Abstract
The 2022 monkeypox outbreak, caused by the zoonotic monkeypox virus, has spread across 6 World Health Organization regions (the Americas, Africa, Europe, Eastern Mediterranean, Western Pacific, and South-East Asia) and was declared a public health emergency of international concern on July 23, 2022. The global situation is especially concerning given the atypically high rate of person-to-person transmission, which suggests viral evolution to an established human pathogen. Pregnant women are at heightened risk of vertical transmission of the monkeypox virus because of immune vulnerability and natural depletion of population immunity to smallpox among reproductive-age women, and because orthopoxviral cell entry mechanisms can overcome the typically viral-resistant syncytiotrophoblast barrier within the placenta. Data on pregnancy outcomes following monkeypox infection are scarce but include reports of miscarriage, intrauterine demise, preterm birth, and congenital infection. This article forecasts the issues that maternity units might face and proposes guidelines to protect the health of pregnant women and fetuses exposed to the monkeypox virus. We review the pathophysiology and clinical features of monkeypox infection and discuss the obstetrical implications of the unusually high prevalence of anogenital lesions. We describe the use of real-time polymerase chain reaction tests from mucocutaneous and oropharyngeal sites to confirm infection, and share an algorithm for the antenatal management of pregnant women with monkeypox virus exposure. On the basis of the best available knowledge from prenatal orthopoxvirus infections, we discuss the sonographic features of congenital monkeypox and the role of invasive testing in establishing fetal infection. We suggest a protocol for cesarean delivery to avoid the horizontal transmission of the monkeypox virus at birth and address the controversy of mother–infant separation in the postpartum period. Obstetrical concerns related to antiviral therapy with tecovirimat and vaccinia immune globulin are highlighted, including the risks of heart rate–corrected QT-interval prolongation, inaccuracies in blood glucose monitoring, and the predisposition to iatrogenic venous thromboembolism. The possibility of monkeypox vaccine hesitancy during pregnancy is discussed, and strategies are offered to mitigate these risks. Finally, we conclude with a research proposal to address knowledge gaps related to the impact of monkeypox infection on maternal, fetal, and neonatal health.
Key words: ACAM2000, antiviral, chickenpox, cidofovir, COVID-19, cowpox, emerging pathogen, miscarriage, monkeypox, MVA-BN, obstetrical management, orthopoxvirus, outbreak, pregnancy, rash, sexual transmission, smallpox, tecovirimat, vaccine, vaccinia immune globulin, vaccinia virus, varicella-zoster, vertical transmission, World Health Organization, zoonosis
Introduction
The global outbreak of human monkeypox—caused by the double-stranded DNA monkeypox virus (MPXV)—was declared a public health emergency of international concern by the World Health Organization (WHO) on July 23, 2022. As of September 7, 2022, the outbreak has resulted in 56,026 laboratory-confirmed infections from 95 nonendemic countries.1 Epidemiologic observations from the ongoing outbreak suggest a high rate of person-to-person transmission of MPXV clade IIb (the formerly designated “West African” clade)2 , 3 through close physical contact, including during oral, anal, and vaginal intercourse.4
Although the outbreak has disproportionately affected gay and bisexual men, MPXV infection is neither confined by gender nor sexual orientation and will likely be reported in pregnancies with time and heightened disease surveillance. We believe pregnant women and their fetuses are especially vulnerable for 3 reasons.
First, the attenuation of cell-mediated immunity by T-helper 1 (Th1) cells because of the physiological shift to a Th2-dominant environment in pregnancy increases maternal susceptibility to viral infections.5 Th1 cytokines, including type 1 interferon (IFN), inhibit viral replication through direct antiviral and indirect immunoregulatory activities by binding to widely expressed heterodimeric receptors on cell surfaces.6 MPXV, however, expresses soluble IFNα/β-binding proteins, which interfere with IFN signaling pathways and broadly inhibit antiviral responses in the host.6 We therefore hypothesize that the combination of a gestational bias toward Th2 dominance and IFN evasion by MPXV-induced binding proteins could mediate both susceptibility and enhanced virulence from monkeypox infection in pregnancy.
Second, the eradication of smallpox (a closely related Orthopoxvirus) and cessation of the global smallpox vaccination program in 1980 created a niche for monkeypox because of waning population immunity: MPXV infections in Africa have increased at least 10-fold since 1970.7 The median age at diagnosis has also increased since vaccinations ended, from young children (4 years) in 1970 to young adults (21 years) from 2010 to 2019.7 This trend is reflected in the current 2022 outbreak, with men with a median age of 36 (interquartile range, 31–43 years) comprising the group with the highest number of infections.8 Taken together, women presently of reproductive age—defined as 15 to 49 years by WHO9—and who are thus unimmunized are at considerable risk of acquiring monkeypox because they lack cross-protective immunity.
Third, vertical transmission and pregnancy loss have been described following MPXV infection.10 , 11 Cross-border transmission of monkeypox within populations with no previous immunity and among immunocompromised individuals (at least 41% of cases in the current outbreak are HIV-positive8) may allow MPXV to acquire mutations that increase virulence and the chance of sustained spread. Monkeypox could thus evolve from a regionally limited zoonosis to a globally endemic infectious disease.8 , 12 Pregnancies, particularly in low- and middle-income countries, could then be at risk, aggravated by the reality that 89% of the estimated 213 million pregnancies yearly occur in resource-limited settings with the highest probability of poor obstetrical and perinatal outcomes.13
This article describes the virology and clinical characteristics of monkeypox infection and discusses the disease’s vertical transmission potential and management in pregnant women. Where gaps exist, we compare the similarities between monkeypox and other infections and draw on lessons learned from past epidemics. We believe this analysis is essential for developing the principles of obstetrical care for pregnant women at risk of MPXV exposure.
Pathogen
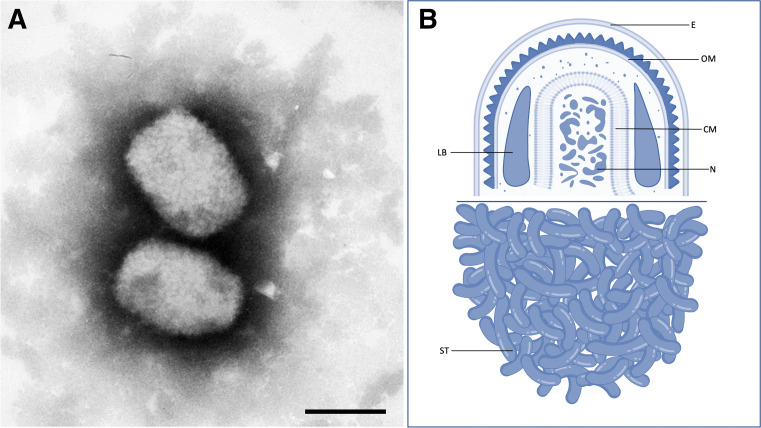
MPXV is a brick-shaped, enveloped, 200 to 250 nm–sized, double-stranded DNA, zoonotic virus (Figure 1 ) of the Orthopoxvirus genus in the Poxviridae family, which includes smallpox (variola), cowpox, and vaccinia viruses.14
Figure 1.
Monkeypox virus
A, Monkeypox virus on transmission electron microscopy, negative staining (bar=200 nm). B, cutaway line drawing of the monkeypox virus. Tecovirimat targets the VP37 protein and inhibits formation of the viral envelope (E). Cidofovir targets DNA polymerase within the viral nucleosome (N) but is teratogenic, unlike tecovirimat. Image credit: Panel A – Andrea Männel 2001/ RKI Robert Koch Institute; Panel B – Authors’ original illustration using Biorender.
