Abstract
Background
Monkeypox virus (MPXV) is currently spreading among men who have sex with men, outside of sub-Saharan Africa, and close contact during sex seems to be one of the key pathways of viral transmission in the current outbreak. Our aim was to describe the distribution of MPXV in the human body, as it might play a role in its dissemination through sexual contact.
Methods
The study population in this case series consisted of patients with confirmed MPXV infection attending the Pitié-Salpêtrière Hospital (Paris, France), who had been sampled from multiple anatomical sites, including skin, anus, throat, blood, urine, and semen, at diagnosis and 2 weeks later. We compared the proportion of positive samples and MPXV viral loads (given as PCR cycle thresholds [Ct]) between anatomical sites, and between day 0 (D0) and D14.
Findings
Overall, 356 samples were collected between May 20 and June 13, 2022, from 50 men with a median age of 34 years (IQR 29–40). 22 (44%) of the 50 men were classified as HIV-negative on day (D)0, and 22 (44%) were living with HIV. At D0, MPXV detection was more frequent from skin (44 [88%] of 50), anus (30 [71%] of 42), and throat (36 [77%] of 47) than from blood (13 [29%] of 45), urine (nine [22%] of 41), or semen (13 [54%] of 24). Viral loads were significantly higher from skin lesions (Ct 19·8) and anal samples (Ct 20·9) than from throat (Ct 27·2), blood (Ct 32·8), urine (31·1), or semen samples (Ct 27·8). When analysing the 107 samples taken from 24 patients at D14, the proportion of positive samples strongly decreased between D0 and D14 at all sites: skin (four [22%] of 18), anus (two [9%] of 22), throat (none of 21), blood (one [5%] of 21), urine (none of 14), and semen (two [9%] of 11).
Interpretation
These data contribute to a better understanding of how the virus might spread between sexual partners over a relatively short period of time. High MPXV viral loads from skin and mucosa, including genital and anal sites, suggest that transmission most likely occurs through direct body contact rather than through the respiratory route or contact with body fluids, which should help to refine the prevention messages delivered to individuals most exposed to the virus.
Funding
None.
Introduction
Monkeypox is a zoonotic disease, discovered in monkeys in 1958. The first human case was confirmed in 1970, when the monkeypox virus (MPXV) was isolated from a child in the Democratic Republic of the Congo.1 MPXV causes a disease in humans that is similar to smallpox, but with a much lower mortality, ranging from 0% to 10% for cases reported after 2000.2, 3 Symptoms are based on the triad of fever, lymphadenopathy, and rash, and complications such as encephalitis or profuse skin and mucous lesions are rarely described.4
Monkeypox is endemic in West Africa and Central Africa,5 with sporadic cases or outbreaks in high-income countries, linked to the exotic pet trade or international travel.6 Two distinct clades of MPXV have been characterised: Congo Basin and West African. The Congo Basin clade is associated with higher mortality than the West African clade and appears to be transmitted more frequently between humans.7 The last epidemic with indigenous transmission in a high-income country occurred in the USA in 2003, with 35 confirmed cases and 36 suspected cases following the importation of infected animals from Ghana.8
Since early May, 2022, more than 60 000 cases of monkeypox have been reported in countries with no historically reported cases, particularly in Europe and the USA, among individuals who have not travelled to endemic areas.9 This current epidemic appears to be concentrated among men who have sex with men, a substantial number of whom have been involved in mass gathering events (eg, the Maspalomas Gay Pride).
MPXV is known to be spread through close contact with lesions, body fluids, and respiratory droplets of infected people or animals.10 However, sexual activity might drive MPXV transmission. First reports show an unexpected number of patients infected with MPXV with anal and genital lesions, which might also be associated with the sexual route of transmission.11, 12, 13 Understanding precisely how MPXV transmits from one individual to another—in the context of sex with partners—is crucial, in order to adapt communication about the disease and help control its spread.
Research in context.
