Dear Editor
We read an article with great interest by Stephen T. Green et al.,1 emphasized the importance of an efficient and effective internationally-supported Zoonosis Surveillance System. Because of the lack of an efficient system for monitoring and responding to zoonotic disease outbreaks worldwide, there is a risk that disease outbreaks might spread beyond national boundaries. As a result, the current outbreak of the Monkeypox virus (MPXV) has sounded the alarm for worldwide public health experts. This paper will thus highlight the present epidemiology and offer several preventative and safety measures to limit the spread of this infectious illness.
MPXV belongs to the family Poxviridae, subfamily chordopoxvirinae, genus orthopoxvirus, with the size of is 200–250 nm; like other poxviruses, they are bricked shape with lipoprotein envelope having linear double-stranded DNA.2 , 3 Poxviruses have all the essential proteins required for replication, transcription, assembly, and release, in their genome but they depend on the host cell for mRNA translation.4 Important enzymes and structural proteins are encoded in the central region of the genome of the MPXV. In contrast, virulence and host range factors are encoded in end regions of genome.5
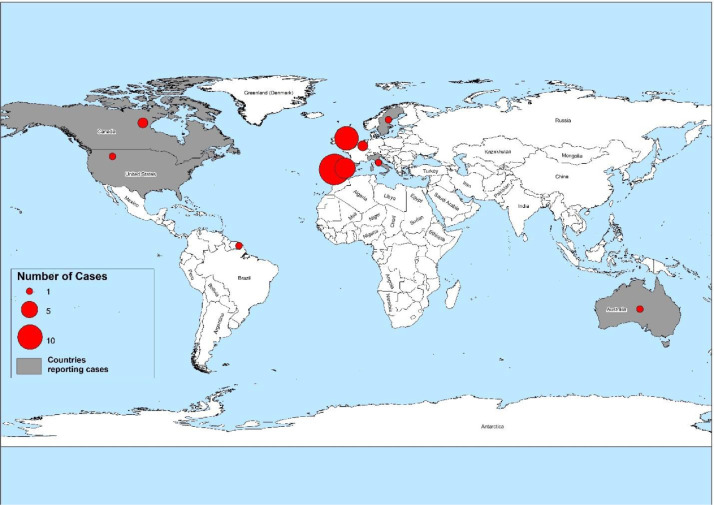
The first human cases of MPXV were detected in 1970 in the Democratic Republic of the Congo (DRC). Since most cases have been documented from rural, rainforest regions of the Congo Basin, mainly the DRC, human cases have surged throughout Central and West Africa. In 1970, 11 African states documented human cases of MPXV. Alarmingly, Nigeria has been experiencing a major epidemic since 2017, with over 500 suspected cases and over 200 confirmed cases, with a case fatality ratio of about 3%. In 2003, the first case of MPXV was reported in the United States of America. Since 2018 several cases have been reported in non-endemic regions like Israel, the United Kingdom (UK), and Singapore. The UK Health Security Agency (UKHSA) recently confirmed a family cluster of two MPXV cases in the United Kingdom (UK) on 14 May 2022, with no history of Nigeria travels. As of 19 May 2022, 37 cases have been confirmed worldwide. Of these, 26 cases have been confirmed in the following EU/EEA countries, as shown in Fig. 1 . Most instances have occurred in young males, many of whom self-identify as male sex with male (MSM), with the lessions predominantly on vaginal or per-genital areas.6
Fig. 1.
Number of confirmed cases of Monkeypox worldwide, as of May 20, 2022. The majority of cases were recorded in European nations. Taking this origin into consideration, it is possible for this virus to be exported all over the world if the necessary precautions are not taken.
