188 | 99.5 | MP/unknown (H2R) |
| 87 | 324 | 89,011 | 88,037 | H3L | 324 | 322 | 99.4 | MP/IMV (H3L) |
| 88 | 795 | 91,399 | 89,012 | H4L | 795 | 791 | 99.5 | RNA pol assoc P 94 (H4L) |
| 89 | 210 | 91,584 | 92,216 | H5R | 213 | 208 | 97.7 | VLTF-4 (H5R) |
| 90 | 314 | 92,217 | 93,161 | H6R | 314 | 313 | 99.7 | DNA topo type I (H6R) |
| 91 | 144 | 93,199 | 93,633 | H7R | 146 | 143 | 99.3 | MP/unknown (H7R) |
| 92 | 845 | 93,677 | 96,214 | E1R | 845 | 843 | 99.8 | Capping enzyme-large (D1R) |
| 93 | 233 | 96,606 | 97,307 | E3R | 233 | 233 | 100.0 | IMV, core (D3R) |
| 94 | 146 | 96,613 | 96,173 | E2L | 146 | 146 | 100.0 | IMV, core (D2L) |
| 95 | 218 | 97,307 | 97,963 | E4R | 218 | 218 | 100.0 | Uracil-DNA glycosylase (D4R) |
| 96 | 785 | 97,995 | 100,352 | E5R | 785 | 784 | 99.9 | N. triphosphat./DNA replication (D5R) |
| 97 | 637 | 100,392 | 102,305 | E6R | 637 | 635 | 99.7 | VETF-small (D6R) |
| 98 | 161 | 102,332 | 102,817 | E7R | 161 | 161 | 100.0 | RNA pol (RPO18) (D7R) |
| 99 | 304 | 103,694 | 102,780 | E8L | 304 | 302 | 99.3 | MP/IMV, attach (D8L) |
| 100 | 213 | 103,736 | 104,377 | E9R | 213 | 213 | 100.0 | MutT-like/unknown (D9R) |
| 101 | 248 | 104,374 | 105,120 | E10R | 248 | 248 | 100.0 | MutT-like/unknown (D10R) |
| 102 | 631 | 107,016 | 105,121 | E11L | 631 | 627 | 99.4 | NPH-I/IMV (D11L) |
| 103 | 287 | 107,914 | 107,051 | E12L | 287 | 287 | 100.0 | Capping enzyme-small (D12L) |
| 104 | 551 | 109,600 | 107,945 | E13L | 551 | 548 | 99.5 | IMV, morphogen, rif resist (D13L) |
| 105 | 150 | 110,076 | 109,624 | A1L | 150 | 150 | 100.0 | VLTF-2 (A1L) |
| 106 | 224 | 110,771 | 110,097 | A2L | 224 | 223 | 99.6 | VLTF-3 (A2L) |
| 107 | 77 | 111,001 | 110,768 | A3L | 77 | 77 | 100.0 | Thioredoxin/-S-S-bond PW (A2.5L) |
| 108 | 644 | 112,950 | 111,016 | A4L | 644 | 644 | 100.0 | IMV, core, precursor of p4b (A3L) |
| 109 | 281 | 113,848 | 113,003 | A5L | 281 | 278 | 98.9 | IMV, matrix, morphogen (A4L) |
| 110 | 161 | 113,886 | 114,371 | A6R | 161 | 161 | 100.0 | RNA pol (RPO19) (A5R) |
| 111 | 372 | 115,486 | 114,368 | A7L | 372 | 372 | 100.0 | Unknown (A6L) |
| 112 | 710 | 117,642 | 115,510 | A8L | 710 | 709 | 99.9 | VETF-large (A7L) |
| 113 | 292 | 117,696 | 118,574 | A9R | 292 | 290 | 99.3 | VITF-3-S (A8R) |
| 114 | 112 | 118,893 | 118,555 | A10L | 100 | 99 | 88.4 | MP/IMV, morphogen (A9L) |
| 115 | 891 | 121,569 | 118,894 | A11L | 891 | 888 | 99.7 | IMV, core, precursor of p4a (A10L) |
| 116 | 318 | 121,584 | 122,540 | A12R | 318 | 318 | 100.0 | MP/unknown (A11R) |
| 117 | 190 | 123,114 | 122,542 | A13L | 190 | 190 | 100.0 | IMV, core (A12L) |
| 118 | 70 | 123,350 | 123,138 | A14L | 70 | 70 | 100.0 | MP/IMV (A13L) |
| 119 | 90 | 123,728 | 123,456 | A15L | 90 | 90 | 100.0 | MP/IMV, morphogen (A14L) |
| 120 | 53 | 123,906 | 123,745 | A15.5L | 53 | 53 | 100.0 | MP/IMV, virulence (A14.5L) |
| 121 | 94 | 124,180 | 123,896 | A16L | 94 | 94 | 100.0 | Unknown (A15L) |
| 122 | 377 | 125,297 | 124,164 | A17L | 377 | 374 | 99.2 | Myristyl P/unknown (A16L) |
| 123 | 204 | 125,914 | 125,300 | A18L | 204 | 203 | 99.5 | MP/IMV, morphogen (A17L) |
| 124 | 492 | 125,929 | 127,407 | A19R | 492 | 489 | 99.4 | IMV, core, DNA helicase (A18R) |
| 125 | 77 | 127,621 | 127,388 | A20L | 77 | 76 | 98.7 | Unknown (A19L) |
| 126 | 426 | 127,968 | 129,248 | A22R | 426 | 425 | 99.8 | DNA pol processivity (A20R) |
| 127 | 115 | 127,969 | 127,622 | A21L | 115 | 114 | 99.1 | SecP/unknown (A21L) |
| 128 | 187 | 129,178 | 129,741 | A23R | 187 | 187 | 100.0 | Holiday junction resolvase (A22R) |
| 129 | 382 | 129,761 | 130,909 | A24R | 382 | 381 | 99.7 | VITF-3L (A23R) |
| 130 | 1164 | 130,906 | 134,400 | A25R | 1164 | 1160 | 99.7 | RNA pol (RPO132) (A24R) |
| 131 | 506 | 139,626 | 138,106 | A28L | 520 | 501 | 96.4 | MP/IMV, P4c IF (BSH A30L) |
| 132 | 110 | 140,009 | 139,677 | A29L | 110 | 108 | 98.2 | MP/IMV (A27L) |
| 133 | 146 | 140,450 | 140,010 | A30L | 146 | 146 | 100.0 | SecP TM/unknown (A28L) |
| 134 | 305 | 141,368 | 140,451 | A31L | 305 | 301 | 98.7 | RNA pol (RPO35) (A29L) |
| 135 | 78 | 141,567 | 141,331 | A32L | 77 | 76 | 97.4 | IMV, matrix, morphogen (A30L) |
| 136 | 142 | 141,727 | 142,155 | A33R | 142 | 140 | 98.6 | Unknown (A31R) |
| 137 | 42 | 141,728 | 141,600 | A32.5L | 42 | 42 | 100.0 | Unknown (A30.5L) |
| 138 | 300 | 143,024 | 142,122 | A34L | 300 | 298 | 99.3 | ATPase/DNA packaging (A32L) |
| 139 | 181 | 143,052 | 143,597 | A35R | 181 | 180 | 99.5 | MP/CEV, EEV (A33R) |
| 140 | 168 | 143,602 | 144,108 | A36R | 168 | 167 | 99.4 | MP/CEV, EEV (A34R) |
| 141 | 176 | 144,152 | 144,682 | A37R | 176 | 175 | 99.4 | Unknown (A35R) |
| 142 | 228 | 144,728 | 145,414 | A38R | 212 | 208 | 98.1 | MP/IEV (A36R) |
| 143 | 268 | 145,466 | 146,272 | A39R | 268 | 264 | 98.5 | Unknown (A37R) |
| 144 | 277 | 147,357 | 146,524 | A40L | 277 | 275 | 99.3 | MP, CD47-like/unknown (A38L) |
| 145 | 221 | 148,758 | 148,093 | A41L | 221 | 219 | 99.1 | SecP/virulence (A41L) |
| 146 | 133 | 148,961 | 149,362 | A42R | 133 | 133 | 100.0 | Profilin-like (A42R) |
| 147 | 196 | 149,400 | 149,990 | A43R | 197 | 193 | 98.5 | MP/unknown (A43R) |
| 148 | 74 | 150,010 | 150,234 | A44R | 74 | 73 | 98.7 | Unknown (MVA-156R) |
| 149 | 346 | 151,370 | 150,330 | A45L | 346 | 345 | 99.7 | Hydroxysteroid DH (A44L) |
| 150 | 125 | 151,417 | 151,794 | A46R | 125 | 125 | 100.0 | Superoxide dismutase-like (A45R) |
| 151 | 240 | 151,784 | 152,506 | A47R | 240 | 239 | 99.6 | Inhibits NF-κB activation (A46R) |
| 152 | 204 | 153,459 | 154,073 | A49R | 204 | 203 | 99.5 | Thymidylate kinase (A48R) |
| 153 | 559 | 154,642 | 156,321 | A50R | 554 | 554 | 100.0 | DNA ligase (A50R) |
| 154 | 334 | 156,362 | 157,366 | A51R | 334 | 331 | 99.1 | Unknown (A51R) |
| 155 | 313 | 160,850 | 161,791 | B2R | 313 | 310 | 99.0 | MP/CEV, EEV, HA (A56R) |
| 156 | 303 | 162,553 | 163,464 | B3R | 299 | 299 | 100.0 | Ser/Thr kinase/unknown (B1R) |
| 157 | 505 | 163,520 | 165,037 | B4R | 503 | 500 | 99.4 | Unknown (B2R/B3R) |
| 158 | 564 | 165,226 | 166,920 | B5R | 561 | 559 | 99.1 | Ankyrin/unknown (B4R) |
| 159 | 317 | 167,024 | 167,977 | B6R | 317 | 316 | 99.7 | MP/CEV, EEV (B5R) |
| 160 | 176 | 168,049 | 168,579 | B7R | 176 | 174 | 98.9 | MP/unknown (B6R) |
| 161 | 182 | 168,617 | 169,165 | B8R | 182 | 180 | 98.9 | ER P/virulence (B7R) |
| 162 | 267 | 169,220 | 170,023 | B9R | 267 | 267 | 100.0 | SecP/IFN-γ BP (B8R) |
