Abstract
The monkeypox virus (MPXV) is the cause of a zoonotic infection similar to smallpox. Although it is endemic to Africa, it has recently begun to circulate in other parts of the world. In July 2022, the World Health Organization declared monkeypox an international public health emergency. This review aims to provide an overview of this neglected zoonotic pathogen. MPXV circulates as two distinct clades, the Central African and West African, with case fatality rates of 10.6% and 3.6%, respectively. The risk of infection is greater for those who work with animals or infected individuals. The virus’ entry into the human body provokes both natural and acquired immunity. Although natural killer cells, CD4 + T cells, and CD8 + T cells play an essential role in eradicating MPXV, there is still a gap in the understanding of the host immune response to the virus. Currently, there are no specific therapeutic guidelines for treating monkeypox; however, some antiviral drugs such as tecovirimat and cidofovir may help to abate the severity of the disease. The use of nonpharmaceutical interventions and immunization can reduce the risk of infection. Increased surveillance and identification of monkeypox cases are crucial to understand the constantly shifting epidemiology of this resurging and intimidating disease. The present review provides a detailed perspective on the emergence and circulation of MPXV in human populations, infection risks, human immune response, disease diagnosis and prevention strategies, and future implications, and highlights the importance of the research community engaging more with this disease for an effective global response.
Keywords: Orthopoxviruses, Smallpox virus, Epidemiology, Immune evasion, Disease outbreaks
Introduction
The monkeypox virus (MPXV), a double-stranded DNA virus, is a member of the genus Orthopoxvirus in the family Poxviridae. It is responsible for a rare zoonotic disease in humans and animals that is similar to smallpox caused by the smallpox virus (SPXV), also an orthopoxvirus [1]. A containment strategy, a very effective vaccine, and the absence of animal or environmental reservoirs led to the eradication of smallpox in 1977 [2]. Following the global eradication of smallpox, monkeypox came to be regarded as one of the most critical orthopoxvirus infections [3]. Monkeypox was first identified in 1958 from lesions on crab-eating monkeys (Macaca fascicularis) kept as laboratory animals in Copenhagen, Denmark. The first case of a human infected with MPXV was identified in the Democratic Republic of the Congo (DRC) in 1970; although the disease was initially thought to be confined to tropical rainforest areas of Africa, new cases were later recorded in Sudan and nearby countries [4]. The active surveillance program for monkeypox ended in 1986, and MPXV infections remained unknown for an extended period. Monkeypox was disseminated outside Africa in 2003 with rodents that were imported from Africa to the midwestern United States [5]. It is difficult to determine the true geographic range of the reservoirs, but zoonotic reservoir species are likely to be associated with the disease and are the primary candidates for disease transfer [6].
Infected animals and humans are the sources of the MPXV transmitted among humans. The virus is transmitted through large respiratory droplets, direct skin-to-skin contact, contaminated fomites, respiratory secretions, and vertical transmission. Although sexual transmission of MPXV through seminal or vaginal fluids is not well established, there is a potential risk of its transfer during sex due to direct body contact [7]. The most common clinical presentation is lymphadenopathy and rash. There is no specific course of treatment against MPXV infections; however, the smallpox vaccination is valuable in preventing the disease [8]. Since 2021, new cases of monkeypox have been reported outside Africa in new countries where a robust surveillance system is required to manage the risk of this disease. The World Health Organization (WHO) has declared the current monkeypox outbreak a high priority and a global health emergency. More than 16,000 confirmed cases and five deaths were reported to the WHO by 75 countries between January and July 2022 [9]. However, not enough attention has been paid to preventing monkeypox outbreaks from growing into an epidemic, despite MPXV being the second most pathogenic poxvirus after SPXV. This review will consider various aspects pertaining to this neglected zoonotic pathogen that have been seldom discussed. It will help to grasp a complete picture of the emergence and spread of human monkeypox in past and recent outbreaks, associated risks, current diagnosis, prevention strategies, therapeutic options, and future implications. In addition, the crosstalk between MPXV and the host immune response will also be discussed in the context of MPXV immunoevasion mechanisms. To ensure that data soundness, we searched PubMed, Google Scholar, WHO bulletins, and the Centers for Disease Control and Prevention (CDC) national public health agency of the United States, using the keywords monkeypox, orthopoxviruses, smallpox virus, epidemiology, immune evasion, immune-modulatory, zoonotic, public health problem, vaccinia virus, host cell response, multi-country outbreaks, and disease outbreaks. Our review did not include duplicate data or studies from preprints.
Outbreaks and re-emergence of monkeypox
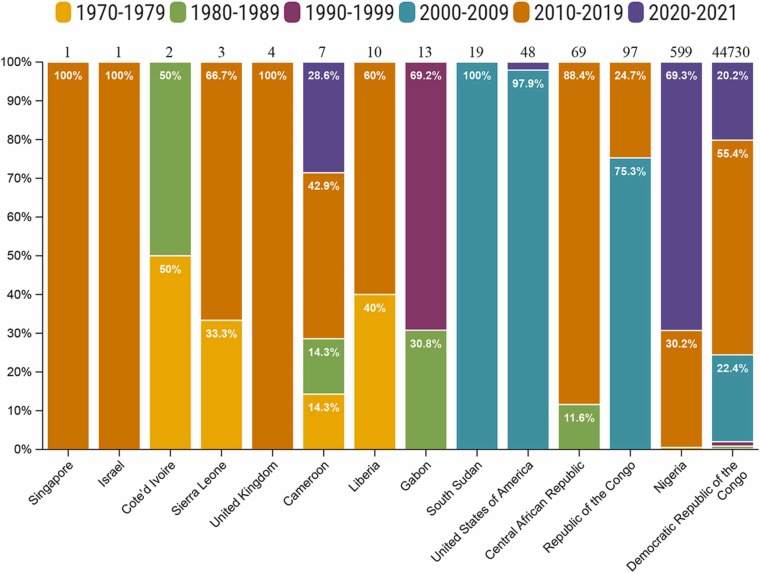
The first human case of MPXV was reported in 1970 in a nine-month-old child who had not been vaccinated against SPXV. Between 1972 and 1978, 35 new cases were reported [10], [11]. Subsequently, the WHO reported 338 confirmed cases of MPXV with 33 deaths between 1981 and 1986 in Africa [12]. A major cluster of 511 cases of monkeypox was reported in 1996–1997 in an outbreak in the Lodja and Katako–Kombe health zones of the DRC [13]. Smaller numbers of cases continued to emerge after 1998, but a considerable outbreak involving 1265 cases was observed between 1998 and 2002, mostly in the equatorial forest regions of the DRC [14]. The first outbreak of MPXV outside Africa occurred in 2003 in the United States following importation of infected animals from Ghana. This outbreak resulted in 47 cases, but without any deaths and person-to-person transmission [8]. From 2005–2007, 760 cases of the disease were confirmed in the most significant forest coverage zones in the DRC and another major outbreak of 587 cases occurred between 2014 and 2016 [15]. The data on the monkeypox cases from 1970 to 2021 reflects the suspected and/or laboratory-confirmed cases. Most of the cases reported in DRC from 2000 to 2021 were suspected cases without any laboratory confirmation ( Fig. 1). There were humanitarian crises in the Democratic Republic of the Congo, such as armed conflicts, tensions between communities, political instability, and population displacement, which resulted in poor surveillance [16], [17].
