Abstract
The detection of >400 Monkeypox virus cases in the month of May 2022 and increase to 57,527.
confirmed cases by September 9th, 2022, across the world, emphasizes the need of new therapeutics for this emerging viral epidemic in humans. Largely the cases seen in Europe, Australia and America are among men who have sex with men making transmission through intimate contact with infectious skin lesions the likely mode of transmission. This implies that this high human-to-human transmission observed in the young Caucasian clusters, and the probable community transmission without any history of travelling to endemic areas would suggest that the epidemic is likely to be sustained human-to-human transmission and unlikely one that would be a short-lasting epidemic. This might necessitate the need for new therapeutic approaches and agents for prophylaxis and treatment of acute infections which is the focus of this review article.
Keywords: Monkeypox, Epidemic, Pandemic, Vaccines, Antiviral drugs
1. Introduction
A recent Monkeypox virus epidemic, a rare zoonotic disease outbreak, was first detected in the UK in May 2022, the first case was reported on May 7 by a traveler coming to the UK from Nigeria [1]. By May 29th, the number had risen to 75 (confirmed and suspected) cases across seven countries (England, Portugal, Spain, United States, Canada, Sweden, and Italy). Later that night, the number had jumped to 107 [1], by September 9th, 2022 the number has risen to 57,527 [2].
In terms of origin, 'Democratic Republic of Congo (formerly Zaire) reported its first case of the Monkeypox virus in 1970, there were no other cases reported until 2017. Between 1st January and August 13th, 2022, the Nigeria CDC has reported a total of 172 confirmed cases with 4 deaths indicating an estimated case-fatality rate of 2.3% [3]. The cases identified in Nigeria are likely underreported, as more cases would have gone under the radar because of lack of testing, index cases not presenting to local health authorities etc. Monkeypox virus cases were reported in the UK in 2018 [4], 2019, 2021, and 2022 in individuals travelling from Nigeria [2].
Though Monkeypox's natural reservoir remains unknown, rodents and non-human primates are believed to harbors the virus to infect humans. There are two distinct subfamilies of the Monkeypox virus: the Congo Basin and the West African subfamilies. The West African variety is reported to be poorly transmissible between humans with a low case-fatality rate (<1%). The Congo basin subfamily is associated with a higher transmission rate and thus has a higher case fatality rate (10%) [5].
Monkeypox virus belongs to the Orthopoxvirus genus. It is endemic to West and Central Africa and transmissible to cause zoonotic infections in humans. Once endemic among human populations, infection occurs as a result of contact with infected humans and can result in transmission [6].
Historically, transmission chains are usually short-lasting resulting in short-lasting epidemics [7], however, researchers hypothesized that changes in population immunity can result in sustained epidemics with an initial reproductive rate (R0) >1 [8]. They also suggested the adaptation of rodents and other animals to urban settings is also likely to be contributory to increased transmission to humans [9].
So far, the virus is spreading from person to person which indicates human-to-human transmission within the community. The virus is believed to be transmitted through contact with a patient's lesions, clothes and bed linens in contact with the lesions. It is presumed that the virus spreads through respiratory droplets. Thus far, the majority of cases recorded have been in young men who have sex with men [2], and none with recent travel history to endemic countries with either smallpox or Monkeypox, Most cases presented with lesions on the genitalia or peri-genital area, highly suggestive that transmission likely occurs during close physical contact during sexual activities, but not a Sexually Transmitted Infection. The majority of cases seen in Europe are among men who have sex with men making transmission through intimate contact with infectious skin lesions remains the likely mode of transmission [10].This implies that this high human-to-human transmission observed in the young European clusters, and the probable community transmission without any history of travelling to endemic areas would suggest that the epidemic is likely to be sustained human-to-human transmission and unlikely one that would be a short-lasting epidemic.