CM, core membrane; E, envelope; LB, lateral body; N, nucleosome; OM, outer membrane; ST, surface tubules.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
The 2 viral clades of monkeypox3—MPXV clade I (corresponding to the previous “Congo Basin” clade) and MPXV clade II (corresponding to the previous “West African” clade, which is further divided into subclades IIa and IIb)—are clinically relevant. Clade I is associated with severe disease and a case fatality rate (CFR) approximately 3 times that of clade II (clade I CFR, 10.6%; 95% confidence interval [CI], 8.4–13.3 vs clade II CFR, 3.6%; 95% CI, 1.7–6.8).7 , 15 Clade IIb comprises the group of variants largely circulating in the 2022 global outbreak.3
Person-to-person transmission of MPXV classically occurs through large respiratory droplets, close contact with skin lesion exudates, and contaminated fomites. Pooled estimates suggest a secondary attack rate of approximately 8% (range, 0%–11%) among household contacts who are unvaccinated against smallpox.16 Sexual transmission might be possible given the detection of MPXV DNA in seminal fluid and the high rate of primary genital and anal mucosal lesions following condomless sexual activity in the 2022 outbreak.17 The caveat, however, is that isolating MPXV in seminal fluid is not necessarily evidence of infectivity because viremia is known to seed the reproductive tract.18 The basic reproduction number (R0) for monkeypox is estimated to be 0.8 but >1 among men who have sex with men.19 For context, SARS-CoV-2 has a strain-dependent R0 of 2.5 (original strain), 7 (Delta variant B.1.617.2), and 10 (Omicron variant), respectively,20 whereas smallpox had an R0 between 3.5 and 6.21
Because of their large DNA (∼197 kb), orthopoxviruses are better at detecting and repairing mutations than RNA viruses (eg, SARS-CoV-2). Consequently, this has previously resulted in only 1 to 2 substitutions per genome per year, which made MPXV a virus with presumably low epidemic potential.22 , 23 However, genomic sequencing studies have revealed that the 2022 MPXV strain contains 6 to 12 times the expected number of single-nucleotide polymorphisms, suggesting accelerated evolution and increased human adaptation.2 These might have contributed to cryptic human transmission of monkeypox for years before the global outbreak was amplified by super-spreading events in 2022.
Pathophysiology
The phases of MPXV viremia correlate with the signs and symptoms of monkeypox infection.24 , 25 Following exposure to MPXV from any route (eg, oropharynx, nasopharynx, intradermal, and possibly anogenital [as seen in the 2022 outbreak]), the virus replicates at the site of infection before spreading to locoregional lymph nodes. From there, MPXV enters the bloodstream, producing a primary viremia that seeds the hematopoietic system. The duration of primary viremia corresponds to the incubation period of monkeypox infection (7–14 days, with an upper limit of 21 days). Further replication of MPXV produces a secondary viremia, which results in a prodrome lasting approximately 2 days, characterized by fever, headache, myalgia, and tender lymphadenopathy. The latter may be cervical or inguinal (1–4 cm in diameter) and is typical of monkeypox infection. Approximately 1 to 3 days following the onset of fever, MPXV seeds the skin and mucous membranes with virions at various stages of assembly within the cytoplasm of keratinocytes. This causes an enanthem (oral cavity lesions) and a skin exanthem because of ballooning degeneration of basal keratinocytes and full-thickness necrosis of the epidermis.26
Clinical features of monkeypox in nonpregnant individuals
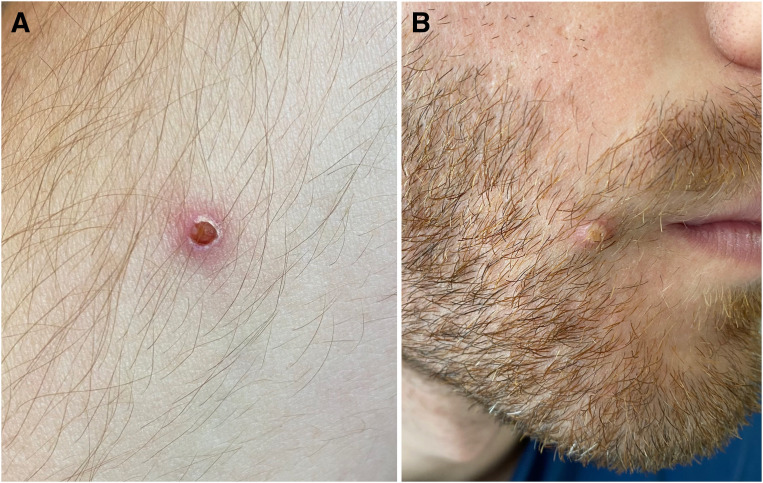
The rash, which is typically centrifugal over the face and extremities, progresses from macules, papules, vesicles, pustules, and finally to crusts (Figure 2 ), and is the most common symptom seen in >90% of patients in the present outbreak.8 Patients are infectious from the onset of fever until the vesicles have scabbed. Extracutaneous manifestations include pneumonia, ocular complications, encephalitis and secondary soft-tissue infections.
Figure 2.
Monkeypox rash
A, Characteristic vesicular and B, pustular lesions in a person with polymerase chain reaction–confirmed human monkeypox infection.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Atypical features of the ongoing outbreak, however, are a high rate of genital, perianal, and oral lesions and rash that does not evolve synchronously, including erythematous maculopapular rash of rapid onset separate from areas of vesicles or pustules (Appendix).27 Among 528 laboratory-confirmed MPXV infections between April and June 2022, 73% had anogenital lesions and 10% presented with only a solitary genital ulcer, which could be easily misdiagnosed as a sexually-transmitted infection and exacerbate community transmission of monkeypox until the correct diagnosis is established.17 It is unclear if MPXV within the anorectum and external genitalia is the consequence of mucosal seeding during viremia or the virus propagating at the site of initial exposure. In addition, lymphadenopathy, although characteristic of monkeypox, was only present in one-third of all cases reported to WHO as of July 22, 2022.8
Clinical features of monkeypox in pregnancy
Information about clinical characteristics, vertical transmission potential, maternal complications, and fetal outcomes of monkeypox infection in pregnancy is limited.
Among 4 pregnant women from the Democratic Republic of the Congo (DRC) with laboratory-confirmed MPXV between March 2007 and July 2011, 1 woman with mild disease delivered a neonate at term with no clinical features of monkeypox infection.10 However, 3 women with moderate-to-severe maternal infection had adverse obstetrical outcomes: 2 had spontaneous first-trimester miscarriages at 6 weeks’ gestation (with a maternal MPXV viral load of 3.5×103 and 7.9×105 gene copies/mL, respectively) and 1 had a second-trimester loss at 18 weeks’ gestation (viral load of 8.9×105 gene copies/mL). The stillborn fetus had a vesicular rash, hepatomegaly, and hydrops with a high viral load (>107 genome copies/mL) detected in fetal tissue, umbilical cord, and placenta, confirming vertical transmission of MPXV. Another woman with maternal infection at 24 weeks’ gestation had a preterm delivery at 30 weeks’ gestation; that neonate had a generalized rash at birth resembling monkeypox.11 Although not reported, we make the assumption that all 5 of these women were probably infected with MPXV clade I given that they were from the DRC. However, the risk factors associated with adverse pregnancy outcomes in monkeypox infection are presently unknown.
How did smallpox compare?
Monkeypox and smallpox (caused by the variola virus) are orthopoxviruses with striking similarities: the clinical features of both infections include an incubation period of approximately 14 days, a 2-day prodrome with fever, and a centrifugal vesiculopustular rash.28 At the molecular level, the central genomic region of MPXV is 96.3% identical to the variola virus, and the amino acid sequences of the virion proteins encoded in this region are up to 99.2% homologous.29 In addition, like MPXV, there are 2 distinct strains of smallpox with varying mortality risks: variola major with a CFR of 30% to 50% and variola minor with a CFR of <2%.30
The overall maternal CFR from smallpox infection in pregnancy was 34.3% (95% CI, 31.4–37.1), and the crude proportion of miscarriage and preterm birth was 39.9% (95% CI, 36.5–43.2).31 In the largest series of 389 pregnant women with smallpox, 75% miscarried before 24 weeks’ gestation, 55% delivered preterm, and 10% suffered stillbirths at term.32 Congenital smallpox occurred in 9% of fetuses and resulted in a neonatal mortality rate of 100%. Maternal mortality from smallpox was the highest in the third trimester of pregnancy; expectant mothers were 2 to 4 times more likely than nonpregnant women to die from the infection, and vaccinated pregnant women were approximately 3 times less likely to succumb than those who were unvaccinated.32 Hemorrhagic smallpox—characterized by petechiae, ecchymoses, profound thrombocytopenia, and multiorgan failure—occurred 7 times more frequently in pregnant women than in men and nonpregnant women regardless of vaccination status and carried a CFR of 100%.33 It is likely that smallpox represented the extreme end of the disease-severity spectrum from orthopox infection during pregnancy.
Importantly, however, monkeypox and smallpox differ in the regions encoding virulence factors (eg, IFN resistance genes and interleukin-1β inhibitors) at the terminal ends of the viral genome, which might explain the variation in clinical presentation and disease severity between the 2 infections.6 In addition, no hemorrhagic form of monkeypox has been described in humans, although MPXV clade I has demonstrated the potential for pulmonary hemorrhage, epistaxis, and impaired coagulation parameters in animal studies.34
What about monkeypox and varicella-zoster coinfection?