Fig. 1.
Suspected/confirmed cases of human monkeypox reported worldwide from 1970 to 2021. The total number of suspected/confirmed monkeypox cases reported during 1970–2021 from each country is given at the top of each bar. The different colors of each bar indicate the percentages of cases in different years in the same country [16], [17], [20], [21], [22], [23], [24].
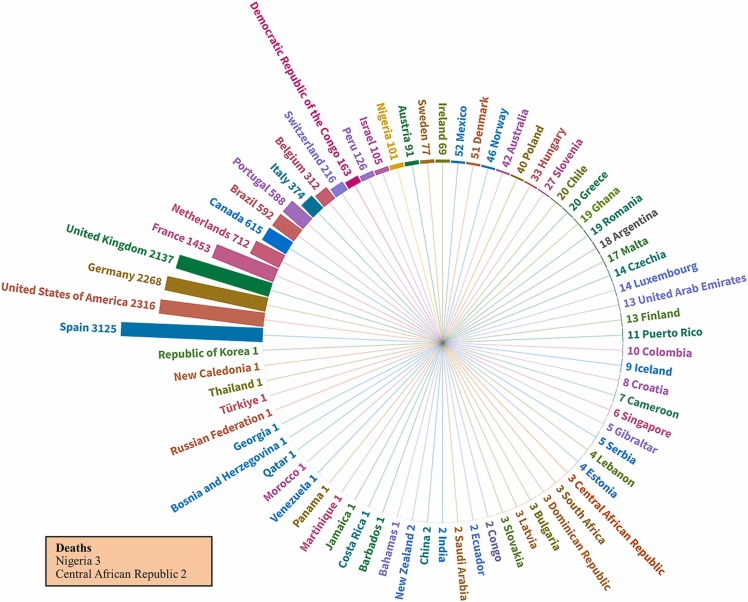
The WHO reported 244 cases of monkeypox resulting in six deaths in 25 Nigerian states between 2017 and 2018. In 2018, 2845 suspected cases were reported in the first 24 weeks of the year in the DRC, with 36 deaths [18]. Monkeypox cases were recorded in the United Kingdom in December 2019, May 2021, and May 2022; in the United States in July and November 2021; and in Singapore in May 2019 [19]. The WHO reported 16,016 confirmed monkeypox cases and five deaths across 75 regions, including 301 cases from Africa, 3772 from the Americas, 21 from the Eastern Mediterranean, 11,865 from Europe, 3 from South-East Asia, and 54 from the Western Pacific region from January 1 to July 25, 2022 during the current outbreak [9]. Data for the 2022 outbreak are presented in Fig. 2.
Fig. 2.
Human monkeypox cases from January 1 to July 25, 2022. The radial tree shows a hierarchy of 75 countries in the current monkeypox outbreak and the number of cases in each country. The deaths in two African countries are shown separately in the box.
Epidemiology
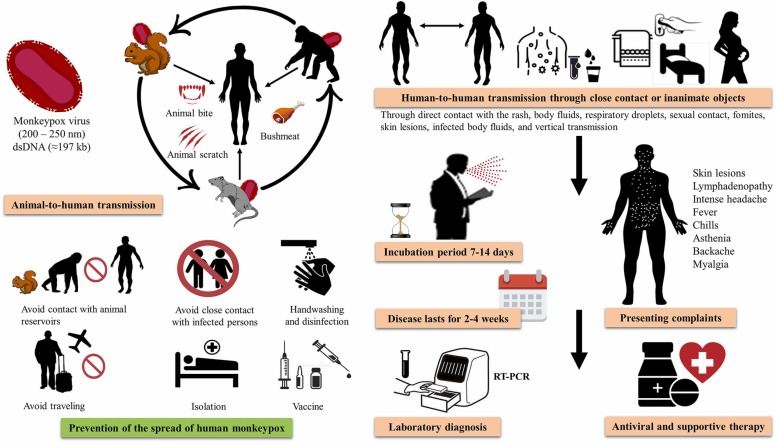
MPXV is transmitted from animals to humans and causes symptoms similar to those of smallpox ( Fig. 3). The usual incubation period of monkeypox is 7–14 days, but a variation of 3–20 days (median 7 days) has also been observed [7], [25]. Several animal species have been identified as reservoirs of the virus, which appears to occur as two distinct clades. The Congo Basin clade causes more severe disease, with a 10.6% case fatality rate (CFR) compared to the West African clade with a 3.6% CFR [19]. A total CFR of about 8.7% has been observed worldwide, while no deaths have been reported outside of Africa [26]. A total of 63 deaths were reported in Africa, with 100% of the deaths in children under 10 years of age in the 1970–1990 s period, whereas 37.5% of the deaths were in children in the same age group in 2000–2019 [27]. Furthermore, a mean age of 27 years was identified for cases of mortality in 2017–2018 in Nigeria [28].
Fig. 3.
Emergence and circulation of monkeypox virus (MPXV). The figure illustrates MPXV transmission from animal to human, human to human, and via fomites; clinical presentations; treatment; and preventive measures.
