Abstract
Introduction
A monkeypox outbreak is spreading in territories where the virus is not generally prevalent. The rapid and sudden emergence of monkeypox in numerous nations at the same time means that unreported transmission may have persisted. The number of reported cases is on a constant increase worldwide. At least 20 non-African countries, like Canada, Portugal, Spain, and the United Kingdom, have reported more than 57662 as of September 9th suspected or confirmed cases. This is the largest epidemic seen outside of Africa. Scientists are struggling to determine the responsible genes for the higher virulence and transmissibility of the virus. Because the viruses are related, several countries have begun acquiring smallpox vaccinations, which are believed to be very effective against monkeypox.
Methods
Bibliographic databases and web-search engines were used to retrieve studies that assessed monkeypox basic biology, life cycle, and transmission. Data were evaluated and used to explain the therapeutics that are under use or have potential. Finally, here is a comparison between how vaccines are being made now and how they were made in the past to stop the spread of new viruses.
Conclusions
Available vaccines are believed to be effective if administered within four days of viral exposure, as the virus has a long incubation period. As the virus is zoonotic, there is still a great deal of concern about the viral genetic shift and the risk of spreading to humans. This review will discuss the virus's biology and how dangerous it is. It will also look at how it spreads, what vaccines and treatments are available, and what technologies could be used to make vaccines quickly using mRNA technologies.
Keywords: Monkeypox, Outbreak, Epidemic, Infectious pathogens, Outbreak investigation, Transmission
Graphical abstract
1. Introduction
Monkeypox is a zoonotic virus that transmits from animal to human and vice versa. The virus belongs to the orthopoxvirus genus and the Poxviridae family with a dsDNA genome. The viral family is among the most diverse but also geographically distributed viral families. The double strands of nucleic acid are connected by palindromic hairpin termini, which replicate and multiply in the cytoplasm of host cells without the help of the cell nucleus [1,2]. However, studies in which the host cell nucleus was removed or inactivated, showed that the cell cytoplasm alone was insufficient to support viral replication [3,4].
The Poxviridae family is split into two subfamilies according to their definitive animal hosts: Entomopoxvirinae and Chordopoxvirinae. The Chordopoxvirinae subgroup infects vertebrates and therefore is organized into 18 genera, encompassing Capripoxvirus, Cervidpoxvirus, Avipoxvirus, Molluscipoxvirus, Orthopoxvirus (OPV), Leporipoxvirus, Suipoxvirus, Yatapoxvirus, and Parapoxvirus, while the Entomopoxviriane subset infects invertebrates and is, therefore, is split into 4 genera Alphaentomopoxvirus, Betaentomopoxvirus, Deltaentomopoxvirus, and Gammaentomopoxvirus [5]. The subfamilies of Poxviridae were classified under genera based on common antigenic homology, activation of serological cross-tolerance, and phylogenetic clustering [6].
Since poxviruses have been documented in mammals, reptiles, birds, and insects, these are also referred to as primordial (ancient) viruses. It is suggested that such viruses created visible “pox” well before the divergence of vertebrates and invertebrates. Once examined by electron microscopy, the poxviruses exhibit brick-shaped or oval geometries that span 150–300 nm in diameter [7]. The virion genome is linear in shape and includes a significant set of genes. The center portion of the genome contains all the essential viral proteins and a core set of genes shared by all poxviruses. Species-specific genes, on the other hand, which are required for host invasion and viral pathogenicity, are placed near the termini [8]. The poxviridae family consists of a variety of historically significant viruses, including the variola virus, which was the etiological agent of smallpox, one of the most lethal human infectious diseases in existence [9]. The first vaccination variant strain to protect against variola is presumed to have been from the cowpox virus, even though the actual sources of the primary smallpox vaccine virus remain unknown. The poxvirus Vaccinia viruses, which are directly related to variola, have been used to vaccine human populations, leading to the global eradication of smallpox and leading to the establishment of the discipline of vaccinology [10,11].
