Dear Editor,
Very recently, a report has been published in Lancet on August 10, 2022, on the first evidence of human-to-dog transmission of monkeypox virus (MPXV), a zoonotic virus affecting humans [1]. During the past three months since May 2022, the world has witnessed a rapid rising of human Monkeypox (MPX) cases in several nations, and the World Health Organization (WHO) declared MPX as a Public Health Emergency of International Concern on 23rd July 2022. Currently more than 42000 confirmed cases from 95 countries have been reported along with 12 deaths as of August 23, 2022 [2]. The virus is transmitted naturally among small mammals and rodents in Central Africa and this is the first time that such a high rate of human-to-human transmission is being observed for this disease, though it has been considered earlier as a neglected/rare disease since it was first identified five decades back in 1970. Human-to–animal transmission of MPXV has not been reported earlier, except the most recent identification of a case of human-to-animal transmission (reverse zoonosis) from Paris. The pet dog (Male Italian Greyhound) of two individuals who were suffering from MPX was also diagnosed to be positive for MPXV. The virus found in the individuals and the dog showed homology on DNA sequencing [1]. This transmission behaviour of the virus is a significant change in the earlier thought of the transmission cycle of MPXV. Of note, Prairie dogs can help us to better understanding of transmission, pathogenesis, and developing newer vaccine and treatment trials due to their susceptibility to severe MPXV infection [3,4].
In the past outbreaks of MPX outside the African continent, no case of human-to-animal transmission was ever seen. During the first outbreak of MPX in the United States of America in the year 2003, wherein 70 human MPX cases were detected, local Prairie dogs were found to be infected with MPXV that picked this viral infection from small rodents/mammals imported from Ghana, Africa [5]. This jumping of the virus from imported animals to prairie dogs was mainly due to close proximity of animals being kept together in cages prior to distribution. Supply of these prairie dogs has led to spread of virus in most of these cases [6]. During that outbreak, no other local or pet animal, except the Prairie dogs was found to be infected with MPXV. In most of the cases, the sick pets were taken to veterinary clinics which could have provided the opportunity for the virus to spill to other animals. In one instance, rabbit that had become ill after exposure to an ill prairie dog at a veterinary clinic was thought to be the as source of disease, but later MPXV was not isolated from that rabbit [7].
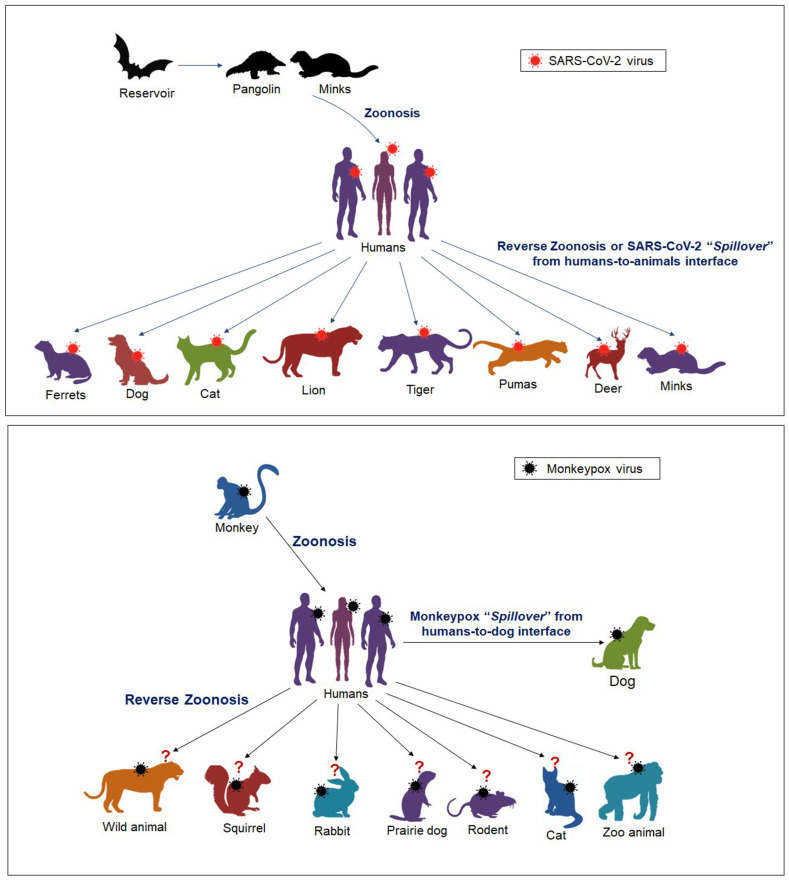
Transmission of virus from humans-to-animals is seen in other orthropox viruses like cow pox and vaccinia virus [8]. The ‘Spillover’ event with change in the host of virus can lead to development of new variants with different characters. Earlier, the novel severe acute respiratory syndrome coronavirus (SARS-CoV-2), the cause of the ongoing COVID-19 pandemic faced in the past more than two and a half year, was seen affecting only humans in a rapid human-to-human transmission, which was initially considered to be spilled over from animals-to-humans (from bats, pangolins) as a zoonotic pathogen. Thereafter, owing