Abstract
Monkeypox is an emerging zoonotic disease caused by monkeypox virus which is a DNA virus. The virus is transmitted to humans as a result of close contact with infected animals, infected humans or contaminated inanimate objects. The disease has a incubation period usually 7–14 days and it causes fever, headache, fatigue, myalgia, widespread body aches, swelling in lymph nodes and skin lesions. It may be difficult to distinguish monkeypox on the basis of clinical presentation alone, especially for cases with an atypical appearance, because of the various conditions that cause skin rashes. Testing should be offered to anyone who falls under the suspected case definition for monkeypox infection. Suitable samples are surface lesion and/or skin materials such as exudates swabs and crusts. Laboratory confirmation of specimens from suspected case is done using nucleic acid amplification testing, such as real-time or conventional polymerase chain reaction. Confirmation of MPXV infection should consider clinical and epidemiological information. Positive detection using an OPXV PCR assay followed by confirmation of MPXV via PCR and/or sequencing, or positive detection using MPXV PCR assay in suspected cases indicates confirmation of MPXV infection. Genetic sequence data (GSD) provide information on the origin and epidemic and characteristics of cases. There is a need to develop a more global and effective laboratory network for this emerging zoonosis, as well as to strengthen laboratory capacity, and international specimens referral capacities.
Keywords: Monkeypox, DNA, Virus, Polymerase chain reaction, Laboratory, Diagnosis
1. Introduction
Monkeypox is an emerging zoonotic disease caused by monkeypox virus (MPXV) a member of the Orthopoxvirus genus in the Poxviridae family; it is a DNA virus related to smallpox virus [[1], [2], [3], [4], [5]]. Two different genetic subtypes are known to cause disease in Central and West Africa. The West African subtype causes milder disease compared to the Central African (Congo Basin) subtype. Initial findings from the current outbreak indicate that the common and most spread form of the virus now is the West African subtype [3,5,6]. It is a disease that is found in rodents such as squirrels, rats and mice rather than monkeys and is transmitted from them to humans. It is called monkeypox because it first caused a smallpox-like disease in monkeys in 1958 [7]. The reservoirs and natural cycle of the virus in nature are not fully known. The virus has been isolated from animals in the wild twice so far: it was first isolated in 1985 from the African squirrel in the Equatorial region of the Democratic Republic of the Congo. The second was isolated in 2012 from a dead baby primate of a monkey species called “mangabey” in the rainforests of the Ivory Coast [3,4,[8], [9], [10], [11]]. As the clinical presentation may be atypical in this outbreak, we should include monkeypox in the differential diagnosis when a patient presents with a sexually transmitted infection (STI)-associated or STI-like rash, even if the rash is localised and not (yet) diffuse [12]. Monkeypox rash can be mistaken for chickenpox, shingles, or herpes, However, by looking at the pattern on the skin and where the rash appears, a board-certified dermatologist can narrow down which disease is causing the rash. If monkeypox is a likely cause, the dermatologist will swab the rash and send the swab to a lab. At the lab, a PCR (polymerase chain reaction) test will be performed. The results from the PCR test will indicate whether the swab contains the monkeypox virus. The recent apparent increase in human monkeypox cases across a wide geographic area and the potential for further spread have raised the level of concern for this emerging zoonosis [1,4,[13], [14], [15]]. The increase in reported incidence of MPX may be partly attributable to decreasing herd immunity in the population following the cessation of the smallpox vaccination program in the early 1980s. Other explanatory factors include changes in the virus itself and modifications of ecosystems that may have caused the natural reservoir's population density to rise and more frequent human-wildlife interactions. It is interesting to note that the strain of the virus in the current monkeypox outbreak in nonendemic countries likely diverged from the monkeypox virus that caused a 2018-19 Nigerian outbreak and has far more mutations than would be expected, several that increase transmission [16]. On July 23, 2022, World Health Organization (WHO), declared the current monkeypox outbreak a Public Health Emergency of International Concern (PHEIC), overriding the WHO Emergency Committee, which decided 6–9 against recommending a PHEIC.1 That decision was justified, with cases in more than 70 countries, most of which are nonendemic, many with no clear epidemiological links and milder nonspecific clinical presentation [17].
