Abstract
Background
Monkeypox is the most prevalent Orthopoxvirus zoonosis infection since the eradication of smallpox. The current multi-country outbreak involves five WHO regions affecting mainly Europe. Accurate clinical and virological aspects of the disease outside endemic areas are needed.
Methods
We performed an observational study of cases diagnosed in Madrid (Spain) (May/June 2022). Confirmation from vesicular lesions swabs, Orthopoxvirus real-time PCR, sequencing, phylogenetic analysis, and direct detection by Electron microscopy was performed. In addition, a structured epidemiological questionnaire was completed systematically to gather sociodemographic, clinical, and behavioral data from all confirmed cases.
Findings
We extracted data from 48 patients, all cisgender men. The median age was 35 years (IQR 29 – 44), and 87.5% were MSM. The most prevalent symptoms were the presence of vesicular-umbilicated and pseudo-pustular skin lesions (93.8%), asthenia (66.6%), and fever (52.1%). In addition, the location of the lesions in the genital or perianal area was related to the role in sexual intercourse (p<0.001). Sequencing analysis indicated the virus circulating in Spain belongs to the western African clade. Like the other European cases in the outbreak, the Spanish isolates are a direct descendant of viruses previously detected in Nigeria, the UK, Singapore, and Israel in 2017–2018.
Conclusions
Monkeypox is an emerging infectious disease in Europe where community transmission is reported, mainly in MSM. The first symptom was skin lesions instead of classical fever and rash. The disease follows a self-limited course, and there have been no cases with a serious presentation or severe complications.
Keywords: Monkeypox, Communicable diseases, Emerging
Introduction
Since the cessation of universal smallpox vaccination after the eradication of smallpox in 1980, Monkeypox (MPX) is the most prevalent orthopoxvirus zoonosis infection in humans.1, 2, 3, 4 As its first identification as a human pathogen in the Democratic Republic of the Congo in 1970, the number of reported Human MPX cases are increasing.1 , 5, 6, 7 The consensus is that this is mostly due to a combination of factors including both increased exposure (deforestation, conflict, and displacement), as well as improved surveillance and diagnostic capacity. Previous evidence shows that MPX does not spread easily between people. Human-to-human transmission occurs through close contact with infectious material from skin lesions of an infected person, respiratory droplets in prolonged face-to-face contact, and fomites.1 There are two different clades of the virus circulating, which results in mortality that differ between regions, from 1% in Western Africa to 11% in Central Africa.2
On 7 May 2022, the United Kingdom (UK) reported an imported case of MPX in a person travelling from Nigeria. The diagnosis was confirmed by PCR on a vesicular swab on 6th May by the UK Health Security Agency Rare and Imported Pathogens Laboratory. On 12th May 2022, the UK reported two further cases of MPX in two family members who were not linked to the imported case. None of the individuals in this cluster had travelled or had contact with anyone with a travel history.8 Recently, community transmission of monkeypox has been reported in UK.9 Since then, more than 28 countries have reported, at least, 1258 confirmed cases without links to endemic areas.10
We describe the clinical presentation of the first monkeypox cases in Spain from a reference clinic for STIs/HIV in Madrid, providing clinical, virological, transmission and diagnostic data, so that this information can be helpful in clinical practice.
Methods
Study design and study population
We performed an observational study of confirmed cases of MPX diagnosed from 18th May until the first weeks of June 2022 at Sandoval Health Center, a reference STI clinic from Hospital Clinico San Carlos (HCSC), Madrid. The inclusion criteria was a laboratory-confirmed case of MPX, defined as positive result by PCR of MPXV from cutaneous lesions swab. The treating physician compiled a complete clinical history of each patient based on the information on sociodemographic, clinical, and behavioral characteristics.
