Background and Objective:
Total mesorectal excision (TME) for rectal cancer (RC) often results in significant bowel symptoms, commonly known as low anterior resection syndrome (LARS). Although pelvic floor muscle training (PFMT) is recommended in noncancer populations for treating bowel symptoms, this has been scarcely investigated in RC patients. The objective was to investigate PFMT effectiveness on LARS in patients after TME for RC.
Methods:
A multicenter, single-blind prospective randomized controlled trial comparing PFMT (intervention; n=50) versus no PFMT (control; n=54) 1 month following TME/stoma closure was performed. The primary endpoint was the proportion of participants with an improvement in the LARS category at 4 months. Secondary outcomes were: continuous LARS scores, ColoRectal Functioning Outcome scores, Numeric Rating Scale scores, stool diary items, and Short Form 12 scores; all assessed at 1, 4, 6, and 12 months.
Results:
The proportion of participants with an improvement in LARS category was statistically higher after PFMT compared with controls at 4 months (38.3% vs 19.6%; P=0.0415) and 6 months (47.8% vs 21.3%; P=0.0091), but no longer at 12 months (40.0% vs 34.9%; P=0.3897). Following secondary outcomes were significantly lower at 4 months: LARS scores (continuous, P=0.0496), ColoRectal Functioning Outcome scores (P=0.0369) and frequency of bowel movements (P=0.0277), solid stool leakage (day, P=0.0241; night, P=0.0496) and the number of clusters (P=0.0369), derived from the stool diary. No significant differences were found for the Numeric Rating Scale/quality of life scores.
Conclusions:
PFMT for bowel symptoms after TME resulted in lower proportions and faster recovery of bowel symptoms up to 6 months after surgery/stoma closure, justifying PFMT as an early, first-line treatment option for bowel symptoms after RC.
Keywords: bowel symptoms, low anterior resection syndrome, pelvic floor muscle training, randomized controlled trial, rectal cancer
Over the years, incorporating neoadjuvant treatment (chemotherapy and radiotherapy) has improved outcome with low local recurrence rates and improved survival.1
Most patients will undergo total mesorectal excision (TME) with a low colorectal or coloanal anastomosis. Despite the nerve-sparing and sphincter-sparing nature of the procedure, up to 60% to 90% of patients will experience cumbersome bowel symptoms, impacting quality of live.2,3 These bowel symptoms can vary greatly and include, but are not limited to: increased frequency of bowel movements, urgency, clustering, and fecal incontinence. The combination of the symptoms and their impact on quality of life has been summarized in an international consensus definition and is referred to as the low anterior resection syndrome (LARS).2,4 The LARS score (LARS questionnaire) was developed as a quick screening tool.5 Other assessment tools, such as the ColoRectal Functioning Outcome (COREFO questionnaire)6 and a stool diary, can provide a more comprehensive means to understand the clinical picture.
The current management of LARS after TME includes antidiarrheal medication, dietary instructions, enemas, or sacral nerve stimulation. However, most patients are instructed that spontaneous improvement will follow. Nevertheless, a pilot study regarding a novel bowel rehabilitation program (BOREAL) in LARS patients was recently published, using a stepwise approach to asses and treat LARS patients, starting with medical management as a first option, followed by pelvic floor physiotherapy, biofeedback, transanal irrigation, then sacral nerve stimulation, antegrade irrigation, and eventually a definitive colostomy.7
Recent treatment guidelines on rectal cancer (RC) do not8 or only briefly9,10 mention pelvic floor muscle training (PFMT) as a conservative treatment option for LARS. However, in noncancer populations, PFMT is highly recommended as a treatment option for fecal incontinence.11
Previous reviews remained inconclusive, partly due to the limited number of studies and the rather low methodological quality (nonrandomized efficacy studies) of these studies.12–14 Furthermore, 2 randomized controlled studies reported on the role of PFMT on fecal incontinence after RC surgery.15,16 Notwithstanding the fact that these were randomized trials, the focus was specifically on PFMT effects for incontinence instead of bowel complaints as a whole complex of symptoms (LARS). Follow-up was only reported until maximally 9 months.15,16 This randomized controlled trial (RCT) aims to evaluate the effects of a comprehensive PFMT program up to 1 year after TME for RC on the comprehensive set of bowel symptoms or LARS, in comparison to patients who received no training.