Poxvirus genomes vary considerably in length, ranging from 135 kbp to more than 300 kbp in size. It might be ovoid (round) or brick-shaped but is architecturally comparable from one poxvirus to another. The arrangement of the poxvirus genome sequence is extremely well preserved (Venkataraman et al., 2018). Palindromic hairpins covalently bind the ends of the linear double-stranded DNA molecule. Situated next to hairpins are highly conserved concatemeric resolution sequence regions that are important in the replication of concatemeric viral genomic sequences, which are then split into the right component portions of the genome. Poxvirus genome termini are duplicated and inverted to form terminal inverted repeat (TIR) structures. Poxviruses are very different in how long they are and how they are put together. Orthopoxviruses, like variola viruses, have many hundreds of nucleotides and don't have any TIR open reading frames (ORFs), while leporipoxviruses have even more than 10 kbp and have duplicate copies of a dozen putative ORFs [12,13]. The poxvirus genetic code is constructed in such a way that genes encoding key conserved viral functionalities, including such transcription, may be identified. The core part of the genome contains structural architectural components for nucleotide synthesis and replication factors. Computational bioinformatics-based investigations have found a core gene cluster comprising 90 genes that are highly conserved in all members of the Chordopoxvirinae subfamily within this approximately 100 kbp central domain of the genome [14,15]. It has been reported that the Entomopoxviruses subfamily contains only 45 genes in this group. There is not a single gene of poxvirus that maps outside the central core that is present in all members [16].
1.1. Poxviridae viral family
The structure of monkeypox virus (MPXV), like that of other orthopoxviruses, displays that virions are brick-shaped (ovoid) particles with a size range between 200 and 250 nm, capsid encompassed by a spatially perforated lipoprotein outer membrane (envelope) [17]. The virus has distinct surface tubules as well as a dumbbell-shaped core component, as shown in Fig. 1 . The monkeypox virus is antigenically linked to the variola and vaccinia viruses [18]. The virion envelope protects the capsid and the tightly packed nucleic acid core that contains enzymes, a double-stranded DNA genome, and transcription factors. The core is described as biconcave because of an electron microscopy fixation artifact, and it has a lateral body along both sides of the genetic material. Housekeeping genes are highly conserved across OPVs and thus are found in the core genomic sequence, whereas virus-host-interacting genes are less conserved and localized in the termini section [19]. Monkeypox viruses were first identified in 1959 as a spontaneous outbreak of a pox-like infection of fever and rash in primates confined to a research center in Copenhagen, Denmark [20]. On September 1, 1970, a nine-month-old boy was hospitalized with smallpox-like symptoms at Basankusu Hospital in the Democratic Republic of the Congo as the first human MPXV case in medical history [21]. Between October 1970 and May 1971, six instances of human MPXV were confirmed in Liberia, Nigeria, and Sierra Leone. Between 1971 and 1978, 10 MPXV cases were confirmed in Nigeria as well [22]. Thousands of human cases of monkeypox have been reported in 15 different countries ever since, including 11 of them occurring in Africa. Monkeypox was transported to the UK, the US, and Singapore [23].
Fig. 1.
Schematic illustration of monkeypox virion showing its unique structural arrangement and its transcription process.
2. Monkeypox virus infection biology
2.1. Epidemiology of monkeypox
The epidemiology of monkeypox is much more complicated than that of other viruses. The zoonotic virus, with two genetically different viral lineages, has been reported, each with distinct and distinct clinical and epidemiologic criteria. The reported monkeypox transmission of human cases in 1970, in African countries from human to human, has been less common than smallpox spreading cases. The incidence of secondary infection in unvaccinated monkeypox instances was reported to be 9.3%, compared to 37–88% for smallpox. Consequently, most cases were obtained via presumed livestock encounters; only 28% of cases were traced to person-to-person transmission. Unvaccinated people had a case-fatality rate of around 10%, with children under the age of 5 accounting for the bulk of mortalities and with the most severe illness presentations [2,24]. Serosurveys revealed that up to 28% of reported cases are due to close contact with monkeypox-infected individuals in some groups who had an asymptomatic infection; such a surprisingly low prevalence might contribute to the scarcity of persistent generations of human-to-human transmission in households and other close-contact contexts [25,26]. Genomic assessments of West and Central African viral strains uncovered a group of potential genes that may be associated with clade virulence differentiation. Such open reading frames are believed to be associated with the viral replication cycle, host species range, immune evasion variations, or virulence factors associated with viral pathogenicity [27,28].