| – | – | – | – | B10Rh | 221 | – | Virulence factor (BR-203) | |
| 163g,i | 98 | 171,559 | 171,855 | – | – | – | – | Unknown (COP-B11R) |
| 164 | 282 | 171,921 | 172,769 | B11R | 282 | 280 | 99.3 | Ser/Thr kinase/unknown (B12R) |
| 165 | 344 | 172,869 | 173,903 | B12R | 344 | 343 | 99.7 | Serpin (SPI-2)/apoptosis (BR-207) |
| 166 | 149 | 174,030 | 174,479 | B13R | 149 | 146 | 98.0 | MP/unknown (B15R) |
| – | – | – | – | B14Rh | 326 | – | – | IL-1β BP (BR-209) |
| 167 | 352 | 176,361 | 177,419 | B16R | 352 | 351 | 99.7 | IFN-α/β BP (B19R) |
| 168 | 787 | 177,488 | 179,851 | B17R | 793 | 781 | 98.5 | Ankyrin/unknown (BSH-B18R) |
| 169 | 397 | 180,861 | 182,054 | B19R | 357 | 352 | 98.6 | Serpin (SPI-1)/unknown (C12L) |
| 170 | 190 | 182,226 | 182,798 | B20R | 190 | 187 | 98.4 | MP/unknown (BR-218) |
| 171 | 1880 | 183,055 | 188,697 | B21R | 1879 | 1861 | 99.0 | MP/unknown (BSH B22R) |
| 172 | 153 | 190,894 | 191,355 | N1R | 153 | 152 | 99.4 | Unknown (B22R) |
| 173 | 176 | 192,104 | 192,634 | N3R | 176 | 174 | 98.9 | Unknown (BR-018) |
| 174 | 437 | 192,749 | 194,062 | N4R/D1L | 437 | 432 | 98.9 | Ankyrin/unknown (BR-017) |
| 175 | 590 | 194,209 | 195,981 | J1R | 587 | 581 | 99.2 | Ankyrin/unknown (BR-006/225) |
| 176 | 349 | 196,071 | 197,120 | J2R | 348 | 345 | 98.9 | SecP/TNF BP (crmB) (BR-005/226) |
| 177 | 246 | 197,247 | 197,987 | J3R | 246 | 246 | 100.0 | SecP/CC chemokine BP (C23L/B29R) |
Abbreviations: SecP, secreted protein; BP, binding protein; CHO, Chinese hamster ovary; P, protein; MG, monoglyceride lipase; R, ribonucleotide; inter, interaction; MP, membrane protein; IEV, intracellular enveloped virion; EEV, extracellular enveloped virion; PP, phosphoprotein; pol, polymerase; PKR, dsRNA-dependent protein kinase; OAS, 2′–5′ oligoadenylate synthetase; VITF, viral intermediate transcription factor; Myristyl P, myristylated protein; PW, pathway; IMV, intracellular mature virion; morphogen, morphogenesis; N triphosphat, nucleotide triphosphatase; CP, cysteine proteinase; VLTF, viral late transcription factor; topo, topoisomerase; VETF, viral early transcription factor; attach, attachment; rif resist, rifampicin resistance; IF, inclusion factor; TM, transmembrane; DH, dehydrogenase; HA, hemagglutinin.
Predictions: secreted proteins by SignalP V1.1; membrane proteins by TMpred.
DNA encoding remnants of CPXV-BR-001, -002, -063, -174, -216, -228, -229, and VACV-COP-C15L were present, but the residual coding sequences were not annotated.
ZAI-96 genome was reannotated as described in the Materials and methods section. This process removes ORFs that are vestiges of conserved ORFs present in other poxviruses or small predicted ORFs on the non-coding strand. We removed 16 fragmented ORFs that were previously annotated, including D2L, D4L, D15L, D16L, D17L, D18L, C3L, A26L, A27L, A48R, B1R, B15L, B18R, K1R, N2R, and R1R.
Length, number of aa in ORF.
Start, first nucleotide of start codon.
Stop, last nucleotide of stop codon.
Orthologus ORF in the VACV-COP, unless otherwise indicated (MOS, ECTV-MOS; BR, CPXV-Brighton Red; BSH, VARV-BSH; TIA, VACV-TIA; MVA, VACV-MVA, and ZAI, ZAI-96).
An ortholog not present in the corresponding region of ZAI-96.
An ortholog is not present in SL-V70.
SL-V70 ORF 163 is the single gene predicted in the SL-V70 isolate, and a number of other OPVs that is not annotated in ZAI-96. Orthologs of this predicted protein range in size from 72–106 due to a highly variable N-terminal region that contains different lengths of an Asp–Thr repeat. A single InDel, approximately one third into the ORF, induces a frameshift that results in an early termination codon in an otherwise complete ZAI-96 gene. Since a promoter has not been characterized for this predicted ORF, it is not possible to predict what polypeptides are likely to be made by the viruses of these two groups. Database searches with these predicted proteins failed to yield any significant matches to non-OPV proteins.
Table 3.
Fragmented ORFs of VL-V70 genome
| Region | Longest OPV ortholog | Motif/putative function |
|---|---|---|
| A/Y | CPXV-BR-016 (764aa) | Ankyrin motif/unknown |
| B/Z | CMLV-M96-006 (237aa) | MAR assoc Pa/unknown |
| C | CPXV-BR-020 (170aa) | Unknown |
| D | CPXV-BR-022 (331aa) | IL-1 receptor antagonistb |
| E | VACV-COP-C8L (184aa) | Unknown |
| F | CPXV-BR-035 (512aa) | Kelch-like/unknown |
| G | VACV-COP-K3L (88aa) | IFN resistance |
| H | CPXV-BR-071 (319aa) | Virosome component |
| I | CPXV-BR-158 (1284aa) | A-type inclusion body |
| J | CPXV-BR-176 (409aa) | Semaphorin |
| K | VACV-COP-A40R (168aa) | Lectin/virulence |
| L | CPXV-BR-185 (244aa) | Unknown |
| M | CPXV-BR-187 (162aa) | Unknown |
| N | CPXV-BR-190 (190aa) | TLR signaling inhibitor |
| O | CPXV-BR-191 (186aa) | TNF binding protein |
| P | CPXV-BR-193 (563aa) | Kelch-like/unknown |
| Q | CPXV-BR-195 (197aa) | Guanylate kinase |
| R | CPXV-BR-203 (225aa) | Virulence factor |
| S | CPXV-BR-204 (501aa) | Kelch-like/unknown |
| T | ECTV-MOS-163 (328aa) | IL-1β binding protein |
| U | CPXV-BR-210 (340aa) | Unknown |
| V | ECTV-MOS-167 (559aa) | Kelch-like/unknown |
| W | CPXV-BR-221 (320aa) | TNF binding protein |
| X | CPXV-GIR-K3R (167aa) | TNF binding protein |
N-methyl-d-aspartate receptor-associated protein.
We identified 170 InDels of a combined length of 9629 nucleotides and 852 substitution mutations out of a total of 193,094 nucleotides when comparing aligned genomes of SL-V70 and ZAI-96. One hundred and forty-seven InDels and 192 substitutions are in intergenic regions and fragmented genes. Five InDels and 321 substitutions occur in 84 genes with functions thought to be essential for virus replication in standard tissue culture cell lines (e.g. functions mainly necessary for transcribing mRNA, replicating the genomic DNA, assembling infectious virions, etc.). These genes are highly conserved in all sequenced OPVs and mainly map to a central conserved region (Table 4 ; see footnote b for map location in SL-V70). A further 16 genes that encode similar functions map to the left and right terminal regions of the genome, and contain 63 substitutions and 4 InDels. Because SL-V70 and ZAI-79 show similar kinetics of replication and cell-to-cell spread in tissue culture (Fig. S1 in Appendix A), we think it less likely that mutations located in these genes are responsible for the virulence difference noted between COP-58 and ZAI-79 in Table 1.