2. How is the virus transmitted to humans?
The monkeypox virus (MPXV) can spread in a number of ways and to anyone through close, personal, often skin-to-skin contact. That includes direct contact (with monkeypox rash, scabs, or body fluids from a person with monkeypox); touching objects, fabrics (clothing, bedding, or towels), and surfaces that have been used by someone with monkeypox; or, contact with respiratory secretions. A pregnant person can spread the virus to their fetus through the placenta. It's also possible for people to get monkeypox from infected animals, either by being scratched or bitten by the animal or by preparing or eating meat or using products from an infected animal [15,[18], [19], [20], [21], [22], [23], [24], [25], [26], [27]]. A person with monkeypox can spread it to others from the time symptoms start until the rash has fully healed and a fresh layer of skin has formed. The illness typically lasts 2–4 weeks. Scientists are still researching to see and improve our understanding: (i) If the virus can be spread when someone has no symptoms, (ii) how often monkeypox is spread through respiratory secretions, or when a person with monkeypox symptoms might be more likely to spread the virus through respiratory secretions, and (iii) whether monkeypox can be spread through semen, vaginal fluids, urine, or feces. Unlike COVID-19, this virus doesn't transmit human to human very efficiently; and, it's also much easier to isolate infected individuals and prevent the spread. Although sexual transmission is not certain, it is possible to be transmitted during sexual intercourse due to close contact with an infected individual [11,21]. On the other hand, air travellers play an important role in spreeding infection [11,14,15,21,28].
3. Clinical manifestations
Monkeypox virus as a member of Orthopoxvirus genus, the clinical presentation is similar to the smallpox [13,18,21,22,24]. The incubation period in human usually is from 7 to 14 days, but it can ranges from 4 to 21 days [8,[19], [20], [21], [22],29]. The disease start with a febrile prodrome for 1–4 days and it's companied by headache, muscle aches and backache and sometimes exhaustion, sweats, fatigue and the cutaneal presentation. Skin rashes appear 1–3 days after the onset of fever. The rash that can appears on the face, inside the mouth, on the hands, feet, chest, genitals, anus and in the eyes. Sometimes, rash is the first symptoms, followed by other symptoms. The number of lesions is variable. Lesions begin as a flat rash (macula) and become raised from the skin (papules); then they get a “vesicle” appearance by filling with clear liquid. The clear liquid inside the vesicles turns into a yellowish liquid and “pustules” form. Pustules, crusts and lesions disappear with the fall of the crusts. After crust fall off, patients are considered noninfectious. The disease is seen to be more severe in people whose immune system is suppressed. The most common outcome following an infection is scarring from the rash. But more serious complications can arise, according to research of monkeypox in humans published in 2009, including pulmonary distress and bronchopneumonia. More severe complications and sequelae are found to be more common among unvaccinated than vaccinated patients and ocular infections can occur and may result in corneal scarring and even permanent vision loss [1,5,8,11,12,21,23,26,27,[30], [31], [32]]. Lymphadenopathy is a distinguishing feature of monkeypox from smallpox. This typically occurs with fever onset, 1–2 days before rash onset, or rarely with rash onset. Usually enlarge lymph nodes are, especially the submental, submandibular, cervical, and inguinal nodes. Exposed people may also develop a sore throat, cough and/or a rash on the mucous membranes of the mouth [1,8,9,[21], [22], [23],25,28,32,33]. However nonspecific clinical presentations, lesions and inflammation of the pharyngeal, conjunctival, and genital mucosae have been observed [8,[21], [22], [23],25,28,33]. Most of these signs and symptoms can be seen in many other viral and nonviral diseases and sometimes it's difficult for clinicians that aren't familiar with diseases that can mimic monkeypox to differentiate it from other viral, bacterial or other diseases. For these reasons the laboratoric diagnosis is of special importance [9,23,[32], [33], [34]].