Variables
A structured epidemiological questionnaire was completed systematically to gather sociodemographic, clinical, and behavioral data, which included gender (male, female, transgender), age, region of origin (Spain, Latin America, Europe, other), sexual orientation (MSM, men who have sex with women, bisexual men, women that have sex with men, bisexual women or other), sex workers, intravenous drug users, HIV status, use of preexposure prophylaxis against HIV (PrEP), vaccination against smallpox, symptom onset date; symptoms at the time of the visit: fever, rash, cutaneous lesions type and location, lymphadenopaties (retroauricular, submandibular, cervical, axillar, inguinal), headache, odynophagia, asthenia, myalgia, urethritis, proctitis, respiratory symptoms (nasal congestion, cough, dyspnea); unprotected sexual practices: oral sex, vaginal sex, insertive or receptive anal intercourse; number of sexual partners during twenty-one days before the symptom onset date, use of recreational drugs for sex (poppers, erectile dysfunction medications, mephedrone, GHB, methamphetamine, ketamine, cocaine, 2C-B, others), participation in sex parties in Madrid and other international regions and a history of international travel to endemic areas.
Laboratory procedures
Samples. Swabs of vesicular lesions in viral transport media were sent refrigerated to the National Center of Microbiology, Instituto de Salud Carlos III, Madrid. Nucleic acids were extracted using either QIAamp MinElute Virus SpinDNA or QIAamp Viral RNA Mini kits from Qiagen (QIAGEN). Inactivation of samples was conducted in a certified Class II Biological Safety Cabinet in a BSL2 laboratory under BSL3 work practices with appropriate PPE.
Nucleic acid detection. A generic Real-time PCR for the Orthopoxvirus genus was used as the screening method11 and a generic conventional validated nested PCR for confirmation.12 Confirmation criteria for the first cases was: (1) positive result in both assays; (2) a positive PCR test in one assay from 2 different extracts with any Ct value. The 450 bp product obtained from the positive cases was sequenced and analyzed phylogenetically. After local outbreak confirmation, only cases from regions with no positive cases were tested in duplicate and only cases with Ct<35 were considered confirmed. Cases with Ct>35 were reextracted and reassayed.
Illumina sequencing. Sequencing libraries were prepared with the tagmentation based Illumina DNA Prep kit and run in a NovaSeq 6000 SP flow cell using 2 × 151 paired-end sequencing.
Viral genetic identification: Sequences were aligned (Muscle into MEGA X software) and a phylogenetic tree was constructed by the Neighbor-Joining (NJ) method based on partial (450 nt) sequences using MEGA X software. Bootstrap confidence limits were based on 1000 replicates.
Genetic data and analysis. Sequencing samples were analysed for viral genomes reconstruction using the viralrecon pipeline version 2.4.1 (https://github.com/nf-core/viralrecon) (https://zenodo.org/record/6320980#.YpB1IZ9BxhE), written in Nextflow (https://www.nextflow.io/) in collaboration between the nf-core community (https://www.nature.com/articles/s41587-020-0439-x) and the Bioinformatics Unit of the Institute of Health Carlos III (https://github.com/BU-ISCIII). Fastq files containing raw reads were analysed for quality using FastQC v0.11.9 http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw reads were trimmed using fastp v.0.20.1 (1), where a sliding window quality filtering approach was performed, scanning the read with a 4-base wide sliding window, cutting 3′ and 5′ ends base when the average quality per base drops below a Qphred33 of 20. Reads shorter than 50 nucleotides and reads with more than 10% of read quality under Qphred 20 were removed. Additionally, poly-X sequences were removed from read ends. Raw reads were assigned by Kraken 2 and human reads were discarded.
Consensus genome sequences were obtained following two approaches, mapping against reference genome and de novo assembly. Viral reads were mapped against two reference genome MPXV-BY-IMB25241 (ON568298.1, 05/23/2022) and MPXV-UK_P2 (MT903344.1, 09/15/2020) with bowtie2 v.2.3.5.1 and de novo assemblies were performed using SPAdes assembler, both approaches included in Viralrecon pipeline. Potential differences among them were manually curated. Consensus genome sequences have been uploaded to ENA with identifiers ERS12168855 to ERS12168868.