Table 4.
Mutation in SL-V70 and ZAI-96a members of the Virulence Ortholog family
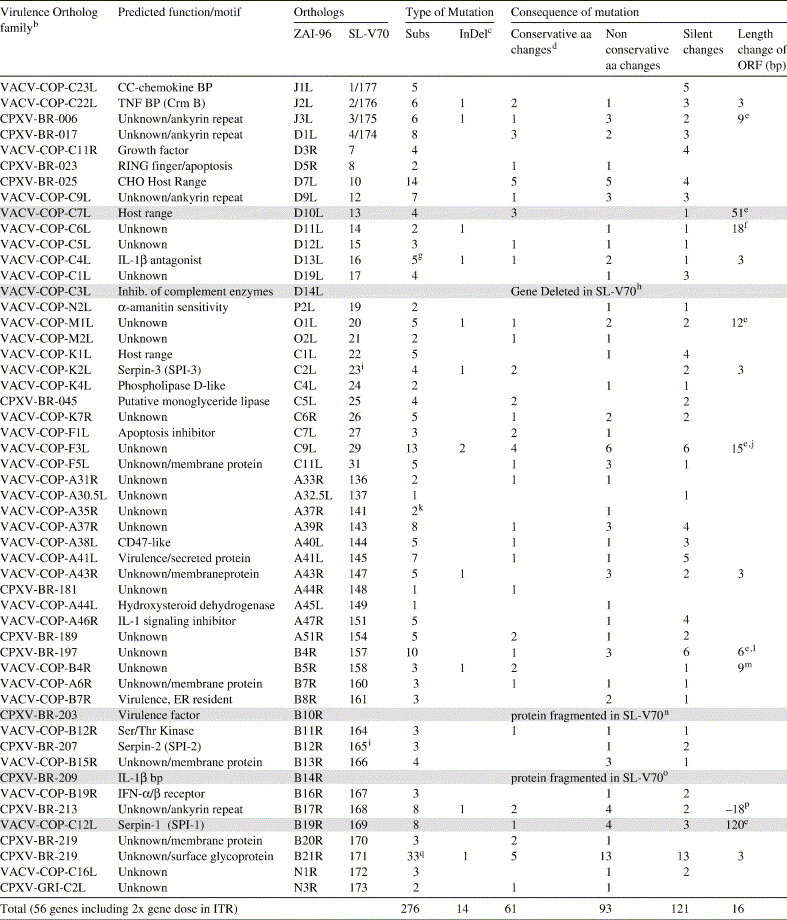
bFunctions thought to be essential for virus replication in standard tissue culture cell lines (e.g. functions mainly necessary for transcribing mRNA, replicating the genomic DNA, and assembling infectious virions, etc.) are highly conserved in all sequenced OPVs, and map to a central conserved region delineated in VACV-COP by gene F6L (position 38,015; in SL-V70 the ortholog is positioned at 35,132) to A25L (position 138,012; in SL-V70 the ortholog is positioned at 134,611). For each OPV, the remainder of the genome contains a mix of genes, some are specifically tailored to the biology of the individual virus in particular cell types or the reservoir host, and others encode functions conserved among OPVs that may be essential for optimal replication and spread in the host. These functions are collectively referred to as the Virulence Ortholog family. This set of genes is listed here minus genes that are or expected to be essential for virus replication in standard tissue culture lines.
cInDel is one deletion or insertion no matter how long of deletion/insertion.
dConservative aa changes among the aa that have the similar structure: small and non-polar (G, C, T, A, S), small and polar (E, D, N, Q), large and non-polar (V, I, M, F, L), or large and polar (K, H, R, W, Y).
eSL-V70 003, 013, 157, and 169 have an N-terminal extension. SL-V70 020 and 029 have C-terminal extensions. These positions show variability in other OPV orthologs.
fSL-V70 014 has 8 copies of repeat element GAT (Asp) near the C-terminus instead of the 3 copies noted in ZAI-96 D11L. The number of repeats varies among OPVs.
gZAI-96 D13L and SL-V70 016 share 1 InDel and 5 substitutions, with 2 proximal substitutions causing 1 aa change.
hZAI-96 D14L is completely absent from the corresponding region of the SL-V70 genome because of a DNA sequence deletion.
iThere are no mutations within the serpin reactive-site loop.
jIn SL-V70 029 has 2 InDels and 3 substitutions near the C-terminus that together not only cause a length change, but also 2 conservative, 3 non-conservative, and 1 silent aa changes.
kSL-V70 141 has 2 subs that cause one aa change.
lOne substitution located 5′ to the N-terminus of ZAI-96 B4R causes M→T change, and therefore cause 2-aa length change. Most OPV orthologs have an N-terminal protein structure similar to SL-V70 157.
mSL-V70 158 has 3 copies of an N-terminal AATTCTTCC repeat element instead of 2 copies found in ZAI-96. This results in a 3 aa extension near the N-terminus extension in ORF 158. The sequence of SL-V70 repeat element is conserved in most OPVs.
nThe DNA sequence of ZAI-96 B10R is conserved in SL-V70 genome; however, a 2-base deletion in the 5′ third of the SL-V70 ortholog causes a frame-shift, splitting this ortholog into 72 aa and 118 aa fragments with the latter fragment out-of-frame.
oDNA sequence of ZAI-96 B14R is conserved in SL-V70 genome; however, a 1-base insertion in SL-V70 near the N-terminus and another 4-base deletion in SL-V70 cause a frame-shift splitting this ortholog into 163 aa and 132 aa fragments.
pSL-V70 168 has 1.5 copies of an ATCTCA repeat element near the C-terminus instead of the 4.5 copies found in ZAI-96 B17R and all other analyzed OPVs.
qThe ortholog of surface glycoprotein in SL-V70 171 has 33 substitutions when compared to ZAI-96; 27 are 1-base substitutions, 3 are 2-base substitutions with one 2-base substitution causing 1 silent and a T→P aa change, and the 2 other 2-base substitutions causing S→L and E→N changes.
The positions of all mutations can be found in the on-line file MPXV.bbb that contains a multiple alignment of four complete MPXV genomes (SL-V70, ZAI-96, COP-58, and WRAIR-61) in Base-By-Base format with supplemental annotation.
The Virulence Ortholog family of genes
We hypothesize the observed virulence phenotype maps to a gene(s) in the terminal regions of the MPXV genome that is important for maximizing virus replication and/or spread within the tested non-human primate species. This group of 56 genes of known and unknown functions is collectively referred to as the Virulence Ortholog family, and includes 3 genes only present in ZAI-96 and 53 genes present in both SL-V70 and ZAI-96. There are 14 InDels and 276 substitutions in the 53 genes common to both isolates. These mutations are responsible for 61 conservative, 93 non-conservative, and 121 silent aa changes and changes in predicted lengths of 16 proteins that are mainly involved N- and C-terminal extensions (Table 4, see foot-notes). The mutational burden in each gene is proportional to length. Although the CPXV-BR-219 ortholog SL-V70 171 has 33 mutations over its 1879 aa length, this is 1.75 mutations per 100 aa that is identical to the 1.75 mutations per 100 aa noted for this group of genes as a whole. It is difficult to evaluate the effect of a mutation(s) on protein function without a detailed understanding of the relationship of protein structure to function, but we have examined in greater detail 5 genes based on a likelihood that their particular mutations may affect function (Table 4, grey highlight).