METHODS
Study Design
The study protocol has previously been published.17 In short, this study was a multicenter, single-blind, prospective RCT. Patients treated for RC were recruited after TME in 3 Belgian hospitals: University Hospitals Leuven, Onze-Lieve-Vrouw (OLV) Hospital in Aalst or General Hospital Groeninge in Kortrijk. Ethics approval was granted by the coordinating Ethical Committee of the University Hospitals Leuven (s59761) and additionally a positive advice from the other local Ethical Committees was obtained. This study applies the principles established in the Declaration of Helsinki and was reported according to the CONSORT guidelines (Supplemental Digital Contents 1, 2, http://links.lww.com/SLA/E106, http://links.lww.com/SLA/E107). This trial was registered at Netherlands Trial Register (NTR6383).
Patients
Inclusion criteria were as follows: (1) patients who had a low anterior resection (LAR) with TME for RC, (2) patients with a minimal LARS score5 of 21/42 (=at least minor LARS) at 1 month after surgery (no ileostomy) or after ileostomy closure, and (3) patients had to be able to come to the hospital once a week during the complete treatment period of 12 weeks. Exclusion criteria have previously been described.17 A written consent form was signed by participants before data collection and obtained by the assessor before the first assessment. All data were deidentified and coded with a unique trial identification number.
Randomization and Blinding
One month after restoration of transit, patients were randomly assigned to the intervention group (receiving 12 weeks of PFMT) or to the control group (not receiving PFMT, standard care). The randomization was computer generated. Sequencing was determined by the date of rectal resection (in case of no ileostomy) or by the date of the ileostomy closing (in case the patient received a temporary ileostomy). The randomization was performed with 8 strata, using 6-size permuted blocks. The strata were a result from 3 binary stratification variables, which were: sex (male vs female), type of anastomosis (stapled vs handsewn), and type of reconstruction (colonic J-pouch/side-to-end coloanal anastomosis vs straight coloanal anastomosis). The assessor was blinded for the allocation of the participants to the 2 groups, and the participants were asked not to discuss the treatment of their bowel symptoms with the assessor. Blinding of the participants or of the therapist who performed the treatments was not possible given the nature of the intervention.
Procedures
The intervention group received 12 weeks of PFMT, consisting of 9 individual treatments: during the first 6 weeks once a week and 3 sessions over the last 6 weeks. Each session was provided by a specialized physiotherapist trained in pelvic re-education and with several years of experience in training these patients. The sessions started with an assessment and evaluation of bowel symptoms with a stool diary, combined with patient education, pelvic floor muscle exercises (focused on strength, endurance, relaxation, proprioception, and coordination), electromyographic-biofeedback/electrical stimulation, and rectal balloon training (improvement of rectal sensation of filling and proper expelling). The content of the treatment has been described in detail in the published protocol.17 The control group did not receive any PFMT. During follow-up, every participant was monitored by the department of abdominal surgery, and no adverse events were expected due to PFMT. If necessary, adverse events could be reported to members of the research team.
Outcomes
All outcome measures were assessed at 1, 4, 6, and 12 months after TME/stoma closure. The primary outcome was defined as the proportion of participants with an improvement in the LARS category at 4 months (from major LARS to minor LARS, from major LARS to no LARS, or from minor LARS to no LARS) compared with the LARS score measured at 1 month postoperatively. The primary outcome was the dichotomous classification of change in the LARS category (1: change in category, 0: no change in category). The LARS score itself (continuous variable) was recorded as a secondary outcome. Other secondary outcomes were bowel symptoms evaluated by (1) the COREFO questionnaire,6 (2) a Numeric Rating Scale (NRS) regarding the subjective bother of bowel symptoms, and (3) a stool diary. A 7-day stool diary assessed: frequency of bowel movements, stool consistency (scored on the Bristol Stool Scale), urgency/incontinence/soiling episodes, fragmentation of stool (clustering). Quality of life was evaluated by the Short Form 12 (SF-12).18
Sample Size Calculation
The primary endpoint was defined as the proportion of patients with an improvement in the LARS category at 4 months (=minimal clinically important difference). The expected proportion of patients with success (improvement) in the control group was assumed to equal 10% after 12 weeks of PFMT, based on expert opinion. It was calculated that 49 subjects per group were needed to detect with at least 80% power a difference of 25% between groups (in the proportion of patients that improved in LARS category), based on expert opinion (thus 10% vs 35%). This calculation was based on a 2-sided Fisher exact test with α equal to 0.05. To anticipate for patient dropout and inclusion of strata (8 strata, resulting from 3 binary stratification variables) in the final analysis (a stratified exact test for proportions), 60 subjects per group were required.