to host jumping and cross-species transmission events, the SARS-CoV-2 was reported to be transmitted from humans-to-animals, and various animal species such as cats, dogs, lions, tigers, white-tailed deer, ferrets, mink and others were reported to be infected and/or susceptible to this virus, and cat-to-cat and ferret-to-ferret transmission could happen via contact and air [9,10]. However, SARS-CoV-2 reverse zoonosis with spillover transmission from animals-to-humans has been significantly documented from mink-to-humans in mink farm workers in Netherlands, wherein rapid transmission of the virus occurred among the mink population, and subsequently a new mink-associated SARS-CoV-2 variant emerged that was recognized in both humans and minks [11]. During the start of COVID-19 pandemic, the variant that first raised alarm globally was isolated in minks in Denmark, named as “Cluster 5” that was thought to be associated with changes in SARS-CoV-2 spike (S) protein and decreased neutralization in convalescent antisera. Various measures like culling of minks and travel restrictions were taken for preventing the spread of that variant to humans [12]. A few other reports of zoonosis and reverse zoonosis of SARS-CoV-2 have been documented, including virus transmission from cat-to-human, human-to-dog, human-to-cat, human-to-minks, human-to-ferrets, human-to-lion, human-to-tiger, human-to-pumas, human-to-deer, human-to-hamsters; however, understanding reverse zoonosis completely needs further explorative research studies and disease investigations [[10], [11], [12], [13], [14], [15]]. An illustration on SARS-CoV-2 and MPXV zoonosis, animal spillover and reverse zoonosis is presented in Fig. 1 .
Fig. 1.
An overview on zoonosis, animal spillover and reverse zoonosis of SARS-CoV-2 and MPXV.
MPXV infected persons are advised to avoid taking care of virus exposed pets. Pets with contact with symptomatic MPX patients should be kept away from other animals for at least 21 days [16]. There is the need for active surveillance and tracking of MPXV among pets and other animals to detect any other reverse zoonosis event(s) of human-to-animal transmission, finding the reservoir of zoonotic pathogen in humans known as zooanthroponosis, studying the virus prevalence in various animal species, throwing more light on zoonotic aspects of this virus, and having vigilance of detecting emergence of any new variant of MPXV [[17], [18], [19]]. There is a dire need to implement one health as a collaborative approach strictly to a wider global level to limit the menace of zoonotic viruses including MPXV, which may ultimately aid in reducing human infections as well as enhancing public health preparedness in the wake of the declined immunity against MPXV in the post-eradication era of smallpox and cessation of smallpox vaccination [19,20].
Apart from all these, COVID-19 Vaccines have been developed for animals including domestic and captive animals, pets such as dogs, zoo animals and susceptible precious exotic and wildlife animals, so as to prevent reverse zoonosis, limiting SARS-CoV-2 circulation in animals and at animal-human interface for confronting any associated zoonosis. Concerning zoonosis and the recently reported concern of reverse zoonosis associated with MPXV, should we be required to develop MPXV vaccines for animals? [19,20].