The possibility of monkeypox and varicella-zoster virus (VZV) coinfection, seen in 10% to 13% of individuals in the DRC,35 , 36 is an epidemiologic observation worth highlighting because women from tropical and subtropical regions are more likely to be nonimmune to VZV. For example, only 80.9% of pregnant women in Tunisia37 have VZV IgG antibodies vs 96.1% and 98.8% of pregnant women in Spain and France, respectively.38 , 39 Coinfection carries important implications for similarly susceptible groups because both viruses carry a risk of vertical transmission. Given that coinfection also modifies the severity of the skin rash, delayed diagnoses and treatment could result in worse perinatal outcomes, particularly in resource-limited settings.4 , 35 , 36
Possible mechanisms and risk of in-utero transmission of monkeypox
There are currently no data demonstrating the mechanisms by which MPXV traverses the maternal-placental-fetal barrier and infects fetal tissues. Multiple pathways are possibly involved in the ability of MPXV to invade placental trophoblast cells. This is especially because MPXV does not express cell-specific receptors facilitating cell tropism, unlike most other viruses that have evolved distinct cell-specific strategies for cell entry and replication within host cells.40 MPXV may reach the fetus via the hematogenous route, arriving at the intervillous space from maternal uterine spiral arteries, binding to trophoblast cells, and consecutively infecting syncytiotrophoblasts, cytotrophoblasts, fetal endothelial cells within the floating or anchored villi, and eventually fetal blood cells. MPXV may also ascend directly from genital lesions via cervical and uterine tissue, directly colonizing the chorionic membranes and decidua.41 In murine models of vaccinia virus infection during pregnancy via intravenous and intraperitoneal routes, harvested placenta initially amplified vaccinia-specific viral messenger RNA (mRNA) only in cells adjacent to maternal blood vessels but not on the fetal side, and only amplified viral mRNA in fetal vessels a few days later, demonstrating the time required for contiguous spread from mother to fetus.42 Cytopathic effects in human syncytiotrophoblasts observed in placenta infected with vaccinia virus include cytoplasmic condensation and cell rounding.43 , 44
Fetal and placental damage following vertical transmission can be additionally inferred from a report of early pregnancy fetal loss in a woman infected with cowpox virus (CPXV), an orthopoxvirus sharing a close genetic relationship with MPXV, and similarly capable of causing zoonotic infections in humans.45 Pregnancy loss in a dairy worker with cowpox occurred at 11 weeks’ gestation as a consequence of viremia46; DNA extracted from maternal blood, pustular areas, and from fetal and placental tissues confirmed CPXV infection by amplification of the A27L (for orthopoxvirus) and D8L/D11L genes (specific to CPXV), and viral cytopathic effects were observed on electron microscopy.47 In vertical transmission of smallpox, stillborn fetuses showed dermal pox signs, and viral particles were isolated from fetal skin and other organs. Placental pathology demonstrated necrotic villi, fibrin deposition, cytopathic effects (inflammatory infiltrates, necrosis), and virions at various stages of assembly on electron microscopy.26 , 41
Taken together, we speculate that MPXV might breach the maternal-placental-fetal barrier via viral fusion with trophoblasts, a process by which viral capsid proteins adhere to target cell-surface receptors initiating configurational changes in the viral capsid, enabling internalization of viral DNA through fusion with syncytiotrophoblast membrane or via transcytosis.48 Internalized viruses can then replicate and cause host cell damage directly (cytopathic effects) or secondarily to local inflammatory and immune reactions from the host. Once the placental barrier is breached, MPXV might be able to infect multiple placental cells, enabling it to reach the fetal bloodstream eventually. Fetal hydrops and hepatomegaly observed in MPXV-infected fetuses may reflect the extent of placental damage and resultant hypoxia from similar effects. It is also unknown whether maternal viral infection with MPXV (particularly in the third trimester) and maternal immune activation during pregnancy—as seen in HIV49 and more recently with SARS-CoV-250—might affect childhood neurodevelopmental milestones in fetuses exposed to monkeypox in-utero.
Approach to the management of monkeypox in pregnancy
Diagnosis
Taking all the above features of monkeypox into consideration, monkeypox infection should be suspected in any pregnant woman who presents with:
-
1.
Unexplained skin rash or genital ulcer (Box 1 contains differential diagnoses) OR
-
2.
One or more symptoms of fever, headache, myalgia, asthenia, or lymphadenopathy AND
-
3.Within the last 21 days:
-
a.A travel history to countries with recently reported cases of monkeypox; OR
-
b.A history of close contact with an infected person; OR
-
c.A history of casual sexual contact during travel.
-
a.
Box 1.
Differential diagnoses of monkeypox-type dermatoses in pregnancy
| Appearance of monkeypox lesion | Possible pregnancy-related causes |
|---|---|
| Maculopapular rash |
|
| Vesiculopustular rash |
|
| Anogenital ulcer |
|
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Because of the atypical features of monkeypox infection in the current outbreak, clinicians must maintain a high index of suspicion and conduct a thorough physical examination with personal protective equipment (PPE) (Table 1 ), including an assessment of oral, vaginal, and perianal regions. Furthermore, given that the monkeypox rash can coexist with sexually transmitted infections17 or be confused with other dermatoses, we suggest broadly excluding common causes of vesiculopustular rash in pregnancy with polymerase chain reaction (PCR) tests, including herpes simplex, varicella zoster, and syphilis.
Table 1.
Infection prevention and control recommendations for staff attending to a pregnant patient with suspected monkeypox infection
| Examples of clinical encounters in obstetrics | Recommended PPE and other IPC measures |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Housekeeping staff with high-risk exposure, eg:
|
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
|
HPV, hydrogen peroxide vaporization; IPC, infection prevention and control; PPE, personal protective equipment; UV-C, ultraviolet C.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Gowns should be fluid-impermeable or Association for the Advancement of Medical Instrumentation level 4-equivalent.
Real-time PCR (RT-PCR) from swabs of vesicle fluid or scabs from at least 2 sites placed in viral transport media is the gold standard for the diagnosis of monkeypox51 because viral DNA will be present within cutaneous lesions because of seeding from secondary viremia. False-negative results might occur because of poor specimen quality, improper handling, or DNA extraction failure. Patients reporting high-risk exposure and experiencing a febrile prodrome before the onset of skin rash can undergo a PCR throat swab. Monkeypox viral load in the upper respiratory tract peaks early in the infection, and thus oropharyngeal sampling in this context demonstrates high detection rates, second only to cutaneous lesions.52 , 53 In contrast, PCR of ethylenediaminetetraacetic acid (EDTA) blood samples may aid, but not replace, mucocutaneous sampling because the duration of monkeypox viremia is short (ie, corresponding with the prodrome, which lasts approximately 2 days), and plasma may thereafter not contain high levels of MPXV. In the current outbreak, a positive PCR result was most commonly obtained from skin or anogenital lesions.17
Antenatal care and fetal surveillance
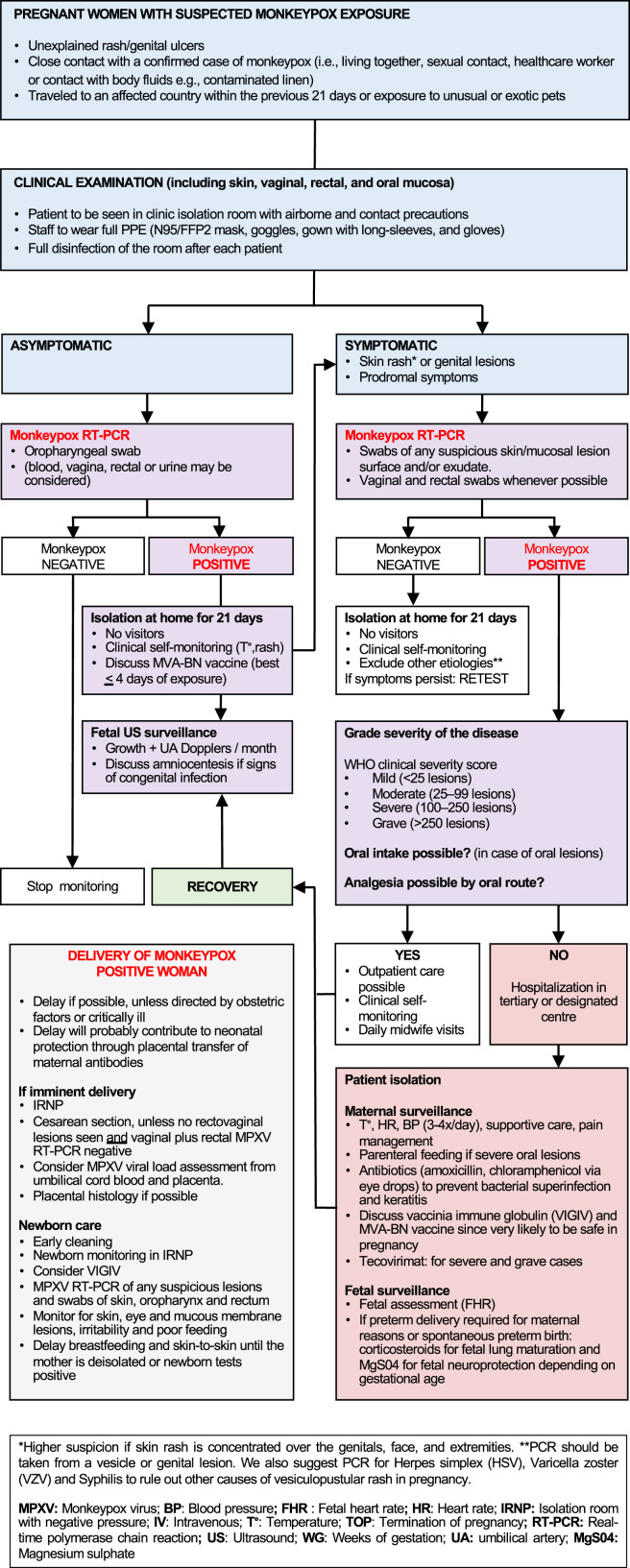
Given the possibility of vertical transmission of MPXV, serial ultrasound surveillance for signs of congenital infection is justified in symptomatic pregnant women with PCR-confirmed disease (Figure 3 ). In addition, we are of the opinion that paucisymptomatic and asymptomatic pregnant women with high-risk monkeypox exposure who test positive on oropharyngeal RT-PCR should also undergo ultrasound screening given the currently unquantifiable risk to the fetus. By extrapolating the known obstetrical outcomes of monkeypox infection, the sonographic features of fetal infection might include hepatomegaly, ascites, hydrops, placental calcifications, and fetal growth restriction.54
Figure 3.