Variant calling and snp matrix generation was performed using snippy v4.4.5 including sequence samples and representative Monkeypox genomes downloaded from NCBI, some of them belonging to the current 2022 outbreak described in this study. SNP matrix with both invariant and variant sites was used for phylogenetic analysis using Iqtree v. 2.1.4-beta using predicted model K3Pu+F + I and 1000 bootstraps replicates.
Electron microscopy. A sample from a vesicular lesion swab was fixed with 2,5% glutaraldehyde and negative staining was performed with 2% phenylpropanoic acid.
Statistical analysis
Qualitative variables are expressed as absolute and relative frequencies. Continuous variables are summarized as mean values and standard deviation (SD) or median and interquartile range (IQR) in case of non-normal distribution. The association between the location of the skin lesions and the role of the patient in anal intercourse was performed using the chi-squared test.
Ethics statement
Data were obtained from a structured epidemiological and clinical questionnaire completed systematically during clinical practice. All data derived from medical histories were fully anonymized prior to access. This study protocol was approved by the Ethics Committee of HCSC (approval number: 22/389-E) and all patients consented to their participation.
Role of the funding source
This study was partially fund by CIBERINFEC.
Results
We extracted data from 48 patients. All of them were cisgender men (n = 48). The median age of the patients was 35 years (IQR 29 – 44). The mean time from the onset of symptoms (see Fig. 1 ) to clinical assessment was 7 days (IQR 4 – 11). 87.5% of the patients self-identified as MSM (n = 42), 4.1% as bisexual men (n = 22), 4.1% as homosexual sex worker (n = 2), 2.1% as homo/bisexual men who were intravenous drug users (n = 1) and 2.1% indicated sex with cisgender women only (n = 1). A total of 25% of the patients (n = 12), were vaccinated against smallpox in their childhood. Regarding HIV serostatus, 39.5% of the patients (n = 19) were people living with HIV (PLWH) and only one of them declared low adherence to antiretroviral treatment. A reported 47.9% of the patients (n = 23) were on daily PrEP with TDF/FTC.
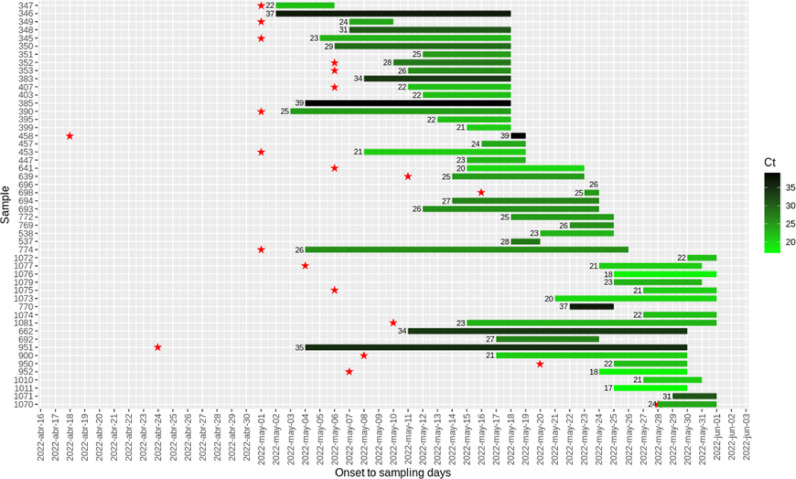
Fig 1.
Distribution of Monkeypox cases, by symptom onset during May/2022. Bars indicate the date of onset and the date of sampling. The Ct is also indicated in a green scale for each patient whose identification is also shown. Red stars indicate the participation in different chemsex events.
Clinical manifestations at the time of visit are collected in Table 1 . Some of the skin lesions are represented in Fig. 2 .
Table 1.