4. Test indications
Monkeypox is a disease that present challenges for public health and healthcare workers (HCW) in terms of surveillance and laboratory capacities. A usually endemic disease, monkeypox is recently being notified as spread to non-endemic areas with the number of cases raising across Europe, North America and Australia [1,4,6,9,11,21,34]. That could be due to infected people traveling more, surveillance measures not in place and also COVID-19 measures more relaxed. Also the smallpox vaccine that provides around 85% protection against monkeypox infection hasn't been applied since 1982. The surveillance process should be assisted by looking at any available epidemiological data. It is known that many of the initial patients reported international travel in the 21 days prior to symptom onset, visiting countries not known to experience endemic monkeypox and participating in large festivals and other activities where close, personal, skin-to-skin contact likely occurred. However, recent travel history does not confirm the person acquired their infection while traveling. Since late June, an increasing number of reported cases have been linked to local community transmission [35]. As mentioned above, clinical sings and symptoms aren't specific. It may be difficult to distinguish monkeypox on the basis of clinical presentation alone, especially for cases with an atypical appearance, because of the various conditions that cause skin rashes, and the clinical presentation may be more often atypical in this outbreak. Therefore, it is important to consider other potential causes of different skin lesions or a widespread rash. Other examples of etiology for skin lesions that appear similar at different stages of development include herpes simplex virus, varicella zoster virus, molluscum contagiosum virus, enterovirus, measles, scabies, syphilis, bacterial skin infections, rickettsia pox, drug allergies, Parapoxviruses or and related conditions) and other diseases [32,[36], [37], [38], [39]]. Clinicians should be alert to patients presenting with a new characteristic rash or if the patient meets one of the epidemiologic criteria and there is a high clinical suspicion for monkeypox. The rash associated with monkeypox can be confused with other rashes encountered in clinical practice including herpes, syphilis, and varicella. Patients co-infected with Monkeypox virus and other infectious agents (e.g., varicella zoster, herpes, syphilis) have been reported [12,18,26,27,32,37,39]. Some countries don't have the capacity (or the capability) to test and the decision to test should be based on both clinical and epidemiological factors, linked to an assessment of the likelihood of being infected. Testing should be offered to anyone who falls under the clinical and epidemiologically suspected case definition for Monkeypox [13,34,36].
5. Specimen collection, shipment and storages
5.1. Safety procedures
It should be ensured that adequate standard operating procedures (SOPs) are used for the collection, storage and handling of Monkeypox specimens. Laboratory personnel should be trained in how to properly do and remove personal protective equipment (PPE) throughout the entire sample handling process. Patient samples taken with the suspicion of monkeypox infection should be considered as possible infective material - care should be taken in terms of the risk of contamination, and all measures should be taken to reduce the risk. It should work with trained and competent personnel. PPE should be used with care and infective aerosol formation should be avoided. Vaccination is recommended among staff if possible. In particular, healthcare workers who are caring for and who are due to start caring for a patient with confirmed monkeypox should be vaccinated (2 doses are normally required). This includes some staff in sexual health clinics who are assessing any suspected cases [40]. Quaternary ammonium compounds and 0.5% (or 200 ppm) bleach (freshly made) can be used [8,28,36,41]. Pre-exposure vaccination should be prioritised for the following workers at high risk of exposure: staff expected to provide care to monkeypox cases in high consequence infectious disease units.