Management of monkeypox during pregnancy
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
In the presence of these features, amniocentesis with RT-PCR could establish the diagnosis of fetal infection. However, the sensitivity of molecular detection of MPXV in amniotic fluid is presently unknown. By analogy with other TORCH (toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex) infections, MPXV is likely shed in the amniotic fluid once sufficient time has elapsed for the virus to breach the placental barrier (typically 6–8 weeks after infection), once the fetal kidneys produce sufficient urine (ie, after 16–18 weeks’ gestation), or fetal skin lesions have developed.55 It is theoretically possible that MPXV might only be transiently detected in-utero (similar to Zika virus in amniotic fluid, placenta, or fetal tissues56), despite a progressive risk of fetal anomalies throughout pregnancy; the kinetics of MPXV within the fetal compartment is an area that warrants further study.
Labor and delivery
Monkeypox infection in the third trimester or during the last 4 weeks of pregnancy should not indicate expediting delivery unless directed by obstetrical factors or clinical urgency in critically ill women. Characterization of acute-phase humoral immunity to monkeypox suggests seroconversion for both IgG and IgM approximately 4 days after the onset of rash in unvaccinated individuals.57 Thus, by additional analogy with varicella-zoster, deferring delivery for at least 7 days after the onset of monkeypox rash might permit the transplacental transfer of maternal IgG antibodies against MPXV.
Cesarean delivery with PPE would be the most reasonable delivery strategy in women with monkeypox infection (Table 2 ). Like neonatal varicella58 (50% risk of transmission, 20% CFR) and neonatal herpes simplex59 (85% risk of transmission, 60% CFR), exposure to anogenital MPXV during vaginal delivery may carry a high risk of fulminant neonatal sepsis, including encephalitis, sight-threatening keratitis, and necrotizing skin infections.28
Table 2.
Delivery protocol for a pregnant patient with monkeypox
| Mode of delivery |
|
| Site of delivery |
|
| Anesthesia and surgical considerations |
|
| Postpartum care |
|
IPC, infection prevention and control; LMWH, low molecular weight heparin; MPXV, monkeypox virus; PCR, polymerase chain reaction; PPE, personal protective equipment; WHO, World Health Organization.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Maternal anesthetic concerns include complications from neuraxial anesthesia (given the risk of transmitting cutaneous MPXV from the trunk into the central nervous system) and intubation (if oropharyngeal lesions are present). In women with widespread rash, extended-spectrum antibiotic cover with cefazolin and azithromycin before skin incision is likely to reduce the risk of postcesarean endometritis and surgical site infection (SSI) to a greater extent than cefazolin alone. Anaphylaxis is an underrecognized but potentially fatal complication following topical exposure to chlorhexidine via broken skin and mucosa.60 Therefore, in women with extensive mucocutaneous involvement in monkeypox, we opine that povidone–iodine for antiseptic skin and vaginal preparation is probably safer, although chlorhexidine–alcohol is more effective in lowering SSI risk after cesarean delivery.61
Management of the newborn depends on the likelihood of vertical transmission, and intravenous vaccinia immune globulin (VIGIV) could be considered in neonates with a high risk of perinatally acquired monkeypox (Table 3 ). Although it is unknown if MPXV is present in breast milk, the infection might be transmitted to the newborn through close contact during breastfeeding. It would therefore be prudent to delay breastfeeding until the mother’s rashes have scabbed over. If, however, the patient chooses to breastfeed, the nipple–areolar complex should be free of lesions, the neonate should be fully swaddled to reduce skin-to-skin contact, and the patient should wear a face mask to reduce droplet transmission because of the close proximity between mother and child. Given the currently unquantifiable and unknown long-term risks to the neonate, we also propose that pediatricians consider neurocognitive phenotyping of the infant to detect developmental disorders of motor function, speech, language, and other deficits related to possible maternal immune activation by MPXV in-utero.
Table 3.
Management of the neonate
| General management |
|
| Management of neonates delivered by cesarean delivery |
|
| |
| |
| |
| Management of neonates delivered vaginally (eg, birth before arrival or precipitate labor in mothers with active or suspected monkeypox infection) |
|
| |
| |
| |
|
MPXV, monkeypox virus; PCR, polymerase chain reaction.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Antiviral treatment and vaccines
Tecovirimat, which inhibits the orthopoxvirus VP37 envelope wrapping protein, is the first-line antiviral recommended by the US Centers for Disease Control and Prevention for the treatment of monkeypox in critically ill pregnant and breastfeeding women.62 Although tecovirimat is not authorized for use during pregnancy, animal studies have shown no embryotoxic or teratogenic effects. VIGIV is also likely to be safe given that immunoglobulins, as a class, have been used widely in pregnancy without adverse outcomes. Given that tecovirimat and VIGIV will feature prominently in the pharmacologic management of monkeypox, clinicians must be aware of the unique obstetrical issues when using these agents (Table 4 ).
Table 4.
Practical prescribing considerations for monkeypox therapy in pregnancy
| Therapy | Dose | Obstetrical precautions |
|---|---|---|
| Tecovirimat | 600-mg oral, twice daily for 2 wk |
|
| ||
| ||
| ||
| ||
| ||
| ||
| Vaccinia immune globulin intravenous (VIGIV) | 6000 units/kg IV infusion For patients >50 kg Time Infusion rate 0 min 0.5 ml/min 30 min 1.0 ml/min 45 min 1.5 ml/min 60 min 2.0 ml/min (max) For patients <50 kg Time Infusion rate 0 min 0.01 ml/kg/min 30 min 0.02 ml/kg/min 45 min 0.03 ml/kg/min 60 min 0.04 ml/kg/min (max) |
Beware the patient with gestational or preexisting diabetes mellitus |
| ||
| ||
| Beware the risk of venous thromboembolism (VTE) | ||
| ||
| ||
| ||
| ||
| Beware the patient with allergies | ||
| ||
| ||
|
BP, blood pressure; BSP, blood sugar profile; CTG, cardiotocogram; ECG, electrocardiogram; HR, heart rate; IV, intravenous; LMWH, low molecular weight heparin; PPROM, preterm prelabor rupture of membranes; QTc, heart rate–corrected QT-interval.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
For pre- and postexposure prophylaxis in pregnancy, WHO recommends the nonreplicating smallpox vaccine (MVA-BN), which confers 85% cross-protective immunity against monkeypox infection.63 To date, 300 pregnant women have received the MVA-BN vaccine without incident.64 In contrast, ACAM2000 is a live, replicating smallpox vaccine that is more heavily stockpiled but carries a risk of fetal vaccinia, which can result in preterm delivery, stillbirth, neonatal death, and adverse maternal reactions.65 Pregnant healthcare workers and others with substantial exposure (eg, pregnant household contacts) must therefore be prioritized for the MVA-BN vaccine when indicated.