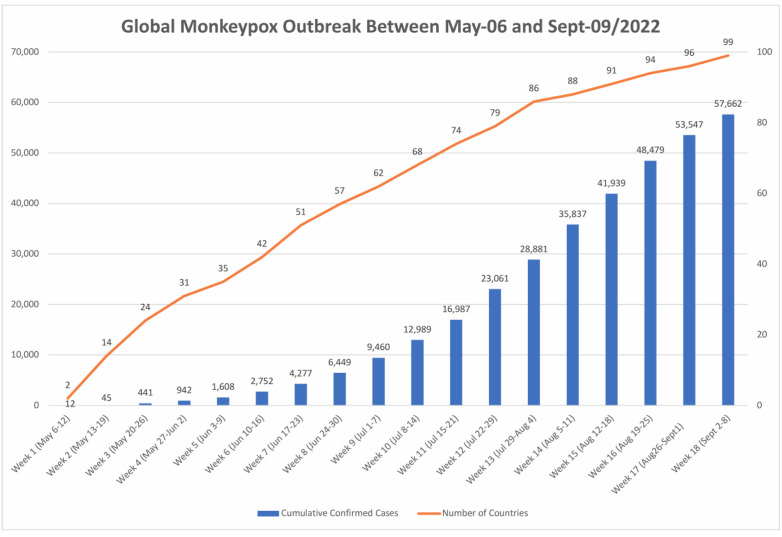
As of May 2022, multination's geographically separated reported an outbreak of monkeypox cases affecting the United Kingdom (UK), and North America. 38 confirmed cases had been reported worldwide, and 37 cases had no history of travel to endemic countries. The UK Health Security Agency confirmed a familial cluster involving two cases of monkeypox in the UK on 14 May 2022. These occurrences have nothing to do with travel-related exposure to any individuals traveling from Nigeria. From the start of the outbreak to June 10th, the number of confirmed cases has increased and been reported as shown in Fig. 2 [29]. Most of the cases identified at the time of writing this review were reported in young people with same-sex sexual partners, and none of the cases had a recent travel history to the endemic area. The clinical manifestation of the infection appeared as lesions on the genitalia indicating transmission occurs during close sexual contact [30]. The monkeypox virus is regarded to be moderately transmissible between people, and it can be spread by aerosol droplets and/or direct contact with infected lesions [31]. Viral acquisition across sexual encounters by physical contact with infected skin lesions during intercourse appeared as being the most common method of spread among same-gender sex partners [32]. Given the very particular intensity of human-to-human transmission seen in this outbreak, as well as the likely community transmission without a history of travel to endemic regions, the chance of future viral dissemination by intimate contacts, such as during sexual activities, is deemed high [33]. Transmission between persons without intimate touch is thought to be unlikely or very low. In Nigeria, the West African lineage, which has so far been identified in confirmed cases in Europe, has a case fatality rate of roughly 3.3% [34] (see Fig. 3).
Fig. 2.
Plot showing the rapid expansion of monkeypox virus cases viruses and the timeline of such reported cases by the time of revision submission on the 9th of September 2022. Data originated from {Mathieu, 2022 #127}.