It is worthy of note that the last natural outbreak of smallpox in the United States was in 1949 [11]. The last cases in the world were in the late 1970s. And, smallpox was declared eradicated during the 33rd World Health Assembly on May 8, 1980 [11]. Monkeypox was first discovered in 1958 when two outbreaks of a pox-like disease occurred in colonies of monkeys kept for research [12]. The first human case of Monkeypox was recorded in 1970 in the Democratic Republic of the Congo [13]. Infectious disease experts globally have more questions than answers thus far, but worrisomely are the increasing number of cases, and countries reporting its emergence are increasing, though it is a relatively mild disease course so far.
1.1. Clinical features
Monkeypox virus infection usually starts with fever, headache, myalgia, malaise, swollen lymphadenopathy, chills, and prostration in extreme cases. Mild to severe cases onset are heralded by fever, and the patient later develops a rash, often beginning on the face, and then spread to other parts of the body. The skin lesions progress through various types of lesions before disappearing namely: macules, papules, vesicles, pustules, and scabs. The acute phase of the disease typically lasts for 2–4 weeks with an incubation period lasting 7–14 days. That is, the time from contracting the disease to onset of symptoms is typically 7–14 days but can extend to 5–21 days. Mortality from Monkeypox is estimated to be as significant as 10% in those who contract the disease in Africa [15], it is too early to determine the western world's mortality ratio..
2. Treatment, early management guidelines adopted in the UK and vaccines prophylaxis
2.1. Monkeypox flowchart
Monkeypox flowchart source: GOV.UK (www.gov.uk) Accessed May 2022 [16].
3. Prophylaxis: vaccines therapeutic options
3.1. Smallpox vaccine
Since there are no clear evidences for effective treatment, prevention measurements are very essential to prevent infection spread [14]. Prevention can be achieved by avoiding direct contact with infected patients especially their skin lesions and clothings used by them. Accordingly, Monkeypox patients should be isolated in single patient rooms, wear surgical masks, and have their skin lesions covered until the lesions heal and formed new skin layer after lesion crusts fall. Health care providers of infected patients should maintain their safety by continuous use of masks, gowns, gloves and eye protection [14,15].
Regarding Monkeypox vaccinations, smallpox vaccinations are being investigated and approved for Monkeypox infections with expected effectiveness of up to 85% [[15], [16], [17]]. The currently approved vaccination by the US. FDA is JYNNEOS, while ACAM2000 vaccine has off- label use [18]. There are no clear evidences about the effectiveness of these two vaccines against current outbreak. However, these vaccinations proved to be effective in previous smallpox outbreaks. Full vaccination is achieved after two weeks from their second shot of JYNNEOS and 4 weeks from their ACAM2000 vaccination. Administration of these vaccines early and immediately can help in aborting the infection and reducing the number of cases as well as reducing the severity and complications of the infection [18]. The vaccines can be given as post exposure prophylaxis to prevent diseases onset after people exposed to virus within few days after exposure. Exposure to patients broken skin, mucous membranes, body fluids, respiratory droplets, or scabs usually warrants post exposure vaccination [14]. On the other hand, vaccine can be given as pre-exposure prophylaxis in people with high risk for exposure like health care professionals [19]. A third vaccine, the Aventis Pasteur Smallpox Vaccine (APSV) could be used for smallpox under an investigational new drug (IND) protocol. In 2019, a novel vaccine based on a modified attenuated Vaccinia virus (Ankara strain) was authorized for Monkeypox prevention [16]. While ACAM2000 vaccine resulted in some side effects in atopic dermatitis patients and immunocompromised patients, the modified vaccinia Ankara (MVA) vaccine is safe to use in those patients [17]. None of these vaccinations is approved for human clinical use yet [20].
Since both smallpox and Monkeypox share same origin from the orthopoxvirus genus— variola virus being the member of the genus known as the causative agent of smallpox [16]. it is believed that the smallpox vaccine should have cross-protection against Monkeypox, some small study data estimate the protection to be as high as 85%. So, mobilization and deployment of smallpox vaccines plans being made in the western world as we watch the events unfolding on this emerging viral disease of increasing concern.