Evidence before this study
Scientific literature on monkeypox dates back to the 1970s, but articles on virological diagnosis are more recent and data on the detection of monkeypox virus (MPXV) from particular anatomical sites are few. We searched PubMed with the terms “monkeypox AND (shedding OR throat OR oropharynx OR nasopharynx OR saliva OR anus OR urine OR semen OR blood OR plasma)” for articles published from database inception up to July 31, 2022, without language restrictions. We also reviewed epidemiological reports from WHO and the US Centres for Disease Control and Prevention. Almost all cases reported before the current monkeypox epidemic in Europe and the USA did not include findings on the distribution of MPXV in the human body, except for a series of seven cases recently reported by English colleagues, providing longitudinal data on MPXV detection from skin, blood, and urine. There are no published prospective cohorts with repeated sampling over time. Since the beginning of the current outbreak (April, 2022), the most notable data have included two series of monkeypox cases. MPXV presence in semen has been investigated at least from one sample in nine and 32 patients, with positivity rates ranging from 78% to 91%. Reports on MPXV detection from the upper respiratory tract (including throat and nasopharynx) are scarce, as well as for MPXV detection from other anatomical sites such as anus, blood, and urine. The most informative recent publication is by Spanish colleagues, showing almost constant detection of MPXV from anal and oral swabs, and inconsistent detection from urine samples (n=12 patients). Data on blood are very few. We did not find any series of monkeypox cases with systematic multiple sampling over time.
Added value of this study
Our case series is representative of the current outbreak of MPXV infection, which is spreading among men who have sex with men in non-endemic countries. The high prevalence of anal and genital lesions suggests that sexual contact is the route of transmission. We present data about MPXV viral loads from 50 French male individuals with confirmed monkeypox, who were sampled from skin, throat, anus, blood, urine, and semen at diagnosis, and 14 days later for 24 of these men. We found a high proportion of MPXV-positive oral and anal swabs (71–77%), a lower proportion of positive blood and urine samples (22–31%), and just over half of positive semen samples at diagnosis. MPXV viral loads were high from skin and anus, intermediate in throat and semen, and low in blood and urine. Viral clearance appeared to be relatively rapid, with most samples being MPXV-negative within 14 days.
Implications of all the available evidence
The detection of MPXV at high concentrations in the anal region, in the mouth, and in semen is consistent with the sexual practices potentially involved in the spread of the virus among men who have sex with men, in addition to skin contact related to sexual or non-sexual proximity. These data should be popularised and disseminated in the community of men who have sex with men, with the aim of promoting safe social and sexual behaviour to curb the epidemic.
To better understand the routes of transmission, investigation of the distribution of MPXV and viral loads according to anatomical locations in the first patients with a confirmed infection is essential. In this Article, we report on a series of French individuals with locally acquired infections of monkeypox, with the aim of describing the distribution of MPXV in the human body, as well as the viral loads associated with different anatomical sites at time of diagnosis and after a 2-week follow-up.
Methods
Study design and participants
This case series includes patients referred to the Pitié-Salpêtrière hospital in Paris, France, with confirmed MPXV infection. For inclusion in the case series, patients were required to undergo testing from at least three different anatomical sites (skin, throat, anus, urine, blood, or semen) at the time of diagnosis (day 0 [D0]). All patients signed a specific written consent form permitting the publication of their clinical and biological data. Data from medical records were aggregated after de-identification. According to the French law (Act 78–17 of Jan 6, 1978, on Computers, Files and Liberties) this study has been conducted in compliance with the CNIL (French National Agency regulating Data Protection, 2085881), and with the reference methodology 004.
Procedures
The infection was considered as confirmed in patients with compatible clinical lesions associated with a positive MPXV PCR from any anatomical site. All patients who attended our centre during this period were offered a test for MPXV from samples obtained from the skin, throat, anus, urine, blood, and semen to increase the sensitivity of the diagnosis, and to provide them with information on the risk of further transmission. Anal swabs were inserted 1–2 cm into the anal canal, regardless of the presence of anal lesions, and oral swabs were rubbed on the tonsils, regardless of the presence of oral lesions. All patients were offered to be tested for MPXV 2 weeks after the diagnosis (day 14 [D14]). The results presented in this case series are from patients who came back for their routine follow-up. Skin samples collected at D14 were from the same lesion tested at D0, for cases in which the lesion was still present. No samples were taken if the lesion was no longer present.
Data on medical background, including HIV pre-exposure prophylaxis (PrEP) use, HIV history for people living with HIV, sexual behaviours, and symptoms evolution, were collected as part of routine care and not as part of any research protocol. All these variables derived from the case definition used in France at time of patients' management.