Statistical Analysis
For the primary analysis, a 2-sided stratified exact Cochran-Mantel-Haenzel-test was used to compare the proportion of patients with a decrease in LARS category between both groups at 4, 6, and 12 months. Tipping point imputation was used for missing data on LARS category changes under MNAR (missing non-at-random) assuming no changes in the intervention group. Data were analyzed according to the intention-to-treat principle. For the secondary outcomes [LARS (continuous), COREFO, NRS, bowel diary frequencies, SF-12], linear mixed-effects models were used to assess changes over time (from 1 to 12 months). To assess different trajectories for patients in both groups, we included random effects (intercept and slope) and fixed effects (time, group, interaction of group, and time) into the model. For the primary and secondary outcomes, the α level was set at 0.05. Analyses were performed by Leuven Biostatistics and Statistical Bioinformatics Centre. The exact Cochran-Mantel-Haenzel test was performed in SAS 9.4, and linear mixed-effects models were fitted in R (v.4.0.3).
RESULTS
Between January 2017 and January 2021, 104 patients entered the study protocol. Initially, the inclusion of 120 patients was foreseen. As the accrual rate was hampered by the COVID-19 restrictions and based upon a lower-than-predicted dropout rate, inclusion was stopped at 49 months (104 patients). Figure 1 gives an overview of the trial. Baseline characteristics are shown in Table 1, and outcome values for the questionnaires in Table 2. At 4 months, after TME/stoma closure, there was a significant difference in the proportion of patients with an improvement in the LARS category (P=0.0415). At 6 months, the difference remained significant (P=0.0091) but no longer at 12 months (P=0.3897) (Table 2, Fig. 2). At 4 months, the continuous LARS score (P=0.0496) and COREFO score (P=0.0369) differed significantly between both groups (Table 2, Fig. 3). Furthermore, all the following items were significantly better in the intervention group at 4 months: the average frequency of bowel movements/24 hours (P=0.0277), the average frequency of solid stool leakage (day: P=0.0241; night: P=0.0496) as well as the average number of clusters per day (P=0.0369), assessed with the stool diary. Other secondary outcome variables (NRS scores, SF-12 scores, and the remaining stool diary items) were not found to be significantly different between the intervention and control group. An overview of the results is further presented in Table 2.
FIGURE 1.
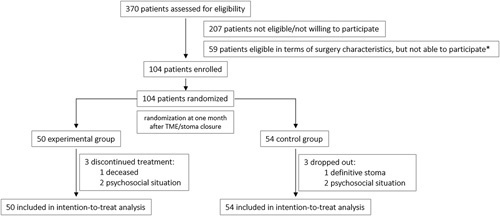
Trial recruitment. *Due to psychosocial circumstances, distance to the hospital or group preference.
TABLE 1.
