Introduction
Cases of West African clade monkeypox (WACM) have been increasing since May 2022.1 As of July 28, 2022, case counts were 4639 cases in the United States and 20,638 internationally.2 Human-to-human transmission is thought to be driving this outbreak, with many early cases reported in the men who have sex with men (MSM) community.2 Close contact with respiratory droplets, monkeypox lesion material, and diseased animals are currently known mechanisms of transmission.1
Atopic dermatitis (AD) compromises the skin barrier, occasionally leading to superimposed infection.3 Several known viral examples include eczema herpeticum (EH), eczema coxsackium, and eczema vaccinatum.3 In particular, EH is a well-described phenomenon in which herpes simplex virus may cause disseminated infection, encephalitis, hepatitis, and death even in immunocompetent patients.4 The causative virus in this case can be spread via direct contact or reactivation.5 Patients with EH tend to display earlier-onset AD, stronger type 2 immune responses, and decreased levels of the expression of antimicrobial peptides, which may contribute to increased susceptibility.4 The pathogenesis of EH can thus provide a framework with which to understand monkeypox transmission in patients with AD.
Here, we present the first described case of WACM associated with eczema. We urge practitioners to keep monkeypox in the differential for not only the MSM community, but also the general population and those with dermatoses that cause barrier compromise.
Case report
A 63-year-old man with AD of the hands since childhood presented to the outpatient dermatology department with painful pustular lesions on the bilateral aspects of the hands and elbows, lower portion of the right extremity, and back. He had been using clobetasol propionate ointment 0.05% and 999 ointment, from China (active ingredients: 1% menthol, 1% synthetic camphor, and 0.075% dexamethasone acetate), as needed for infrequent flares. The patient is a correctional officer at a hospital that recently treated 2 cases of monkeypox on a floor separate from that on which he works. He denied any history of direct contact with patients with monkeypox, travel, contact with animals, sexual encounters with men, sexual activity outside his marriage, or immunocompromising conditions. Two days prior to the onset of the rash, the patient had developed subjective fevers, chills, myalgias, headaches, and fatigue. The patient then noted vesicular and pustular eruptions initially on his fingers and palms, prompting him to seek care.
Examination showed swollen hands with overlying eczematous changes, including erythematous scaling, purulent and serous oozing, and crusted patches (Fig 1). Erythematous papules and pustules were seen on the hands, arms, back, and legs (Fig 2). The mucosa, face, and genitals were spared. There was no lymphadenopathy.
Fig 1.
A, Palmar surface of the bilateral hands with scattered pustules. B, Dorsal surface of the bilateral hands with pustules overlaid on erythema and eczematous scaling. C, Palmar surface of the hand with pustules on the third finger and lateral aspect of the thumb. D, Dorsal surface of the hand demonstrating open pustules, crusting, and scaling.
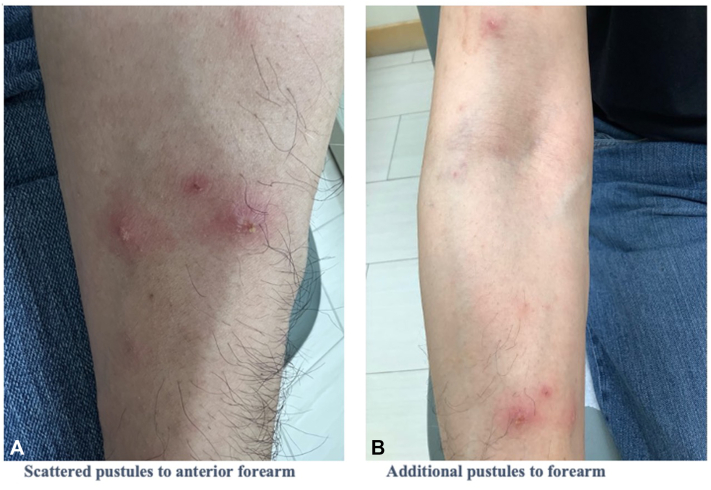
Fig 2.
A, Scattered pustules on the anterior aspect of the forearm. B, Additional pustules on the forearm.
A biopsy of the anterior proximal right arm was consistent with a viral process (Fig 3). Nonvariola orthopoxvirus DNA polymerase chain reaction of the left palm lesion was performed, which was reported reactive 6 days later. The patient was advised at the clinic to self-isolate until resolution of the lesions, which occurred 3 weeks later (Fig 4).