All specimens were transported using triple security packaging and handled in our biosafety level 3 virology laboratory, according to French guidelines. DNA extraction was performed on clinical samples using NucliSENS EasyMAG instrument (BioMérieux, Lyon, France). The real-time MPXV PCR assay was performed on the LightCycler 480 platform (Roche Diagnostics, Meylan, France). PCR reactions were carried out with the LightCycler DNA Hybridization Probes Master mix (Roche Diagnostics). The PCR protocol was developed after slight adaptations from the initial protocol previously reported.14 Results are given as cycle threshold (Ct) values allowing the estimation of the viral load: detection of MPXV was categorised as negative (Ct≥40), weakly positive (35≤Ct<40), or positive (Ct<35). MPXV was also sequenced from several clinical samples by next generation sequencing (MinION, Oxford Nanopore Technologies, Oxford, UK) to determine the MPXV clade.
The primary outcome was the proportion of MPXV-positive samples at D0. The secondary outcomes were the proportion of MPXV-positive samples at D14, as well as the MPXV Ct values from all MPXV-positive samples, at D0 and D14. The determination of the MPXV clade using sequencing was planned as an exploratory investigation.
Statistical analysis
Aggregate data is presented to maintain case confidentiality. χ2 tests were used to compare the proportions of positive and negative samples according to anatomical sites. Kruskal-Wallis tests were used to compare the Ct values according to anatomical sites. Wilcoxon tests were used to compare the Ct values from paired samples, between D0 and D14. A p value of less than 0·05 was considered significant. Analyses were done with SPSS (version 25).
Role of the funding source
There was no funding source for this study.
Results
Between May 20, and June 13, 2022, 50 people with confirmed monkeypox provided 356 clinical samples. All participants were men, with a median age of 34 years (IQR 29–40), and all except one were classified as men who have sex with men (table 1 ). 22 (44%) of the 50 men were HIV-negative on PrEP, six (12%) were HIV-negative and not on PrEP, and 22 (44%) were classified as people living with HIV. All people living with HIV had a recent (<6 months) undetectable HIV plasma viral load on antiretroviral treatment, except for one individual, in whom HIV infection was discovered concurrently with MPXV infection. This patient is the only one who was managed as an inpatient, to complete the initial investigation of HIV infection, and because of severe anal pain. No participants received specific antiviral treatment for MPXV infection. MPXV genomes were sequenced from 24 distinct samples and all viruses belonged to the West African hMPXV-1 clade, lineage B.1.
Table 1.
Baseline characteristics
| Participants (n=50) | |
|---|---|
| Sex | |
| Male | 50 (100%) |
| Age, years | |
| Median (IQR) | 34 (29–40) |
| Anti-smallpox vaccination | |
| Vaccinated | 6 (12%) |
| Unvaccinated | 44 (88%) |
| Suspected transmission route, n (%) | |
| Sex with men | 49 (98%) |
| Sex with women | 1 (2%) |
| HIV co-infection | |
| Yes | 22 (44%) |
| No | 28 (56%) |
| Pre-exposure prophylaxis (in HIV-negative patients) | |
| Yes | 22 (44%) |
| No | 6 (12%) |
| Number of sex partners in the previous 3 weeks | |
| Median (IQR) | 5 (2–10) |
| Chemsex use in the previous 3 weeks | |
| Yes | 15 (30%) |
| No | 35 (70%) |
| Attendance at a sex-on-site event in the previous 3 weeks | |
| Yes | 23 (46%) |
| No | 27 (54%) |
| Medical setting at first admission | |
| Infectious diseases department | 15 (30%) |
| Emergency department | 9 (18%) |
| General practitioner | 14 (28%) |
| Sexual health clinic | 4 (8%) |
| Other hospital | 8 (16%) |
Data are n (%), unless otherwise specified.
All patients presented with skin lesions, with genital or anal involvement in about half of the cases (table 2 ). Most eruptions were paucilesional, with less than ten lesions. Eight patients (16%) presented with tonsillitis, which was associated in some cases with intense odynophagia.
Table 2.