Clinical manifestations of monkeypox confirmed cases at the time of visit.
| Symptoms referred during anamnesis and observed after initial clinical assessment | N (48) | % |
|---|---|---|
| Vesicular-umbilicated skin lesions location | 45 | 93.8 |
| – Genitals | 26 | 54.2 |
| – Upper extremities | 20 | 41.7 |
| – Perianal | 17 | 35.4 |
| – Trunk | 16 | 33.3 |
| – Facial | 12 | 25 |
| – Periorally | 9 | 18.8 |
| – Lower extremities | 10 | 20.8 |
| – Palms and soles | 2 | 4.2 |
| Fever | 25 | 52.1 |
| Asthenia | 32 | 66.6 |
| Myalgia | 25 | 52.1 |
| Inguinal lymphadenopathies | 30 | 62.5 |
| – Painful -Not painful |
25/30 5/30 |
83.3 16.7 |
| Other location of lymphadenopathies | 9 | 18.8 |
| – Submandibular | 4/9 | 44.4 |
| – Cervical | 4/9 | 44.4 |
| – Retroauricular | 1/9 | 11.1 |
| Headache | 25 | 52.1 |
| Proctitis | 13 | 27.1 |
| Urethritis | 7 | 14.6 |
| Rash | 4 | 8.3 |
| Nasal congestion | 4 | 8.3 |
| Cough | 8 | 16.7 |
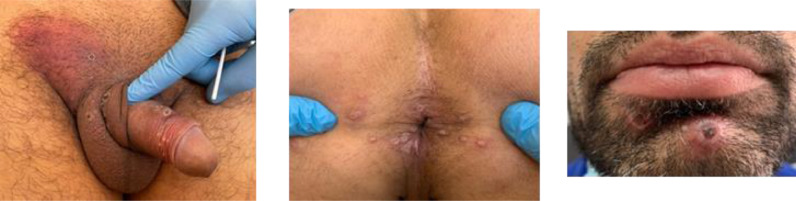
Fig. 2.
Pictures of inguinal lymphadenopathies and vesicular-umbilicated genital lesions (A); perianal (B) and perioral lesions (C).
The first symptom was identified in 39 patients, 53.8% of whom described skin lesions as the first symptom (21) followed by 17.9% who had fever first (7), respiratory symptoms (5) 12.8%, 5.1% headache (2), 5.1% rash (2), asthenia (1) 2.6% and proctitis 2.6% (1). A total of 14.5% of the patients knew other people living in the same household with similar symptoms (n = 7): Five of them were sexual partners, but one was a cohabitant not sex partner, and one was a friend. None of the patients had travelled or had contact with people from endemic areas and 83.3% of the patients had not travelled outside of Spain three weeks before the onset of the symptoms.
Regarding sexual intercourse, 89.5% of the patients had unprotected sex in the three weeks before the onset of the symptoms. The median number of different sexual partners for 21-days before the onset of the symptoms was 5 per person (IQR 4–16). The percentage of people practicing unprotected oral sex was 81.2% (n = 39), unprotected anal intercourse 85.4% (n = 41) and unprotected vaginal sex 4.1% (n = 2). Of those practicing anal intercourse, 43.9% had a versatile role (n = 18), 31.7% had only insertive anal sex (n = 13) and 24.3% only receptive anal sex (n = 10). A total of 7 out of 10 patients that had receptive anal sex had perianal lesions compared to 1 of 13 of the active patients (p< 0.001). Also, 12 of 13 of the active patients presented genital lesions compared to 1 of 10 of the passive patients (p<0.001).
Within the 21-days before the onset of symptoms, 50% of the patients had participated in a chemsex session (24), which is defined as the consumption of drugs during sex with multiple partners for several hours or days (Fig 1). Of those, the most common drug used was mephedrone (66.7%, n = 16), followed by GHB (58.3%, n = 13) and poppers (45.8%, n = 11).
A total of 12 cases (25%) were diagnosed with concomitant STIs: six cases of gonorrhea (with pharyngeal and/or urethral location), four cases of syphilis (primary state), a new diagnosis of HIV and one Mycoplasma genitalium (proctitis).