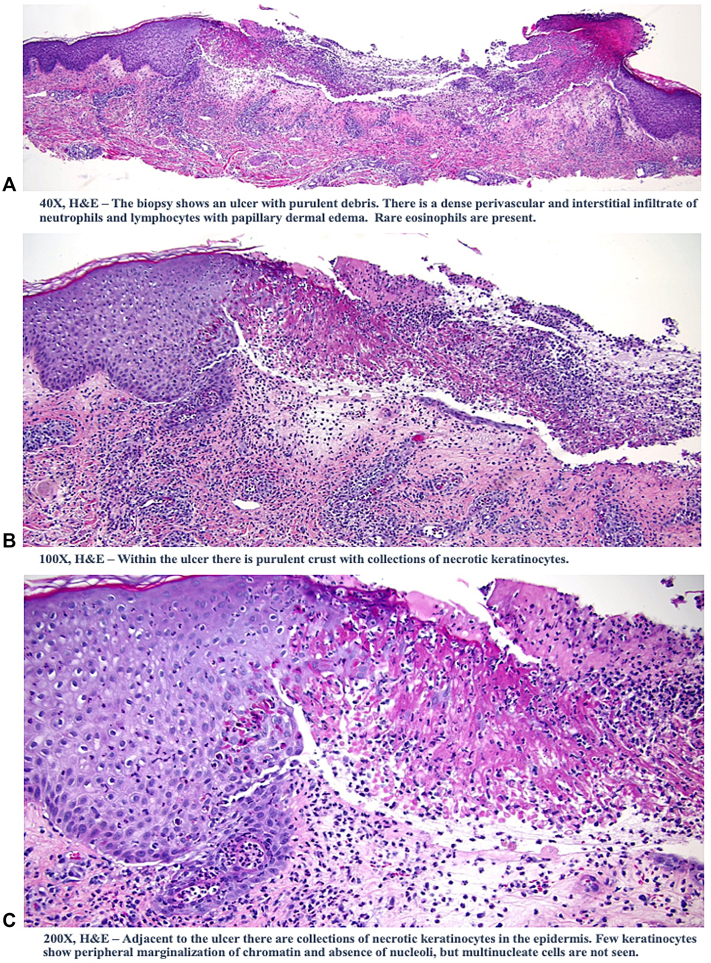
Fig 3.
A, The biopsy showed an ulcer with purulent debris. There was dense perivascular and interstitial infiltrate of neutrophils and lymphocytes with papillary dermal edema. Rare eosinophils were present. B, Within the ulcer, there was purulent crust with collections of necrotic keratinocytes. C, Adjacent to the ulcer, there were collections of necrotic keratinocytes in the epidermis. Few keratinocytes showed peripheral marginalization of the chromatin and the absence of nucleoli; however, multinucleate cells were not seen. (A-C, Hematoxylin-eosin stain; original magnifications: A, ×40; B, ×100; C, ×200.)
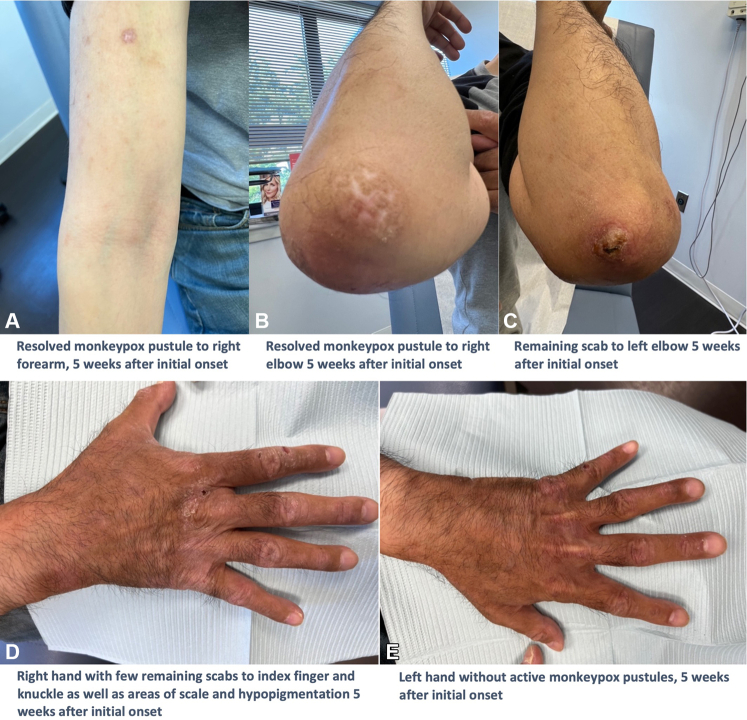
Fig 4.
A, Resolved monkeypox pustule on the right forearm 5 weeks after initial onset. B, Resolved monkeypox pustule on the right elbow 5 weeks after initial onset. C, Remaining scab on the left elbow 5 weeks after initial onset. D, Right hand with few remaining scabs on the index finger and knuckle as well as areas of scale and hypopigmentation 5 weeks after initial onset. E, Left hand without active monkeypox pustules 5 weeks after initial onset.