Patients' clinical presentation
| Participants (n=50) | |
|---|---|
| Reported clinical features | |
| Skin lesions | 50 (100%) |
| Fever | 26 (52%) |
| Exhaustion | 27 (54%) |
| Lymphadenopathy | 27 (54%) |
| Headache | 23 (46%) |
| Myalgia | 19 (38%) |
| Rectitis* | 16 (32%) |
| Tonsillitis | 8 (16%) |
| Cough | 3 (6%) |
| Prodromes (general signs before skin lesions) | |
| Yes | 30 (60%) |
| No | 20 (40%) |
| Number of skin lesions at time of monkeypox diagnosis | |
| 1 | 5 (10%) |
| 2–5 | 16 (32%) |
| 6–10 | 15 (30%) |
| 11–50 | 13 (26%) |
| >50 | 1 (2%) |
| Localisation of skin lesions at time of monkeypox diagnosis | |
| Body | 36 (72%) |
| Face | 22 (44%) |
| Palms or soles | 12 (24%) |
| Genitals | 25 (50%) |
| Anal margin | 23 (46%) |
Data are n (%).
Includes anal pain, bleeding, and diarrhoea.
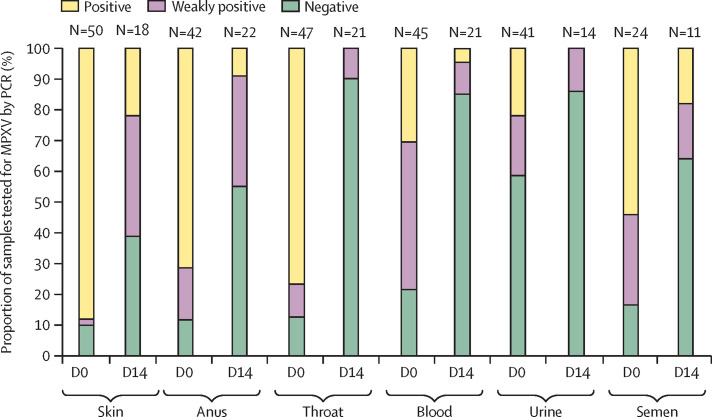
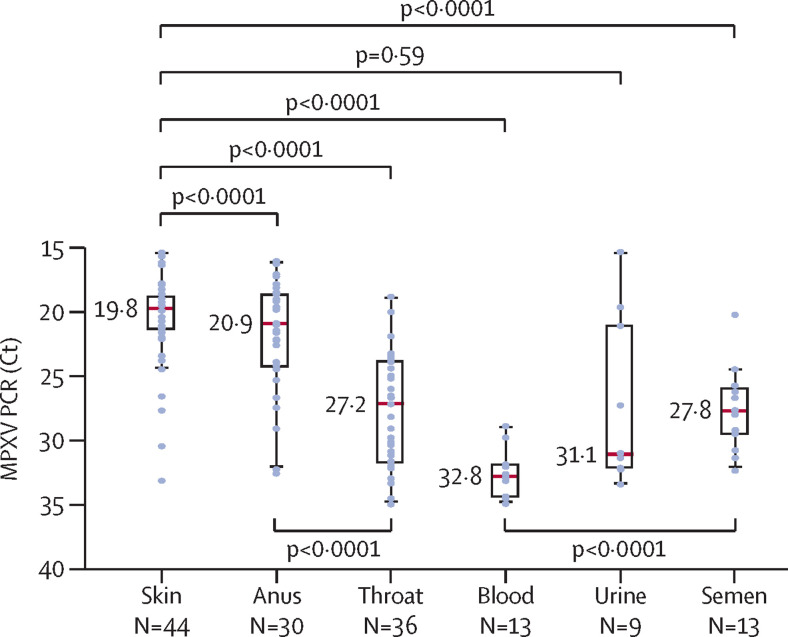
Overall, 249 samples were collected at the time of diagnosis, with a median duration from the onset of symptoms of 5 days (IQR 3–6). MPXV detection was more frequent from skin (44 [88%] of 50), anus (30 [71%] of 42), and throat (36 [77%] of 47) than from blood (13 [29%] of 45), urine (nine [22%] of 41), or semen (13 [54%] of 24; figure 1 ). The five patients with undetectable MPXV from skin samples were positive from samples collected from the throat or the anus, or both. Regarding body fluids, MPXV was undetected or weakly detected in more than two thirds of blood and urine samples, whereas it was detected in 13 (54%) of 24 semen samples. For cases in which MPXV was detectable, viral loads, inversely reflected by Ct values, were significantly higher from skin lesions (Ct 19·8) than from throat (Ct 27·2), blood (Ct 32·8), or semen (Ct 27·8) and higher from anal samples (Ct 20·9) than from throat samples (figure 2 ). MPXV viral load was higher in the anal samples of patients with anal ulcers (Ct 19·6 vs 25·3, p=0·0073), higher in throat samples of patients with tonsillitis (Ct 23·5 vs 29·0, p=0·0076), and higher in semen samples of patients with genital lesions (Ct 26·3 vs 30·2, p=0·022). There were no significant differences in MPXV PCR Ct values according to HIV status.