Only one patient needed hospitalization for 32 h, due to high fever and rash, but he recovered without sequelae. This patient was still PCR-positive 12 days after the appearance of the skin lesions. All the patients were isolated at home while they had skin lesions and none of them had medical complications.
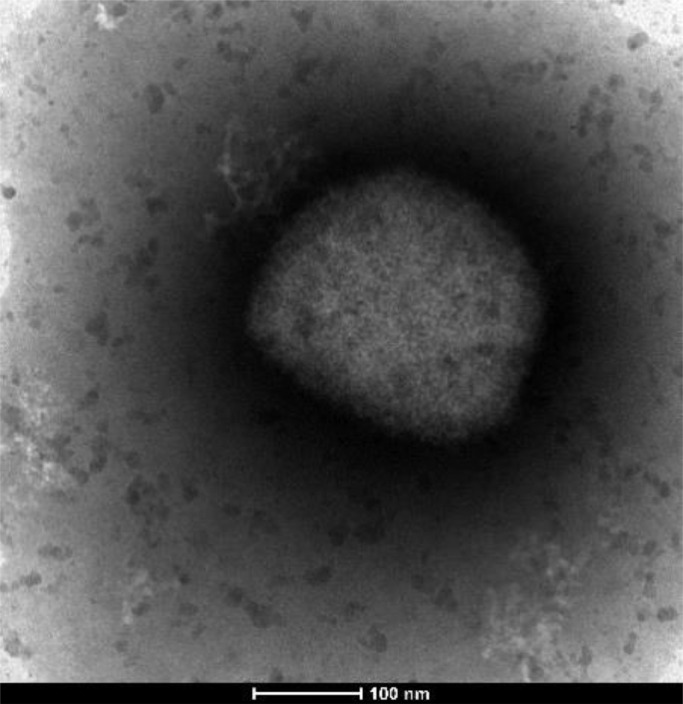
MPX infection was confirmed by complete or partial sequencing in the first 14 cases and an image from an orthopoxvirus was obtained by electron microscopy from one sample (Fig 3 ). The highest viral loads (Ct <25) were seen in samples obtained in the first nine days after onset (Fig 1). From 14 samples with a Ct less than 30, complete genome sequences were obtained obtaining more than 99% of reference genome coverage.
Fig 3.
Image of the virus obtained by negative staining from a clinical sample by electron microscopy.
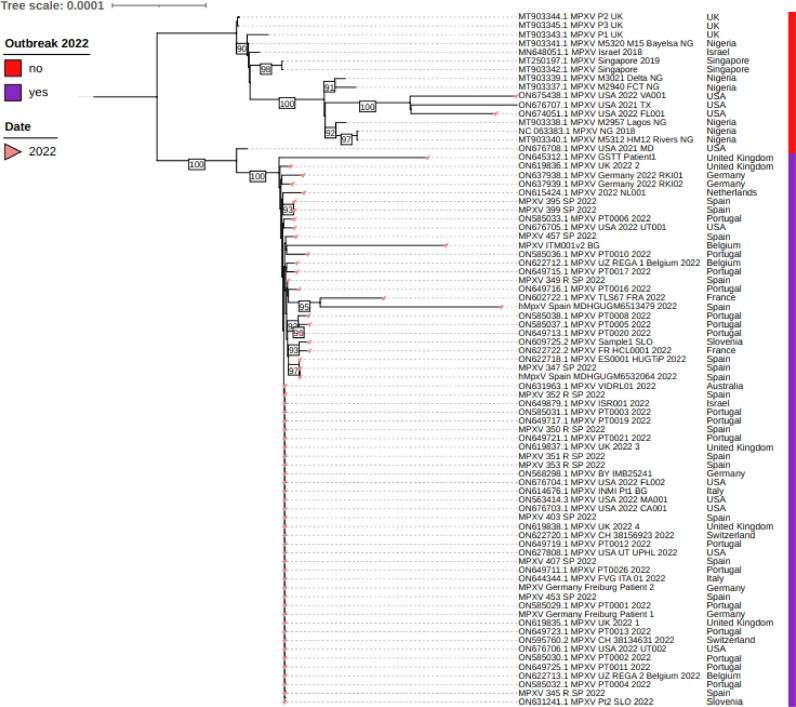
The phylogenetic tree indicates that the virus circulating in Spain belongs to the western Africa clade (Fig 4 ). The signature of changes observed clearly link the Spanish isolates to the rest of the strains circulating in Portugal13), UK,9 Italy14, 15 and Australia16 and to viruses circulating in USA in 2022.15
Fig. 4.