ZAI-96 D10L, the ortholog of SL-V70 013, encodes a host-range function necessary for optimal VACV and ECTV replication in certain tissue culture lines, but was not important for ECTV pathogenesis in the A strain of mouse (Chen et al., 1993, Gillard et al., 1985). SL-V70 013 has a 4 bp deletion starting 29 bp upstream of the predicted start codon. This mutation brings another upstream ATG in frame and suggests the possibility of an N-terminal extension to the SL-V70 protein that could affect function. This upstream ATG, however, is 5′ of a predicted promoter sequence immediately upstream of the putative initiator ATG that is conserved among OPV orthologs, so it is unknown whether there will be an N-terminal extension for SL-V70 as compared to ZAI-96. D14L encodes VCP-MPXV, an ortholog of VACV complement-binding protein (VCP; VACV-COP C3L). As compared to VCP-VACV, the VCP-MPXV gene has a single nucleotide deletion leading to a stop codon that terminates the predicted protein 13 aa into the fourth complement control protein (CCP) module also known as a short consensus repeat (Uvarova and Shchelkunov, 2001). All Congo basin MPXV isolates (CNG-8, ZAI-V70, ZAI-77, ZAI-96, and ZAI-V79) that were acquired over a 26-year period have an identical VCP-MPXV gene (Uvarova and Shchelkunov, 2001). This gene is completely absent from the genomes of the three West African viruses due to large DNA deletions. ZAI-96 B10R encodes a 221 aa protein in the myxoma virus M-T4 virulence factor family characterized by a C-terminal KDEL-like motif in a potential ER-anchoring domain; it has orthologs in a variety of poxviruses, but a frameshift mutation removes the C-terminal two-thirds of the predicted protein in SL-V70. In poxviruses, this protein is thought to play a role in abrogating apoptosis of infected cells (Barry et al., 1997, Hnatiuk et al., 1999). ORF B10R has orthologs in a variety of poxviruses, but a frameshift mutation removes the C-terminal two-thirds of the predicted protein in SL-V70. ZAI-96 B14R encodes for an IL-1 binding protein that is encoded by most OPVs. The role of this ortholog in pathogenesis may be dependent on the route of inoculation as one study found a vaccinia virus strain lacking the B14R ortholog showed less virulence by the intracranial route, while a second study using a similar mutant observed enhanced virulence by the intranasal route of infection (Spriggs et al., 1992, Alcami and Smith, 1992). This ortholog in SL-V70 is disrupted by two frameshifts. ZAI-96 B19R (SPI-1 gene) and SPI-1 orthologs of several OPVs contain an unusual tandem repeat of CATTATATA immediately upstream of the initiator ATG. SL-V70 169 has 7 copies of the repeat compared with 37, 27, 16 identical copies of the sequence in the WRAIR-61, COP-58, and ZAI-96 genomes, respectively. These repeats are positioned between the predicted promoter region and the initiating ATG codon of the SPI-1 genes; although there appears to be an in-frame ATG upstream of the ZAI-96 ortholog, our promoter prediction and primer extension data (Kettle et al., 1995, Kettle et al., 1997) indicate that the mRNA initiates 3′ of this ATG. It is not known, however, what effect the variable lengths of 5′ untranslated mRNA, containing these repeats will have (if any) on the level of SPI-1 protein production. Several other OPV genomes (CPXV, VARV, CMLV, and VACV-WR) possess a monomer of a similar sequence (CATTATTTA) that may be related to the ancestral sequence of the SL-V70 repeat. The SPI-1 gene also has a Val→Ala mutation at the P12 position of the reactive center loop that would not be expected to affect Serpin activity.
Members of the Virulence Ortholog family conserved in a Congo basin isolate of MPXV and VARV
Since human monkeypox caused by Congo basin isolates of MPXV is almost clinically indistinguishable from smallpox, we further compared genomic sequences of MPXV and VARV to determine if ZAI-96 D10L, D14L, B10R, B14R, and B19R genes are conserved in VARV (Shchelkunov et al., 1995). Because ZAI-96 D10L and B19R orthologs are conserved in both West African and Congo Basin isolates of MPXV as well as VARV-BSH-75 (Table 5 ), and the consequences of their genetic differences among the viruses are predicted to be minor, D10L and B19R are not considered leading candidate as a determinant of virulence for non-human primates and humans. The absence of orthologs of ZAI-96 B10R and B14R in West African isolates and VARV-BSH-75 also argues against a role of these genes in ZAI-96 virulence. Thus, the presence of VCP-MPXV orthologs in ZAI-96 and VARV-BSH-75, and its absence in three West African viruses, makes VCP-MPXV a leading candidate to explain the virulence difference between West African and Congo basin isolates. Further inspection of Table 5 indicates that 11 genes, which are lacking or truncated in VARV-BSH-75 or in the virulent ZAI-96 may not be essential for orthopoxvirus virulence in humans (grey highlight). And finally, all of the remaining virulence genes that are conserved in both VARV-BSH-75 and ZAI-96 may indicate a subgroup of OPV virulence genes important for human infections.
Table 5.
Presence of OPV Virulence Ortholog family members in monkeypox and variola viruses
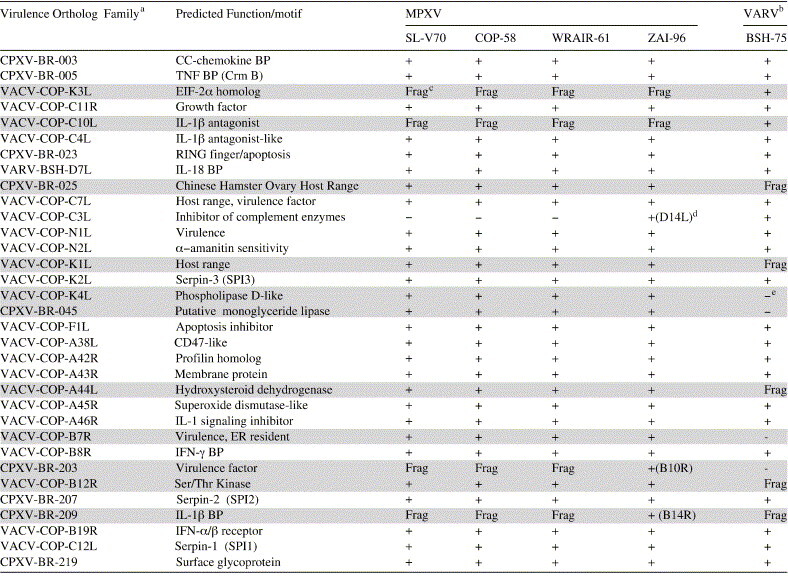
aSee footnote b of Table 4.
bWe removed 29 fragmented ORFs that were previously annotated in VARV-BSH-75 genome, including A26L, A27L, A28L, A29L, A39L, A40R, A42R, A43R, A47L, C1L, C7L, D17L, D16L, D13L, D10L, D9L, D8L, D1L, B20R, B19R, B14L, B11R, B7R, B4L, B3L, B2L, E7L, O3L, and J6R. The reannotated VARV-BSH genome contains 162 ORFs. Our analysis suggests the VARV-BSH ORF D3L was likely not functional thus this ORFs was omitted from our reannotation. The original annotation was described by Massung et al. (1994). These updated annotations are available from the POCsdb (http://athena.bioc.uvic.ca).
cFragment.
dVCP-MPXV has a single nucleotide deletion leading to a stop codon that terminates the protein 13 aa into the fourth CCP module and 43 aa from the C terminus.
eGene is missing.
VCP-MPXV, a candidate MPXV virulence gene
The poxvirus inhibitors of complement enzymes, which include VCP-MPXV, mimic the biologic activity of complement regulatory proteins (CRPs) that interact with C3b and C4b to inhibit C3 and C5 convertases of the cascades (Liszewski et al., 1996). This prevents a variety of events downstream of complement activation including the deposition of large amounts of C3 fragments, the release of the anaphylactoid and chemotactic mediators (C3a and C5a), and the formation of the membrane attack complex (Isaacs et al., 1992, Kotwal and Moss, 1988, McKenzie et al., 1992, Sahu et al., 1998). Poxviral complement inhibitors consist entirely of a series of four repeating CCP domains; the CCPs are 30–40% identical to the human CRPs including membrane co-factor protein (MCP; CD46), C4b-binding protein, Factor H, and decay-accelerating factor (DAF; CD55) and contain the regulatory-sites for C3b and C4b (Herbert et al., 2002). Recently, Rosengard et al. (2002) demonstrated that the VARV ortholog, VCP-VARV, was nearly 100-fold and 6-fold more potent than VCP-VACV at inactivating C4b and C3b, respectively, a finding that was generally confirmed by others (Sfyroera et al., 2005). VCP-MPXV, however, is unique among OPV orthologs in that it has a truncated fourth CCP module (Fig. 3A). In spite of this truncation, one preliminary study using a sheep red blood cell hemolysis assay suggested that VCP-MPXV from a Congo basin isolate had some complement enzyme inhibitory activity (Smith et al., 2000). To more fully establish the role of VCP-MPXV as a possible virulence determinant for the Congo basin MPXV isolates, its inhibitory activity for human complement proteins was characterized.
Fig. 3.