Figure 1.
Proportion of clinical samples found to be positive, weakly positive, and negative for MPXV at diagnosis, by use of PCR
D0=day 0. D14=day 14. MPXV=monkeypox virus.
Figure 2.
MPXV viral loads given as cycle thresholds, according to sampled sites
Clinical samples found to be weakly positive (35≤Ct<40) or negative (Ct≥40) using PCR were excluded for this analysis. Results are given as box plots in which red lines represent median Ct. Ct=cycle threshold. MPXV=monkeypox virus.
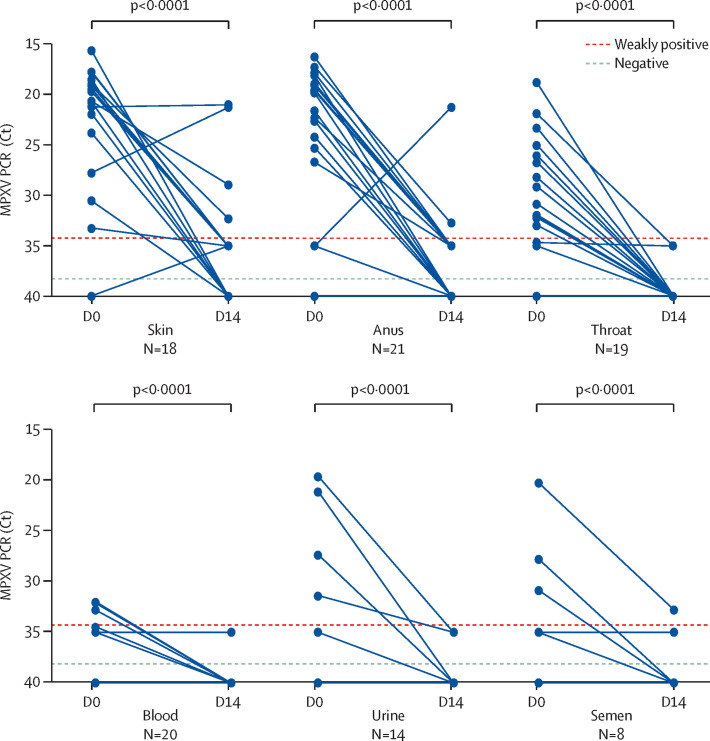
Sampling was repeated in 24 patients 2 weeks after monkeypox diagnosis, with 107 samples collected at a median of 19 days (IQR 18–21) from the onset of symptoms, 100 of which were paired (ie, taken from the same site in the same individual on both D0 and D14). The proportion of positive samples, as well as MPXV viral loads, strongly decreased during this interval (Figure 1, Figure 3 ). In the 107 samples taken at D14, tests were negative or weakly positive for MPXV in 20 (91%) of 22 anal samples, in 21 (100%) of 21 throat samples, in 14 (100%) of 14 blood samples, in 20 (95%) of 21 urine samples, and in nine (82%) of 11 semen samples. Four (22%) of 18 paired skin swabs remained positive; skin lesions had fully disappeared and swabbing was not possible in six of 24 patients at D14. Of note, one anal sample was highly positive at D14 (Ct 21·3) but weakly positive at D0 in a patient who presented with anal ulcers at time of diagnosis. We assume that the sample taken at D0 was of poor quality. Of the 107 samples taken at D14, only six of 24 patients had samples with a Ct value of less than 35 at D14 (of which four were skin samples, two were anal samples, and two were semen samples). Two patients were HIV co-infected, one with a CD4 cell count of 768 cells per mm3 on antiretroviral therapy, and the other with a CD4 cell count of 55 cells per mm3 without antiretroviral therapy, as he had just tested positive for HIV. Neither of these two patients had other concomitant diseases.