Sample to be collected. Suitable examples for monkeypox are surface and/or skin materials such as exudate swabs, lesion crusts [8,16,19,20,23,31,32,36,41]. The swab should be rubbed vigorously against the lesion to obtain sufficient viral DNA (Table 1). If possible, samples should be taken from more than one location and different lesion, and these swab samples should be transported to the laboratory in a dry or a Viral Transport Medium (VTM). Two lesions of the same type may be placed in a single tube, but lesions, crusts, and vesicular fluids should not be transported in the same tube. As part of making a MPX diagnosis, there is a risk of either a false negative test result (meaning the test says the person does not have monkeypox, but they do have monkeypox), or a false positive test result (meaning the test says the person has monkeypox, but they do not have monkeypox). The U.S. Food and Drug Administration (FDA) is advising the personel to use swab samples taken directly from a lesion (rash or growth) when testing for the monkeypox virus. The FDA is not aware of clinical data supporting the use of other sample types, such as blood or saliva, for monkeypox virus testing. Testing samples not taken from a lesion may lead to false test results [37,42]. Depending on the clinic, urine, semen, rectal and/or genital swab samples may also be taken. A blood sample with EDTA may facilitate the diagnosis of MPXV, but the viremia stage is short, and the virus may not be as high as in the lesions. Macular stage biopsy should be taken by highly experienced personnel [19,20,[41], [42], [43], [44]]. Plasma/serum antibody test alone is not sufficient in the diagnosis of monkeypox. Detection of IgM in acute infection or IgG increase in two different samples taken 21 days apart helps the diagnosis. Vaccination affects serological test results [5,19,41].
Table 1.
Sample, collection material and storage temperature for MPXV diagnostic and differential purpose.
| Sample type | Collection materials | Storage temperature | Collection purpose |
|---|---|---|---|
Skin lesion material, including:
|
It should be taken with a dry swab or VTM (Dacron or polyester flocked swabs should be used) | After the samples are collected, they should be stored in the refrigerator (2–8 °C) until they are sent to the laboratory and should be sent in the cold chain. | It is recommended for diagnosis. |
| Oropharyngeal swab | It should be taken with a dry swab or VTM(Dacron or polyester flocked swabs should be used) | After the samples are collected, they should be stored in the refrigerator (2–8 °C) until they are sent to the laboratory and should be sent in the cold chain. | Recommended for diagnosis, if feasible, in addition to skin lesion material. |
| Serum | Serum separator tubes | After the samples are collected, they should be stored in the refrigerator (2–8 °C) until they are sent to the laboratory and should be sent in the cold chain. | It should be considered for serology for use in differential diagnosis and to aid research. |
6. Sample collection and storage
6.1. Transport of clinical specimens
Specimens should be stored refrigerated or frozen within an hour of collection and transported to the laboratory as soon as possible after collection. Correct handling and storage of specimens during transportation is essential for accurate diagnostic testing. Specimens collected for MPXV investigation should be refrigerated (2–8 °C) or frozen (−20 °C or lower) within 1 h after collection. If transport exceeds 7 days for the sample to be tested, specimens should be stored at −20 °C or lower. Longer term specimen storage (>60 days from collection) is recommended at 70 °C [41]. Samples must be transported in accordance with national and/or international regulations. For international transport, specimens from suspected probable or confirmed cases of MPXV, including clinical specimens, viral isolates and cultures, must be transported as Category A, UN2814 “infectious material affecting humans”. All remotely transported samples must have appropriate triple packaging, labeling and information documents. Longer term samples storage is recommended at −70 °C. Viral DNA from skin lesion material is relatively stable if kept in a dark, cool environment, which can be considered when cold chain is not readily. Repeated freeze-thaw cycles should be avoided [19,20,36,[41], [42], [43], [44], [45], [46]].
6.2. Laboratory testing methods and algorithm
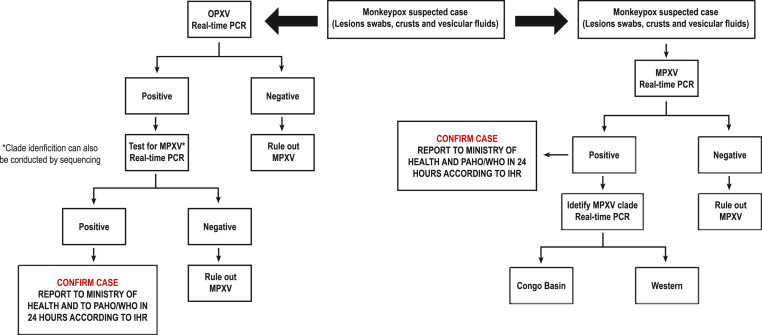
MPXV diagnostic tests should be performed in a suitably equipped laboratory by technicians trained in technical and safety procedures. Real-time or conventional Polymerase Chain Reaction (PCR) systems should be used to detect the MPXV agent and/or detect specific viral DNA sequences. Post-PCR sequencing can also be done. Several groups have developed validated PCR protocols for the detection of orthopoxvirus (OPXV), and more specifically MPXV, some of which include the separation of Congo Basin and West African clades (Fig. 1 ) [8,19,32,41,[44], [45], [46],46,47].
