The world is facing a public health emergency of international concern [1] associated with the spread of human monkeypox in more than 90 countries and territories. Monkeypox is a zoonotic orthopoxvirus infection clinically similar to smallpox. There is emerging evidence that most cases in this multi-country outbreak have been reported among men who have sex with men, with a high prevalence of lesions in the anogenital and oropharyngeal regions [2]. Although most lesions may initially appear at the inoculation site based on the close skin-to-skin contact, recent evidence has found monkeypox virus DNA in the seminal fluid in 60% of individuals with the disease [3], which could indicate the possibility of sexual transmission.
Confirmation of monkeypox infection is based on the presence of virus DNA by polymerase chain reaction (PCR) or next-generation sequencing of a clinical specimen (https://www.who.int/publications/m/item/monkeypox-outbreak-toolbox). Swabs from clinical rash samples have been recommended for diagnostic purposes (https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1), but other biological materials have been evaluated in research protocols. Besides, studies are needed to compare the infectivity between different samples. Here, we present the pooled results from studies that evaluated the presence of monkeypox DNA in different biological samples from patients diagnosed with the disease during the 2022 outbreak (through August 23, 2022).
We included studies that evaluated at least two different biological samples (skin lesions, rectal swab, naso-oropharyngeal swab, faeces, saliva, semen, plasma, or urine). We excluded single case reports, publications with potential overlapping reports, and studies from which data extraction was not possible. Our outcomes of interest were the PCR positivity and cycle threshold (Ct) values in positive samples. The PCR positivity rate for monkeypox virus DNA was calculated using the variance-stabilizing Freeman-Tukey double-arcsine transformation with an inverse-variance random-effects model. Because of differences in PCR assays, differences in Ct values were explored in a head-to-head comparison using the standardized mean difference as the effect size with 95% confidence interval (CI). Cohen's classification was used to interpret the magnitude of the effect size, and a SMD >0.8 was considered a large effect size. Between-study heterogeneity was quantified by the I2 index. A random-effects model was used to pool the results, and a 2-tailed p < 0.05 was used to determine significance. Analyses were conducted in RStudio (version 0.98.1083) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (https://prisma-statement.org/).
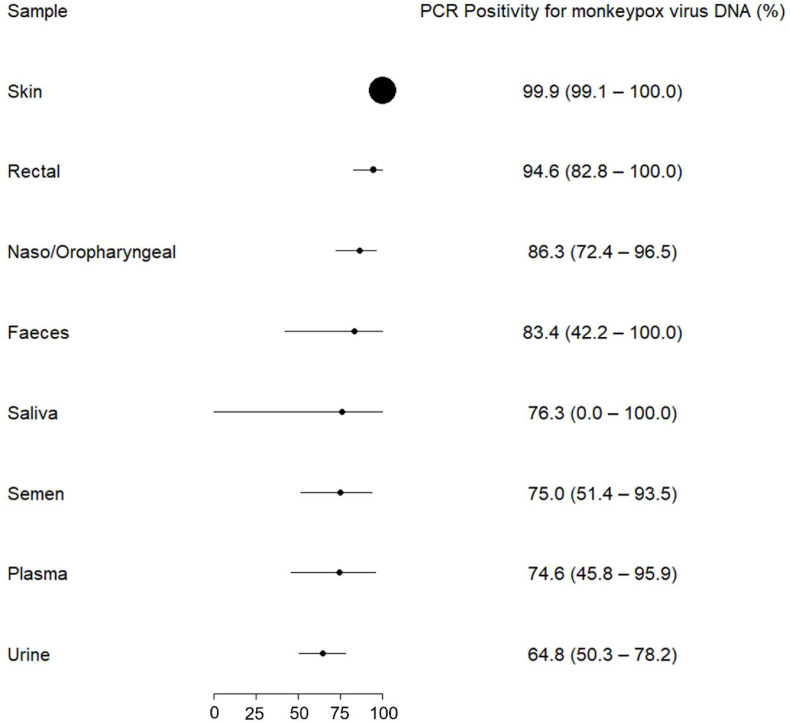
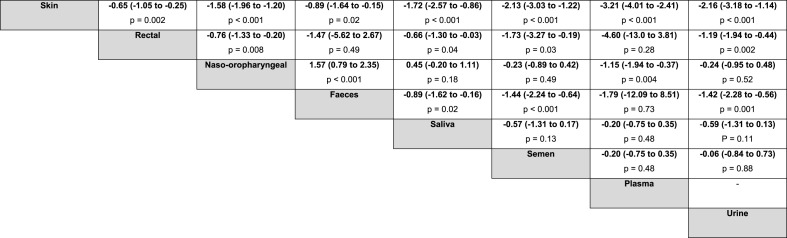
Using a systematic strategy (“monkeypox” OR “monkey pox” OR “monkeypox virus”) in PubMed, Web of Science, Scopus, and Google Scholar, we found eight (supplementary file) relevant studies that met the eligibility criteria. Data from 569 monkeypox patients from Spain, Italy, France, Germany, and Israel were analyzed. We estimated a PCR positivity rate for monkeypox virus DNA from skin lesions of 99.9% (95% CI 99.1 to 100.0; I2 = 0%), followed by rectal swab (94.6%, 95% CI 82.8 to 100.0; I2 = 73.7%) and naso-oropharyngeal swab (86.3%, 95% CI 72.4 to 96.5; I2 = 81.6%). The positivity rates in saliva and semen samples were 76.3% (95% CI 0.0–100.0; I2 = 89.7%) and 75% (95% CI 51.4 to 93.5; I2 = 43.1%), respectively. The lowest rate was found in urine (64.8%, 95% CI 50.3 to 78.2; I2 = not available) (Fig. 1 ). Ct values from skin samples were lower compared to all other specimens, and the magnitude of effect was considered large. In addition, we found that samples from rectal swabs had lower Ct values than those from naso-oropharynx, saliva, semen, and urine with a moderate to large effect size, but no differences were observed in relation to faeces and plasma. Naso-oropharyngeal samples showed higher Ct values than faecal samples. The magnitude of the difference in Ct values between biological samples from patients with monkeypox is detailed in a league table (Table 1 ).