Figure 3.
Change in MPXV viral load between D0 and D14 according to sampled sites
Only 21 patients with paired samples (taken at both D0 and D14 from the same site) were retained for this analysis; 100 paired samples are presented here. Ct=cycle threshold. D0=day 0. D14=day 14. MPXV=monkeypox virus.
Discussion
Most patients infected with MPXV during the current outbreak in Europe and the USA are men who have sex with men, as was the case with all patients in our series, except one. In the 21 days before the onset of symptoms, which corresponds to the maximal incubation period of monkeypox,4 patients in our series reported multiple sex partners. All previous reports of the current outbreak have identified close sexual contact as the main risk factor of MPXV dissemination, emphasising the crucial role of understanding the route of transmission of the virus. Transmission of MPXV has been shown to occur through direct contact with skin lesions, body fluids, or respiratory droplets from infected animals or humans, or indirectly through contaminated fomites.10 By nature, sexual activity involves intimate contact, which one would expect to increase the likelihood of transmission, regardless of sexual orientation and irrespective of the mode of transmission. However, there has been little research into whether MPXV can be transmitted sexually until now (ie, through sexual secretions containing the virus, and through oral, genital or anal mucosa). Herein, we provide data on 356 clinical samples, collected at D0 and D14 from 50 individuals with confirmed monkeypox in Paris.
We detected MPXV in semen from most men (83% at diagnosis) with confirmed monkeypox, in line with other recent reports,13, 15, 16 suggesting a possible risk of human-to-human transmission through male sexual secretions. This detection of the virus in semen is concordant with previous zoonotic viruses including the Zika virus17, 18 and Ebola virus;19, 20 however, sexual contact might not be the dominant transmission pathway. We also detected MPXV from the oropharynx and anus of almost all the patients reported here (87% in the oropharynx and 88% in the anus at diagnosis), which supports evidence of transmission through oral and anal sex. A recent Spanish study showed a high rate of MPXV detection in saliva (100%), the nasopharynx (83%), and the anus (92%), coming to similar conclusions as our study did, and supporting the transmission of MPXV through breathing and kissing.16
The detection of MPXV was rarer in blood and urine than in other sampled sites. Our English and Spanish colleagues showed that the detection of MPXV in urine is not constant (never detected in three of seven patients in blood samples and in three of 12 patients in urine samples).16, 21 The presence of viraemia associated with monkeypox symptoms has been studied by a few teams. In the 2003 US monkeypox epidemic, only three of 12 patients investigated were viraemic,22 and of the two cases in Nigeria in 2018, both were viraemic.23 In the English series, MPXV was detected in the blood in six of seven patients at least once, at the beginning of the disease, and viraemia seemed to be undetectable within 1–4 weeks.21 In our series, MPXV was undetectable in urine from more than half of patients at diagnosis, and undetectable in blood from about 20% of patients.
We also found differences in MPXV viral loads depending on the anatomical sites and body fluids collected: they were significantly higher from skin than from throat, blood, and semen; significantly higher from the anus than from the throat; and significantly higher from semen than from blood. MPXV detection from clinical samples taken 14 days after the diagnosis supported a rapid viral clearance in body fluids and anatomical compartments.
We speculate that there might be a short viraemic phase (accompanied by viral shedding in body fluids such as urine and semen) at the beginning of the symptomatic phase of monkeypox, and that systemic viral circulation ends before healing of the skin lesions. However, studies about Ebola and Zika viruses have shown that viral shedding in semen can continue for several months after the viraemic phase and after the end of symptoms, with a risk of late secondary sexual transmission.17, 18, 19, 20
Our findings should be taken with caution. We only had one sample per anatomical site, and differences in sampling procedures could alter the outcome (especially for skin scrabbing). We also did not have samples at D14 for half of the patients, which could lead to a bias in the interpretation of the results, and not having samples available after D14 limits the ability to gather more information about viral kinetics. Furthermore, a molecular biology test investigates DNA, and we did not have evidence of the presence of infectious viral particles, which is a key point when making conclusion about the potentiality of sexual transmission of this disease. However, very recent data argue in favour of the infectivity of MPXV in semen.24 Lapa and colleagues24 have shown the viral viability of MPXV isolated from semen (Ct 29·3) in a case of monkeypox, showing a clear cytopathic effect 48 h after inoculation of semen in Vero E6 cells. These results should precede longitudinal cohort studies exploring viral secretion in different body fluids over time, associated with assessment of viral infectivity using cell culture.