The number of monkeypox cases has increased in African and other countries over time. In the 1970 s, six African countries, Liberia, Sierra Leone, Cameroon, the DRC, Nigeria, and Côte d′Ivoire, reported 48 cases, with the highest number in the DRC [1]. A nine-fold increase in monkeypox cases was observed in the DRC and four other African countries, with the DRC reporting 511 cases and Gabon 9 cases during 1990–1999 [29], [30]. Only three African countries, South Sudan, the Republic of the Congo (ROC), and the DRC, reported cases of monkeypox during 2000–2009, but a resurgence of the disease was seen in the next decade, with seven countries, Cameroon, Central African Republic, the DRC, ROC, Liberia, Nigeria, and Sierra Leone, reporting cases during 2010–2019 [21]. The recent outbreak of monkeypox infection has led to five deaths in African countries [9].
Between the periods 1970–1980 s and 2000–2010 s, more males were affected by monkeypox (≥50%) than females in Africa [20]. Between 2014 and 2016, all suspected monkeypox cases in the DRC were males younger than 15 years old, with 57.8% aged 15–18 years. In the ROC, 60% of the infected were children below the age of 15 years, while 51% were females [22]. The WHO data on the outbreak in 2022 indicate that 99% of the infected cases are male, with a median age of 36 years [9]. The disease is most prevalent among adult males due to their outdoor activities, and they are most likely to be carriers of the disease to females. Male children between the ages of 15 and 18 engaging in outdoor play are also affected by the disease.
Animal reservoirs of MPXV
The definite animal reservoir of MPXV is still unknown, but small mammals, such as African dormice (Graphiurus spp.), sun squirrels (Heliosciurus spp.), rope squirrels (Funisciurus spp.), and giant pouched rats (Cricetomys spp.), are thought to spread the virus to humans in Central and West Africa [31]. MPXV is transmitted from animals to humans during trapping, hunting, processing infected animals, and dealing with their body fluids (Fig. 3). Non-human primates can become infected with MPXV and fall ill, while small mammals can be asymptomatic carriers of the virus [32]. Domesticated prairie dogs in the United States also become infected with the virus when they share caging and bedding with infected mammals from West Africa [32]. Rodents, squirrels, and non-human primates have been found with monkeypox infection on serological examination in Africa. Wild animals from forest habitats are more likely to become infected. MPXV was isolated from Thomas’s rope squirrel (Funisciurus anerythrus) in 1985 in the DRC and the sooty mangabey (Cercocebus atys) in 2012 in Cote d′Ivoire, thus suggesting that these species may be reservoirs of the virus [33].
Several primate (Cercocebus atys, Macaca mulatta, M. fascicularis) and rodent (Cricetomys gambianus, Heliosciurus, Jaculus) species have been identified as susceptible to MPXV infection [31]. Since it is not yet known which animal species are prone to illness from monkeypox, as a precautionary measure, it is best to assume that all mammals are susceptible to the disease. Transmission from one animal to another or to humans can occur through the animal's respiratory droplets, inhalation of aerosolized virus or organic matter containing virus particles, skin or eye abrasions, or ingestion of infected animal tissue [31], [32]. Animal-to-animal and animal-to-human transmission highlights the importance of separating affected or exposed individuals and infected animals to limit the danger of secondary infections to and from animals.
Transmission and risk factors
In the 1980 s, 72.5% of monkeypox cases in the DRC were due to viral transmission by animals, while 27.5% were due to transmission by humans [12]. However, this trend was reversed in the 1990 s, with 78% of the reported cases being infected with the virus through human-to-human contact, and 22% cases through non-human transmission [34]. In the Nigerian outbreak of 2017–2018, 78.3% of the cases were a result of human-to-human contact and 8.2% due to animal-to-human transmission [28]. All monkeypox cases outside Africa prior to the current outbreak occurred as a result of animal-to-human transmission, except in the UK, where human-to-human transmission of the disease was confirmed in healthcare workers taking care of monkeypox-infected patients [35]. Females are at risk of the disease because of the virus being transmitted by their children, while males are infected because they tend to come into contact with rodents and carcasses of rodents in the field. The risk of congenital monkeypox is associated with vertical transmission of the virus to the fetus through the placenta or at birth [19]. During the 1970 s and 1980 s, individuals vaccinated against smallpox showed a lower incidence of infection with MPXV, and in one study, only 13% of individuals with monkeypox had a history of smallpox vaccination [12].
Several risk factors are associated with contracting monkeypox. Human-to-human transmission of the virus occurs while sleeping in the same room or bed, drinking or eating from the same dish, and living in the same household [36]. Of 5561 monkeypox cases reported to the WHO for which sexual orientation was known, 98% were men who had sex with men; in addition, of the 4614 monkeypox-infected patients with known HIV status, 41% were HIV-positive. Apart from African nations, monkeypox primarily affects gay, bisexual, and other men who have sex with other men and have recently had sex with one or more partners [9]. Several cases of monkeypox have been documented without any previous travel history to endemic countries, and many of the patients had histories of homosexuality with males, which may be related to direct contact with lesions and secretions [37]. Animal-to-human transmission of MPXV is a risk for people who sleep outside, live near or work in forests, deal with rodents, or care for animals [38]. The 2003 outbreak in the United States indicated that people dealing with sick animals or cleaning their bedding or cages were at risk of infection. Almost all cases in that outbreak were as a result of viral transmission from animals to humans except for some healthcare workers who became infected after treating patients with monkeypox [39]. Moreover, a scratch or accidental touching of serum from an infected animal may also be risk factors as both could lead to the transmission of MPXV (Fig. 3).
Clinical characteristics of monkeypox infection
Severe lesions on the skin, which last for 2–4 weeks, are the characteristic clinical manifestation of the disease [19]. Symptoms in the early phase of infection (0–5 days) include back pain, intense asthenia, myalgia, lymphadenopathy, intense headache, and fever (Fig. 2). Although monkeypox may initially appear similar to smallpox, measles, or chickenpox, a distinctive characteristic of the disease is lymphadenopathy, the swelling of lymph nodes [8]. After 1–3 days of continuous fever, skin eruption begins; in 95% of cases lesions appear on the face, in 75% on soles and palms, in 70% on oral mucous membranes, in 30% on the genitalia, and in 20% on conjunctivae and cornea [19]. In severe rashes, vesicles, papules, macules, pustules, and sometimes crusts form, which vary in number from person to person; they rarely coalesce until a large area of the skin is affected, at which point the skin is sloughed off [40].