Fig. 1.
Algorithms for OPXV initial PCR or for MPXV specific initial PCR.
* Explanations: A positivity with an OPXV PCR test requires confirmation of MPXV by another PCR and/or sequencing, or for suspected cases in endemic/non-endemic areas, a positivity with MPXV PCR provides confirmation of MPXV. Genetic sequence data (GSD) provide information on the origin and epidemic and characteristics of cases.
6.3. Interpretation of laboratory results
In the confirmation of MPXV infection, clinical and epidemiological information should be primarily evaluated. A positivity obtained with an OPXV PCR test also needs to be confirmed for MPXV by PCR and/or sequencing. Confirmation of MPXV infection should consider clinical and epidemiological information. Positive detection using an OPXV PCR assay followed by confirmation of MPXV via PCR and/or sequencing, or positive detection using MPXV PCR assay in suspected cases indicates confirmation of MPXV infection. While it is preferable to perform MPXV specific confirmatory testing, positive detection using OPXV PCR assay is considered sufficient for laboratory confirmation of suspected cases. Member States are requested to immediately notify WHO of laboratory confirmed cases [2,32,33,48]. Laboratory-confirmed cases should be reported to WHO. In the case when both the epidemiology and clinical signs suggest that a patient has been infected with MPXV despite negative PCR results, serological testing may be useful for epidemiological purposes to further investigate previous infection. PCR false negatives can be caused by a number of factors such as sample quality, incorrect test setup, and technical reasons or failures in sample transport (e.g., DNA extraction failure).
Sequencing is used to clarify the agent in diagnosis, as well as to obtain information about the origin of the agent. Genetic sequence data (GSD) also helps to understand the origin, epidemiology and characteristics of the virus, whether cases originates from a single origin or from multiple different entries. At this stage, it is recommended to sequence MPXV from as many positive samples as possible from different patients and sites. WHO urges countries and laboratories to share GSDs, including raw data whenever possible - usually through existing publicly accessible databases. GSD can be generated using Sanger or Next-Generation Sequencing (NGS) methods [36]. Some protocols are two-step, while detecting OPXV in the first PCR reaction, a second step using a PCR sequence with type-specific primers is required to determine which strain it contains. Tests should be run by trained personnel with validated and/or verified test methods [36,41]. Reagents should be stored under appropriate conditions; there is a set of primers and probe sequences published in the literature for PCR tests for OPXV and especially MPXV, which can be used for in-house development of tests in laboratories with appropriate capacities (5–7). PCR kits to detect OPXV, or specifically MPXV, are under development, but commercial PCR or serology kits are not widely available at present. Positive control material for PCR tests can be ordered from private enterprises [23,41,49]. Best practice is that the positive control should be included at a low (above the detection limit) but easily detectable concentration. The quality control step should give us information on sample quality, nucleic acid quality and process quality. PCR can be extremely sensitive, so efforts should be made to either limit its contamination or ensure that contamination hasn't occurred. Sample integrity controls (e.g., RNase P), extraction, positive and inhibition controls can help distinguish false negative from true negative. Controls should be used after the laboratory checks the Standart Operating Procedures (SOPs). If any check fails, the test should be repeated [41,48,49].
Disposal of Waste. All waste that may contain MPXV must first be decontaminated and then destroyed by an approved method such as autoclaving or chemical disinfection may be carried out in accordance with specially approved laboratory procedures [36].
Electron microscope. It can be used to detect the presence of a potential smallpox virus in the sample, but despite the successes of molecular testing, this system is not routinely applicable given the high technical skill and facility requirements of this system.
Viral cell culture and isolation. Not recommended for routine, only applicable in laboratories with appropriate experience and containment/safety facilities.