3.2. Novel mRNA monkeypox vaccine
Over decades, conventional vaccines were proven to be successful prophylaxes against various diseases such as hepatitis, chickenpox, influenza, and many others [21]. Until recently, these conventional vaccines are based on attenuated virus or viral protein. While these vaccine types are considered very effective in preventing the spread of various viral infections, the development of these vaccines might be difficult against certain viral infections with adaptive immune response [22]. Moreover, the process of conventional vaccine development might take several years which will not result in the availability of these vaccines in a reasonable time during an emerging viral pandemic, as the large-scale development of these vaccines is a challenging process.
Another vaccination option that has been emerged in the 1990s is based on the use of nucleic acids components as vaccines rather than attenuated viruses or viral parts. This concept is based on the administration of messenger RNA (mRNA) molecules that encode specific crucial viral proteins [23]. Following the administration, these mRNA molecules should be translated into the encoded protein and then the human will recognize these transcripted viral proteins and start to develop antibodies against these proteins. This will ensure the protection of the future viral infection following viral exposure [24,25].
Over the past years, several efforts have been done in an attempt to improve vaccine development using this mRNA vaccination technology for the protection of various diseases. This is because of the promising potential of this vaccine type as an alternative to conventional vaccines. Nucleic acids vaccines are based on using highly precise and specific mRNA molecules which can result in triggering a specific immune response and limit the common side effects of the traditional vaccines as they are a more precise and targeted approach [26].
In the event of pandemics, the need for effective vaccines in a very short period of time is necessary as it will help in minimizing the virus burden on individuals and nations such as death, hospital bed need, economic burden, and social impacts [27,28]. The concept of mRNA vaccines can ensure the very fast development of viral vaccines once the sequence of the vital protein in the virus has been determined. However, although the application of mRNA vaccines looks promising, still their development and use are challenging. This is mainly to the limited stability of the mRNA molecules and their high rate of degradation by the nucleases enzymes in the body following their administration, along with their limited cellular uptake because of their negative charge [29,30].
Therefore, the development of effective mRNA vaccines was based on the efficient utilization of the nanotechnology drug carriers which help in overcoming most of the nucleic acids limitations [31,32]. This concept is based on using the carrier in which the mRNA molecules will be loaded inside it. This will result in protecting these mRNA molecules and minimize their rapid elimination which will result in increasing the half-life of these mRNA molecules and enhance their distribution to the target cells [33,34]. Moreover, these nanocarriers will also enhance the cellular uptake of the mRNA molecules into the cytoplasm of the target cells where the process of translation and protein production starts [35].
The proper utilization and production of mRNA vaccines emerged following the COVID-19 pandemic in the late 2019 where this pandemic resulted in many health and economic consequences and showed the urgent need for rapid development of effective vaccines to limit the viral burden worldwide [36]. These consequences of COVID-19 have forced the pharmaceutical companies to explore all the vaccine development options including conventional and non-conventional vaccines to develop effective COVID-19 vaccines in the shortest possible time. As mentioned above, the development of conventional vaccines might take seral years and waiting this long time was not possible in the event of COVID-19 pandemic and the high mortality rates, therefore, the non-conventional vaccines and specifically, the mRNA vaccines proved to be the most logical option at that time for rapid development and deployment worldwide [[37], [38], [39]].
Several pharmaceutical companies were working on the development of such vaccines which were based on delivering mRNA molecules that encode for the viral spike protein of the virus, which is the protein that facilitates the viral entry into the human cells. The translation of these mRNA molecules into the viral spike protein will help in triggering an immune response by producing antibodies against this protein which can provide immune responses against the following viral exposures [40]. This has led to the fast development and the urgent approval of two mRNA-based vaccines developed by Pfizer and Moderna. Both vaccines are based on the use of lipid nanoparticles to encapsulate and deliver the mRNA molecules into the target cells after immunization [41]. These urgent approvals represent the first vaccines in history that has been developed and approved in a very short period of time. This means that in the event of pandemics, mRNA-based vaccines would remain a viable option [42]. Moreover, since the high rate of COVID-19 spread was associated with the development of viral mutations, this can be easily overcome by continuous modification of the mRNA sequence to combat these mutations. This concept can theoretically be applied to various types of viral infections simply by changing the type and the sequence of the mRNA molecules to suit individual vaccine development [43,44].