Children and adults with compromised immune systems are most likely to be affected by monkeypox. The smallpox vaccination has proven successful in controlling monkeypox. However, individuals less than 40–50 years of age are more susceptible to monkeypox attacks since the smallpox vaccination program ended after the global eradication of the disease. Several secondary infections, such as sepsis, bronchopneumonia, and encephalitis, may cause complications in monkeypox. Monkeypox is regarded as a self-limited disease, with a CFR of 0–11% in children and 3–6% in adults [19].
The evolution of MPXV
One of the factors contributing to the resurgence of monkeypox may be the genetic development of MPXV. MPXV exists as two distinct clades—the West African clade and the Congo Basin clade. Most people in South Sudan, the DRC, and Central African Republic are infected with the Congo Basin clade [41]. In contrast, the 2003 outbreak in the United States and the 2017 outbreak in Nigeria were caused by the West African clade [28], which has also been found in Israel, Sierra Leone, the United Kingdom, and Singapore as a result of transmission by travelers [26], [35].
Transportation of animals, such as Graphiurus spp., Funisciurus spp., and Cricetomys spp., is the primary route of transmission of the virus from Africa to the United States, and genetic analysis has confirmed that the pathogen belongs to the West African clade and does not cause mortality [40]. Four distinct lineages have been identified within the Central African clade based on the viral genomic diversity of 60 samples obtained from humans with primary and secondary infections in Sankuru District, DRC. A gene loss was also detected in 17% of the samples, which appeared to be linked to human-to-human transmission [42]. The monkeypox strains isolated in Sudan have close genetic relationships with those isolated in the Congo Basin, where the virus is known to be endemic [41]. A genome sequence of 10.8 kb shows one mutation in the entire genome suggesting that northern DRC is the ultimate source of the virus for the Congo Basin clade [14], [43]. The virus has continued to mutate, and the strains isolated in the ROC in 2010 are similar to those isolated in 2003, with fewer similarities to those isolated in 1979 and 1996 [44]. Moreover, a strain isolated in 2010 in the Central African Republic was identical to the strain isolated during an outbreak of the disease at the border between the Central African Republic and the DRC in 2001; the disease was contracted as a result of hunting and eating bemba (a wild rodent) [45]. In 2017, the ROC reported another outbreak of monkeypox, and a phylogenetic analysis showed that the isolates from this outbreak were related to the two Nigerian isolates from the 1971 outbreak [46]. Based on the first MPXV genome sequences derived from monkeypox cases in 2022, it was determined that these MPXV descended from a clade sampled from monkeypox cases between 2017 and 2019 [47]. These data indicate that each disease outbreak of monkeypox began with the animal-to-human transmission of the virus with limited human-to-human, which appears to have accumulated mutations, thereby complicating the problem.
Genomic and proteomic comparison with smallpox
The monkeypox virions are ovoid or brick-shaped, 200–250 nm in size, and have a corrugated lipoprotein outer membrane [48]. The outer membrane protects the enzyme-packed core, double-stranded DNA, and transcription factors. Due to an artifact of fixation, electron microscopy images show the core of the MPXV as similar to that of SPXV, biconcave, and with lateral bodies on each side [31]. The monkeypox viral genome is a linear double-stranded DNA (197 kb) covalently connected at its ends by palindromic hairpins. The inverted terminal repeats consist of hairpin loops, tandem repeats, and some open reading frames.
Monkeypox is similar to smallpox, but its life cycle occurs within infected cells [31]. The viral genome encodes all proteins needed for DNA replication, transcription, virion assembly, and egress. The genes encoding housekeeping tasks are highly conserved in SPXV and MPXV and are in the genome's core region. Those encoding for virus–host interactions are less conserved and are in the termini regions [49]. The internal mature virus (IMV) and the extracellular-enveloped virus (EEV) are two infectious virions formed in poxvirus-infected cells. These virions are found in both SPXV- and MPXV-infected cells. It is believed that the rapid and long-distance dissemination of the viruses within an infected host is due to the fact that IMV is released during cell lysis, and EEV is released from cells via contact with actin tails. Both of these events take place simultaneously. Even though the characteristics mentioned above are of SPXV, all orthopoxviruses probably share them [50].
Monkeypox virus battles with the human immune system
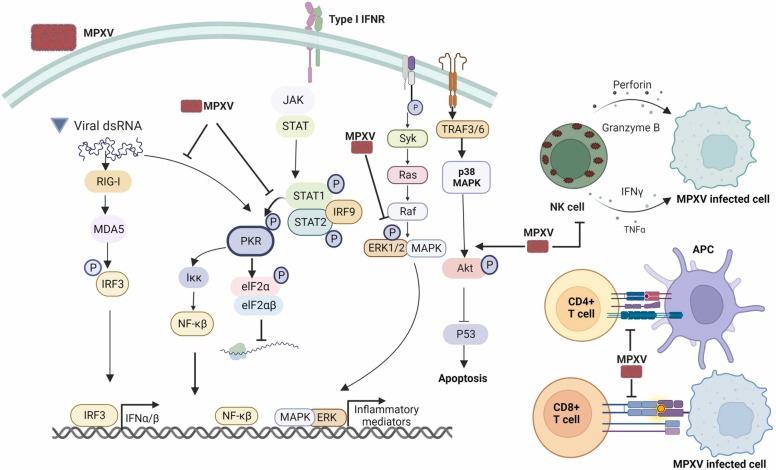
The replication of viruses inside host cells acts as viral propagation since viruses are host-dependent and target cell development and immunoregulation. MPXV inhibits or delays apoptosis, suppresses antiviral host defense, and exploits the host cell machinery [51]. The elimination of viral infections requires both arms of the immune system—innate and adaptive immune responses. Nevertheless, research on the strategies of MPXV to modulate effector host immune defenses to promote survival within the host remain in its infancy. MPXV can suppress macrophage response to chemokines in in-vivo and ex-vivo models. Viral chemokine binding protein is secreted extracellularly from MPXV-infected cells and can repress the chemotactic function of macrophage inflammatory protein-1 [52]. Transcriptomic data for an in vitro infection model showed that MPXV can negatively regulate the expression of innate immune signaling components such as the mitogen-activated protein kinase (MAPK-ERK) pathway [51]. Likewise, a tissue section from a rhesus monkey infected with MPXV was found to reduce the phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) ( Fig. 4) [53]. However, MPXV can induce high cytokine production, including granulocyte–monocyte colony-stimulating factor (GM-CSF), interleukin 10, and interleukin 2 receptors, in severely ill patients [54]. Type-I interferon is a crucial innate antiviral cytokine that interferes with viral replication by mediating viral mRNA degradation and inhibiting translation [55]. MPXV can repel antiviral activity of type-I interferon by hindering phosphorylation of the protein kinase R pathway (Fig. 4) [56]. Moreover, MPXV compromises protein kinase R pathway activation by synthesizing a lower amount of double-stranded RNA than that produced by Vaccinia virus [57]. The virulent Congo Basin MPXV strain induces imbalance of host signaling unlike the avirulent West African strain. It can increase phosphorylation of p38 MAPK and the Akt pathway, which reduces phosphorylation of p53 (Fig. 4), thereby leading to the suppression of cell apoptosis [58]. This evidence thus suggests that MPXV modulates innate immune signaling via the phosphorylation process.