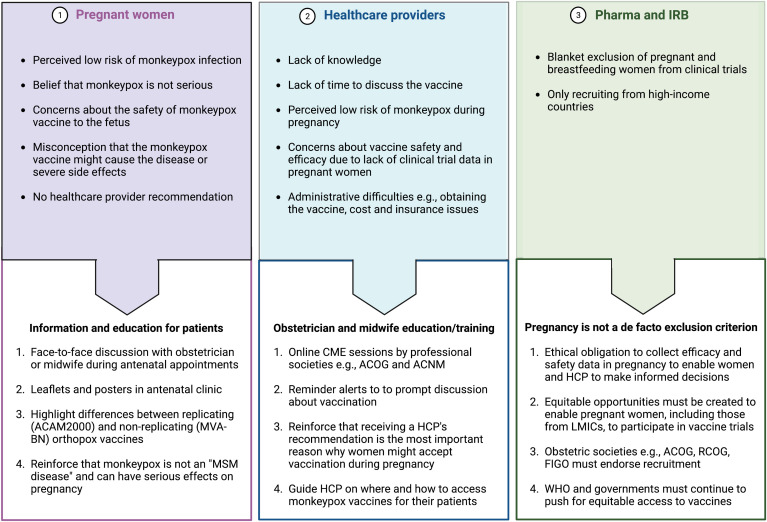
However, as with influenza, pertussis, and COVID-19 vaccination,66, 67, 68 we anticipate barriers to vaccine acceptance, and so we propose strategies aimed at the pregnant woman, healthcare provider, and institutional review board to improve the uptake of monkeypox vaccination during pregnancy (Figure 4 ). The Monitored Emergency Use of Unregistered and Experimental Interventions (MEURI) framework from the WHO69 and the PREVENT Working Group70 roadmap should be used by healthcare systems to guide the ethical use of expanded-access drugs and facilitate the deployment of vaccines in pregnancy.
Figure 4.
Possible barriers to monkeypox vaccination during pregnancy
ACNM, American College of Nurse-Midwives; ACOG, American College of Obstetricians and Gynecologists; FIGO, International Federation of Gynecology and Obstetrics; HCP, healthcare provider; IRB, institutional review board; LMIC, low- and middle-income countries; RCOG, Royal College of Obstetricians and Gynaecologists.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Conclusions and recommendations
For years, the scientific community has warned that monkeypox could emerge as the most crucial orthopoxvirus infection in humans.7 , 12 The disease will be a challenge for pregnant individuals, who represent a vulnerable population during any public health emergency of international concern. For now, much of the obstetrical management will be based on consensus and best-practice recommendations. We propose the following research priorities for clinicians and health systems (Box 2 ) to supplement WHO’s recommendations to guide the global effort to tackle monkeypox—now and in the future.71
Glossary of terms.
-
•
Centrifugal: concentrated on the face and extremities rather than over the trunk
-
•
Clade: group of organisms believed to comprise all the evolutionary descendants of a common ancestor
-
•
MPXV: monkeypox virus—the pathogen that causes human monkeypox infection, first identified in the Democratic Republic of the Congo in 1970 and largely confined to Central and Western Africa before 2022
-
•
MPXV clade 1: previously designated as the “Congo Basin” clade
-
•
MPXV clade 2: previously designated as the “West African” clade
-
•
Negative pressure room: room that maintains lower air pressure inside the treatment area than that of the surrounding environment, thus preventing internal air from circulating back out
-
•
Public health emergency of international concern: an extraordinary event that is determined to constitute a public health risk to other states through the international spread of disease and to potentially require a coordinated international response
-
•
R0: average number of people that a single infected person can be expected to transmit a disease to in a population where all individuals are susceptible to that infection
-
•
TORCH: Toxoplasmosis, Others (including parvovirus B19, syphilis, varicella-zoster virus, HIV, hepatitis B and C, Chikungunya, and Zika virus), rubella, cytomegalovirus (CMV), and herpes simplex virus
-
•
WHO: World Health Organization
-
•
Zoonosis: an infectious disease that has jumped from an animal to humans; zoonotic pathogens may be bacterial, viral, or parasitic, and can be transmitted to humans through direct contact or through food, water, or the environment
Box 2.
Knowledge gaps and research priorities for monkeypox in pregnancy
| Clinical features |
|---|
|
| Maternal–fetal and neonatal transmission |
|
| Therapeutics and vaccines |
|
MPXV, monkeypox virus; WHO, World Health Organization.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Footnotes
The authors report no conflict of interest.
A.R. is Director of the affiliated University of California, Los Angeles–Democratic Republic of the Congo (UCLA-DRC) Health Research and Training Program in the Democratic Republic of the Congo. This work did not receive any funding.
Supplementary Data
XXX
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Appendix
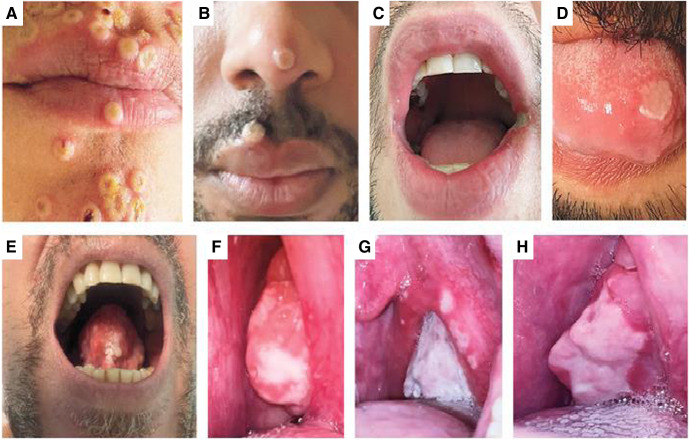
Supplemental Figure 1.
Oral and perioral lesions in monkeypox
A, Perioral umbilicated lesions. B, Perioral vesicular lesion on day 8, positive PCR test result. C, Ulcer on the left corner of the mouth on day 7, positive PCR test result. D, Tongue ulcer. E, Tongue lesion on day 5, positive PCR test result. F, G, and H, Pharyngeal lesions on days 0, 3, and 21, respectively, positive PCR test result on days 0 and 3 and negative PCR test result on day 21. Reproduced, with permission, from Thornhill et al.1
PCR, polymerase chain reaction.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
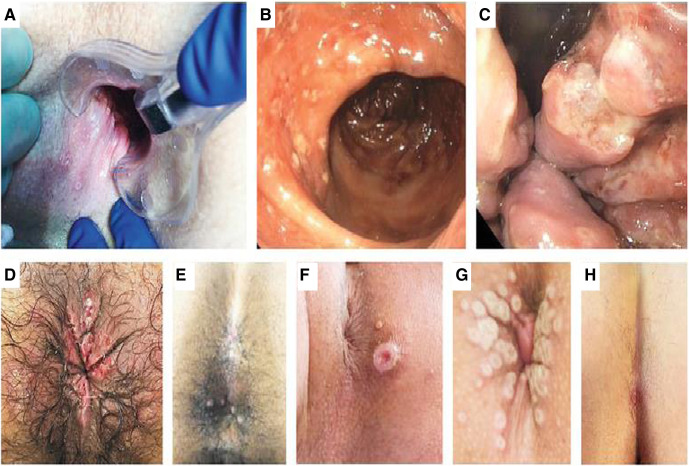
Supplemental Figure 2.
Perianal, anal, and rectal lesions in monkeypox
A, Anal and perianal lesions on day 6, positive PCR test result. B and C, Rectal and anal lesions in a single person, positive PCR test result. D, Perianal ulcers, positive PCR test result. E, Anal lesions. F, Umbilicated perianal lesion on day 3, positive PCR test result. G, Umbilicated perianal lesions on day 3, positive PCR test result. H, Perianal ulcer on day 2, positive PCR test result. In the context of pregnancy, these lesions might be confused with genital herpes, syphilis, or lymphogranuloma venereum. Reproduced, with permission, from Thornhill et al.1
PCR, polymerase chain reaction.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Supplemental Figure 3.