Discussion
The initial prevalence of WACM in the MSM population was most likely attributable to an early cluster of infections in this group, as well as ascertainment bias.6 As noted by the Centers for Disease Control and Prevention, any individual in close contact with monkeypox patients is at risk of transmission.2 Treatment is supportive, although antiviral agents, such as tecovirimat, may be recommended for children under the age of 8 years and immunocompromised individuals.2 Two Food and Drug Administration-licensed vaccines (JYNNEOS and ACAM2000) are available in the United States and can be administered as postexposure prophylaxis.2 JYNNEOS is a live-attenuated, replication-deficient vaccine, whereas ACAM2000 is a replication-competent, live vaccinia virus. Inadvertent inoculation may occur with ACAM2000 but not with JYNNEOS.2 Severe immunodeficiency, AD, and other barrier-disruptive conditions are contraindications for ACAM2000 due to the risk of progressive vaccinia and eczema vaccinatum.2 For exposed patients under these categories, JYNNEOS is the vaccine of choice, with similar rates of adverse reactions reported between these groups and controls.2 The pediatric safety and efficacy of either vaccine have not been studied. However, per current Centers for Disease Control and Prevention guidelines, postexposure prophylaxis should not be withheld in children or adolescents who are otherwise eligible.2 These individuals should be evaluated for vaccination based on their risk of exposure and severity of the disease. Consideration regarding the prevention of spread, including prevention of scratching and autoinoculation, should also be taken in children.2
Uniquely, our patient did not report any of the common mechanisms of exposure. His disease was likely transmitted occupationally via fomites, which his AD made him particularly susceptible to. Although monkeypox typically first manifests in the mucosa and face,7 initial symptoms developed on the hands of our patient. AD likely caused barrier impairment and obscured the initial morbiliform stages of the rash, leading him to present once it was already pustular. A review of monkeypox transmission prior to this current pandemic helped identify 1 case of healthcare-associated transmission related to changing bedding without the use of personal protective equipment8; unlike this case, our patient would not have been considered to be at a high risk of exposure. Another study in Germany quantified hospital surface contamination with monkeypox virus and did indeed note traces of viral DNA in hallways outside of monkeypox isolation rooms.9 As such, our case highlights the importance of appropriate personal protective equipment and disinfection in preventing transmission in health care settings. The current Centers for Disease Control and Prevention guidelines recommend gowns, gloves, eye protection, and National Institute for Occupational Safety and Health-approved particulate respirators equipped with N95 filters or higher for health care personnel.2
This case represents the first reported case of eczema monkeypoxicum in the literature, illustrating the relative susceptibility of patients with AD to transmission and the potential environmental stability of the virus. In the similar phenomenon of Kaposi's varicelliform eruption, superinfection with herpes simplex virus is seen in patients with psoriasis, rosacea, epidermolysis bullosa simplex, ichthyosis, and other conditions that cause a disrupted barrier, with sequelae that may be life threatening.4 The effect of disseminated monkeypox due to superinfection of AD on severity of the disease is unclear. Although WACM is typically self-limited, a case fatality of up to 3% has been reported in the overall population,10 generally impacting young children and the immunocompromised.7 With the relative prevalence of AD in children and the use of immunosuppressing medications used to treat severe AD, these groups may be at a greater risk of contracting monkeypox and adverse events than previously realized. Finally, we would like to address that the risk of monkeypox is not limited to any 1 demographic, and a characteristic rash should prompt thorough evaluation.
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Harris E. What to know about monkeypox. JAMA. 2022;327(23):2278–2279. doi: 10.1001/jama.2022.9499. [DOI] [PubMed] [Google Scholar]
- 2.Monkeypox signs and symptoms. Centers for Disease Control and Prevention. https://www.cdc.gov/poxvirus/monkeypox/index.html
- 3.Wollenberg A., Wetzel S., Burgdorf W.H., Haas J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112(4):667–674. doi: 10.1016/j.jaci.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Traidl S., Roesner L., Zeitvogel J., Werfel T. Eczema herpeticum in atopic dermatitis. Allergy. 2021;76(10):3017–3027. doi: 10.1111/all.14853. [DOI] [PubMed] [Google Scholar]
- 5.Kim E.L., Hohmuth B. Eczema herpeticum in early pregnancy. CMAJ. 2017;189(13) doi: 10.1503/cmaj.151544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minhaj F.S., Ogale Y.P., Whitehill F., et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71(23):764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryer JS, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatological primer. Preprint. Posted online July 8, 2022. J Am Acad Dermatol. 10.1016/j.jaad.2022.07.007 [DOI] [PMC free article] [PubMed]
- 8.Zachary K.C., Shenoy E.S. Monkeypox transmission following exposure in healthcare facilities in nonendemic settings: low risk but limited literature. Infect Control Hosp Epidemiol. 2022;43(7):920–924. doi: 10.1017/ice.2022.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nörz D., Pfefferle S., Brehm T.T., et al. Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022. Euro Surveill. 2022;27(26) doi: 10.2807/1560-7917.ES.2022.27.26.2200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monkeypox. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/monkeypox Updated May 19, 2022.