Fig. 4.
Manipulation of the host immune response by the monkeypox virus. MPXV can escape from the protein kinase R pathway through reduction of mRNA synthesis and ablation of type-I interferon mediating protein kinase R phosphorylation. MPXV can also reduce the production of inflammatory mediators by interfering with phosphorylation of the MAPK/ERK1/2 pathways. It suppresses cell apoptosis by increasing phosphorylation of the ATK pathway. MPXV can prevent natural killer cells from killing virus-infected cells and secreting inflammatory cytokines. MPXV can also interfere with the adaptive immune response by hindering T cell receptor trans-signaling.
Natural killer cells are essential players in the innate host defense against viral infections and contribute to the augmentation of cell-mediated immunity through cytokine secretion. MPXV infection in rhesus macaque enhanced natural killer cell proliferation and increased natural killer cell count in blood and lymph nodes. Despite the high number of natural killer cells, the early response to chemokines, secretion of tumor necrosis factor alpha and interferon gamma, and cytotoxic activity (Fig. 4) were severely compromised [59]. A recent study demonstrated that administration of interleukin 15 to MPXV-susceptible CAST mice promoted interferon gamma expression and the killing capabilities of natural killer cells [60].
The cell-mediated immune response involves helper T lymphocytes (CD4 + T cells), and cytotoxic T lymphocytes (CD8 + T cells) are the leading eradicator of viral infections, including poxvirus infections [61]. A previous study showed that MPXV can subvert CD4 + and CD8 + T cells responses and inflammatory cytokine production [62]. MPXV encodes B22 protein, which can interfere with the T cell receptor response to the MHC complex and other stimuli, accelerating viral replication and disease severity in a non-human primate model (Fig. 4) [63]. Further studies are needed to understand the host immune response against MPXV and explore the immunomodulatory molecules of MPXV, which will open avenues for the development of antiviral drugs and vaccines.
Diagnosis of monkeypox
Monkeypox is characterized by lesions and rashes on the skin with mild fever at the beginning of the disease. Other rash disorders that must be investigated in the differential diagnosis include chickenpox, measles, bacterial skin infections, scabies, syphilis, and medication-associated allergies. Lymphadenopathy in the prodromal stage of sickness can be used to differentiate monkeypox from chickenpox and smallpox. Swabs taken from two to three lesions on the skin or genitals are typically used to confirm laboratory diagnosis of monkeypox. The use of throat or nasopharyngeal swabs and the testing of blood are less common. Collection of anal swabs is recommended from patients who present with proctitis. MPXV DNA has also been detected in the semen of 90.6% of cases tested; however, the replication competence of the DNA is not confirmed [25]. The specimens for laboratory testing should be handled at least at Biosafety Level 2 (BSL-2) and more stringently in a BSL-3 laboratory, while a BLS-3 facility is recommended for MPXV cultures [7].
In routine diagnostic laboratories, real-time polymerase chain reaction (RT-PCR) is recommended as the most reliable method of detecting MPXV DNA. RT-PCR is a highly sensitive technique and shows positivity from 2 to 20 days (median 5 days) after the onset of clinical symptoms [25]. MPXV is characterized by whole-genome sequencing, but next-generation sequencing is expensive, and downstream processing requires massive computing power [31]. MinION nanopore sequencing technology has produced sufficient sequence quality for complete sequencing of MPXV directly from lesions [64]. It is not possible to confirm monkeypox by antigen or antibody detection methods because orthopoxviruses are serologically cross-reactive [19]. The detection of IgM and IgG antibodies in a patient's serum usually takes > 8 days after the onset of symptoms. A positive IgM finding indicates recent exposure to orthopoxviruses in both vaccinated and unvaccinated people, whereas a positive IgG indicates previous exposure to orthopoxviruses from either immunization or infection [31]. The presence of IgM in vaccinated individuals living in MPXV-endemic areas suggests recent infection.
Treatment
Most monkeypox infections need supportive care and symptomatic treatment. Patients should drink plenty of fluids and have a nutritious diet. There is no particular treatment available for monkeypox, although cidofovir and vaccinia immune globulin can help in better patient recovery [25]. The antivirals initially developed for treating patients with smallpox might be helpful in treating monkeypox [7]. Tecovirimat is an antiviral drug originally formulated against smallpox and has been licensed in the European Union for treatment of monkeypox patients, particularly those who are immunocompromised [7], [19]. It is important to treat secondary bacterial infections according to their severity with empirical antibiotics.
Nonpharmaceutical interventions: a smart preventive strategy
Nonpharmaceutical interventions (NPIs) are actions, other than vaccination and medication, to prevent an infection. During the COVID-19 pandemic, NPIs were widely implemented as the only option available to diminish the spread of the virus in the population [65]. However, monkeypox does not require the implementation of the very stringent NPIs (e.g., closure of offices and educational institutions) put in place in the first wave of the COVID-19 pandemic. The mainstay of monkeypox prevention is to adopt NPI strategies in daily life. During an outbreak, NPIs should be applied routinely at home and work, while traveling, in the community, and at social gatherings. The first important step is to educate individuals and communities to decrease the risk of exposure to MPXV.
Effective handwashing practices and social distancing play a crucial role in protecting against several infections, including monkeypox. Viruses can stick to surfaces and inanimate objects for several hours and can be transmitted to people after touching the objects (Fig. 2). Orthopoxviruses have been observed to survive on environmental surfaces for several weeks. Environmental surface cleaning is one of the most effective forms of NPI [7]. Most human infections result through direct animal-to-human transmission over time. Contact with wild animals, especially the sick or dead and their meat, blood, and other body components, must be avoided [36].