Fig. 1.
PCR positivity for monkeypox virus DNA from different biological samples.
Table 1.
Square matrix showing the magnitude of the difference in cycle threshold values between biological samples from patients with monkeypox.
To the best of our knowledge, this is the first study to synthesize the PCR positivity rates in patients with monkeypox in the current outbreak and to compare Ct values between samples of different biological materials. Although monkeypox is not a new virus, the results of the present study are important to guide clinical practice and protocols for laboratory diagnosis. According to Huggett el al. [4], pre-analytical factors including specimen and swab choice can influence the diagnostic performance and the protocols that use heterogeneous biological samples remain an important and challenging area of research. Furthermore, the correlation between the amount of virus in different samples and infectivity needs to be better understood if PCR is also used for risk assessment.
Ct values indicate the number of cycles required to amplify the viral genetic material to a detectable level and have been used as a proxy for viral load and infectivity. In general, lower Ct values indicate a higher viral load and increased infectivity. Although further evidence is warranted to confirm the clinical value of the quantification cycle in patients with monkeypox, a recent study [5] found a strong correlation between viral DNA content and infectivity in skin and oropharynx specimens, with a Ct value ≥ 33 representing a poorly or non-infectious sample. However, it was shown that most patients had lower Ct values (higher viral loads) in skin lesions than in the oropharynx.
In our meta-analysis, we found a PCR positivity rate very close to 100% in samples from skin lesions and a trend towards increasing differences in Ct values in relation to samples with lower positivity rates. These findings indicate a high risk of infection from close contact with skin lesions. A person with lesions associated with monkeypox is considered infectious until the lesions crust over and fall off [6]. In addition, the imprecision of estimates obtained from rectal, naso-oropharyngeal, and fecal samples seems to limit their indication for diagnostic purposes. Current protocols have recommended the pharyngeal swab for people who have had close personal contact with confirmed cases of monkeypox and for suspected cases without cutaneous manifestations. Interestingly, the studies included in this meta-analysis that evaluated semen, plasma, and urine samples had mean Ct values close to or above 33, which suggests that these body fluids have low infectivity despite the detection of monkeypox DNA.
Our findings suggest that skin lesion swabs are the most effective means of detecting monkeypox DNA using the PCR technique. Besides, our study reinforces the evidence for the role of direct mucocutaneous contact in virus transmission. Studies assessing the sensitivity and specificity for different biological samples are still needed, and the potential for transmission of the monkeypox virus through semen and saliva remains to be further clarified.
Authors contributions
All authors contributed equally to this manuscript.
Financial source
This study did not receive financial source.
Declaration of competing interest
The authors declare they have no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2022.102448.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ghebreyesus T.A. Why the monkeypox outbreak constitutes a public health emergency of international concern. BMJ. 2022 doi: 10.1136/bmj.o1978. o 1978. [DOI] [PubMed] [Google Scholar]
- 2.Philpott D., Hughes C.M., Alroy K.A., Kerins J.L., Pavlick J., Asbel L., et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, may 17–july 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raccagni A.R., Candela C., Mileto D., Canetti D., Bruzzesi E., Rizzo A., et al. Monkeypox infection among men who have sex with men: PCR testing on seminal fluids. J Infect. 2022 doi: 10.1016/j.jinf.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huggett J.F., French D., O'Sullivan D.M., Moran-Gilad J., Zumla A. Monkeypox: another test for PCR. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.32.2200497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paran N., Yahalom-Ronen Y., Shifman O., Lazar S., Ben-Ami R., Yakubovsky M., et al. Monkeypox DNA correlates with virus infectivity in clinical samples. bioRxiv. 2022 doi: 10.1101/2022.08.02.502454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Z., Sun J., Zhang L., Yan S., Li D., Zhang C., et al. Laboratory diagnostics for monkeypox: an overview of sensitivities from various published tests. Trav Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.