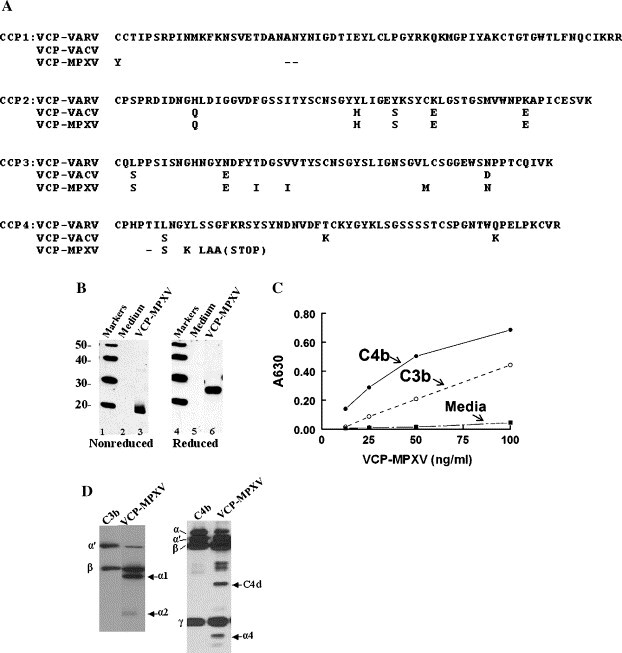
VCP-MPXV structure and function. (A) Amino acid alignment of VCP-VARV and VCP-MPXV without signal peptides illustrating amino acid differences and the premature termination of VCP-MPXV. (B) Western blot of non-reduced and reduced VCP-MPXV. Concentrated CHO supernatants containing VCP-MPXV were electrophoresed in a 10% SDS-PAGE, transferred to nitrocellulose and developed with 1:5000 rabbit anti-VCP-VACV antibody. (C) VCP-MPXV binds human C4b and C3b. A representative binding curve is shown. Ligands were coated onto microtiter plates followed by incubations with media or VCP-MPXV. Binding was detected with rabbit anti-VCP-VACV antibody (1:5000). VCP-MPXV was quantified in an ELISA (Materials and methods). (D) VCP-MPXV possesses cofactor activity for human C3b and C4b. Chemiluminescent cofactor assays were performed (with or without 10 ng VCP-MPXV), biotinylated human C3b and C4b and 100 ng of human factor I followed by Western blot analysis. Arrows denote some of the major cleavage fragments. Controls of VCP-MPXV without factor I did not show cleavage fragments (data not shown).
To ascertain if VCP-MPXV retained complement regulatory function, recombinant VCP-MPXV was expressed and purified. A Western blot demonstrated the predicted electrophoretic pattern including the faster relative mobility of the non-reduced form (Fig. 3B). In the functional assessments, VCP-MPXV retained regulatory activity for human C3b and C4b. It bound human C3b and C4b (Fig. 3C), and also demonstrated cofactor activity for both proteins (Fig. 3D) (Liszewski et al., 1998). The latter assay analyzes the ability of VCP-MPXV to serve as a cofactor for the serine protease factor I (human) to cleave and thereby inactivate C3b and C4b. The limited proteolytic cleavage fragments generated through VCP-MPXV's cofactor activity are similar in pattern to those generated by mammalian homologs (Rosengard et al., 2002). Indeed, two of these complement regulatory proteins CD55 and CD46, also possess four CCPs, but require the fourth CCP for complement regulatory function (Liszewski and Atkinson, 1998). Thus, it is somewhat surprisingly that VCP-MPXV, which lacks most of the fourth CCP, retains complement regulatory activity. These results, therefore, support VCP-MPXV as a candidate for a major MPXV virulence gene in non-human primates.
The lack of a VCP-MPXV ortholog could make MPXV virions and infected cells more susceptible to antibody and complement lysis, which could diminish virus spread, and lead to less severe disease. Consistent with this hypothesis, patients in the U.S. 2003 outbreak, as compared to those infected in the Congo basin, had significantly fewer skin lesions (Damon, unpublished data). Also, the lesions presented with a unique focal hemorrhagic necrosis, possibly due to uncontrolled complement-mediated tissue destruction at the site of infection (Reed et al., 2004). Increased local tissue destruction was also noted in experimental studies in mice with the CPXV mutants lacking a VCP-MPXV ortholog (Miller et al., 1995, Miller et al., 1997, Kotwal et al., 1998). Formal proof that VCP-MPXV is a virulence gene will require its deletion in a Congo basin MPXV isolate and pathogenesis studies in non-human primates.
Materials and methods
Viruses and cells
MPXV-SL-V70-I-266 (SL-V70) was obtained from the crusts of lesions from a single case in Sierra Leone in 1970 (Lourie et al., 1972). The virus was passaged twice in BSC-40 cells. The MPXV-WRAIR-7-61 (WRAIR-61) isolate was deposited with the American Type Culture Collection (ATCC, catalogue number VR-267 and NIH bei collection from which registered users can receive the virus) in May of 1962 by Major Stewart J. McConnell of WRAIR. WRAIR-61 was isolated from a female cynomolgus monkey, B-39, that was observed with a poxvirus-like infection 45 days following whole-body irradiation of 350 rads (McConnell et al., 1962). B-39 died 12 days after onset of disease. WRAIR-61 was isolated from tissue culture in monkey kidney cells, plaque-purified three times in rabbit kidney cells, and passaged a further eight more times in rabbit kidney cells prior to accession into the ATCC. At the ATCC, the virus was further passage twice in Vero cells. ZAI-V79-I-005 (ZAI-V79) was obtained from scab material of a severe case of human monkeypox in Zaire in 1979, and was passaged sequentially once in LLCMK2 cells, twice in BSC-40 cells, and two or three times in Vero cells (Zaucha et al., 2001). MPXV-COP-58 (COP-58) was isolated in 1958 from scrapings of several papules on an infected cynomolgus monkey from an outbreak of a vesicular eruptive disease in a primate holding facility (von Magnus et al., 1959). The virus was passaged an unknown number of times on the chorioallantoic membrane of the chick egg and in FL, LLCMK2, and BSC-40 cells. Vero, BSC-1, and BSC-40 cells were grown in Eagle's minimum essential medium (EMEM; Bio-Whittaker, Walkersville, MD) containing 10% fetal bovine sera (Hyclone, Logan, UT), 2 mM l-glutamine (GIBCO, Grand Island, NY), 100 U/ml of penicillin (GIBCO, Grand Island, NY), and 100 μg/ml of streptomycin (GIBCO, Grand Island, NY). Virus plaque assays were carried out on BSC-1 cell monolayers as previously described (Chen et al., 1992). Comet assays were carried out as for the plaque assay except 1% carboxyl methyl cellulose was omitted from the overlay medium.
Monkey challenge experiment
Juvenile to adult, 1.6 to 4.7 kg, cynomolgus monkeys (Macaca fascicularis) were challenged by small particle aerosol (mass median diameter of 1.2 μm) as described previously (Zaucha et al., 2001). The husbandry and experimental protocols were in accordance with Guide for the Care and Use of Laboratory Animals. The facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Purification of monkeypox virus genomic DNA and DNA sequencing
Five to eight monolayer cultures containing 2 × 107 Vero, BSC-40 or BSC-1 cells were infected with 0.1–5 pfu/cell of each MPXV isolate in 5 ml of EMEM. After ∼1 h at 37 °C, cultures were supplemented with a further 10 ml of prewarmed EMEM and incubated until maximum cytopathic effect was observed (∼36 h). MPXV genomic DNA was extracted from virions as described previously (Dhar et al., 2004, Chen et al., 2003).
The genomes of SL-V70, WRAIR-61, and COP-58 were sequenced as described previously for ectromelia virus except that sequencing primers for the sequences of the variable left and right-hand terminal regions were based on ZAI-96 (Chen et al., 2003). Sequencing primers were chosen approximately 450 bp apart on each strand to ensure adequate overlap of sequencing reads. Both strands of each fragment were sequenced and any gaps were closed by primer-walking. Sequencing reactions were carried out using CEQ 2000 Dye Terminator Cycle Sequencing with Quick Start Kit (Beckman Coulter, Inc., Fullerton, CA) and run on a CEQ 2000XL DNA Analysis System (Beckman Coulter, Inc., Fullerton, CA).
Cloning and sequencing of heterogeneous regions in WRAIR-61
The genome contains four extended runs of dA or dT nucleotides that are heterogeneous in nature and therefore are refractory to sequencing using PCR derived template DNA. To resolve this problem, we cloned the heterogeneous amplicons containing these regions and sequenced approximately 10 clones for each region. These four regions were PCR-amplified using the following primers. MLEA: MLEA-5′ (5′-ATAAGAATGCGGCCGCGTGTCTAGAAAAAAATGTGTGACC-3′) and MLEA-3′ (5′-TCCCCCGGGCATAGAACAGTGTCATCTATCG-3′); M1A: M1A-5′ (5′-ATAAGAATGCGGCCGCGTTTAAGATAGTATATTCTCTAG-3′) and M1A-3′ (5′-TCCCCCGGGCATGAGGACTCTACTTATTAG-3′); M18A: M18A-5′ (5′-ATAAGAATGCGGCCGCGGAAAATACAAGTATAGATACAACG-3′) and M18A-3′ (5′-TCCCCCGGGTACTCCGTATTCATACTCG-3′); M18B: M18B-5′ (5′-ATAAGAATGCGGCCGCGGAACTAACTATTACCATGAATC-3′) and M18B-3′ (5′-TCCCCCGGGTGTCT AGAAAAAAATGTGTGACC-3′). The 5′ and 3′ primers for each region contained NotI and SmaI recognition sites (underlined), respectively. After digestion with NotI and SmaI, each region was cloned into similarly digested pKT1012-gpt−. pKT1012-gpt− was derived from pKT1012 (kindly provided by Dr. Kangla Tsung, University of California, San Francisco) by removal of the fragment containing E. coli guanine phosphoribosyltransferase gene (gpt) under the control of P7.5 early VACV promoter by digestion with NdeI and EcoRV, followed by Klenow-blunting and self-ligation. Eleven plasmid clones for the region MLEA, M1A, and M18B and 10 clones for the region M18A were selected and separately sequenced by primer walking.