Every infected individual does not require admission to hospital and can be isolated at home. Infected individuals can transmit MPXV to pets during close contact. It is important that pets are kept away during a person’s infectious period. MPXV can be transmitted from person to person by direct contact with lesions, infected body fluids, or by exposure to respiratory secretions. Infected individuals should avoid all sexual activity, including touching and kissing, during the infectious period. An individual is considered infectious until all scabs have separated and a fresh layer of skin has formed. Infected people living in communal settings should be isolated and individuals they come into contact with should be medically monitored [7]. WHO advised bisexuals and homosexuals to isolate themselves if they have symptoms and avoid skin contact with those suffering from symptoms. It is important to improve hygiene, avoid sexual activity, physical contact, and wear masks. A person should not be stigmatized for having a disease. It is possible for anyone, regardless of their sexual orientation, to contract monkeypox or spread it to others [66]. In order to prevent infection with MPVX, healthcare workers and those caring for monkeypox patients should follow the standard infection control precautions.
Immunization
Researchers are currently investigating the suitability and feasibility of vaccination for monkeypox prevention and control. Some countries have policies in place or are in the process of adopting policies to provide vaccines to people who are at risk, including laboratory personnel, rapid response teams, and health workers [19]. The smallpox vaccine suppresses MPXV infection, and it is recommended that vaccinated individuals provide care for monkeypox patients while taking all other precautions [67]. Routine vaccination against smallpox with vaccinia-based vaccines has been discontinued in every country for at least the last 40 years. Since immunization also protects against monkeypox in West and Central Africa, individuals who have not been vaccinated are now more likely to be infected by MPXV [20].
Properly administered prior vaccination against smallpox has nearly 85% efficacy in preventing monkeypox [19]. Currently, two approved brands of vaccine against orthopoxviruses are available that protect against monkeypox. ACAM2000 is derived from calf lymph and is a type of active immunization; it is manufactured by Sanofi Pasteur Biologics, LLC (Cambridge, United States) and is administered as a single dose. JYNNEOS (Imvanex/Imvamune in the European Union) is manufactured by Bavarian Nordic (Tuborg Havn, Denmark) and is approved in the United States and Canada as a Modified Vaccinia Ankara (MVA) vaccine, which is a live attenuated vaccine, and is recommended as two doses. A limited supply of vaccines is available for people at risk [7]. Even though naturally occurring smallpox is no longer a threat, new vaccinations, diagnostics, and antiviral medications are now being developed to guarantee that the whole world is prepared in the event that smallpox reappears. Such products may also provide effective protection against monkeypox.
Future prospects and strategies
The basic method of disease control in infectious disease epidemics is to prevent further transmission. It is possible to lower the risk of transmission of animal infections to humans with even the simplest of biosecurity measures and social distancing of infected people. The advice during both the 2009–2010 H1N1 pandemic and the COVID-19 pandemic cautioned people to “cough hygienically” into their sleeves.
The possibility that MPXV circulates among various naturally occurring and incidental animal species without a natural reservoir should also be explored in future studies. The evolutionary divergence of the two MPVX clades should be investigated because they may have different reservoir species [31]. The smallpox vaccine confers immunity against MPXV despite the difference in several proteins produced by MPXV. Vaccines against these specific proteins may more efficiently target MPXV and develop monkeypox-specific immunity [49].
As a result of globalization, contagious diseases are becoming more prevalent and pose a threat to public health. Consequently, humans and animals are exposed to new diseases against which they have little or no natural immunity. The advances in transportation and increased freedom of movement have resulted in the unprecedented spread of monkeypox. There is no way to predict the next newly emerging infection; however, it is apparent that we have lessons to learn from previous outbreaks, and the implementation of routine infection control practices may serve to reduce the impact of such an outbreak. It is evident from this review that data on monkeypox epidemiology is still limited and fragmented, which will cause outbreaks of the disease to be underestimated. There is an urgent need for the research community to engage more with this disease, follow up on it, and conduct more research on the topic.
Conclusion
A decline in immunity against smallpox due to the cessation of smallpox vaccination has paved the way for the resurgence of monkeypox and its dissemination in non-endemic parts of the world. MPXV is a serious reemerging pathogen that is circulating globally and jeopardizing public health. The incubation period of 7–14 days after exposure to MPXV in a natural setting underlines the need to sharpen our understanding of the risk of transmission of the virus, effective mechanisms for disease surveillance, and measures for prevention such as isolation. MPXV is generally transmitted by infected animals, but human-to-human transmission has come to prevail in recent outbreaks. There is a significant increase in the number of individuals infected with monkeypox in the current outbreak, with the highest rates among males. Globally, the combined CFR of the West African and Congo Basin MPXV clades is approximately 8.7%. The most apparent and common presentations are skin lesions and lymphadenopathy. The human immune system elicits both innate and adaptive immunity against MPXV, but the role of immunomodulatory molecules has not yet been explored in depth. The identification of IgM and IgG does not add much value to the diagnosis of MPXV cases, while RT-PCR is the standard technique of choice. Antiviral therapy with tecovirimat or cidofovir is helpful for rapid recovery of patients with weak immune systems. The use of NPIs is a smart strategy to prevent the population from circulating MPXV. The two smallpox vaccines, JYNNEOS (Imvanex/Imvamune) and ACAM2000, provide 85% efficacy in preventing monkeypox. The attack rate should be reduced by implementing measures such as family-based education in hygiene and isolation of patients. Global implementation of comprehensive strategies to deal with monkeypox is crucial in order to strengthen health systems over the long term to respond to large-scale outbreaks of the disease.
Funding
This work was funded by the Deanship of Scientific Research at Jouf University under Grant Number (DSR2022-RG-0154).
Ethical approval
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The authors are incredibly thankful to the Deanship of Scientific Research at Jouf University, Saudi Arabia for the tremendous support.
References
- 1.Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58(2):165–182. [PMC free article] [PubMed] [Google Scholar]
- 2.Fenner F, Henderson D, Arita I, Jezek Z, Ladnyi I. Smallpox and its eradication. Geneva Switzerland. 〈https://apps.who.int/iris/handle/10665/39485〉; 2022 [Accessed 30 July 2022].