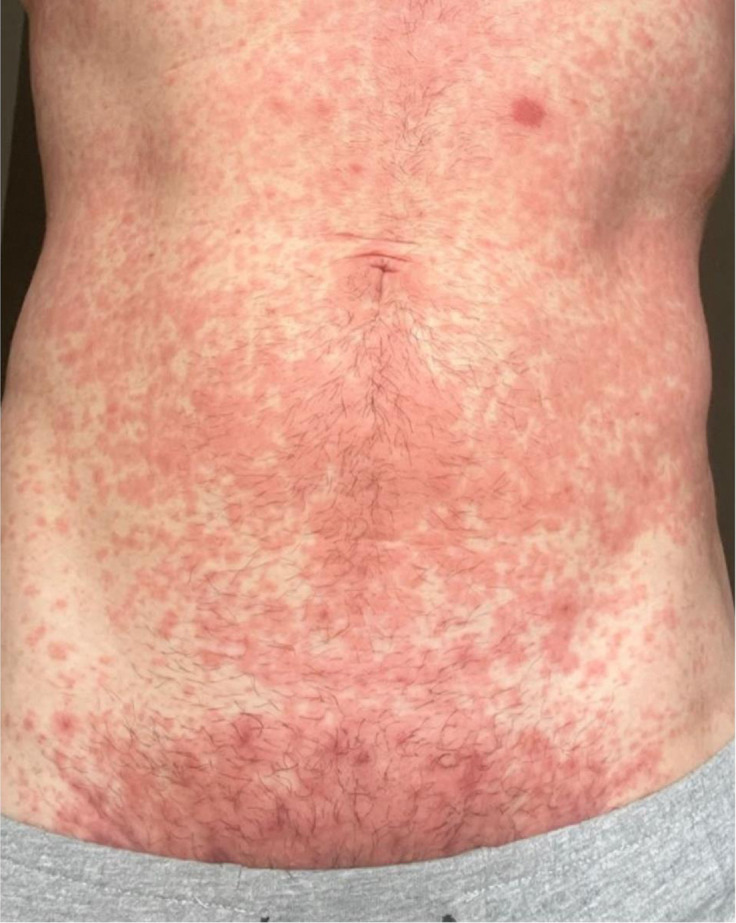
Maculopapular rash in monkeypox
In the context of pregnancy, a maculopapular rash such as this might be confused with pruritic urticarial papules and plaques of pregnancy. Reproduced, with permission, from Thornhill et al.1
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
Supplemental Table.
Current and novel therapies for monkeypox and their safety in pregnancy
| CDC and WHO options | Licensed indication | Effectiveness data against monkeypox in humans | Reproductive safety data in animals | Reproductive safety data in humans | Notes |
|---|---|---|---|---|---|
| Small molecules | |||||
| Tecovirimat | 1. Authorization by the EMA for the treatment of orthopoxvirus infections (cowpox, monkeypox, and smallpox) and to treat complications because of replication of vaccinia virus after vaccination against smallpox in adults and children with a body weight of at least 13 kg 2. Authorization by the FDA is limited to human smallpox disease in adults and pediatric patients weighing at least 13 kg. Therefore, the CDC provides a nonresearch EA-IND protocol that allows its use for treatment of nonvariola orthopoxvirus infections, including monkeypox, in adults and children of all ages |
None2; effectiveness relies on animal studies | Reproductive risk assessment based on available animal reproductive toxicity studies are inconclusive as the chosen dosage margins were questionable3 | None | May be safe in pregnancy Effectiveness data are required for a risk-benefit assessment |
| Cidofovir | 1. Authorization by the EMA and the FDA for the treatment of CMV retinitis in adult patients with AIDS without renal impairment, when other therapies are considered inappropriate Medical countermeasure in case of smallpox or monkeypox bioterrorism attack by the US government |
None2; effectiveness relies on animal studies | Studies have shown that cidofovir is clastogenic in vitro and is embryotoxic in rats and rabbits at doses below the one used in human therapeutics4 | None | Best avoided in pregnancy unless critically ill (teratogenic potential) Renal toxicity resulting in kidney damage is the major dose-limiting side effect of cidofovir |
| Brincidofovir | 1. In 2016, orphan designation (EU/3/16/1697) was granted by the European Commission for the treatment of adenovirus infection in immunocompromised patients 2. Authorization in 2021 by the FDA for the treatment of smallpox in adult and pediatric patients, including neonates |
None2; effectiveness relies on animal studies | Studies have shown that brincidofovir is clastogenic in vitro and is embryotoxic in rats and rabbits at doses below the one used in human therapeutics5 | None | Best avoided in pregnancy unless critically ill (teratogenic potential) Brincidofovir is a prodrug of cidofovir with a lipid conjugate that improves drug delivery to the target cells and greatly reduces nephrotoxicity |
| Intravenous immunoglobulin | |||||
| VIGIV | Authorization by the FDA for the treatment of complications from vaccinia virus vaccination | None | Carcinogenicity, genotoxicity, and fertility studies have not been conducted with VIGIV6 | None specific to VIGIV; evidence of relative safety of other intravenous immunoglobulin treatments therapies used in pregnancy (eg, anti-D immunoglobulin and varicella zoster immunoglobulin) | Very likely safe in pregnancy |
| Vaccines | |||||
| ACAM2000: second-generation smallpox vaccine | FDA approved live vaccinia virus vaccine to prevent smallpox May be used under nonresearch EA-IND protocol for the treatment of monkeypox |
Smallpox effectiveness based on 2 pivotal clinical trials that demonstrated noninferiority to Dryvax (a first-generation vaccine used to eradicate smallpox) As MPXV is closely related to the smallpox virus, the smallpox vaccine is considered cross-protective against monkeypox with an 85% effectiveness rate7 |
None found | As a live vaccinia virus, it can cause fetal vaccinia, a rare (ranges from 1/10,000 to 1/100,000) but serious complication of exposure during pregnancy that often results in fetal or neonatal death8,9 Data from a meta-analysis of 12,201 pregnant women showed that live smallpox vaccination was not associated with an increased risk of congenital defects (pooled RR, 1.25; 95% CI, 0.99–1.56) or fetal vaccinia in any trimester of pregnancy10 |
Best avoided in pregnancy Administered percutaneously using the multiple puncture technique and creating a lesion in case of successful inoculation (ie, called a “take”). Unvaccinated persons who have close contact with the inoculation site can be infected with the vaccinia virus More highly stockpiled by countries than the newer MVA-BN and LC16 vaccines11 |
| MVA-BN (also called Imvanex, Jynneos, or Imvamune): third-generation smallpox vaccine | The FDA (2019) and the EMA (2013) approved a replication-deficient vaccine (Ankara vaccine) for the prevention of smallpox The FDA approved (2019) for the prevention of monkeypox as well |
Effectiveness relies on comparative immunogenicity and protection studies in animal studies, but effectiveness rate is considered similar to ACAM2000 | Studies assessing fertility and embryofetal and postnatal toxicity did not reveal any particular risk to humans | 300 exposed pregnancies with follow-up without safety signal12 Replication-deficient virus technology carries a low risk of fetal vaccinia |
Likely safe in pregnancy Administered subcutaneously as 2 doses separated by 4 wk (1 dose at week 0 and a second dose at week 4) for primary vaccinees and 1 dose for individuals previously vaccinated against smallpox |
| LC16: third-generation smallpox vaccine | Japan (1975) approved live attenuated (minimally replicating) smallpox vaccine for the prevention of smallpox The FDA (2014) provided a nonresearch EA-IND protocol for smallpox13 |
Effectiveness relies on comparative immunogenicity and protection studies in animal studies14 | None found | None found | Theoretically less risk of developing fetal vaccinia than ACAM2000 Administered similar to ACAM2000 |
| Novel agents for repurposing or in the development stage | |||||
| Imatinib15 | The FDA and the EMA approved for the treatment of cancer | None; antiviral activity against orthopoxvirus in in vitro infection models | Studies have shown that imatinib is clastogenic in vitro and is teratogenic in rats and rabbits at the maximal doses used in human therapeutics | Case reports showing normal and abnormal outcomes16 | Best avoided in pregnancy (teratogenic) |
| Olomoucine15 | Preclinical research stage | None; antiviral activity against orthopoxvirus in in vitro infection models | None found | None | NA |
| Terameprocol15 | Clinical research phase 1 | None; antiviral activity against orthopoxvirus in in vitro infection models | None found | None | NA |
| Mitoxantrone15 | The FDA and the EMA approved for the treatment of cancer | None; antiviral activity against orthopoxvirus in in vitro infection models | Studies have shown that mitoxandrone is clastogenic and mutagenic in vitro and is fetotoxic in rats and rabbits at doses below the one used in human therapeutics17 | Considered a potential human teratogen because of its mechanism of action | Best avoided in pregnancy (teratogenic) |
| Bisbenzimide derivatives15 | Preclinical research stage | None; antiviral activity against orthopoxvirus in in vitro infection models | None found | None | NA |
| Resveratrol18 | Preclinical research stage | None; antiviral activity against orthopoxvirus in in vitro infection models | None found | None | NA |
CDC, Centers for Disease Control and Prevention; CI, confidence interval; CMV cytomegalovirus; EA-IND, Expanded Access for an Investigational New Drug; EMA European Medicines Agency; FDA, Food and Drug Administration; MPXV, monkeypox virus; NA, not available; RR, risk ratio; VIGIV, vaccinia immune globulin intravenous; WHO, World Health Organization.