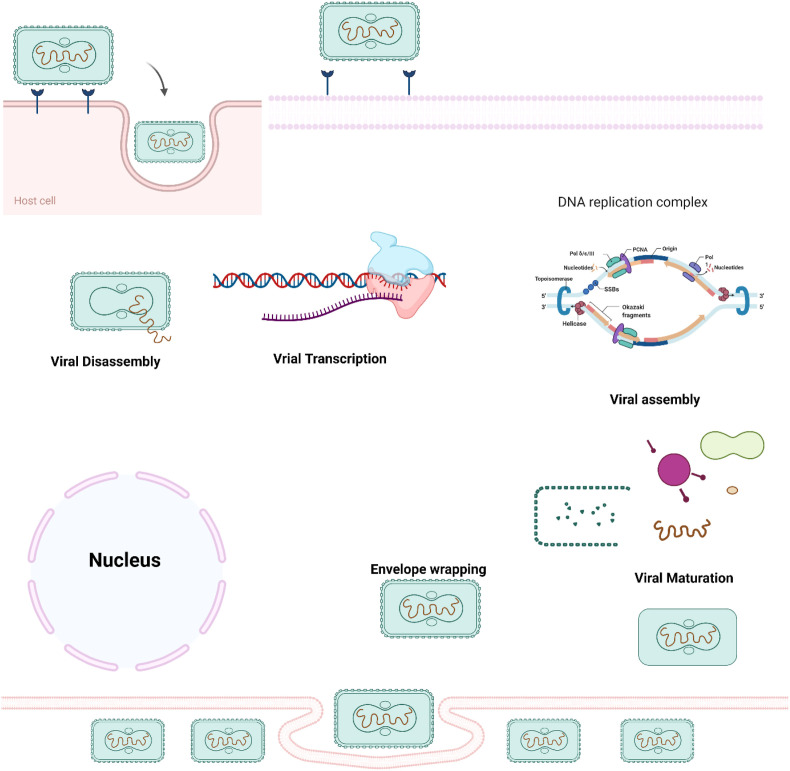
Fig. 3.
The life cycle of a poxvirus is depicted schematically. The viral capsid is discharged after the virion fuses with the host cell membrane. When a virion adheres to and fuses with a cell membrane, the viral particle is released into the host cell's cytoplasm. Transcription is set in motion by enzymes and substances transported by the nucleus itself. Most viruses get encapsulated in the scab's protein matrix after remaining in the cytoplasm as intracellular mature virions. The remaining virions migrate to the host cell membrane, where they adhere and take on a new envelope, becoming known as intracellular enveloped virions. The virus spreads from cell to cell via enveloped virions attached to cell surfaces, whereas enveloped virions (EEVs) released from infected cells might contribute to the virus's systemic dissemination.
3. Clinical manifestations
The clinical manifestation of the viral infection appears on the skin as distinctive rashes and fever. When the virus and the disease are still prevalent, an asymptomatic incubation period of 10–14 days is accompanied by a fever that promptly escalates to 38°–40 °C, occasionally associated with cutaneous dermal petechia. Headaches, backaches, nausea, vomiting, and prostration were among the related clinical manifestations. A widespread systemic rash with a distinct centrifugal pattern (i.e., higher lesions just in the oral mucosal cavity, face, and extremities than in the body) emerged a day or two after contracting the infection. A prodrome of fever, headaches, backaches, fatigue, and weariness develops after an incubation period of 7–17 days (with a mean of 12 days). As the rash appears, the fever usually abates. The eruption of the cutaneous lesion progresses similarly to smallpox infection. Lesions may progress from macules, papules, and vesicles to pustules in any region of the body and eventually develop into a crust and scar. After remission of the rash, hypopigmentation of the scarred lesions is accompanied by hyperpigmentation of the scarred regions. Clinically, severe cervical, postauricular, submandibular, and inguinal lymphadenopathy differentiates monkeypox from smallpox.