Cloning and sequencing of heterogeneous regions in SL-V70
Four heterogeneous regions (MSLEA, MS1A, MS2A, and MS18A) of SL-V70 were PCR-amplified using the following primers. MSLEA: MSLEA-5′ (5′-ATAAGAATGCGGCCGCGTGTCTAGAAAAAAATGTGTGACC-3′) and MSLEA-3′ (5′-TCCCCCGGGCATAGAACAGTGTCATCTATCG-3′); MS1A: MS1A-5′ (5′-ATAAGAATGCGGCCGCGTACATCACTGTAAGCATGTCC-3′) and MS1A-3′ (5′-TCCCCCGGGATATGTAGCACAGACCAATTTC-3′); MS2A: MS2A-5′ (5′-ATAAGAATGCGGCCGCCTTTTATGTCAAGAAGGCACTGG-3′) and MS2A-3′ (5′-TCCCCCGGGTATCCCAATTTACGAGCCCGTTAACAAG-3′); MS18A: MS18A-5′ (5′-ATAAGAATGCGGCCGCGGAACTAACTATTACCATGAATC-3′) and MS18A-3′ (5′-TCCCCCGGGTGTCTAGAAAAAAATGTGTGACC-3′). The 5′ and 3′ primers for each region contained NotI and SmaI recognition sites (underlined), respectively. After digestion with NotI and SmaI, each region was cloned as described above. Eleven clones for the region MSLEA, 10 clones for the region MS1A, 8 clones for the region MS2A, and 12 clones for the region MS18A were selected, and their plasmid DNA inserts were sequenced.
Cloning and sequencing of heterogeneous regions in COP-58
Six heterogeneous regions (MCLEA, MC1A, MC15A, MC16A, MC18A, and MC18B) of the genome of COP-58 were PCR-amplified using the following primers. MCLEA: MCLEA-TOPO-5′ (5′-CACCGAATGCGGCCGCGTGTCTAGAAAAAAATGTGTGACC-3′) and MCLEA-3′ (5′-TCCCCCGGGCATAGAACAGTGTCATCTATCG-3′); MC1A: MC1A-TOPO-5′ (5′-CACCGAATGCGGCCGCGTTTAAGATAGTATATTCTCTAG-3′) and MC1A-3′ (5′-TCCCCCGGGCATGAGGACTCTACTTATTAG-3′); MC15A: MC15A-TOPO-5′ (5′-CACCGAATGCGGCCGCGATGAAAATCTTTGGATGGTTGC-3′) and MC15A-3′ (5′-TCCCCCGGGTAACCATCGTTAATTGGTCTTGC-3′); MC16A: MC16A-TOPO-5′ (5′-CACCGAATGCGGCCGCGAGTTCGGAAGTATGTCTGAC-3′) and MC16A-3′ (5′-TCCCCCGGGTAATCGATATTGGTCGTGTAG-3′); MC18A: MC18A-TOPO-5′ (5′-CACCGAATGCGGCCGCGGAAAATACAAGTATAGATACAACG-3′) and MC18A-3′ (5′-TCCCCCGGGTACTCCGTATTCATACTCG-3′); MC18B: MC18B-TOPO-5′ (5′-CACCGAATGCGGCCGCGGAACTAACTATTACCATGAATC-3′) and MC18B-3′ (5′-TCCCCCGGGTGTCTAGAAAAAAATGTGTGACC-3′). Each PCR product was cloned into pcDNA3.1D/V5-His-TOPO using pcDNA3.1 Directional TOPO Expression Kit according to the manufacture's instructions (Invitrogen, Carlsbad, California 92008). Fourteen clones for the region MCLEA, 12 clones for the region MC1A, 11 clones for the region MC15A, 12 clones for the region MC16A, 12 clones for the region MC18A, and 12 clones for the region MC18B were selected, and their plasmid DNA inserts were sequenced as described above.
DNA sequence analysis
Assembly of the raw sequence data from the chromatograms for WRAIR-61 was performed using the Staden software package on a Linux platform (Dear and Staden, 1991). Data were processed using Pregap4 (Ewing and Green, 1998) and a consensus sequence was assembled and edited using Gap4 (Bonfield and Staden, 1996, Bonfield et al., 1995). The raw sequence data for SL-V70 and COP-58 was assembled using ContigExpress in Vector NTI Suite 8 (Invitrogen, Carlsbad, CA). An ORF was defined as a continuous sequence that translated into a polypeptide initiated by a methionine residue and extended for 60 or more amino acids (aa) prior to a termination codon. Artemis software (Mural, 2000) and Poxvirus Orthologous Clusters database (POCsdb; (Ehlers et al., 2002) were used to detect and annotate ORFs. Early and late promoter sequences in genomes were identified using promoter sequence models based upon sequence alignments of experimentally-verified early and late vaccinia virus promoters (Wang and Lefkowitz, unpublished data). BLASTP (Altschul et al., 1997) was used to detect SL-V70 orthologs in other poxvirus genomes contained in the POCsdb. In addition, BLASTN, TBLASTN, and BLASTP searches were carried out at the NCBI website to annotate some ORFs. Viral Genome Organizer (VGO) software (Upton et al., 2000) and Nucleotide-Amino Acid Alignment Program (NAP) (Huang and Zhang, 1996) were used to compare nucleotide sequences of fragmented ORF regions against the corresponding predicted protein sequences of the longest Chordopoxvirus ortholog. To calculate the primary nucleic acid sequence identity shared between any two genomes, complete genomic sequence alignments of every possible pair-wise genomic combination of all available orthopoxvirus sequences were constructed using the alignment program LAGAN (Brudno et al., 2003). Percent identity was calculated using the following formula: 100 × (Number of identical residues) / [(Number of identical residues) + (Number of Mismatches) + (Number of InDels)]. An InDel was defined as a single insertion–deletion event independent of the length of the resultant gap. Poxvirus genomes used for comparison are available at www.poxvirus.org. The accession numbers are: NC_002520, Amsacta moorei entomopoxvirus; NC_005337, Bovine papular stomatitis virus; NC_003391, Camelpox virus, M-96; AY009089, Camelpox virus, CMS; NC_005309, Canarypox virus (ATCC VR-111); NC_003663, Cowpox virus, Brighton Red; X94255, Cowpox virus, GRI-90; NC_004105, Ectromelia virus, Moscow; NC_002188, Fowlpox virus; AJ581527, Fowlpox virus, HP-438 Munich; NC_003027, Lumpy skin disease virus, Neethling 2490; AF409137, Lumpy skin disease virus, Neethling Warmbaths LW; AF409138, Lumpy skin disease virus, Neethling vaccine LW 1959; NC_001993, Melanoplus sanguinipes entomopoxvirus; NC_001731, Molluscum contagiosum virus; NC_003310, Monkeypox virus, Zaire-96-I-16; NC_001132, Myxoma virus, Lausanne; NC_005336, Orf virus, OV-SA00; AY386263, Orf virus, OV-IA82; NC_001266, Rabbit fibroma virus; AY484669, Rabbitpox virus, Utrecht; AX754989, sequence 1 from Patent WO03006654; NC_004002, Sheeppox virus, TU-V02127; NC_003389, Swinepox virus, 17077-99; NC_001559, Vaccinia virus, strain Copenhagen; U94848, Modified vaccinia virus, strain Ankara; AY243312, Vaccinia virus, strain Western Reserve; L22579, Variola major virus, Bangladesh-1975; NC_001611, Variola major virus, India-1967; NC_005179, Yaba monkey tumor virus; NC_002642, Yaba-like disease virus; Y16780, Variola minor virus, Garcia-1966.
Sequence alignment and phylogenetic analysis
Sequence alignments were generated using a combination of the programs MAVID and Multi-LAGAN (Bray and Pachter, 2004, Brudno et al., 2003). Extensive hand editing was also used to optimize the alignment. Phylogenetic analysis was carried out using an ∼138 kbp conserved central region of completely sequenced genomes of various orthopoxvirus species. The left end of the alignment extends from gene C7L of vaccinia virus strain Copenhagen (VACV-COP, position 18,805 of the genome) to A51R at the right end (position 157,688). Evolutionary relationships were solved using the Branch-and-Bound search method with maximum parsimony as the optimality criterion. Bootstrap resampling confidence values on 1000 replicates were also calculated using Branch-and-Bound with maximum parsimony. Branch lengths are proportional to the number of sequence changes along each branch. All evolutionary relationships were estimated using the program PAUP* version 4.0b10 (http://paup.csit.fsu.edu/).