6.4. An effective laboratory network
It is very important for the MPXV diagnosis that the samples taken from the suspected cases are delivered to any laboratory in a timely and correct manner; the valid tests are carried out and concluded properly; and, the records are regularly updated and shared. All countries, and in particular those that have already reported MPX cases, should have access to reliable testing, either nationally or by referral to laboratories in other countries that are willing and capable to diagnose OPXV or MPXV.
WHO can help Member States access tests by referral via their existing Regional Offices. When performed appropriately and safely, inactivation of samples at the local laboratory can facilitate shipping and ease logistical challenges. All countries with reported cases are encouraged to share ranking data for a better understanding of the current outbreak, to the following organizations: The US Centers for Disease Control and Prevention (CDC), the WHO Collaborating Center for Smallpox and Other Poxvirus Infections (United States) and the Federal Budgetary Research Agency - State Research Center for Virology and Biotechnology, “VECTOR” (Russian Federation), WHO. Collaborative Center for Orthopoxvirus Diagnosis and Repository for Variola Virus Strains and DNA [36,50].
7. Conclusion
The recent apparent increase in human monkeypox cases across a wide geographic and non-endemic area and the potential for further spread have raised the level of concern for this emerging disease. The current monkeypox outbreak was declared a Public Health Emergency of International Concern (PHEIC) by the World Health Organization (WHO) director-general on July 23, 2022. As of August 9, 2022, nearly 32 000 confirmed cases of monkeypox had been reported across 82 nonendemic countries [51]. It is now well known how the monkeypox virus (or MPXV) can spread. More research is required though to better understand if the MPXV can be spread when someone has no symptoms, the frequency of its spread through respiratory secretions, the likelihood of a symptomatic individual spreading the virus through respiratory secretions, and other ways of transmission (i.e. through semen, vaginal fluids, urine, or feces). The most common outcome following an infection is scarring from the rash – however, more serious complications can arise, according to some research of monkeypox in humans. The evidence suggest that more severe complications and sequelae are found to be more prevalent in particular among unvaccinated patients, which highlights the need to raise awareness and also the need to vaccinate all categories at risk including healthcare and sexual health workers.
In terms of sampling, the advice is that it is more effective to use swab samples taken directly from a lesion (rash or growth) when testing for the monkeypox virus. Testing samples not taken from a lesion may lead to false test results – something labs should try to minimise. Specimens collected for MPXV investigation should be refrigerated (2–8 °C) or frozen (−20 °C or lower) within 1 h after collection. Samples must be transported in accordance with national and/or international regulations. Confirmation of MPXV infection should consider clinical and epidemiological information. Positive detection using an OPXV PCR assay followed by confirmation of MPXV via PCR and/or sequencing, or positive detection using MPXV PCR assay in suspected cases indicates confirmation of MPXV infection. While it is preferable to perform MPXV specific confirmatory testing, positive detection using OPXV PCR assay is considered sufficient for laboratory confirmation of suspected cases.
Member States are requested to immediately notify WHO of laboratory confirmed cases. New confirmed cases should be reported to WHO, on a weekly basis and through channels established under the provision of the IHR, probable and confirmed cases of monkeypox, including using the minimum data set contained in the WHO Case Report Form (CRF). In terms of having a more global and effective laboratory network for this emerging zoonosis, there is a need to strengthen laboratory capacity, and international specimens referral capacities as needed, for the diagnosis of monkeypox virus infection, and related surveillance, based on the use of nucleic acid amplification testing (NAAT), such as real time or conventional polymerase chain reaction (PCR).