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.
References
- 1.Centers for Disease Control and Prevention 2022 monkeypox and orthopox virus outbreak global map. 2022. Available at. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 2.Isidro J., Borges V., Pinto M., et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28:1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Monkeypox: experts give virus variants new names. 2022. Available at. https://www.who.int/news/item/12-08-2022-monkeypox-experts-give-virus-variants-new-names
- 4.World Health Organization Clinical management and infection prevention and control for monkeypox. Interim rapid response guidance. 2022. Available at. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
- 5.Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 6.Fernández de Marco Mdel M., Alejo A., Hudson P., Damon I.K., Alcami A. The highly virulent variola and monkeypox viruses express secreted inhibitors of type I interferon. FASEB J. 2010;24:1479–1488. doi: 10.1096/fj.09-144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunge E.M., Hoet B., Chen L., et al. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Multi-country outbreak of monkeypox, External situation Report #2. 2022. Available at. https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox-external-situation-report-2--25-july-2022
- 9.World Health Organization Maternal, newborn, child and adolescent health and ageing. 2022. https://www.who.int/data/maternal-newborn-child-adolescent-ageing/indicator-explorer-new/mca/women-of-reproductive-age-(15-49-years)-population-(thousands) Available at.
- 10.Mbala P.K., Huggins J.W., Riu-Rovira T., et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 11.Kisalu N.K., Mokili J.L. Toward understanding the outcomes of monkeypox infection in human pregnancy. J Infect Dis. 2017;216:795–797. doi: 10.1093/infdis/jix342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sklenovská N., Van Ranst M. Emergence of monkeypox as the most important Orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bardají A., Sevene E., Cutland C., et al. The need for a global COVID-19 maternal immunisation research plan. Lancet. 2021;397:e17–e18. doi: 10.1016/S0140-6736(21)00146-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker S., Nuara A., Buller R.M.L., Schultz D.A. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Otu A., Ebenso B., Walley J., Barceló J.M., Ochu C.L. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe. 2022;3:e554–e555. doi: 10.1016/S2666-5247(22)00153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med 2022 [Epub ahead of print]. [DOI] [PubMed]
- 18.Le Tortorec A., Matusali G., Mahé D., et al. DM. From ancient to emerging infections: the odyssey of viruses in the male genital tract. Physiol Rev. 2020;100:1349–1414. doi: 10.1152/physrev.00021.2019. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization Second meeting of the International Health Regulations (2005) (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox. 2022. https://www.who.int/news/item/23-07-2022-second-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-regarding-the-multi-country-outbreak-of-monkeypox Available at.
- 20.Burki T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. 2022;10 doi: 10.1016/S2213-2600(21)00559-2. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gani R., Leach S. Transmission potential of smallpox in contemporary populations. Nature. 2001;414:748–751. doi: 10.1038/414748a. [DOI] [PubMed] [Google Scholar]
- 22.Firth C., Kitchen A., Shapiro B., Suchard M.A., Holmes E.C., Rambaut A. Using time-structured data to estimate evolutionary rates of double-stranded DNA viruses. Mol Biol Evol. 2010;27:2038–2051. doi: 10.1093/molbev/msq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds M.G., Carroll D.S., Karem K.L. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr Opin Virol. 2012;2:335–343. doi: 10.1016/j.coviro.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff A.J., Chapman J., Foster C., et al. A novel respiratory model of infection with monkeypox virus in cynomolgus macaques. J Virol. 2011;85:4898–4909. doi: 10.1128/JVI.02525-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mucker E.M., Wollen-Roberts S.E., Kimmel A., Shamblin J., Sampey D., Hooper J.W. Intranasal monkeypox marmoset model: prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayer-Garner I.B. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28–34. doi: 10.1111/j.0303-6987.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel A., Bilinska J., Tam J.C.H., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shchelkunov S.N., Totmenin A.V., Babkin I.V., et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shchelkunov S.N., Totmenin A.V., Loparev V.N., et al. Alastrim smallpox variola minor virus genome DNA sequences. Virology. 2000;266:361–386. doi: 10.1006/viro.1999.0086. [DOI] [PubMed] [Google Scholar]
- 31.Nishiura H. Smallpox during pregnancy and maternal outcomes. Emerg Infect Dis. 2006;12:1119–1121. doi: 10.3201/eid1207.051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao A.R., Prahlad I., Swaminathan M., Lakshmi A. Pregnancy and smallpox. J Indian Med Assoc. 1963;40:353–363. [PubMed] [Google Scholar]
- 33.Suarez V.R., Hankins G.D.V. Smallpox and pregnancy: from eradicated disease to bioterrorist threat. Obstet Gynecol. 2002;100:87–93. doi: 10.1016/s0029-7844(02)02048-3. [DOI] [PubMed] [Google Scholar]
- 34.Sbrana E., Xiao S.Y., Newman P.C., Tesh R.B. Comparative pathology of North American and Central African strains of monkeypox virus in a ground squirrel model of the disease. Am J Trop Med Hyg. 2007;76:155–164. [PubMed] [Google Scholar]
- 35.Hughes C.M., Liu L., Davidson W.B., et al. A tale of two viruses: coinfections of monkeypox and varicella zoster virus in the Democratic Republic of Congo. Am J Trop Med Hyg. 2021;104:604–611. doi: 10.4269/ajtmh.20-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoff N.A., Morier D.S., Kisalu N.K., et al. Varicella coinfection in patients with active monkeypox in the Democratic Republic of the Congo. EcoHealth. 2017;14:564–574. doi: 10.1007/s10393-017-1266-5. [DOI] [PubMed] [Google Scholar]
- 37.Hussey H., Abdullahi L., Collins J., Muloiwa R., Hussey G., Kagina B. Varicella zoster virus-associated morbidity and mortality in Africa - a systematic review. BMC Infect Dis. 2017;17:717. doi: 10.1186/s12879-017-2815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plans P., Costa J., Espuñes J., Plasència A., Salleras L. Prevalence of varicella-zoster antibodies in pregnant women in Catalonia (Spain). Rationale for varicella vaccination of women of childbearing age. BJOG. 2007;114:1122–1127. doi: 10.1111/j.1471-0528.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 39.Saadatian-Elahi M., Mekki Y., Del Signore C., et al. Seroprevalence of varicella antibodies among pregnant women in Lyon-France. Eur J Epidemiol. 2007;22:405–409. doi: 10.1007/s10654-007-9136-z. [DOI] [PubMed] [Google Scholar]
- 40.Zaga-Clavellina V., Diaz L., Olmos-Ortiz A., Godínez-Rubí M., Rojas-Mayorquín A.E., Ortuño-Sahagún D. Central role of the placenta during viral infection: immuno-competences and miRNA defensive responses. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166182. doi: 10.1016/j.bbadis.2021.166182. [DOI] [PubMed] [Google Scholar]
- 41.Fuentes-Zacarías P., Murrieta-Coxca J.M., Gutiérrez-Samudio R.N., et al. Pregnancy and pandemics: interaction of viral surface proteins and placenta cells. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166218. doi: 10.1016/j.bbadis.2021.166218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benning N., Hassett D.E. Vaccinia virus infection during murine pregnancy: a new pathogenesis model for vaccinia fetalis. J Virol. 2004;78:3133–3139. doi: 10.1128/JVI.78.6.3133-3139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nørskov-Lauritsen N., Zachar V., Petersen P.M., Hager H., Aboagye-Mathiesen G., Ebbesen P. In vitro infection of human placental trophoblast by wild-type vaccinia virus and recombinant virus expressing HIV envelope glycoprotein. Res Virol. 1992;143:321–328. doi: 10.1016/s0923-2516(06)80120-2. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt F.I., Bleck C.K.E., Helenius A., Mercer J. Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture. EMBO J. 2011;30:3647–3661. doi: 10.1038/emboj.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourquain D., Dabrowski P.W., Nitsche A. Comparison of host cell gene expression in cowpox, monkeypox or vaccinia virus-infected cells reveals virus-specific regulation of immune response genes. Virol J. 2013;10:61. doi: 10.1186/1743-422X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrier A., Frenois-Veyrat G., Schvoerer E., et al. Fatal cowpox virus infection in human fetus, France, 2017. Emerg Infect Dis. 2021;27:2570–2577. doi: 10.3201/eid2710.204818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maksyutov R.A., Gavrilova E.V., Meyer H., Shchelkunov S.N. Real-time PCR assay for specific detection of cowpox virus. J Virol Methods. 2015;211:8–11. doi: 10.1016/j.jviromet.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 48.León-Juárez M., Martínez-Castillo M., González-García L.D., et al. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog Dis. 2017;75 doi: 10.1093/femspd/ftx093. ftx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wedderburn C.J., Weldon E., Bertran-Cobo C., et al. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6:393–408. doi: 10.1016/S2352-4642(22)00071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edlow A.G., Castro V.M., Shook L.L., Kaimal A.J., Perlis R.H. Neurodevelopmental outcomes at 1 year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization WHO Laboratory testing for the monkeypox virus: interim guidance. 2022. https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 Available at.