4. Viral transmission or virus life cycle
One of the most extensively researched members of the Orthopoxvirus genus of the Poxviridae is the Vaccinia virus, which is closely linked to variola and monkeypox viruses. Viruses have a substantial genome of approximately 200,000 bp, a cytoplasmic replication locus that is highly regulated for early, intermediate, and late expression levels of viral genes, and a complicated assembly mechanism that requires numerous viral membrane proteins [35,36]. Poxvirus virions contain approximately 200 genes and an intricate life cycle. Replication occurs in the host cell's cytoplasm after entry, encoding its replication machinery, including transcription factors, as shown in Fig. 2. Cho and Wenner (1973) say that studying viral replication cycles could help us learn a lot about the molecular biology of new viruses and find new drug targets. The viral cell cycle starts with the binding of the surface receptor and enters the host cell with the release of the viral nucleocapsid (cores) in the cellular cytoplasm. Cellular binding to the surface has not been well studied or documented in the viral family. A17L-specific antibody restricts viral entry, and a second intracellular mature viral protein, myristoylated transmembrane protein (L1R; 250 residues essential for viral maturation), has also been shown to become a target of neutralizing antibodies [37]. Only immunization with the B5R or A33R genes, two of the six proteins identified specifically for the EEV outer envelope of the EEV, was demonstrated to be preventative in an animal model and resulted in the development of neutralizing antibodies [17]. The viral cores unravel, and initial gene expression proceeds with the help of component proteins contained within the virion. Viral cores enter the cytoplasm after virus particles adhere to the cell membrane in an undefined mechanism that is not always fusion-mediated, as newly delivered viral cores have also been shown to preserve transmembrane features at least spontaneously. Uncoating exposes the viral DNA genome within viral cores to deoxyribonuclease that is mediated by viral function.
The expression of early genes results in the formation of intermediate gene transcriptional regulatory factors and the start of viral DNA synthesis. Intermediate transcription factors comprise a group of late transcription factors that induce late transcription. Mature protein molecules and enzymes are essential for virion production and assembly, particularly in electron-rich parts of the cytoplasm, including for recruiting earlier transcription factors designated to be packed into developing virions [38]. Newly emerging genomic DNA is organized into unit-length genomes and packed into immature virions in the form of very large concatemeric precursors. Proteolytic processes promote the development of immature components in IMVs, and a fraction of them go through a subsequent envelope in which more glycoproteins are acquired. Most mature intracellular virions are released by lysis of the host cell [21]. Most mature internalized virions develop extra membranes before exiting via the host cell membrane. They could stay on the surface of the cell or depart like an extracellular-enveloped virus. Fragmentation of the outer membrane, which can be caused by mechanical stress or by parts of the host's complement system, can also cause mature intracellular virions to leak out of the cell [39].
5. Treatment
Currently, the US FDA provides regulatory oversight to the use of vaccines/therapeutics previously tested for the treatment of monkeypox infection. However, there are three antivirals (i.e., Tecovirimat, Cidofovir, and Brincidofovir) that are expected to be effective based on in vivo and animal module investigations. Tecovirimat and Brincidofovir have been approved by the US FDA for the treatment of smallpox and are expected to be effective against monkeypox, as they are genetically closely related [40]. The FDA approved these two antivirals based on studies that didn't involve people. This is because it's not ethical to make a person sick with variola (this is called the “Animal modules”). Their applications were granted fast track, priority review, and orphan drug designation by the FDA. Cidofovir, on the other hand, is only licensed to treat cytomegalovirus retinitis in patients with AIDS.
5.1. Tecovirimat
Tecovirimat (previously known as ST-246) is an antiviral that inhibits P37 protein, which is highly conserved and present in all Orthopoxviruses (e.g., smallpox, cowpox vaccinia, ectromelia, rabbitpox, and monkeypox) [41]. P37 protein interacts with components of late endosome-derived transport vesicles (i.e., Rab9, GTPase, and TIP47). This interaction results in the formation of the virus-specific wrapping complex for enveloped virions [41,42]. Therefore, P37 is responsible for the virulence through the formation and egress of enveloped virions. This makes Tecovirimat effective in treating orthopoxviruses [41].