References
- 1.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peter O.J., Kumar S., Kumari N., Oguntolu F.A., Oshinubi K., Musa R. Transmission dynamics of Monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2022;8:3423–3434. doi: 10.1007/s40808-021-01313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.León-Figueroa D.A., Bonilla-Aldana D.K., Pachar M., Romaní L., Saldaña-Cumpa H.M., Anchay-Zuloeta C., et al. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Trav Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mileto D., Riva A., Cutrera M., Moschese D., Mancon A., Meroni L., et al. New challenges in human monkeypox outside Africa: a review and case report from Italy. Trav Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Neglected Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnus P von, Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 8.Petersen E., Kantele A., Koopmans M., Asogun D., Yinka-Ogunleye A., Ihekweazu C., et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin. 2019;33:1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Neglected Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds M.G., Doty J.B., McCollum A.M., Olson V.A., Nakazawa Y. Monkeypox re-emergence in Africa: a call to expand the concept and practice of One Health. Expert Rev Anti Infect Ther. 2019;17:129–139. doi: 10.1080/14787210.2019.1567330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaughan A., Aarons E., Astbury J., Brooks T., Chand M., Flegg P., et al. Human-to-Human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zambrano P.G., Acosta-España J.D., Mosquera Moyano F., Altamirano Jara J.B. Sexually or intimately transmitted infections: a look at the current outbreak of monkeypox in 2022. Trav Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S., et al. Emergence of monkeypox — west and central Africa, 1970–2017. MMWR Morb Mortal Wkly Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puca E., Shapo L. For how long can monkeypox reach the Balkan region? Trav Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farahat R.A., Ali I., Al-Ahdal T., Benmelouka A.Y., Albakri K., El-Sakka A.A., et al. Monkeypox and human transmission: are we on the verge of another pandemic? Trav Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isidro J., Borges V., Pinto M., Sobral D., Santos J.D., Nunes A., et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28:1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuzzo J.B., Borio L.L., Gostin L.O. The WHO declaration of monkeypox as a global public health emergency. JAMA. 2022;328:615–617. doi: 10.1001/jama.2022.12513. [DOI] [PubMed] [Google Scholar]
- 18.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., et al. Diagnosis and assessment of monkeypox virus (MPXV) infection by quantitative PCR assay: differentiation of Congo Basin and West African MPXV strains. Jpn J Infect Dis. 2008;61:140–142. [PubMed] [Google Scholar]
- 21.Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022:27. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sklenovská N., Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 24.Grant R., Nguyen L.-B.L., Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98:638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelo K.M., Petersen B.W., Hamer D.H., Schwartz E., Brunette G. Monkeypox transmission among international travellers-serious monkey business? J Trav Med. 2019;26 doi: 10.1093/jtm/taz002. taz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdelaal A., Serhan H.A., Mahmoud M.A., Rodriguez-Morales A.J., Sah R. Ophthalmic manifestations of monkeypox virus. Eye. 2022 doi: 10.1038/s41433-022-02195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patrocinio-Jesus R., Peruzzu F. Monkeypox genital lesions. N Engl J Med. 2022;387:66. doi: 10.1056/NEJMicm2206893. [DOI] [PubMed] [Google Scholar]