In conclusion, the recent report of Seang et al. on the first evidence of human-to-dog transmission of MPXV urges a priority demand of enhancing surveillance and conducting further explorative and in-depth disease investigations, and strengthening researches on this zoonotic virus [1]. This will facilitate finding out the real magnitude of MPXV reverse zoonosis and spillover from other animal species, including pets like cats, zoo, wildlife and experimental animals to humans. Any emergence of MPXV variant must be considered to be tracked and monitored with a high degree of vigilance, like the emerging variants of SARS-CoV-2 that have been reported immediately from time to time. This should paved ways for advancements in finding out more effective vaccines, promoting booster vaccine shots and discovering newer and effective therapeutics to counteract COVID-19 pandemic in a better way. Urgent focus should be given to formulate pro-active control measures and strategic future preparedness plans to avoid what has happened to the SARS-CoV-2 ongoing pandemic, which now seems to be a never endemic pandemic despite high global efforts of developing few potential vaccines in emergency and ramping up of massive COVID-19 vaccination campaigns. These facts may well be due to the risk factor the virus being circulating continuously in the animal population.
Provenance and peer review
Not commissioned, internally peer-reviewed.
Ethical approval
Not applicble
Please state any sources of funding for your research
No funding received.
Author contribution
RS, VH and AM design and draw the original draft, NZNA, SC, MB, CC and KD reviewed the literature and critically edit the article. All authors read and approve for the final version of the manuscript.
Research registration Unique Identifying number (UIN)
1. Name of the registry:
2. Unique Identifying number or registration ID:
3. Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Ranjit Sah.
Declaration of competing interest
No conflicts of interest.
References
- 1.Seang S., Burrel S., Todesco E., Leducq V., Monsel G., Le Pluart D., Cordevant C., Pourcher V., Palich R. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;(22):1487–1488. doi: 10.1016/S0140-6736(22)01487-8. Aug 10S0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC (2022) Monkeypox outbreak global map. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 3.Kile J.C., Fleischauer A.T., Beard B., Kuehnert M.J., Kanwal R.S., Pontones P., Messersmith H.J., Teclaw R., Karem K.L., Braden Z.H., Damon I., Khan A.S., Fischer M. Transmission of monkeypox among persons exposed to infected prairie dogs in Indiana in 2003. Arch. Pediatr. Adolesc. Med. 2005 Nov;159(11):1022–1025. doi: 10.1001/archpedi.159.11.1022. [DOI] [PubMed] [Google Scholar]
- 4.Guarner J., Johnson B.J., Paddock C.D., Shieh W.J., Goldsmith C.S., Reynolds M.G., Damon I.K., Regnery R.L., Zaki S.R., Veterinary Monkeypox Virus Working Group Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 2004 Mar;10(3):426–431. doi: 10.3201/eid1003.030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson K., Heymann D., Brown C.S., Edmunds W.J., Elsgaard J., Fine P., Hochrein H., Hoff N.A., Green A., Ihekweazu C., Jones T.C., Lule S., Maclennan J., McCollum A., Mühlemann B., Nightingale E., Ogoina D., Ogunleye A., Petersen B., Powell J., Quantick O., Rimoin A.W., Ulaeato D., Wapling A. Human monkeypox - after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020 Jul 14;38(33):5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 2004 Jan;4(1):15–25. doi: 10.1016/s1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds M.G., Cono J., Curns A., Holman R.C., Likos A., Regnery R., Treadwell T., Damon I. Human monkeypox. Lancet Infect. Dis. 2004 Oct;4(10):604–605. doi: 10.1016/S1473-3099(04)01139-9. discussion 605 PMID: 15451482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddad N. The presumed receptivity and susceptibility to monkeypox of European animal species. Infect. Dis. News. 2022 Aug;52(5):294–298. doi: 10.1016/j.idnow.2022.06.006. Epub 2022 Jun 23. PMID: 35753629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhama K., Patel S.K., Sharun K., Pathak M., Tiwari R., Yatoo M.I., Malik Y.S., Sah R., Rabaan A.A., Panwar P.K., Singh K.P., Michalak I., Chaicumpa W., Martinez-Pulgarin D.F., Bonilla-Aldana D.K., Rodriguez-Morales A.J. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Trav. Med. Infect. Dis. 2020 Sep-Oct;37 doi: 10.1016/j.tmaid.2020.101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharun K., Dhama K., Pawde A.M., Gortázar C., Tiwari R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., de la Fuente J., Michalak I., Attia Y.A. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet. Q. 2021 Dec;41(1):181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., Bouwmeester-Vincken N., Harders F., Hakze-van der Honing R., Wegdam-Blans M.C.A., Bouwstra R.J., GeurtsvanKessel C., van der Eijk A.A., Velkers F.C., Smit L.A.M., Stegeman A., van der Poel W.H.M., Koopmans M.P.G. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021 Jan 8;371(6525):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garigliany M., Van Laere A.S., Clercx C., Giet D., Escriou N., Huon C., van der Werf S., Eloit M., Desmecht D. SARS-CoV-2 natural transmission from human to cat, Belgium, march 2020. Emerg. Infect. Dis. 2020 Dec;26(12):3069–3071. doi: 10.3201/eid2612.202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Račnik J., Kočevar A., Slavec B., Korva M., Rus K.R., Zakotnik S., Zorec T.M., Poljak M., Matko M., Rojs O.Z., Županc T.A. Transmission of SARS-CoV-2 from human to domestic ferret. Emerg. Infect. Dis. 2021 Sep;27(9):2450–2453. doi: 10.3201/eid2709.210774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sila T., Sunghan J., Laochareonsuk W., Surasombatpattana S., Kongkamol C., Ingviya T., Siripaitoon P., Kositpantawong N., Kanchanasuwan S., Hortiwakul T., Charernmak B., Nwabor O.F., Silpapojakul K., Chusri S. Suspected cat-to-human transmission of SARS-CoV-2, Thailand, july-September 2021. Emerg. Infect. Dis. 2022 Jul;28(7):1485–1488. doi: 10.3201/eid2807.212605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivero R., Garay E., Botero Y., Serrano-Coll H., Gastelbondo B., Muñoz M., Ballesteros N., Castañeda S., Patiño L.H., Ramirez J.D., Calderon A., Guzmán C., Martinez-Bravo C., Aleman A., Arrieta G., Mattar S. Human-to-dog transmission of SARS-CoV-2, Colombia. Sci. Rep. 2022 May 12;12(1):7880. doi: 10.1038/s41598-022-11847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. 2022. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes [Google Scholar]
- 17.Bonilla-Aldana D.K., Rodriguez-Morales A.J. Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet. Q. 2022 Dec;42(1):148–150. doi: 10.1080/01652176.2022.2088881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty C., Bhattacharya M., Nandi S.S., Mohapatra R.K., Dhama K., Agoramoorthy G. Appearance and re-appearance of zoonotic disease during the pandemic period: long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Vet. Q. 2022 Jun 7;42(1):119–124. doi: 10.1080/01652176.2022.2086718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pramod R.K., Nair A.V., Tambare P.K., Chauhan K., Kumar T.V., Rajan R.A., Mani B.M., Asaf M., Pandey A.K. Reverse zoonosis of coronavirus disease-19: present status and the control by one health approach. Vet. World. 2021 Oct;14(10):2817–2826. doi: 10.14202/vetworld.2021.2817-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ga E., Won Y., Hwang J., Moon S., Yeom M., Lyoo K., Song D., Han J., Na W. A COVID-19 vaccine for dogs prevents reverse zoonosis. Vaccines (Basel) 2022 Apr 24;10(5):676. doi: 10.3390/vaccines10050676. [DOI] [PMC free article] [PubMed] [Google Scholar]