Baseline Characteristics (N=104)
| Value | ||
|---|---|---|
| Variables | Intervention Group (n=50) n (%) | Control Group (n=54) n (%) |
| Age [mean (SD)/median (IQR)] (y) | 58.8 (12.7) | 57.1 (10.9) |
| ≤49 | 11 (22.0) | 14 (25.9) |
| 50–69 | 29 (58.0) | 35 (64.8) |
| ≥70 | 10 (20.0) | 5 (9.3) |
| Sex | ||
| Male | 36 (72.0) | 35 (64.8) |
| Female | 14 (28.0) | 19 (35.2) |
| BMI [mean (SD)/median (IQR)] (kg/m2) | 24.6 (4.0) | 24.1 (3.7) |
| <25.0 | 28 (56.0) | 30 (55.6) |
| 25.1–30.0 | 19 (38.0) | 16 (29.6) |
| >30.0 | 3 (6.0) | 8 (14.8) |
| Partner | ||
| Yes | 46 (92.0) | 48 (88.9) |
| No | 4 (8.0) | 6 (11.1) |
| Employment status | ||
| Retired | 23 (46.0) | 17 (31.5) |
| Employed/unemployed | 27 (54.0) | 27 (68.5) |
| Tumor height* | ||
| Low (0–5 cm) | 29 (58.0) | 31 (59.3) |
| Mid (5.1–10 cm) | 14 (28.0) | 16 (29.6) |
| High (10.1–15 cm) | 7 (14.0) | 7 (11.1) |
| Type of reconstruction | ||
| Straight coloanal anastomosis | 36 (72.0) | 31 (57.4) |
| Side-to-end coloanal anastomosis | 5 (10.0) | 16 (29.6) |
| Colon pouch-anal anastomosis/J-pouch | 9 (18.0) | 7 (13.0) |
| Anastomosis | ||
| Handsewn | 15 (30.0) | 17 (31.5) |
| Stapled | 35 (70.0) | 37 (68.5) |
| Neoadjuvant therapy | ||
| No | 15 (30.0) | 16 (29.6) |
| Chemotherapy and/or radiotherapy | 35 (70.0) | 38 (70.4) |
| Adjuvant therapy | ||
| No | 26 (52.0) | 28 (51.9) |
| Chemotherapy | 23 (46.0) | 25 (46.2) |
| Chemoradiotherapy | 1 (2.0) | 1 (1.9) |
| Stoma | ||
| Yes | 43 (86.0) | 47 (87.0) |
| No | 7 (14.0) | 7 (13.0) |
From the anal verge.
BMI indicates body mass index; IQR, interquartile range.
TABLE 2.
Overview of Results
| 1 mo | 4 mo | 6 mo | 12 mo | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E (N=50) | C (N=54) | E (N=50) | C (N=54) | Statistics | E (N=50) | C (N=54) | Statistics | E (N=50) | C (N=54) | Statistics | |||||||
| Primary Outcome* | — | Proportion (%) | Adjusted Proportion Difference | Wald CI | P | Proportion (%) | Adjusted Proportion Difference | Wald CI | P | Proportion (%) | Adjusted Proportion Difference | Wald CI | P | ||||
| LARS category improvement | — | — | 38.30 | 19.61 | −0.2040 | −0.365; −0.042 | 0.0415 | 47.83 | 21.28 | −0.2630 | −0.042; −0.105 | 0.0091 | 40.00 | 34.88 | −0.1000 | −0.273; 0.074 | 0.3897 |
| Secondary outcomes † | Mean (SD) | Mean (SD) | Value | Standard Error | P | Mean (SD) | Value | Standard Error | P | Mean (SD) | Value | Standard Error | P | ||||
| LARS score | 36.20 (5.70) | 37.20 (4.42) | 29.9 (8.09) | 33.80 (7.70) | −3.1340 | 1.5888 | 0.0496 | 26.80 (10.30) | 30.60 (8.89) | −3.3765 | 1.8174 | 0.0643 | 29.40 (9.69) | 29.70 (9.09) | 0.1836 | 1.9579 | 0.9254 |
| COREFO score | 45.10 (19.40) | 45.30 (18.90) | 31.1 (17.00) | 36.30 (18.40) | −5.3905 | 2.5699 | 0.0369 | 28.40 (17.80) | 29.00 (15.80) | −1.0715 | 2.5962 | 0.6801 | 27.10 (16.90) | 28.20 (15.10) | −1.1730 | 2.7912 | 0.6746 |