Phlylogenetic tree obtained with sequence samples and representative Monkeypox genomes downloaded from NCBI and those sequenced in this work. Accession numbers, country of origin and years are indicated. Sequences from the current outbreak are shown by a red bar. Phylogenetic analysis was performed by using Iqtree v. 2.1.4-beta using predicted model K3Pu+F+I and 1000 bootstraps replicates.
Discussion
These cases represent a change in the geographic distribution of the MPX-related disease. The presentation in Spain and in other European countries reinforces the notion that Monkeypox is a locally transmitted and a community-acquired emerging infectious disease in Europe. None of the patients had travelled to or had contact with people from endemic regions. The epidemiological data indicates local community transmission also outside of the MSM community and compatible with the classical presentation of monkeypox through close contact transmission.
One important observation is that most of the patients had, as the first symptom, skin lesions instead of fever and rash, which are usually described in African countries3 perhaps due to the immediate access to health care systems in developed countries. The typical skin lesions are small vesicles surrounded by an erythematous halo and nodular pseudo-pustules plaques, mainly in the genital, perianal and mucosal areas. Laboratory confirmation was done by using generic orthopoxvirus real-time PCRs. Although initially a confirmatory assay and sequencing was also performed, after confirmation of local transmission a positive result for orthopoxvirus amplification was considered as confirmation criteria. Sequence analysis obtained by analyzing the complete genome shows a close phylogenetic relationship with strains circulating in Europe that are direct descendant of strains that were first described in Nigeria, UK, Singapore, and Israel since 2017.17, 18, 19, 20, 21
Only one of the patients required hospitalization and none of them had encephalitis, pneumonia, or corneal ulcers, as it has been previously reported from endemic countries. These findings might be explained in part by the fact that the virus responsible for this global outbreak is similar to those previously circulating in west African countries, that has been reported with lower mortality and morbidity rates. However, the morbidity rates observed here appear to be even lower which could highlight the fact that improving basic and immediate health access in endemic countries might have an impact in morbidity.
Most cases diagnosed in our cohort is among MSM or bisexual men. There is a lack of understanding of the route of introduction of MPXV in MSM communities. The location of the lesions in most cases suggests that transmission occurred during sexual intercourse, and there is a statistical relation between the location of the lesions and the role of the patients regarding sexual practices. A recent publication has established the presence of MPXV in seminal fluid of four patients in Italy.14 This does not mean that this is a sexually transmitted infection per se, but it might be important in future studies to determine the viral culture of MPXV in human fluids to support the theory that MPX could be defined as an emerging STI.
We only used swabs from skin lesions since it is the best non-invasive sample for diagnostic. It shows very high viral loads in the first 10 days of infection. Unfortunately, it cannot be used for quantitative viral load comparison since sampling is not homogeneous.
Prior studies have shown that sexualized drug use is spread among MSM and bisexual men.22 , 23 Chemsex is also related with low condom use, multiple sexual partners and therefore, a higher incidence of STIs, including syphilis, chlamydia, and gonorrhea.24 These sex parties are also related to a higher prevalence of SARS-COV-2 virus, especially in PrEP users.25 These conditions might also favor the spread of MPXV.