- 52.Adler H., Gould S., Hine P., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noe S., Zange S., Seilmaier M., et al. Clinical and virological features of first human monkeypox cases in Germany. Infection. 2022 doi: 10.1007/s15010-022-01874-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dashraath P., Nielsen-Saines K., Mattar C., Musso D., Tambyah P., Baud D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet. 2022;400:21–22. doi: 10.1016/S0140-6736(22)01063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khalil A., Sotiriadis A., Chaoui R., et al. ISUOG practice guidelines: role of ultrasound in congenital infection. Ultrasound Obstet Gynecol. 2020;56:128–151. doi: 10.1002/uog.21991. [DOI] [PubMed] [Google Scholar]
- 56.Schaub B., Vouga M., Najioullah F., et al. Analysis of blood from Zika virus-infected fetuses: a prospective case series. Lancet Infect Dis. 2017;17:520–527. doi: 10.1016/S1473-3099(17)30102-0. [DOI] [PubMed] [Google Scholar]
- 57.Karem K.L., Reynolds M., Braden Z., et al. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauerbrei A., Wutzler P. Neonatal varicella. J Perinatol. 2001;21:545–549. doi: 10.1038/sj.jp.7210599. [DOI] [PubMed] [Google Scholar]
- 59.Looker K.J., Magaret A.S., May M.T., et al. M. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health. 2017;5:e300–e309. doi: 10.1016/S2214-109X(16)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose M.A., Garcez T., Savic S., Garvey L.H. Chlorhexidine allergy in the perioperative setting: a narrative review. Br J Anaesth. 2019;123:e95–e103. doi: 10.1016/j.bja.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 61.Tuuli M.G., Liu J., Stout M.J., et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:647–655. doi: 10.1056/NEJMoa1511048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention Clinical considerations for monkeypox in people who are pregnant or breastfeeding. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/pregnancy.html Available at.
- 63.World Health Organization Vaccines and immunisation for monkeypox. Interim guidance. 2022 https://www.who.int/publications/i/item/WHO-MPX-Immunization-2022.2-eng Available at. [Google Scholar]
- 64.European Medicines Agency Summary of product characteristics - Imvanex. https://www.ema.europa.eu/en/documents/product-information/imvanex-epar-product-information_en.pdf Available at.
- 65.Kozlov M. Monkeypox vaccination begins - can the global outbreaks be contained? Nature. 2022;606:444–445. doi: 10.1038/d41586-022-01587-1. [DOI] [PubMed] [Google Scholar]
- 66.Qiu X., Bailey H., Thorne C. Barriers and facilitators associated with vaccine acceptance and uptake among pregnant women in high income countries: a mini-review. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.626717. 626717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dashraath P., Nielsen-Saines K., Madhi S.A., Baud D. COVID-19 vaccines and neglected pregnancy. Lancet. 2020;396 doi: 10.1016/S0140-6736(20)31822-5. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuckelberger S., Favre G., Ceulemans M., et al. SARS-CoV-2 vaccine willingness among pregnant and breastfeeding women during the first pandemic wave: a cross-sectional study in Switzerland. Viruses. 2021;13:1199. doi: 10.3390/v13071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Health Organization Emergency use of unproven clinical interventions outside clinical trials: ethical considerations. 2022. https://apps.who.int/iris/rest/bitstreams/1416840/retrieve Available at.
- 70.Krubiner C.B., Faden R.R., Karron R.A., et al. Pregnant women and vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine. 2021;39:85–120. doi: 10.1016/j.vaccine.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Health Organinzation Meeting on monkeypox research priorities. 2022. https://cdn.who.int/media/docs/default-source/blue-print/day-2_meeting-summary_monkeypox-meeting_03june2022.pdf?sfvrsn=f4ec1066_3 Available at.
Supplemental References
- 1.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Treatment Information for Healthcare Professionals. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#Tecovirimat Available at.
- 3.European Medicines Agency Assessment report – tecovirimat. https://www.ema.europa.eu/en/ documents/overview/ tecovirimat-siga-epar- medicine-overview_en.pdf Available at.
- 4.Gilead Sciences VISTIDE® (cidofovir injection) 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/1999/020638s003lbl.pdf Available at.
- 5.Brincidofovir summary of product characteristics. 2022. https://www.chimerix.com/wp-content/uploads/2021/06/TEMBEXA-USPI-and-PPI-04June2021.pdf Available at.
- 6.VIGIV prescribing information 2022. https://www.fda.gov/media/77004/download Available at.
- 7.Centers for Disease Control and Prevention Monkeypox and smallpox vaccine guidance. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html Available at.
- 8.Suarez V.R., Hankins G.D. Smallpox and pregnancy: from eradicated disease to bioterrorist threat. Obstet Gynecol. 2002;100:87–93. doi: 10.1016/s0029-7844(02)02048-3. [DOI] [PubMed] [Google Scholar]
- 9.Levine M.M. Live-virus vaccines in pregnancy. Risks and recommendations. Lancet. 1974;2:34–38. doi: 10.1016/s0140-6736(74)91363-4. [DOI] [PubMed] [Google Scholar]
- 10.Badell M.L., Meaney-Delman D., Tuuli M.G., et al. Risks associated with smallpox vaccination in pregnancy: a systematic review and meta-analysis. Obstet Gynecol. 2015;125:1439–1451. doi: 10.1097/AOG.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozlov M. Monkeypox vaccination begins - can the global outbreaks be contained? Nature. 2022;606:444–445. doi: 10.1038/d41586-022-01587-1. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency Summary of product characteristics – Imvanex. https://www.ema.europa.eu/en/documents/product-information/imvanex-epar-product-information_en.pdf Available at.
- 13.Otu A., Ebenso B., Walley J., Barceló J.M., Ochu C.L. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe. 2022;3:e554–e555. doi: 10.1016/S2666-5247(22)00153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenner J., Cameron F., Empig C., Jobes D.V., Gurwith M. LC16m8: an attenuated smallpox vaccine. Vaccine. 2006;24:7009–7022. doi: 10.1016/j.vaccine.2006.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugert J.J., Hucke F., Zanetta P., Bassetto M., Brancale A. Antivirals in medical biodefense. Virus Genes. 2020;56:150–167. doi: 10.1007/s11262-020-01737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolai T.K., Bhargava R., Mahapatra M., et al. Is imatinib safe during pregnancy? Leuk Res. 2009;33:572–573. doi: 10.1016/j.leukres.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency Summary of product characteristics – mitoxantrone. https://www.ema.europa.eu/en/documents/referral/novantrone-article-30-referral-annex-iii_en.pdf Available at.
- 18.Cao S., Realegeno S., Pant A., Satheshkumar P.S., Yang Z. Suppression of poxvirus replication by resveratrol. Front Microbiol. 2017;8:2196. doi: 10.3389/fmicb.2017.02196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
XXX
Dashraath. Monkeypox and pregnancy. Am J Obstet Gynecol 2022.