| NRS | 5.50 (2.80) | 6.06 (2.61) | 4.21 (2.42) | 4.96 (2.80) | −0.2449 | 0.4919 | 0.6190 | 4.00 (2.32) | 3.96 (2.69) | 0.5335 | 0.4928 | 0.2800 | 4.04 (2.70) | 4.00 (2.29) | 0.6364 | 0.6294 | 0.3129 |
| BM/24 h‡ | 6.81 (3.85) | 6.16 (3.33) | 3.97 (2.39) | 5.12 (4.10) | −1.6624 | 0.7503 | 0.0277 | 4.08 (2.37) | 4.76 (3.98) | −1.0709 | 0.7256 | 0.1414 | 3.83 (2.20) | 4.06 (3.02) | −0.9298 | 0.7699 | 0.2284 |
| BM during day‡ | 6.01 (4.03) | 5.04 (2.96) | 3.51 (2.32) | 4.11 (3.70) | −1.3622 | 0.7282 | 0.0627 | 3.64 (2.41) | 3.93 (3.17) | −1.0811 | 0.7205 | 0.1349 | 2.95 (1.84) | 3.28 (1.83) | −1.3320 | 0.6837 | 0.0526 |
| BM during night‡ | 0.77 (0.93) | 0.72 (0.66) | 0.41 (0.68) | 0.67 (0.86) | −0.3158 | 0.1909 | 0.0994 | 0.33 (0.43) | 0.60 (1.10) | −0.2606 | 0.1971 | 0.1875 | 0.41 (0.62) | 0.33 (0.46) | −0.0241 | 0.1998 | 0.9040 |
| SL/24 h‡ | 2.02 (3.15) | 1.55 (2.88) | 0.36 (0.84) | 0.83 (2.11) | −0.9447 | 0.5651 | 0.0959 | 0.54 (1.30) | 0.35 (0.61) | −0.4342 | 0.5186 | 0.4034 | 0.24 (0.42) | 0.27 (0.54) | −0.5676 | 0.5531 | 0.3059 |
| Liquid SL during day‡ | 0.79 (1.82) | 0.64 (1.67) | 0.09 (0.22) | 0.32 (1.28) | −0.3760 | 0.3710 | 0.3119 | 0.26 (1.00) | 0.13 (0.33) | −0.0783 | 0.3488 | 0.8226 | 0.04 (0.11) | 0.11 (0.27) | −0.2918 | 0.3386 | 0.3897 |
| Solid SL during day‡ | 0.80 (1.53) | 0.41 (0.83) | 0.17 (0.54) | 0.37 (0.80) | −0.5854 | 0.2579 | 0.0241 | 0.16 (0.49) | 0.11 (0.36) | −0.3910 | 0.2592 | 0.1327 | 0.09 (0.23) | 0.10 (0.27) | −0.4155 | 0.2472 | 0.0941 |
| Liquid SL during night‡ | 0.17 (0.45) | 0.11 (0.36) | 0.02 (0.07) | 0.02 (0.11) | −0.0559 | 0.0835 | 0.5037 | 0.03 (0.10) | 0.05 (0.12) | −0.0638 | 0.0848 | 0.4522 | 0.03 (0.12) | 0.01 (0.05) | −0.0565 | 0.0846 | 0.5051 |
| Solid SL during night‡ | 0.21 (0.53) | 0.06 (0.18) | 0.06 (0.30) | 0.10 (0.31) | −3.1340 | 1.5888 | 0.0496 | 0.08 (0.32) | 0.02 (0.09) | −3.3765 | 1.8174 | 0.0643 | 0.08 (0.25) | 0.01 (0.09) | 0.1836 | 1.9579 | 0.9254 |
| Clusters/day§ | 1.01 (1.4) | 0.92 (1.13) | 0.55 (0.90) | 0.85 (1.26) | −5.3905 | 2.5699 | 0.0369 | 0.47 (0.71) | 0.63 (0.76) | −1.0715 | 2.5962 | 0.6801 | 0.50 (0.70) | 0.57 (0.65) | −1.1730 | 2.7912 | 0.6746 |
| Urgency episodes/day§ | 3.08 (3.46) | 2.85 (3.05) | 0.86 (1.65) | 1.45 (1.73) | −0.2449 | 0.4919 | 0.6190 | 0.91 (1.68) | 1.13 (1.71) | 0.5335 | 0.4928 | 0.2800 | 0.86 (1.76) | 1.06 (1.46) | 0.6364 | 0.6294 | 0.3129 |
| SF-12, PCS | 33.80 (4.32) | 33.4 (3.63) | 31.70 (3.88) | 33.10 (3.76) | −1.7625 | 0.9471 | 0.0639 | 32.60 (3.50) | 32.20 (3.44) | 0.0776 | 0.8622 | 0.9284 | 32.50 (3.33) | 33.50 (3.78) | −1.6075 | 0.9298 | 0.0850 |
| SF-12, MCS | 28.00 (4.55) | 27.8 (4.48) | 27.50 (4.60) | 27.20 (3.96) | 0.0346 | 1.0086 | 0.9727 | 27.10 (4.53) | 26.60 (3.94) | 0.3599 | 0.9893 | 0.7163 | 27.60 (4.32) | 27.40 (4.34) | 0.5113 | 1.0286 | 0.6195 |
Bold values indicate statistically significant P < 0.005.