4. Prophylactic measures and antivirals therapeutic options
Since there are no clear evidences for effective treatment currently, prevention measurements are very essential to prevent infection spread [45]. Accordingly, Monkeypox infected patients should be isolated in single patient rooms, they should follow recommended personal protective equipment (PPE) guidelines such as wearing gloves, gown, medical mask and eye protection – goggles or face shield. Ideally, they should wear a medical mask when they come into close contact (under 1 m) with health workers or other patients [15]. In addition, a bandage, sheet or gown can be used to cover lesions in order to minimize potential contact with lesions and cover their skin lesions until the lesions heal and form a new skin layer after lesion crusts fall. Health care providers of infected patients should adhere to standard precautions include strict adherence to hand hygiene, appropriate handling of contaminated medical equipment, laundry, waste and cleaning and disinfection of in-contact vicinity surfaces (see Fig. 1).
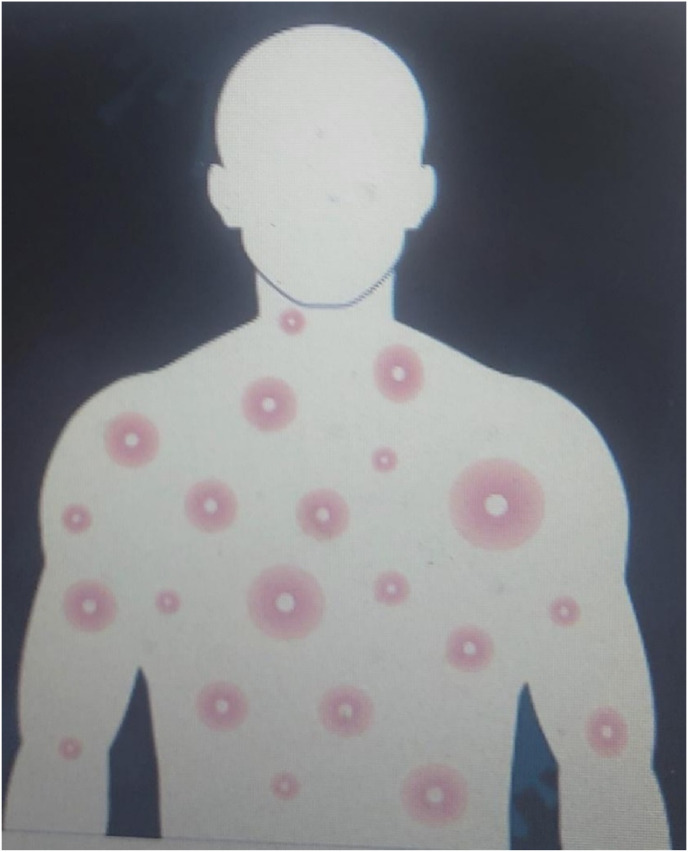
Fig. 1.
Depicting typical pustules of Monkeypox human infection. Source: https://nigeriahealthwatch.com/accessed May 9th, 2022.
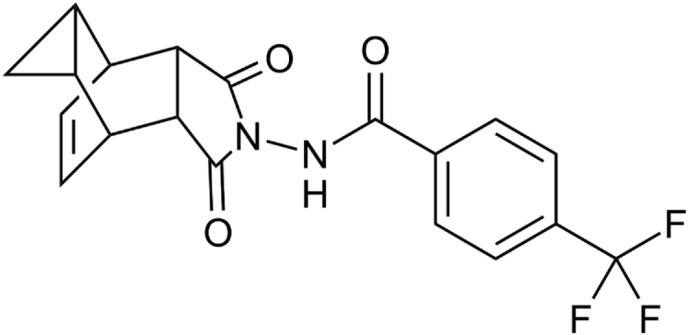
In terms of therapeutics, Tecovirimat with the brand name Tpoxx (TPOXX, ST-246) was approved for smallpox in 2018 as the first approved small molecule drug against smallpox by FDA [16]. Its chemistry compromises of carbotricycle moiety as well as a hydrazine (Fig. 2 ). It is approved for both adults and children weighing 3 kg or more [46]. It acts as p37 protein inhibitor which prevents the formation of viral envelope [15]. Thus, as envelope protein inhibitor, it prevents the virus from leaving the infected cell and the spread of virus in the host body [17]. The drug can be given orally or parenterally and shown to be safe and well tolerated. CDC holds Expanded Access–Investigational New Drug (EA-IND) protocol for the use of this antiviral in non-variola orthopoxviruses such as Monkeypox virus. Recently, tecovirimat was approved by European Medical Association for treatment of Monkeypox in 2022 [16]. Its effectiveness against Monkeypox in human has not been approved yet. However, it was found to be effective in preclinical studies in Monkeypox infected animal models.