As for the transmission is concerned, animal-to-human (zoonotic) risk of transmission by close contact with infested animals' blood, body fluids, or cutaneous or mucosal sores. According to the estimated 72.5% of cases, MPXV is transmitted via an animal source. Different factors like— sleeping outside, increased contact with the ground, visiting a forest, or residing near a forest and other factors might enhance exposure animals. Human-to-human transmission occurs in 27.5% due to variables such as sharing a room or bed, physical contact, living in the same residence, or using the same utensils.7 MPXV enters through the nasopharyngeal, oropharyngeal, or intradermal route and replicate at the inoculation site and gain access to local lymph nodes; after that, viremia will spread to other organs.4 Unlike fever, myalgia, and common lesions in orthopoxviruses, lymphadenopathy can differentiate MPXV from chickenpox, measles, and smallpox. After 1–3 days of fever, the onset of rashes that can range from a few to several thousand will affect the face, limbs, mucous membrane, soles/palms, and genitalia. The rash progresses successively from freckle to pustule to blister and finally to boils which layer, dries, and falls off that, can be a risk for transmission.8 , 9
Despite the approval of a vaccine (MVA-BN) and a treatment (tecovirimat) for MPXV in 2019 and 2022, these countermeasures are not yet readily accessible. The Variola virus (smallpox) is closely related to MPXV—hence smallpox vaccination is likewise 85% effective against this virus.7 Because earlier smallpox vaccination programs no longer provide immunity for individuals younger than 40 or 50 years old, only the elderly will benefit from cross-protective immunity from the smallpox vaccine. However, in the absence of a licensed vaccination for MPXV, the third generation of smallpox vaccine should be administered to prevent pre-and post-exposure to smallpox and MPXV.10
For this epidemic to be managed, the following action must be taken. First, the primary preventative method for MPXV is raising public knowledge of risk factors and informing them of the strategies they may take to decrease their exposure to the virus. Second, scientific research should be conducted to determine the feasibility and suitability of vaccination for preventing and controlling MPXV. Third, these human illnesses have frequently emerged through animal-to-human transmission over time; thus, sick or dead wild animals, the proper handling of potential animal reservoirs, the isolation of diseased animals, and animal flesh must not be handled without protection. Fourth, public health specialists should develop strategies to vaccinate and train medical personnel at risk, including laboratory personnel, frontline practitioners, and nurses. In addition, travel restrictions should be imposed on endemic nations, and adequate screening should be implemented at all national entrance and exit ports to prevent the spread of the virus.
Authors' contribution
UAA, SR and WN conceived and designed the study, analyzed and interpreted the data. SR, WJ, AAK and SK were involved in the writing of the first draft, statistical analysis of the manuscript and interpretation of results. UAA, SK and AAK supervised did the final correction of the manuscript. All authors reviewed and approved the final version of the manuscript.
Declaration of Competing Interest
We declare no conflict of interest.
Funding
There is no role of any funding source for this manuscript.
Footnotes
Edited by: R. Read
References
- 1.Green S.T., Cladi L. High time for an efficient and effective internationally-supported Zoonosis Surveillance System? J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahy B.W. 3rd ed. Elsevier Inc; 2008. Encyclopedia of Virology. [Google Scholar]
- 3.Moore M., Zahra F. StatPearls Publishing; 2021. Monkeypox. StatPearls. [PubMed] [Google Scholar]
- 4.Moore M., Zahra F. StatPearls Publishing; 2021. Monkeypox. StatPearls. [Internet] [PubMed] [Google Scholar]
- 5.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epidemiological update: monkeypox outbreak [Internet]. 2022 [cited 20-05-2022]. Available from: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-monkeypox-outbreak.
- 7.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobson G., Adamson J., Adler H., Firth R., Gould S., Houlihan C., et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom. Eurosurveillance. 2021;26(32) doi: 10.2807/1560-7917.ES.2021.26.32.2100745. May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monkypox [Internet]. 2022 [cited 22-05-2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/monkeypox.
- 10.Multi-country monkeypox outbreak in non-endemic countries [Internet]. 2022 [cited 21-05-2022]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385#:∼:text=Historically%2C%20vaccination%20against%20smallpox%20had,are%20not%20yet%20widely%20available.