All the patients were monitored until the submission of this article and none of them showed moderate or severe illness. Only one patient needed hospitalization due to high fever and rash that improved spontaneously in about four days. None of the health workers that attended him got ill. COVID-19 measures might have helped to avoid the spread of the disease to health personnel due to the extended use of masks and gloves in this pandemic era, compared to the human-to-human transmission in the UK in 2018.26
This study has some limitations: the small number of patients included and the lack of information about MPX in other locations like semen, urine, or blood, that might be relevant to determining the evolution of the disease and the potential transmissibility.
Diagnostic studies on some samples (seminal fluid, exhalated air) are needed to assure that there are no changes that favor human-to-human transmission virus and other routes of transmission like sexual or aerosol routes.
As a conclusion, this study presents the first cases of MPX diagnosed in Spain, most of them MSM who had unprotected sexual practices. Most of the skin lesions are in genital area, perianal region and perioral, together with other symptoms such as lymphadenopathies, asthenia and fever. There is a correlation between the location of the skin lesions and the role in anal intercourse that suggest that transmission occurred during sexual act. The presentation of fever appeared mostly after the skin lesions. Fortunately, our data show a low severity of the disease in terms of morbidity and mortality, although follow up studies with larger numbers of patients are needed to confirm this trend.
Funding
This study was partially funded by CIBERINFEC.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgments
We want to thank the support of the general director of the ISCIII, Cristobal Belda MD, for his collaboration and support in this project.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.07.005.
Contributor Information
On behalf of Grupo Viruela del Simio Madrid CNM/ISCIII/HCSC/Sandoval:
Juan Ballesteros, Begoña Baza, Juan Carlos Carrió, Cynthia Chocron, Giovanni Fedele, Cristina García-Amil, Laura Herrero, Reynaldo Homen, Alberto Mariano, Teresa Martínez-Burgoa, Francisca Molero, Maria Luisa Navarro, Maria José Núñez, Juncal Perez-Somarriba, Teresa Puerta, Javier Rodríguez-Añover, Esperanza Pérez Pastrana, Mercedes Jiménez, Leticia de la Vega, Jorge Vergas, and Isabel Zarza
Appendix. Supplementary materials
References
- 1.European Center For Disease Prevention and Control. Rapid Risk Assessment. Monkeypox multi-country outbreak. 23 May 2022. Available at: https://www.ecdc.europa.eu/en/publications-data/risk-assessment-monkeypox-multi-country-outbreak.
- 2.Durski K.N., McCollum A.M., Nakazawa Y., et al. Emergence of monkeypox—West and central Africa, 1970–2017. Morb Mortal Wkly Rep. 2018;67(10):306. doi: 10.15585/mmwr.mm6710a5. https://www.cdc.gov/mmwr/volumes/67/wr/mm6710a5.htm Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. https://academic.oup.com/cid/article-abstract/58/2/260/335791 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson K., Heymann D., Brown C.S., et al. Human monkeypox–after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladnyj I., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972;46(5):593. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2480792/ Available at: [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) WHO; Geneva: 2019. Monkeypox Fact Sheet.https://www.who.int/news-room/fact-sheets/detail/monkeypox Available at: [Google Scholar]
- 7.MacNeil A., Reynolds M.G., Braden Z., et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin Infect Dis. 2009;48(1):e6–e8. doi: 10.1093/cid/cit703. https://academic.oup.com/cid/article-abstract/48/1/e6/291721 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UK Health Security Agency (UKHSA) UKHSA; London: 2022. Monkeypox Cases Confirmed in England –Latest Updates.https://www.gov.uk/government/news/monkeypox-cases-confirmed-in-england-latest-updates Available at: [Google Scholar]
- 9.Roberto Vivancos, Charlotte Anderson, Paula Blomquist, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200422. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Multi-country Monkeypox outbreak: Situation Update. Wordl Health Organization, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON392.