Cochran-Mantel-Haenzel.
Linear mixed-effect models.
Average frequency.
Average number.
C indicates control; E, experimental; BM, bowel movements; CI, confidence interval; MCS, Mental Component Score; PCS, Physical Component Score; SL, stool leakage.
FIGURE 2.
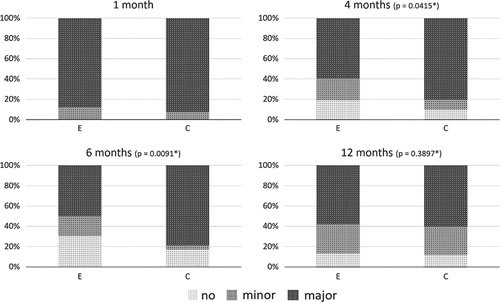
Representation of the LARS categories for the experimental and control group at each timepoint. *P-values correspond to the results of the exact proportion test of the proportion differences, based on the 1 month timepoint.
FIGURE 3.
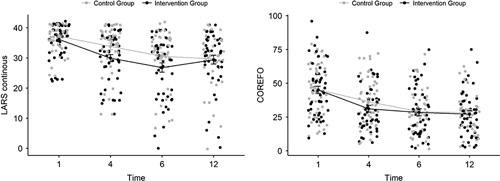
Representation of the trajectories of the LARS and COREFO scores over time.
No serious adverse events related to PFMT were reported. No RC patients were withdrawn because of harm related to the intervention.
DISCUSSION
This is the first RCT justifying the use of PFMT to improve bowel symptoms in the early care pathway of RC patients. PFMT resulted in a significantly higher proportion of patients with an improvement in LARS at 4 and 6 months. At 4 months, the total LARS and COREFO scores were significantly decreased in the intervention group, and PFMT had a beneficial effect on stool frequency, incontinence, and clustering.
There is a natural tendency for functional improvement over time.19 This study shows that PFMT can accelerate this process, as twice as many patients reached acceptable function at 4 months. The lack of improvement in the quality of life or the lack of differences between the groups regarding this aspect might be linked to the choice of a questionnaire rather than the intervention that is falling short. After all, the LARS questionnaire was developed as a short questionnaire for bowel dysfunction after LAR on the basis of symptoms and impact on quality of life. Seeing that PFMT was shown to have a significant influence on the LARS score, we can further perpetuate the foregoing reasoning regarding the choice in the quality of life questionnaires. We therefore propose that all patients with LARS symptoms at 1 month should receive PFMT for 12 weeks.
The pathophysiology of LARS is most likely multifactorial and results from a complicated interplay between anatomical, neurological, physiological, and psychological factors.4,10 PFMT can only interact with some of these aspects, which explains that only 38% of patients benefited from this approach. To date, we do not know which factors influence the success or failure of PFMT. Therefore, we would advise to implement PFMT early on in the care pathway before exploring more invasive and often costly treatment options or waiting to see whether or not spontaneous recovery occurs. This is in line with the findings Harji et al7 regarding their bowel rehabilitation program, in which they demonstrated that the median time to achieve good bowel function was significantly lower in patients following the program, which also included PFMT.
Previous reviews did not reach a consensus regarding the effectiveness of PFMT for bowel symptoms after RC due to several limitations of included trials, such as: retrospective design,20,21 small or heterogeneous patient groups20,22–24 and the fact that treatment was either too short or showed a lack of uniformity.21–26 Furthermore, due to lack of evidence, PFMT was scarcely mentioned as a treatment option in current guidelines.8–10
Previously, 2 RCT’s investigating the role of PFMT were published. Lin et al15 showed a short-term effect of PFMT on fecal incontinence (as measured by the Wexner score) after LAR, which are partially in line with the results from the present study. The second RCT16 could not demonstrate an effect of PFMT on Wexner incontinence scores in RC patients.16 Analogous to the present study, a specialized physiotherapist provided PFMT, and the same treatment modalities were performed.16 However, important differences should be noted. First of all, timing and duration of the interventions differed, that is, PFMT in the current study started 1 month after TME/stoma closure and lasted 9 sessions, compared with a start at 3 months after LAR/6 weeks after stoma closure and 12 sessions in the study by van der Heijden et al16 Similar to the study of Lin et al,15 the primary outcome focused on incontinence only. In addition, baseline Wexner incontinence scores differed significantly between groups sessions in the study by van der Heijden et al.16 No differences after PFMT were found for the whole group; but subgroup analyses showed significant differences after PFMT for those patients who reported urgency or at least moderate incontinence at baseline.16 These subgroup analyses are in line with our results, as incontinence-related items were also significantly better at the end of the treatment phase. However, the benefit for patients with urgency could not be confirmed.