- 3.Parker S., Buller R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8(2):129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damon I.K., Roth C.E., Chowdhary V. Discovery of monkeypox in Sudan. N Engl J Med. 2006;355(9):962–963. doi: 10.1056/NEJMc060792. [DOI] [PubMed] [Google Scholar]
- 5.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 6.Khodakevich L., Szczeniowski M., Nambu ma D., Jezek Z., Marennikova S., Nakano J., et al. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop Geogr Med. 1987;39(1):56–63. [PubMed] [Google Scholar]
- 7.CDC Monkeypox. Atlanta, Georgia. 〈https://www.cdc.gov/poxvirus/monkeypox/index.html〉; 2022 [Accessed 9 August 2022].
- 8.Reynolds M.G., Yorita K.L., Kuehnert M.J., Davidson W.B., Huhn G.D., Holman R.C., et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Multi-country outbreak ofmmonkeypox, External situation report #2. Geneva, Switzerland. 〈https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--2---25-july-2022〉; 2022 [Accessed 9 August 2022].
- 10.Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 11.Breman JG. Human monkeypox: update 1978. Geneva, Switzerland. 〈http://apps.who.int/iris/bitstream/handle/10665/68294/SME_78.15.pdf?sequence=1&isAllowed=y〉; 2022 [Accessed 9 August 2022].
- 12.Jezek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66(4):459–464. [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Human monkeypox in Kasai Oriental, Democratic Republic of the Congo (former Zaire): Preliminary report of October 1997 investigation. Geneva, Switzerland. 〈http://apps.who.int/iris/handle/10665/230323?locale-attribute=pt&〉; 2022 [Accessed 9 August 2022]. [PubMed]
- 14.Sklenovská N., Van, Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Weekly bulletin on outbreaks and other emergencies: Week 31. Geneva Switzerland. 〈https://apps.who.int/iris/bitstream/handle/10665/273631/OEW31-2873082018.pdf?sequence=1&isAllowed=y〉; 2022 [Accessed 9 August 2022].
- 17.WHO. Weekly bulletin on outbreaks and other emergencies: Week 1. Geneva Switzerland. 〈https://apps.who.int/iris/bitstream/handle/10665/278952/OEW01-29122018-04012019.pdf?sequence=1&isAllowed=y〉; 2022 [Accessed 9 August 2022].
- 18.WHO. Weekly bulletin on outbreaks and other emergencies: Week 26. Geneva Switzerland. 〈http://apps.who.int/iris/bitstream/handle/10665/272981/OEW26-2329062018.pdf〉; 2022 [Accessed 9 August 2022].
- 19.WHO. Monkeypox. Geneva, Switzerland. 〈https://www.who.int/news-room/fact-sheets/detail/monkeypox〉; 2022 [Accessed 9 August 2022].
- 20.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., et al. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoff N.A., Doshi R.H., Colwell B., Kebela-Illunga B., Mukadi P., Mossoko M., et al. Evolution of a disease surveillance system: an increase in reporting of human monkeypox disease in the Democratic Republic of the Congo, 2001-2013. Int J Trop Dis Health. 2017;25(2):35885. doi: 10.9734/ijtdh/2017/35885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Weekly bulletin on outbreaks and other emergencies: Week 18. Geneva Switzerland. 〈http://apps.who.int/iris/bitstream/handle/10665/255272/OEW18-294552017.pdf?sequence=1〉; 2022 [Accessed 9 August 2022].
- 23.WHO. Weekly bulletin on outbreaks and other emergencies: Week 31. Geneva Switzerland. 〈https://apps.who.int/iris/bitstream/handle/10665/326159/OEW31-290704082019.pdf?sequence=1&isAllowed=y〉; 2022 [Accessed 9 August 2022].
- 24.WHO. Weekly bulletin on outbreaks and other emergencies: Week 1. Geneva, Switzerland. 〈https://apps.who.int/iris/bitstream/handle/10665/330353/OEW01-05012020.pdf?sequence=1&isAllowed=y〉; 2022 [Accessed 9 August 2022].
- 25.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022:1–14. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 26.Erez N., Achdout H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Weekly bulletin on outbreaks and other emergencies: Week 4. Geneva, Switzerland. 〈https://apps.who.int/iris/handle/10665/338891〉; 2022 [Accessed 9 August 2022].
- 28.Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCollum A., Disu Y., et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/s1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monkeypox Monkeypox. Gabon. Wkly Epidemiol Rec 1992. 1991;67(14):101–102. [PubMed] [Google Scholar]
- 30.Meyer A., Esposito J.J., Gras F., Kolakowski T., Fatras M., Muller G. First appearance of monkey pox in human beings in Gabon. Med Trop. 1991;51(1):53–57. [PubMed] [Google Scholar]
- 31.Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC. Monkeypox in animals. Atlanta, Georgia. 〈https://www.cdc.gov/poxvirus/monkeypox/veterinarian/monkeypox-in-animals.html〉; 2022 [Accessed 9 August 2022].