Fig. 2.
Chemical structure of Tecovirimat.
Tpoxx orally is used according to the patient's weight: For patients 40 kg to <120 kg: 600 mg orally twice a day for 14 days. While for obese patients ≥120 kg: 600 mg orally thrice a day for 14 days. It is preferable to be taken within 30 min after meal. For those that cannot tolerate orally, use as an IV infusion, with a dosing regimen also based on the patient's weight: 35 kg to <120 kg: 200 mg IV over 6 h every 12 h for 14 days and for obese patients with weight ≥120 kg: 300 mg IV over 6 h every 12 h for 14 days.
Additionally, Cidofovir is another antiviral that is approved for cytomegalovirus (CMV) retinitis in patients with Acquired Immunodeficiency Syndrome (AIDS). It is an acyclic nucleoside phosphate that is available only as intravenous formulation [46]. Upon reaching the host cells, cidofovir is activated by phosphorylation into Cidofovir-diphosphate (CDV-PP) which acts by inhibiting viral DNA synthesis through the inhibition of DNA polymerase [17]. However, Cidofovir can be used for Monkeypox with caution. However, it is associated with severe toxicities including renal damage. Administration of probenecid and intravenous normal saline is usually needed during Cidofovir treatment [46].
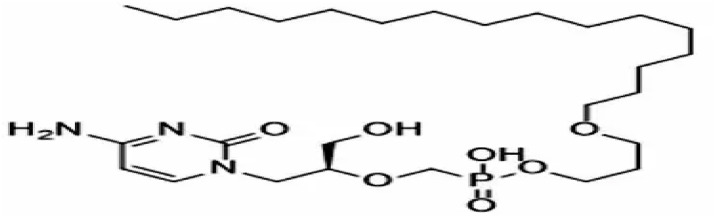
On the other hand, a lipid conjugate of Cidofovir known as Brincidofovir (TEMBEXA) developed as a pro-drug was approved for the treatment of smallpox in 2021 as smallpox was considered a potential bio-weapon. Brincidofovir has better safety and antiviral activity compared to Cidofovir (Fig. 3 ) [47]. It can be used in the treatment of a variety of viral infections such as adenovirus, BK virus, herpes viruses including cytomegalovirus, Ebola, and papilloma virus as it is a relatively broad-spectrum antiviral. To be strongly efficacious against dsDNA viruses, such as orthopoxvirus like smallpox and now Monkeypox it was designed as a lipid pro-drug that releases Cidofovir intracellularly to inhibit DNA replication.
Fig. 3.
Chemical structure of Brincidofovir.
Brincidofovir can be tried in patients with complicated Monkeypox infections and it has improved safety profile compared to Cidofovir [48]. Gastrointestinal adverse effects including diarrhea, nausea, vomiting, and abdominal pain are the most commonly encountered adverse events in patients. Liver function test is usually needed before and during the treatment of Brincidofovir. Accordingly, the CDC holds an EA-IND to use Brincidofovir for Monkeypox infection.