Production and functional assessment of VCP-MPXV activity
The VCP-MPXV gene was PCR amplified using genomic DNA from Congo basin isolate MPXV-ZAI-V70-I-823 using the following primers: VCP-MPXV-5′ (5′-ATGAAGGTGGAGAGCGTGACGTTCCTGACATTGTTGG-3′) and VCP-MPXV-3′ (5′-TTAAGCCGCTAGAAGTTTTCCGTTTGATATAG-3′) and cloned into the pCR®-Blunt vector (Invitrogen, Carlsbad, CA). A clone was isolated and the insert was shown by DNA sequencing to be identical to the VCP-MPXV gene of ZAI-96. The DNA was further subcloned into the EcoR1 site of plasmid pSG5 (Stratagene, La Jolla, CA). A construct also was prepared in pSG5 that added a cleavable (enterokinase: Asp–Asp–Asp–Asp–Lys) 6× histidine tag using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) with the following two complementary oligonucleotides each underlining the area of recognition (5′-CGGAAAACTTCTAGCGGCTGACGATGA CGATAAGCATCATCATCATCATCAT TAACCTGAATTCGGATCCAG-3′ and 5′-CTGGATCCGAATTCAGGTTAATGATGATGATGATGATGCTTATCGTCATCGTCAGCCGCTAGAAGTTTTCCG-3′). DNAs from the clones in pSG5 with the enterokinase/6× histidine tag (VCP-MPXV-EH) and without (VCP-MPXV) were expressed transiently in Chinese hamster ovary (CHO) cells and the supernatants concentrated. Tagged and untagged versions of VCP-MPXV had similar activity. VCP-MPXV-EH supernatants were purified on ProBond™ Resin (Invitrogen, Carlsbad, CA). VCP-MPXV protein concentrations were estimated in an ELISA assay. Briefly, the capture antibody (5A10, a cross reacting VCP-VARV mAb, gift of Ariella Rosengard) was coated at 5 μg/ml overnight at 4 °C and then blocked for 1 h at 37 °C (1% BSA and 0.1% Tween-20 in PBS). Dilutions of concentrated samples and VCP-VACV (as a standard) were incubated for 1 h at 37 °C and then washed with PBS containing 0.05% Tween 20. Rabbit anti-VCP-VACV antiserum (1:5000; gift of Girish Kotwal) was applied for 1 h at 37 °C. After washing, horseradish peroxidase-coupled donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was added and incubated for 1 h at 37 °C. After washing, TMB substrate (Pierce, Rockford, IL) was added and absorbance (630 nm) assessed in an ELISA reader. For Western blot assays, the supernatants or purified proteins were electrophoresed in non-reduced and reduced 10% SDS-PAGE, transferred to nitrocellulose, and probed with 1:5000 rabbit anti-VCP-VACV antibody, followed by horseradish peroxidase goat anti-rabbit IgG and developed with ECL Plus (Amersham, Piscataway, NJ). To characterize VCP-MPXV binding to human C4b and C3b, the ligands were coated onto microtiter plates (5 μg/ml in PBS) followed by incubations with media or VCP-MPXV. Binding was detected with rabbit anti-VCP-VACV antibody (1:5000) as described above. Chemiluminescent cofactor assays were performed in the presence or absence of 10 ng VCP-MPXV-EH, biotinylated human C3b and C4b and human factor I in 10 mM Tris pH 7.4 with 25 mM sodium chloride (Liszewski et al., 1998). Following 1 h incubation at 37 °C, samples were reduced, run on 10% SDS-PAGE, transferred to nitrocellulose, and probed with avidin-horseradish peroxidase. Final signal development used ECL Plus.
Sequence availability
The genomic sequences have the following accession numbers in GenBank: WRAIR-61, AY603973; SL-V70, AY741551, and COP-58, AY753185.
Acknowledgments
This work was funded through four grants: NIAID/DARPA U01 AI48706 (E. J. L), NIAID/DARPA U01 AI48653-02 (R. M. L. B. and C.U.), Canadian NSERC grant OPG0155125-01 (C.U.), and NIAID U54 AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (MRCE; R.M.L.B., M.K.L. and J.P.A.). We would like to thank: Monica Allen for administrative assistance; Angelika Ehlers, Ryan Brodie, and Ross Gibbs for their work in developing the software tools used for analysis of this genome (www.poxvirus.org; www.virology.ca), and Scott Sammons, Christine Wylie, and Drew Lichtenstein for help with bioinformatic analyses and discussions.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virol.2005.05.030.
Appendix A. Supplementary data
Comparison of the protein coding regions of the genomes of isolates SL-V70, COP-58, and WRAIR-61.
Comparison of nucleotide differences between SL-V70, COP-58, and WRAIR-61.
Fig. S1.
Comparison of single-cycle replication yields of COP-58 and ZAI-V79. Monolayer cultures of BSC-1 cells were infected with COP-58 or ZAI-V79 at approximately 1 PFU/cell. At 1, 4, 12.5, 24, 34.5, and 46 h post-infection, 4 cultures were harvested for each virus. Cells were scrapped into the cultures supernatant, frozen and thawed 3 times, and infectivity was measured by plaque assay on BSC-1 monolayers. Plaque titers are presented as means with error bars indicating 1 standard deviation of the mean. The inset shows a typical COP-58 and ZAI-V79 plaque stained at 4 days post-infection.
References
- Alcami A., Smith G.L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita I., Gispen R., Kalter S.S., Wah L.T., Marennikova S.S., Netter R., Tagaya I. Outbreaks of monkeypox and serological surveys in nonhuman primates. Bull. World Health Organ. 1972;46:625–631. [PMC free article] [PubMed] [Google Scholar]
- Barry M., Hnatiuk S., Mossman K., Lee S.F., Boshkov L., McFadden G. The myxoma virus M-T4 gene encodes a novel RDEL-containing protein that is retained within the endoplasmic reticulum and is important for the productive infection of lymphocytes. Virology. 1997;239:360–377. doi: 10.1006/viro.1997.8894. [DOI] [PubMed] [Google Scholar]
- Bonfield J.K., Staden R. Experiment files and their application during large-scale sequencing projects. DNA Seq. 1996;6:109–117. doi: 10.3109/10425179609010197. [DOI] [PubMed] [Google Scholar]
- Bonfield J.K., Smith K., Staden R. A new DNA sequence assembly program. Nucleic Acids Res. 1995;23:4992–4999. doi: 10.1093/nar/23.24.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N., Pachter L. MAVID: constrained ancestral alignment of multiple sequences. Genome Res. 2004;14:693–699. doi: 10.1101/gr.1960404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie R., Smith A.J., Roper R.L., Tcherepanov V., Upton C. Base-By-Base: single nucleotide-level analysis of whole viral genome alignments. BMC Bioinformatics. 2004;5:96. doi: 10.1186/1471-2105-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno M., Do C.B., Cooper G.M., Kim M.F., Davydov E., Green E.D., Sidow A., Batzoglou S. LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 2003;13:721–731. doi: 10.1101/gr.926603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Drillien R., Spehner D., Buller R.M. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 1992;187:433–442. doi: 10.1016/0042-6822(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Chen W., Drillien R., Spehner D., Buller R.M. In vitro and in vivo study of the ectromelia virus homolog of the vaccinia virus K1L host range gene. Virology. 1993;196:682–693. doi: 10.1006/viro.1993.1525. [DOI] [PubMed] [Google Scholar]
- Chen N., Danila M.I., Feng Z., Buller R.M., Wang C., Han X., Lefkowitz E.J., Upton C. The genomic sequence of ectromelia virus, the causative agent of mousepox. Virology. 2003;317:165–186. doi: 10.1016/s0042-6822(03)00520-8. [DOI] [PubMed] [Google Scholar]
- Dear S., Staden R. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 1991;19:3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A.D., Werchniak A.E., Li Y., Brennick J.B., Goldsmith C.S., Kline R., Damon I., Klaus S.N. Tanapox infection in a college student. N. Engl. J Med. 2004;350:361–366. doi: 10.1056/NEJMoa031467. [DOI] [PubMed] [Google Scholar]
- Douglass N.J., Richardson M., Dumbell K.R. Evidence for recent genetic variation in monkeypox viruses. J. Gen. Virol. 1994;75:1303–1309. doi: 10.1099/0022-1317-75-6-1303. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Osborne J., Slack S., Roper R.L., Upton C. Poxvirus orthologous clusters (POCs) Bioinformatics. 2002;18:1544–1545. doi: 10.1093/bioinformatics/18.11.1544. [DOI] [PubMed] [Google Scholar]
- Esposito J.J., Fenner F. In: Fields Virology. Knipe D.M., Howley P.M., editors. Lippincott Williams and Wilkins; New York: 2001. Poxvirus; pp. 2885–2921. [Google Scholar]
- Esposito J.J., Knight J.C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143:230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Ewing B., Green P. Base-calling of automated sequencer traces using phred: II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyj I.D. World Health Organization; Geneva: 1988. Smallpox and Its Eradication. [Google Scholar]
- Fenner F., Wittek R., Dumbell K.R. Academic Press; San Diego: 1989. The Orthopoxviruses. [Google Scholar]
- Foster S.O., Brink E.W., Hutchins D.L., Pifer J.M., Lourie B., Moser C.R., Cummings E.C., Kuteyi O.E., Eke R.E., Titus J.B., Smith E.A., Hicks J.W., Foege W.H. Human monkeypox. Bull. World Health Organ. 1972;46:569–576. [PMC free article] [PubMed] [Google Scholar]