Major strengths of this study were the randomized trial design and reporting the effect of PFMT on a range of bowel symptoms in the short (4 months), middle (6 months) as well as the long term (12 months). A broad range of bowel symptoms treated was investigated through reliable and valid questionnaires as well as a stool diary since LARS represents a myriad of bowel symptoms. In addition, a low dropout rate was observed, and all PFMT sessions were provided by experienced specialized physiotherapists using highly standardized procedures. Last, potential sources of bias were addressed by using a computer-generated and sequenced randomization process. A limitation of the present study was the fact that this study was stopped prematurely because of the COVID-19 pandemic. Also, a nonvalidated stool questionnaire was used to evaluate bowel symptoms.
Future research should determine whether PFMT should be started as soon as 1 month after TME/stoma closure and whether an extension of supervised PFMT sessions up to 6 months or even 12 months could enhance the outcomes.
To conclude, PFMT for bowel symptoms after TME for RC resulted in a lower proportion and faster recovery of bowel symptoms up to 6 months after surgery/stoma closure, justifying PFMT as a first-line option for the improvement of bowel symptoms after RC. Since there are no side effects or risks attached to PFMT, we advise that PFMT should be offered to all patients with bowel symptoms, starting ∼1 month after surgery/stoma closure.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to the trial participants and thank all participating centers and collaborating physiotherapists (MW, LDW, LV, RVH) of this trial for their contributions. They also thank Kim Sterckx, Hilde Lemkens, and Lynn Debrun especially, for all of their contributions.
Footnotes
Supported by a grant of the Research Foundation—Flanders (FWO-TBM) (T000216N) (Fonds Wetenschappelijk Onderzoek—Vlaanderen, Egmontstraat 5, 1000 Brussel 1000, Belgium).
A.D.G. is a postdoctoral research fellow of the FWO-Flanders.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Anne Asnong, Email: anne.asnong@kuleuven.be.
André D’Hoore, Email: andre.dhoore@uzleuven.be.
Marijke Van Kampen, Email: marijke.van.kampen@telenet.be.
Albert Wolthuis, Email: albert.wolthuis@uzleuven.be.
Yves Van Molhem, Email: Yves.Van.Molhem@olvz-aalst.be.
Bart Van Geluwe, Email: bart.vangeluwe@azgroeninge.be.
Nele Devoogdt, Email: nele.devoogdt@kuleuven.be.
An De Groef, Email: an.degroef@kuleuven.be.
Ipek Guler Caamano Fajardo, Email: ipek.guler@kuleuven.be.
Inge Geraerts, Email: inge.geraerts@kuleuven.be.
REFERENCES
- 1.Borstlap WAA, Deijen CL, den Dulk M, et al. Benchmarking recent national practice in rectal cancer treatment with landmark randomized controlled trials. Colorectal Dis. 2017;19:O219–O231. [DOI] [PubMed] [Google Scholar]
- 2.Bryant CL, Lunniss PJ, Knowles CH, et al. Anterior resection syndrome. Lancet Oncol. 2012;13:e403–e408. [DOI] [PubMed] [Google Scholar]
- 3.Kupsch J, Jackisch T, Matzel KE, et al. Outcome of bowel function following anterior resection for rectal cancer-an analysis using the low anterior resection syndrome (LARS) score. Int J Colorectal Dis. 2018;33:787–798. [DOI] [PubMed] [Google Scholar]
- 4.Keane C, Fearnhead NS, Bordeianou L, et al. International consensus definition of low anterior resection syndrome. Colorectal Dis. 2020;22:331–341. [DOI] [PubMed] [Google Scholar]
- 5.Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922–928. [DOI] [PubMed] [Google Scholar]
- 6.Bakx R, Sprangers MA, Oort FJ, et al. Development and validation of a colorectal functional outcome questionnaire. Int J Colorectal Dis. 2005;20:126–136. [DOI] [PubMed] [Google Scholar]
- 7.Harji D, Fernandez B, Boissieras L, et al. A novel bowel rehabilitation programme after total mesorectal excision for rectal cancer: the BOREAL pilot study. Colorectal Dis. 2021;23:2619–2626. [DOI] [PubMed] [Google Scholar]
- 8.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN). Rectal cancer, version 2.2021; 2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed October 28, 2021.