- 33.Falendysz E.A., Lopera J.G., Doty J.B., Nakazawa Y., Crill C., Lorenzsonn F., et al. Characterization of monkeypox virus infection in African rope squirrels (Funisciurus sp.) PLoS Negl Trop Dis. 2017;11(8) doi: 10.1371/journal.pntd.0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC. Human monkeypox -- Kasai Oriental, Democratic Republic of Congo, February 1996-October 1997. MMWR Morb Mortal Wkly Rep; 46(49); 1997. pp. 1168–71. [PubMed]
- 35.Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Eur Surveill. 2018;23(38) doi: 10.2807/1560-7917.Es.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolen L.D., Osadebe L., Katomba J., Likofata J., Mukadi D., Monroe B., et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93(2):410–415. doi: 10.4269/ajtmh.15-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaheen N., Diab R.A., Meshref M., Shaheen A., Ramadan A., Shoib S. Is there a need to be worried about the new monkeypox virus outbreak? A brief review on the monkeypox outbreak. Ann Med Surg. 2022;81 doi: 10.1016/j.amsu.2022.104396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guagliardo S.A.J., Doshi R.H., Reynolds M.G., Dzabatou-Babeaux A., Ndakala N., Moses C., et al. Do monkeypox exposures vary by ethnicity? Comparison of Aka and Bantu suspected monkeypox cases. Am J Trop Med Hyg. 2020;102(1):202–205. doi: 10.4269/ajtmh.19-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds M.G., Davidson W.B., Curns A.T., Conover C.S., Huhn G., Davis J.P., et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007;13(9):1332–1339. doi: 10.3201/eid1309.070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boumandouki P., Bileckot R., Ibara J.R., Satounkazi C., Wassa Wassa D., Libama E., et al. Simian smallpox (or monkey smallpox): study of 8 cases observed at Impfondo Hospital in Republic of Congo. Bull Soc Pathol Exot. 2007;100(1):17–21. [PubMed] [Google Scholar]
- 41.Formenty P., Muntasir M.O., Damon I., Chowdhary V., Opoka M.L., Monimart C., et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg Infect Dis. 2010;16(10):1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kugelman J.R., Johnston S.C., Mulembakani P.M., Kisalu N., Lee M.S., Koroleva G., et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20(2):232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakazawa Y., Mauldin M.R., Emerson G.L., Reynolds M.G., Lash R.R., Gao J., et al. A phylogeographic investigation of African monkeypox. Viruses. 2015;7(4):2168–2184. doi: 10.3390/v7042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Learned L.A., Reynolds M.G., Wassa D.W., Li Y., Olson V.A., Karem K., et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73(2):428–434. [PubMed] [Google Scholar]
- 45.Berthet N., Nakouné E., Whist E., Selekon B., Burguière A.M., Manuguerra J.C., et al. Maculopapular lesions in the Central African Republic. Lancet. 2011;378(9799):1354. doi: 10.1016/s0140-6736(11)61142-2. [DOI] [PubMed] [Google Scholar]
- 46.Faye O., Pratt C.B., Faye M., Fall G., Chitty J.A., Diagne M.M., et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect Dis. 2018;18(3):246. doi: 10.1016/s1473-3099(18)30043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Toole A, Rambaut A. Initial observations about putative APOBEC3 deaminase editing driving short-term evolution of MPXV since 2017. University of Edinburgh, UK. 〈https://virological.org/t/initial-observations-about-putative-apobec3-deaminase-editing-driving-short-term-evolution-of-mpxv-since-2017/830〉; 2022 [Accessed 9 August 2022].
- 48.Moore MJ, Rathish B, Zahra F. Monkeypox. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing LLC.; 2022.
- 49.Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remichkova M. Poxviruses: smallpox vaccine, its complications and chemotherapy. Virus Adapt Treat. 2010;2:41–46. doi: 10.2147/VAAT.S8563. [DOI] [Google Scholar]
- 51.Bourquain D., Dabrowski P.W., Nitsche A. Comparison of host cell gene expression in cowpox, monkeypox or vaccinia virus-infected cells reveals virus-specific regulation of immune response genes. Virol J. 2013;10:61. doi: 10.1186/1743-422x-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones J.M., Messauodi I., Estep R.D., Orzechowska B., Wong S.W. Monkeypox virus viral chemokine inhibitor (MPV vCCI), a potent inhibitor of rhesus macrophage inflammatory protein-1. Cytokine. 2008;43(2):220–228. doi: 10.1016/j.cyto.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sood A., Sui Y., McDonough E., Santamaría-Pang A., Al-Kofahi Y., Pang Z., et al. Comparison of multiplexed immunofluorescence imaging to chromogenic immunohistochemistry of skin biomarkers in response to monkeypox virus infection. Viruses. 2020;12(8):787. doi: 10.3390/v12080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston S.C., Johnson J.C., Stonier S.W., Lin K.L., Kisalu N.K., Hensley L.E., et al. Cytokine modulation correlates with severity of monkeypox disease in humans. J Clin Virol. 2015;63:42–45. doi: 10.1016/j.jcv.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng X., Schoggins J., Rose L., Cao J., Ploss A., Rice C.M., et al. C7L family of poxvirus host range genes inhibits antiviral activities induced by type I interferons and interferon regulatory factor 1. J Virol. 2012;86(8):4538–4547. doi: 10.1128/jvi.06140-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arndt W.D., Cotsmire S., Trainor K., Harrington H., Hauns K., Kibler K.V., et al. Evasion of the innate immune Type I interferon system by monkeypox virus. J Virol. 2015;89(20):10489–10499. doi: 10.1128/jvi.00304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arndt W.D., White S.D., Johnson B.P., Huynh T., Liao J., Harrington H., et al. Monkeypox virus induces the synthesis of less dsRNA than vaccinia virus, and is more resistant to the anti-poxvirus drug, IBT, than vaccinia virus. Virology. 2016;497:125–135. doi: 10.1016/j.virol.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kindrachuk J., Arsenault R., Kusalik A., Kindrachuk K.N., Trost B., Napper S., et al. Systems kinomics demonstrates Congo Basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol Cell Proteom. 2012;11(6) doi: 10.1074/mcp.M111.015701. M111.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song H., Josleyn N., Janosko K., Skinner J., Reeves R.K., Cohen M., et al. Monkeypox virus infection of rhesus macaques induces massive expansion of natural killer cells but suppresses natural killer cell functions. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Earl P.L., Americo J.L., Moss B. Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses. PLoS Pathog. 2020;16(4) doi: 10.1371/journal.ppat.1008505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ando J., Ngo M.C., Ando M., Leen A., Rooney C.M. Identification of protective T-cell antigens for smallpox vaccines. Cytotherapy. 2020;22(11):642–652. doi: 10.1016/j.jcyt.2020.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammarlund E., Dasgupta A., Pinilla C., Norori P., Früh K., Slifka M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci USA. 2008;105(38):14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alzhanova D., Hammarlund E., Reed J., Meermeier E., Rawlings S., Ray C.A., et al. T cell inactivation by poxviral B22 family proteins increases viral virulence. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vandenbogaert M., Kwasiborski A., Gonofio E., Descorps-Declère S., Selekon B., Nkili Meyong A.A., et al. Nanopore sequencing of a monkeypox virus strain isolated from a pustular lesion in the Central African Republic. Sci Rep. 2022;12(1):10768. doi: 10.1038/s41598-022-15073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haug N., Geyrhofer L., Londei A., Dervic E., Desvars-Larrive A., Loreto V., et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav. 2020;4(12):1303–1312. doi: 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- 66.WHO. Monkeypox: public health advice for gay, bisexual and other men who have sex with men. Geneva, Switzerland, 〈https://www.who.int/news/item/25-05-2022-monkeypox--public-health-advice-for-gay--bisexual-and-other-men-who-have-sex-with-men〉; 2022 [Accessed 8 September 2022].
- 67.Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]