In 2018, oral Tecovirimat was the first drug approved by the US FDA to treat smallpox [43,44], and Health Canada approved it for the same indication in December 2021 [45]. A month later (January 20,22), Tecovirimat received approval from the European Medicines Agency (EMA) to treat smallpox, monkeypox, cowpox, and severe adverse reactions resulting from vaccination with the vaccinia virus [46]. On 19 May 2022, the US FDA approved the intravenous (IV) formulation of Tecovirimat as an alternative for smallpox patients who cannot swallow the oral capsules of Tecovirimat. However, the US FDA has not yet approved Tecovirimat for monkeypox treatment [47]. A recent retrospective observational study reported that a monkeypox patient who received Oral Tecovirimat 600 mg twice daily had a shorter duration of viral shedding, hospitalization, and illness compared to patients who received Brincidofovir or received no antiviral therapy [48].
5.2. Cidofovir
Cidofovir (l-[(S)-3-hydroxy-2-(phosphonomethoxy)-propyl]cytosine dihydrate) is an acyclic nucleoside monophosphate and analogue cytosine [49]. It is phosphorylated twice intracellularly to its active form, Cidofovir diphosphate [50]. Cidofovir diphosphate competes with cytosine, resulting in the termination of the DNA chain and inhibiting the synthesis of DNA in viruses. Cidofovir was approved by the FDA in 1996 to treat cytomegalovirus retinitis in patients with AIDS [51]. However, in vitro studies showed that Cidofovir is effective in a wide range of dsDNA viruses such as herpes viruses, iridovirus, papilloma, polyoma, hepadnavirus pox, and adenoviruses in cell culture [[52], [53], [54]].
Cidofovir's efficacy to treat monkeypox virus using different routes of administration were investigated in animal models [55]. Intraperitoneal administration of Cidofovir in monkeys starting one day after infection was found to be effective in reducing the number of monkeypox lesions and reducing mortality [56]. Similarly, the intravenous administration of Cidofovir in monkeys before the infection was found to be protective against signs of illness and virus replication [57]. Moreover, animal model studies found that monkeypox virus treatment with Cidofovir was more effective in reducing mortality when compared to smallpox vaccination following lethal intratracheal monkeypox virus infection [57]. Furthermore, the co-administration of the Cidofovir and smallpox vaccine in infected monkeys was found to reduce the side effects associated with the vaccine, in addition to reducing vaccine immunity against the monkeypox virus [58]. Evidence from animal model studies suggests that Cidofovir could be an effective therapy for pre- and post-exposure to monkeypox virus infections in humans; however, there is no clinical evidence to support this claim [59,60]. Moreover, the use of Cidofovir is limited due to its nephrotoxicity which can manifest as Fanconi-type syndrome with proximal tubular injury and renal failure. Therefore, it needs to be administered with probenecid and intravenous saline hydration to minimize the risk of kidney damage [61].
5.3. Brincidofovir
Brincidofovir (hexadecyloxypropyl-cidofovir) is a lipid conjugate prodrug for Cidofovir that has enhanced cellular uptake and superior conversion to the active form (that is, Cidofovir diphosphate) by intracellular enzymes [62]. Brincidofovir is also superior to Cidofovir in terms of renal safety profile and oral bioavailability [40,63]. It has been approved by the US FDA to treat smallpox; however, animal studies suggest it can be effective to treat other orthopoxvirus infections (e.g., monkeypox and cowpox) in humans [40,64]. Crump et al. suggested that treatment with Brincidofovir should be initiated as soon as the following exposure to monkeypox virus as Brincidofovir efficacy decreases when the virus load increases [65]. While in vitro and animal studies suggested promising results for Brincidofovir against monkeypox, a recent report from the UK reported that Brincidofovir has no evidence of any clinical benefit when used in three patients infected with monkeypox. In addition, liver enzymes were elevated in the three patients who received Brincidofovir, resulting in precautionary decisions to stop therapy [66].