- 10.Christensen P, Im Baeten C, Espín‐Basany E, et al. Management guidelines for low anterior resection syndrome—the MANUEL project. Colorectal Dis. 2021;23:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2012:CD002111. [DOI] [PubMed] [Google Scholar]
- 12.Lin KY, Granger CL, Denehy L, et al. Pelvic floor muscle training for bowel dysfunction following colorectal cancer surgery: a systematic review. Neurourol Urodyn. 2015;34:703–712. [DOI] [PubMed] [Google Scholar]
- 13.Visser WS, Te Riele WW, Boerma D, et al. Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: a systematic review. Ann Coloproctol. 2014;30:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maris A, Devreese AM, D’Hoore A, et al. Treatment options to improve anorectal function following rectal resection: a systematic review. Colorectal Dis. 2013;15:e67–e78. [DOI] [PubMed] [Google Scholar]
- 15.Lin YH, Yang HY, Hung SL, et al. Effects of pelvic floor muscle exercise on faecal incontinence in rectal cancer patients after stoma closure. Eur J Cancer Care (Engl). 2016;25:449–457. [DOI] [PubMed] [Google Scholar]
- 16.van der Heijden J, Kalkdijk-Dijkstra A, Pierie J, et al. Pelvic floor rehabilitation after rectal cancer surgery: a multicentre randomised clinical trial (FORCE Trial). Ann Surg. 2021;276:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asnong A, D’Hoore A, Van Kampen M, et al. Randomised controlled trial to assess efficacy of pelvic floor muscle training on bowel symptoms after low anterior resection for rectal cancer: study protocol. BMJ Open. 2021;11:e041797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendel CS, Grant M, Herrinton L, et al. Reliability and validity of a survey to measure bowel function and quality of life in long-term rectal cancer survivors. Qual Life Res. 2014;23:2831–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croese AD, Lonie JM, Trollope AF, et al. A meta-analysis of the prevalence of low anterior resection syndrome and systematic review of risk factors. Int J Surg. 2018;56:234–241. [DOI] [PubMed] [Google Scholar]
- 20.Bartlett L, Sloots K, Nowak M, et al. Biofeedback therapy for symptoms of bowel dysfunction following surgery for colorectal cancer. Tech Coloproctol. 2011;15:319–326. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Yu CS, Yoon YS, et al. Effectiveness of biofeedback therapy in the treatment of anterior resection syndrome after rectal cancer surgery. Dis Colon Rectum. 2011;54:1107–1113. [DOI] [PubMed] [Google Scholar]
- 22.Ho YH, Chiang JM, Tan M, et al. Biofeedback therapy for excessive stool frequency and incontinence following anterior resection or total colectomy. Dis Colon Rectum. 1996;39:1289–1292. [DOI] [PubMed] [Google Scholar]
- 23.Ho YH, Tan M. Biofeedback therapy for bowel dysfunction following low anterior resection. Ann Acad Med Singapore. 1997;26:299–302. [PubMed] [Google Scholar]
- 24.Liu CH, Chen CH, Lee JC. Rehabilitation exercise on the quality of life in anal sphincter-preserving surgery. Hepatogastroenterology. 2011;58:1461–1465. [DOI] [PubMed] [Google Scholar]
- 25.Allgayer H, Dietrich CF, Rohde W, et al. Prospective comparison of short- and long-term effects of pelvic floor exercise/biofeedback training in patients with fecal incontinence after surgery plus irradiation versus surgery alone for colorectal cancer: clinical, functional and endoscopic/endosonographic findings. Scand J Gastroenterol. 2005;40:1168–1175. [DOI] [PubMed] [Google Scholar]
- 26.Laforest A, Bretagnol F, Mouazan AS, et al. Functional disorders after rectal cancer resection: does a rehabilitation programme improve anal continence and quality of life? Colorectal Dis. 2012;14:1231–1237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