- 28.Ogoina D., Izibewule J.H., Ogunleye A., Ederiane E., Anebonam U., Neni A., et al. The 2017 human monkeypox outbreak in Nigeria—report of outbreak experience and response in the Niger Delta University teaching Hospital, bayelsa state, Nigeria. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel A., Bilinska J., Tam J.C.H., Da Silva Fontoura D., Mason C.Y., Daunt A., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radonić A., Metzger S., Dabrowski P.W., Couacy-Hymann E., Schuenadel L., Kurth A., et al. Fatal monkeypox in wild-living sooty mangabey, côte d'Ivoire, 2012. Emerg Infect Dis. 2014;20:1009–1011. doi: 10.3201/eid2006.131329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown K., Leggat P.A. Human monkeypox: current state of knowledge and implications for the future. Trav Med Infect Dis. 2016;1:E8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes C.M., Liu L., Davidson W.B., Radford K.W., Wilkins K., Monroe B., et al. A tale of two viruses: coinfections of monkeypox and varicella zoster virus in the democratic Republic of Congo. Am J Trop Med Hyg. 2020;104:604–611. doi: 10.4269/ajtmh.20-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxena S.K., Ansari S., Maurya V.K., Kumar S., Jain A., Paweska J.T., et al. Re-emerging human monkeypox: a major public-health debacle. J Med Virol. 2022 doi: 10.1002/jmv.27902. [DOI] [PubMed] [Google Scholar]
- 35.Technical Report: Multi-National Monkeypox Outbreak, United States Monkeypox | Poxvirus | CDC 2022. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/technical-report.html
- 36.Surveillance, case investigation and contact tracing for Monkeypox: interim guidance n.d. https://www.who.int/publications-detail-redirect/WHO-MPX-Surveillance-2022.2
- 37.Lewis A., Josiowicz A., Hirmas Riade S.M., Tous M., Palacios G., Cisterna D.M. Introduction and differential diagnosis of monkeypox in Argentina, 2022. Emerg Infect Dis. 2022:28. doi: 10.3201/eid2810.221075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffiths-Acha J., Vela-Ganuza M., Sarró-Fuente C., López-Estebaranz J.L. Monkeypox: a new differential diagnosis when addressing genital ulcer disease. Br J Dermatol. 2022 doi: 10.1111/bjd.21834. [DOI] [PubMed] [Google Scholar]
- 39.Bothra A., Maheswari A., Singh M., Pawar M., Jodhani K. Cutaneous manifestations of viral outbreaks. Australas J Dermatol. 2021;62:27–36. doi: 10.1111/ajd.13421. [DOI] [PubMed] [Google Scholar]
- 40.Monkeypox: waiting for your vaccination. https://www.gov.uk/government/publications/monkeypox-vaccination-resources/monkeypox-waiting-for-your-vaccination GOVUK n.d.
- 41.Laboratory-Testing-Guidelines-for-Diagnosis-of-Monkeypox-Virus-Final.pdf n.d.
- 42.Dumont C., Irenge L.M., Magazani E.K., Garin D., Muyembe J.-J.T., Bentahir M., et al. Simple technique for in field samples collection in the cases of skin rash illness and subsequent PCR detection of orthopoxviruses and varicella zoster virus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrahão J.S., Lima L.S., Assis F.L., Alves P.A., Silva-Fernandes A.T., Cota M.M.G., et al. Nested-multiplex PCR detection of Orthopoxvirus and Parapoxvirus directly from exanthematic clinical samples. Virol J. 2009;6:140. doi: 10.1186/1743-422X-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davi S.D., Kissenkötter J., Faye M., Böhlken-Fascher S., Stahl-Hennig C., Faye O., et al. Recombinase polymerase amplification assay for rapid detection of Monkeypox virus. Diagn Microbiol Infect Dis. 2019;95:41–45. doi: 10.1016/j.diagmicrobio.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maksyutov R.A., Gavrilova E.V., Shchelkunov S.N. Species-specific differentiation of variola, monkeypox, and varicella-zoster viruses by multiplex real-time PCR assay. J Virol Methods. 2016;236:215–220. doi: 10.1016/j.jviromet.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shchelkunov S.N., Shcherbakov D.N., Maksyutov R.A., Gavrilova E.V. Species-specific identification of variola, monkeypox, cowpox, and vaccinia viruses by multiplex real-time PCR assay. J Virol Methods. 2011;175:163–169. doi: 10.1016/j.jviromet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder K., Nitsche A. Multicolour, multiplex real-time PCR assay for the detection of human-pathogenic poxviruses. Mol Cell Probes. 2010;24:110–113. doi: 10.1016/j.mcp.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Laboratory testing for the monkeypox virus: interim guidance n.d. https://www.who.int/publications-detail-redirect/WHO-MPX-laboratory-2022.1
- 49.Laboratory guidelines for the detection and diagnosis of monkeypox virus infection - PAHO/WHO | Pan American health organization. https://www.paho.org/en/documents/laboratory-guidelines-detection-and-diagnosis-monkeypox-virus-infection n.d.
- 50.Epidemiological update: Monkeypox multi-country outbreak European centre for disease prevention and control. 2022. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-monkeypox-multi-country-outbreak
- 51.Del Rio C., Malani P.N. COVID-19-New insights on a rapidly changing epidemic. JAMA. 2020;323:1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]