We hope that these findings, in addition to other recent reports, will contribute to the debate on sexual transmission of MPXV and a better understanding of the risk of MPXV dissemination to sexual partners, and will help define the best communication and prevention strategies for the most affected communities. All sexually active men who have sex with men should be informed about the presence of MPXV on the skin, in the throat, in the anus, and in body fluids in case of infection, to help decrease transmission via sexual contact or physical promiscuity during festive events.
Data sharing
Individual participant data that underlie the results reported in this Article can be shared, after de-identification, for individual participant data meta-analysis. Proposals can be submitted up to 36 months following Article publication and should be directed to the corresponding author (RP).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank the patients, the nurses, and all the clinical staff of Le 190 Sexual Health Center and of the infectious diseases and virology departments of the Pitié-Salpêtrière hospital who provided care for the patients and performed virological investigations. We thank Christine Katlama, Luminita Schneider, Roland Tubiana, and Marc-Antoine Valantin for helping with the clinical management.
Contributors
RP, SB, GM, AN, A-GM, and VP contributed to conceptualisation of the study. RP, SB, GM, AN, AB, SS, VB, CB, AG, YW, NG, AF, YT, TG, MO, ET, VL, SM, VC, A-GM, and VP contributed to data collection and performance of virological tests for the study. RP, SB, A-GM, and VP supervised these investigations. RP, SB, A-GM, and VP wrote the original draft. GM, AN, AB, SS, VB, CB, AG, YW, NG, AF, YT, TG, MO, ET, VL, SM, and VC reviewed and edited the manuscript. All authors had full access to the data. RP, SB, A-GM, and VP accessed the original data and verified the underlying data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 2.Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13:e0007791. doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox— a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16:e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown K, Leggat PA. Human Monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:E8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis Off Publ Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sklenovská N, Van Ranst M. Emergence of Monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Centers for Disease Control and Prevention Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:642–646. [PubMed] [Google Scholar]
- 9.US Centers for Disease Control and Prevention 2022 monkeypox outbreak global map. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 10.Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- 11.Patrocinio-Jesus R, Peruzzu F. Monkeypox genital lesions. N Engl J Med. 2022;387:66. doi: 10.1056/NEJMicm2206893. [DOI] [PubMed] [Google Scholar]
- 12.Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhao H, Wilkins K, et al. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27:2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveill. 2022;27:2200503. doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mead PS, Duggal NK, Hook SA, et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med. 2018;378:1377–1385. doi: 10.1056/NEJMoa1711038. [DOI] [PubMed] [Google Scholar]
- 18.D'Ortenzio E, Matheron S, de Lamballerie X, et al. Evidence of sexual transmission of Zika Virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 19.Fischer WA, Wohl DA. Confronting Ebola as a sexually transmitted infection. Clin Infect Dis. 2016;62:1272–1276. doi: 10.1093/cid/ciw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vetter P, Fischer WA, Schibler M, Jacobs M, Bausch DG, Kaiser L. Ebola virus shedding and transmission: review of current evidence. J Infect Dis. 2016;214:S177–S184. doi: 10.1093/infdis/jiw254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 23.Eseigbe EE, Akude C, Osagie IA, Eseigbe P. Human monkey pox virus infection in Plateau State, North Central Nigeria: a report of two cases. West Afr J Med. 2021;38:1242–1246. [PubMed] [Google Scholar]
- 24.Lapa D, Carletti F, Mazzotta V, et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect Dis. 2022;22:1267–1269. doi: 10.1016/S1473-3099(22)00513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this Article can be shared, after de-identification, for individual participant data meta-analysis. Proposals can be submitted up to 36 months following Article publication and should be directed to the corresponding author (RP).