- 11.Fedele C.G., Negredo A., Molero F., et al. Use of internally controlled real-time genome amplification for detection of variola virus and other orthopoxviruses infecting humans. J Clin Microbiol. 2006;44(12):4464–4470. doi: 10.1128/JCM.00276-06. DecEpub 2006 Oct 25. PMID: 17065259; PMCID: PMC1698395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez-Seco M.P., Hernández L., Eiros J.M., et al. Detection and identification of orthopoxviruses using a generic nested PCR followed by sequencing. Br J Biomed Sci. 2006;63(2):79–85. doi: 10.1080/09674845.2006.11732725. PMID: 16872000. [DOI] [PubMed] [Google Scholar]
- 13.Perez Duque M., Ribeiro S., Martins J.V., et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200424. JunPMID: 35656830; PMCID: PMC9164676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antinori A., Mazzotta V., Vita S., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200421. JunPMID: 35656836; PMCID: PMC9164671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costello V., Sowash M., Gaur A., et al. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022 May;28(5):1002–1005. doi: 10.3201/eid2805.220292. Epub 2022 Mar 9. PMID: 35263559; PMCID: PMC9045429. [DOI] [PMC free article] [PubMed]
- 16.Hammerschlag Y., MacLeod G., Papadakis G., et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200411. JunPMID: 35656835; PMCID: PMC9164678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faye O., Pratt C.B., Faye M., et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect Dis. 2018;18(3):246. doi: 10.1016/S1473-3099(18)30043-4. MarEpub 2018 Jan 18. Erratum in: Lancet Infect Dis. 2018 Mar;18(3):244. PMID: 29361427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan A., Aarons E., Astbury J., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38) doi: 10.2807/1560-7917.ES.2018.23.38.1800509. SepPMID: 30255836; PMCID: PMC6157091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yong S.E.F., Ng O.T., Ho Z.J.M., et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. AugEpub 2020 Apr 27. PMID: 32338590; PMCID: PMC7392406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng O.T., Lee V., Marimuthu K., et al. A case of imported monkeypox in Singapore. Lancet Infect Dis. 2019;19(11):1166. doi: 10.1016/S1473-3099(19)30537-7. NovPMID: 31657773; PMCID: PMC7129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen-Gihon I., Israeli O., Shifman O., et al. Identification and whole-genome sequencing of a monkeypox virus strain isolated in Israel. Microbiol Resour Announc. 2020;9(10) doi: 10.1128/MRA.01524-19. Mar 5e01524-19PMID: 32139560; PMCID: PMC7171222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell S., Shahmanesh M., Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74–89. doi: 10.1016/j.drugpo.2018.11.014. JanEpub 2018 Dec 1. PMID: 30513473. [DOI] [PubMed] [Google Scholar]
- 23.Curtis T.J., Rodger A.J., Burns F., et al. Patterns of sexualised recreational drug use and its association with risk behaviours and sexual health outcomes in men who have sex with men in London, UK: a comparison of cross-sectional studies conducted in 2013 and 2016. Sex Transm Infect. 2020;96(3):197–203. doi: 10.1136/sextrans-2019-054139. MayEpub 2019 Nov 19. PMID: 31744928; PMCID: PMC7167300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacGregor L., Kohli M., Looker K.J., et al. Chemsex and diagnoses of syphilis, gonorrhoea and chlamydia among men who have sex with men in the UK: a multivariable prediction model using causal inference methodology. Sex Transm Infect. 2021;97(4):282–289. doi: 10.1136/sextrans-2020-054629. JunEpub 2021 Jan 15PMID: 33452129. [DOI] [PubMed] [Google Scholar]
- 25.Ayerdi O., Puerta T., Clavo P., et al. Preventive efficacy of tenofovir/emtricitabine against severe acute respiratory syndrome coronavirus 2 among pre-exposure prophylaxis users. Open Forum Infect Dis. 2020;7(11):ofaa455. doi: 10.1093/ofid/ofaa455. Sep 25PMID: 33200081; PMCID: PMC7543639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughan A., Aarons E., Astbury J., et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26(4):782–785. doi: 10.3201/eid2604.191164. AprEpub 2020 Apr 17. PMID: 32023204; PMCID: PMC7101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.