- Gillard S., Spehner D., Drillien R. Mapping of a vaccinia host range sequence by insertion into the viral thymidine kinase gene. J. Virol. 1985;53:316–318. doi: 10.1128/jvi.53.1.316-318.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A., O'leary J., Krych-Goldberg M., Atkinson J.P., Barlow P.N. Three-dimensional structure and flexibility of proteins of the RCA family—A progress report. Biochem. Soc. Trans. 2002;30:990–996. doi: 10.1042/bst0300990. [DOI] [PubMed] [Google Scholar]
- Hnatiuk S., Barry M., Zeng W., Liu L., Lucas A., Percy D., McFadden G. Role of the C-terminal RDEL motif of the myxoma virus M-T4 protein in terms of apoptosis regulation and viral pathogenesis. Virology. 1999;263:290–306. doi: 10.1006/viro.1999.9946. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhang J. Methods for comparing a DNA sequence with a protein sequence. Comput. Appl. Biosci. 1996;12:497–506. doi: 10.1093/bioinformatics/12.6.497. [DOI] [PubMed] [Google Scholar]
- Hutin Y.J., Williams R.J., Malfait P., Pebody R., Loparev V.N., Ropp S.L., Rodriguez M., Knight J.C., Tshioko F.K., Khan A.S., Szczeniowski M.V., Esposito J.J. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001;7:434–488. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S.N., Kotwal G.J., Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. U.S.A. 1992;89:628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek Z., Fenner F. vol. 17. Karger; 1988. (Human Monkeypox in Monographs in Virology). [Google Scholar]
- Jezek Z., Arita I., Mutombo M., Dunn C., Nakano J.H., Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am. J. Epidemiol. 1986;123:1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- Kettle S., Blake N.W., Law K.M., Smith G.L. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode M(r) 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology. 1995;206:136–147. doi: 10.1016/s0042-6822(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Kettle S., Alcami A., Khanna A., Ehret R., Jassoy C., Smith G.L. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J. Gen. Virol. 1997;78(Pt. 3):677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- Kluczyk A., Siemion I.Z., Szewczuk Z., Wieczorek Z. The immunosuppressive activity of peptide fragments of vaccinia virus C10L protein and a hypothesis on the role of this protein in the viral invasion. Peptides. 2002;23:823–834. doi: 10.1016/s0196-9781(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Kotwal G.J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988;335:176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Kotwal G.J., Miller C.G., Justus D.E. The inflammation modulatory protein (IMP) of cowpox virus drastically diminishes the tissue damage by down-regulating cellular infiltration resulting from complement activation. Mol. Cell. Biochem. 1998;185:39–46. doi: 10.1023/a:1006844624825. [DOI] [PubMed] [Google Scholar]
- Liszewski M.K., Atkinson J.P. In: The Complement System. Rother K., Till G.O., Hansch G.M., editors. Springer-Verlag; 1998. Membrane confactor protein (CD46) and decay accelerating factor (CD55) pp. 146–162. [Google Scholar]
- Liszewski M.K., Farries T.C., Lublin D.M., Rooney I.A., Atkinson J.P. Control of the complement system. Adv. Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- Liszewski M.K., Leung M.K., Atkinson J.P. Membrane cofactor protein: importance of N- and O-glycosylation for complement regulatory function. J. Immunol. 1998;161:3711–3718. [PubMed] [Google Scholar]
- Lourie B., Bingham P.G., Evans H.H., Foster S.O., Nakano J.H., Herrmann K.L. Human infection with monkeypox virus: laboratory investigation of six cases in West Africa. Bull. World Health Organ. 1972;46:633–639. [PMC free article] [PubMed] [Google Scholar]
- Massung R.F., Liu L.I., Qi J., Knight J.C., Yuran T.E., Kerlavage A.R., Parsons J.M., Venter J.C., Esposito J.J. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology. 1994;201:215–240. doi: 10.1006/viro.1994.1288. [DOI] [PubMed] [Google Scholar]
- McConnell S.J., Herman Y.F., Mattson D.E., Erickson L. Monkey pox disease in irradiated cynomologous monkeys. Nature. 1962;195:1128–1129. (9–15) [Google Scholar]
- McKenzie R., Kotwal G.J., Moss B., Hammer C.H., Frank M.M. Regulation of complement activity by vaccinia virus complement-control protein. J. Infect. Dis. 1992;166:1245–1250. doi: 10.1093/infdis/166.6.1245. [DOI] [PubMed] [Google Scholar]
- Miller C.G., Justus D.E., Jayaraman S., Kotwal G.J. Severe and prolonged inflammatory response to localized cowpox virus infection in footpads of C5-deficient mice: investigation of the role of host complement in poxvirus pathogenesis. Cell. Immunol. 1995;162:326–332. doi: 10.1006/cimm.1995.1086. [DOI] [PubMed] [Google Scholar]
- Miller C.G., Shchelkunov S.N., Kotwal G.J. The cowpox virus-encoded homolog of the vaccinia virus complement control protein is an inflammation modulatory protein. Virology. 1997;229:126–133. doi: 10.1006/viro.1996.8396. [DOI] [PubMed] [Google Scholar]
- Mukinda V.B., Mwema G., Kilundu M., Heymann D.L., Khan A.S., Esposito J.J. Re-emergence of human monkeypox in Zaire in 1996. Monkeypox Epidemiologic Working Group [letter] Lancet. 1997;349:1449–1450. doi: 10.1016/S0140-6736(05)63725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mural R.J. ARTEMIS: a tool for displaying and annotating DNA sequence. Brief. Bioinform. 2000;1:199–200. doi: 10.1093/bib/1.2.199. [DOI] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., Swain G.R., Olson V.A., Sargent E.K., Kehl S.C., Frace M.A., Kline R., Foldy S.L., Davis J.P., Damon I.K. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Rosengard A.M., Liu Y., Nie Z., Jimenez R. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8808–8813. doi: 10.1073/pnas.112220499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A., Isaacs S.N., Soulika A.M., Lambris J.D. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 1998;160:5596–5604. [PubMed] [Google Scholar]
- Sfyroera G., Katragadda M., Morikis D., Isaacs S.N., Lambris J.D. Electrostatic modeling predicts the activities of orthopoxvirus complement control proteins. J. Immunol. 2005;174:2143–2151. doi: 10.4049/jimmunol.174.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchelkunov S.N., Massung R.F., Esposito J.J. Comparison of the genome DNA sequences of Bangladesh-1975 and India-1967 variola viruses. Virus Res. 1995;36:107–118. doi: 10.1016/0168-1702(94)00113-q. [DOI] [PubMed] [Google Scholar]
- Shchelkunov S.N., Totmenin A.V., Babkin I.V., Safronov P.F., Ryazankina O.I., Petrov N.A., Gutorov V.V., Uvarova E.A., Mikheev M.V., Sisler J.R., Esposito J.J., Jahrling P.B., Moss B., Sandakhchiev L.S. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchelkunov S.N., Totmenin A.V., Safronov P.F., Mikheev M.V., Gutorov V.V., Ryazankina O.I., Petrov N.A., Babkin I.V., Uvarova E.A., Sandakhchiev L.S., Sisler J.R., Esposito J.J., Damon I.K., Jahrling P.B., Moss B. Analysis of the monkeypox virus genome. Virology. 2002;297:172–194. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.A., Mullin N.P., Parkinson J., Shchelkunov S.N., Totmenin A.V., Loparev V.N., Srisatjaluk R., Reynolds D.N., Keeling K.L., Justus D.E., Barlow P.N., Kotwal G.J. Conserved surface-exposed K/R-X-K/R motifs and net positive charge on poxvirus complement control proteins serve as putative heparin binding sites and contribute to inhibition of molecular interactions with human endothelial cells: a novel mechanism for evasion of host defense. J. Virol. 2000;74:5659–5666. doi: 10.1128/jvi.74.12.5659-5666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M.K., Hruby D.E., Maliszewski C.R., Pickup D.J., Sims J.E., Buller R.M., VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992;71:145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Upton C., Hogg D., Perrin D., Boone M., Harris N.L. Viral genome organizer: a system for analyzing complete viral genomes. Virus Res. 2000;70:55–64. doi: 10.1016/s0168-1702(00)00210-0. [DOI] [PubMed] [Google Scholar]
- Uvarova E.A., Shchelkunov S.N. Species-specific differences in the structure of orthopoxvirus complement-binding protein. Virus Res. 2001;81:39–45. doi: 10.1016/S0168-1702(01)00332-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Magnus P., Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Path. Microbiol. Scand. 1959;46:156–176. [Google Scholar]
- Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab. Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Davison A.J., Moss B. Structure of vaccinia virus late promoters. J. Mol. Biol. 1989;210:771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Payne L.G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the protein coding regions of the genomes of isolates SL-V70, COP-58, and WRAIR-61.
Comparison of nucleotide differences between SL-V70, COP-58, and WRAIR-61.