A study in UK retrospectively tracked the 7 cases of Monkeypox that were encountered between 2018 and 2021. Three patients were treated with oral Brincidofovir with a three once a week doses of 200 mg. Due to the limited number of cases, the association was not made between the drug doses and the clinical or virological parameters and subsequently the clinical prognosis of the disease. All three patients showed elevated alanine transaminase [49]. Patient 7 in the same study was isolated for 35 days and received a 2-weeks course of oral tecovirimat (600 mg twice daily). The goal of the treatment was to prevent severe complications in this patient and aid faster recovery from the viral infection. The patient PCR samples of blood and respiratory tract became negative 48 h remained negative at 72 h [49,50]. the patient developed no toxicities while on tecovirimat therapy.
Another example is the Vaccinia Immune Globulin Intravenous (VIGIV). VIGIV is approved for skin conditions and infections induced by vaccinia virus. It might be used in severe cases of Monkeypox infections [15]. However, the effectiveness of VIGIV is still not established in patients with Monkeypox [16]. Additionally, VIGIV can be considered a post-exposure prophylactic agent in immunocompromised individuals that potentially exposed to Monkeypox and who are contraindicated to other smallpox vaccine as a post-exposure preventive measure [47]. Other suggested potential future antivirals that could have anti-Monkeypox activities include C-CA3-ADO and C3-NPC A as S-adenosylhomocysteine hydrolase inhibitors as well as Ribavirin and Tiazofurin as inosine monophosphate dehydrogenase (IMP) inhibitors [17].
5. Conclusions
In this review, we have looked at the use of Tecovirimat also known as Tpoxx and Brincidofovir the prodrug of Cidofovir as available antivirals for treatment of acute Monkeypox infections. We also hypothesized the development of novel Monkeypox mRNA vaccines as possible prophylaxis vaccines option should this epidemic continue to spread around the world unabated.
In terms of clinical metabolomics, it would be interesting to see if biomarkers like LDH (lactate dehydrogenase), Ferritin, CRP (C-Reactive Protein), thrombocytosis, leukopenia, lymphopenia, monocytosis, depleted glutathione, malondialdehyde, F2 Isoprostanes levels, transient elevated liver enzymes (AST, ALT) levels in patients with Monkeypox virus infection would emerge as relevant biomarkers as more cases spread globally. The detection of nearly 58,000 Monkeypox virus cases as at early September 2022 across the world, emphasizes the need of new therapeutics of this emerging virus epidemic in humans. So far, the cases seen in Europe, Australia and America are mainly among men who have sex with men making transmission through intimate contact with infectious skin lesions the likely mode of transmission and the probable community transmission without any history of travelling to endemic areas. This would suggest that the epidemic is likely to be sustained human-to-human transmission and unlikely one that would be a short-lasting epidemic, necessitating the need for new therapeutic approaches and agents for prophylaxis and treatment of acute infections.
The spread of the COVID-19 pandemic with all its consequences has afforded us a template to develop a viable strategy to combat future spread of similar viral pandemics. Since the cases of Monkeypox infections are increasing day by day, the same scenario of COVID-19 could be applied. The increase of the Monkeypox infection potentially could warrant huge efforts for the development of effective vaccines in case sudden mutations occur to turn this epidemic into a pandemic. Therefore, based on the gained experience from COVID-19, fast and effective mRNA vaccines development would be a viable option should infection rates take a turn for the worse. Then the best option of Monkeypox vaccines development would be to utilize this mRNA concept by determining the vital surface protein on the virus surface that is responsible for the virus internalization into the human cells. Knowing these key surface protein components can then help in the development of mRNA vaccine against the Monkeypox infections in the same manner used in the development of COVID-19 vaccines. Pharmaceutical companies can start by using similar nanoparticles used in the COVID-19 vaccines with proper modification as carriers of mRNA molecules against the Monkeypox virus. This will save efforts and time in the case the Monkeypox epidemic turns into a pandemic.
CRediT authorship contribution statement
Mohammad A. Obeid: Writing – review & editing, Writing – original draft, Validation, Methodology, Data curation, Conceptualization. Haneen Amawi: Writing – review & editing, Writing – original draft, Resources, Conceptualization. Ahmed Alshehri: Writing – review & editing. Adedapo Adesokan: Writing – original draft, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vivancos R., et al. Community transmission of monkeypox in the United Kingdom, April to may 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.government U. 2022. Monkeypox Cases Confirmed in England – Latest Updates. [Google Scholar]
- 3.Nigeria, C., Situation Report: Monkeypox (MPX) in Nigeria.
- 4.Petersen E., et al. Monkeypox—enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int. J. Infect. Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Likos A.M., et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86(10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 6.Durski K.N., et al. Emergence of monkeypox—west and central Africa, 1970–2017. Morb. Mortal. Wkly. Rep. 2018;67(10):306. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine P., et al. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 8.Grant R., Nguyen L.-B.L., Breban R. Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 2020;98(9):638. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albery G.F., et al. Urban-adapted mammal species have more known pathogens. Nature Ecology & Evolution. 2022:1–8. doi: 10.1038/s41559-022-01723-0. [DOI] [PubMed] [Google Scholar]
- 10.Control, E.C.f.D.P.a., Monkeypox Cases Reported in UK and Portugal.
- 11.OCTOBER O. 2003. 25th Anniversary of the Last Case of Naturally Acquired Smallpox. [PubMed] [Google Scholar]
- 12.Magnus P.v., et al. A pox‐like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959;46(2):156–176. [Google Scholar]
- 13.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 2004;4(1):15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore M., Zahra F. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; Treasure Island (FL: 2022. Monkeypox. [Google Scholar]
- 15.Guarner J., Del Rio C., Malani P.N. JAMA; 2022. Monkeypox in 2022—What Clinicians Need to Know. [DOI] [PubMed] [Google Scholar]
- 16.Saxena, S.K., et al., Re-emerging human monkeypox: a major public-health debacle. J. Med. Virol.. n/a(n/a). [DOI] [PubMed]
- 17.Gong Q., et al. Monkeypox virus: a re-emergent threat to humans. Virol. Sin. 2022 doi: 10.1016/j.virs.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adalja A.A., Inglesby T.V. Annals of Internal Medicine; 2022. A Novel International Monkeypox Outbreak. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, N.C.f.E.a.Z.I.D.N. Vaccination; Monkeybox: 2022. Division of High-Consequence Pathogens and Pathology (DHCPP) [Google Scholar]
- 20.Martín-Delgado M.C., et al. Rev Esp Quimioter; 2022. Monkeypox in Humans: a New Outbreak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajj Hussein I., et al. Vaccines through centuries: major cornerstones of global health. Front. Public Health. 2015;3:269. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adesokan A., Obeid M.A., Lawal A.F. SARS-CoV-2: vaccinology and emerging therapeutics; challenges and future developments. Ther. Deliv. 2022;13(3):187–203. doi: 10.4155/tde-2021-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aljabali A.A., et al. Multidisciplinary Science and Advanced Technologies. Nova Science Publishers, Inc.; 2021. Viral nanoparticles where we are heading; pp. 85–91. [Google Scholar]
- 24.Pantelić I., et al. Lipid nanoparticles employed in mRNA-based COVID-19 vaccines: an overview of materials and processes used for development and production. Arch. Pharm. 2022;72(Notebook 1):20–35. [Google Scholar]
- 25.Hussain A., et al. mRNA vaccines for COVID-19 and diverse diseases. J. Contr. Release. 2022 doi: 10.1016/j.jconrel.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan M.J., Pardi N. mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 2022;73:17–39. doi: 10.1146/annurev-med-042420-112725. [DOI] [PubMed] [Google Scholar]
- 27.Williams J., et al. How should we conduct pandemic vaccination? Vaccine. 2021;39(6):994–999. doi: 10.1016/j.vaccine.2020.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aljabali A.A., Obeid M.A. Inorganic-organic nanomaterials for therapeutics and molecular imaging applications. Nanosci. Nanotechnol. - Asia. 2020;10(6):748–765. [Google Scholar]
- 29.Rubin E.J., Longo D.L. Mass Medical Soc; 2022. Covid-19 mRNA Vaccines—Six of One, Half a Dozen of the Other; pp. 183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sangboonruang S., et al. Potentiality of melittin-loaded niosomal vesicles against vancomycin-intermediate Staphylococcus aureus and Staphylococcal skin infection. Int. J. Nanomed. 2021;16:7639. doi: 10.2147/IJN.S325901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obeid M.A., et al. Melanoma. Springer; 2021. Sirna delivery to melanoma cells with cationic niosomes; pp. 621–634. [DOI] [PubMed] [Google Scholar]
- 32.Obeid M.A., et al. Advanced Drug Delivery Systems in the Management of Cancer. Elsevier; 2021. Targeting siRNAs in cancer drug delivery; pp. 447–460. [Google Scholar]
- 33.Gregoriadis G. Liposomes and mRNA: two technologies together create a COVID-19 vaccine. Medicine in Drug Discovery. 2021;12 [Google Scholar]
- 34.Aljabali A.A., et al. Applications of Nanomaterials in Human Health. Springer; 2020. Application of nanomaterials in the diagnosis and treatment of genetic disorders; pp. 125–146. [Google Scholar]
- 35.Obeid M.A., Alsaadi M., Aljabali A.A. Recent updates in curcumin delivery. J. Liposome Res. 2022:1–12. doi: 10.1080/08982104.2022.2086567. [DOI] [PubMed] [Google Scholar]
- 36.Andreano E., et al. SARS-CoV-2 escape from a highly neutralizing COVID-19 convalescent plasma. Proc. Natl. Acad. Sci. USA. 2021;118(36) doi: 10.1073/pnas.2103154118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21(2):73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebril A., et al. Mucosal and systemic immune responses following mucosal immunisation of tetanus toxoid entrapped in lipid nanoparticles prepared by microwave reactor. Eur. J. Pharm. Biopharm. 2022;171:11–18. doi: 10.1016/j.ejpb.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Aljabali A.A., et al. A potential MRI agent and an anticancer drug encapsulated within CPMV virus-like particles. Comb. Chem. High Throughput Screening. 2021;24(10):1557–1571. doi: 10.2174/1386207323666200914110012. [DOI] [PubMed] [Google Scholar]
- 40.Du L., et al. Recent advances in nanotechnology-based COVID-19 vaccines and therapeutic antibodies. Nanoscale. 2022 doi: 10.1039/d1nr03831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szabó G.T., Mahiny A.J., Vlatkovic I. Molecular Therapy; 2022. COVID-19 mRNA Vaccines: Platforms and Current Developments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A., et al. Status report on COVID-19 vaccines development. Curr. Infect. Dis. Rep. 2021;23(6):1–12. doi: 10.1007/s11908-021-00752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbasi J. COVID-19 and mRNA vaccines—first large test for a new approach. JAMA. 2020;324(12):1125–1127. doi: 10.1001/jama.2020.16866. [DOI] [PubMed] [Google Scholar]
- 44.Alyamani H., et al. Exosomes: fighting cancer with cancer. Ther. Deliv. 2019;10(1):37–61. doi: 10.4155/tde-2018-0051. [DOI] [PubMed] [Google Scholar]
- 45.Moore M., Zahra F. StatPearls Publishing; 2022. Monkeypox. StatPearls. Treasure Island (FL) [Google Scholar]
- 46.Rizk J.G., et al. Prevention and treatment of monkeypox. Drugs. 2022:1–7. doi: 10.1007/s40265-022-01742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afshar Z.M., et al. Authorea Preprints; 2022. The Reemergence of Monkeypox as a New Potential Health Challenge: A Critical Review. [Google Scholar]
- 48.Centers for Disease Control and Prevention, N.C.f.E.a.Z.I.D.N. 2022. Division of High-Consequence Pathogens and Pathology (DHCPP), Monkeybox: Prevention and Treatment. [Google Scholar]
- 49.Adler H., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022 doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obeid M.A., et al. Examination of the effect of niosome preparation methods in encapsulating model antigens on the vesicle characteristics and their ability to induce immune responses. J. Liposome Res. 2020:1–8. doi: 10.1080/08982104.2020.1768110. [DOI] [PubMed] [Google Scholar]