6. Vaccination
6.1. Traditional vaccine
Monkeypox virus is antigenically related to the variola and vaccinia viruses which cause smallpox. The genomes of the monkeypox and variola viruses are approximately 96% identical in the central regions [67]. Based on that, smallpox vaccines could be used to protect people from acquiring monkeypox. Data from Africa shows that the smallpox vaccine is at least 85% effective in preventing monkeypox [30]. The availability of a highly effective and inexpensive live vaccine derived from the closely related vaccinia virus contributed to eradicating smallpox by the international community under the support of the World Health Organization [68,69]. Following smallpox eradication, vaccination was discontinued throughout the world, resulting in a growing population that is completely unprotected from the variola virus as well as related orthopoxviruses. Therefore, the cessation of smallpox vaccination appears to have increased the vulnerability of humans to severe monkeypox. Approximately 70% of the world's population is no longer protected against smallpox, and through cross-immunity, to closely related orthodox viruses such as monkeypox [30]. In some countries, young non-vaccinated individuals exceed 75% of the population. These individuals are almost certainly susceptible to monkeypox virus infection [70]. People vaccinated before 1980 had a five-fold lower risk of monkeypox corresponding to those who had not been vaccinated [71].
The smallpox vaccine is not currently available to the public. However, for specific populations at high risk of vocational vulnerability to orthopoxviruses, Advisory Committee on Immunization Practices (ACIP) advises routine vaccination against the disease. Therefore, the Centers for Disease Control and Prevention (CDC) provide smallpox vaccines to these recommended individuals as needed. Dryvax was a live virus smallpox vaccine used for smallpox eradication efforts and provided successful immunogenicity in approximately 95% of vaccinated people. After world health authorities announced that smallpox had been eradicated, Wyeth stopped making the vaccine in 1980. A stockpile of Dryvax was kept by the U.S. Centers for Disease Control and Prevention (CDC) for use in case of emergency and helped contain an outbreak of monkeypox in the United States in 2003. However, its supply was replaced by ACAM2000, a more modern product manufactured by Sanofi Pasteur.
ACAM2000 is a replication-competent vaccine FDA-licensed for active immunization against smallpox disease in persons defined to be at high risk for smallpox infection. ACAM2000 does not contain the variola virus, but it contains the live vaccinia virus, which belongs to the poxvirus family, the genus Orthopoxvirus. ACAM2000 is administered as a single dose by the percutaneous route (scarification) using 15 jabs of a bifurcated needle. ACIP guidance recommends in 2015 routine vaccination of laboratory personnel who directly handle cell cultures or animals infected with other orthopoxviruses, including monkeypox. Therefore, ACAM2000 can be used in people exposed to monkeypox if used under new drug protocols. The Aventis Pasteur Smallpox Vaccine (APSV) is another replication-competent vaccinia virus vaccine available for use in circumstances where ACAM2000 is depleted, not readily available, or on a case-by-case basis where ACAM2000 is contraindicated.
A vaccine, JYNNEOS (also known as Imvamune or Imvanex), has been approved in the United States to prevent monkeypox and smallpox [72]. JYNNEOS can cross-react and generate immune protection against monkeypox, therefore it is believed that vaccination after a monkeypox exposure may help prevent the disease or make it less severe. Unlike ACAM2000 and APSV, JYNNEOS is an attenuated live virus vaccine [73,74]. It has a safer profile and could be given to people without needing detailed health screening which led the FDA to approve it for the general population. In November 2021, the U.S. CDC's vaccine committee voted in favor of JYNNEOS as an alternative to ACAM2000 for primary vaccination. JYNNEOS is administered subcutaneously as two doses separated by 4 weeks (one dose at week 0 and a second dose at week 4) for primary vaccines. People who had previously been vaccinated against smallpox obtained only one dose. The modified JYNNEOS vaccine is licensed as a third-generation vaccine against smallpox. The new vaccine, based on modified attenuated vaccinia virus (Ankara strain), was approved for the prevention of monkeypox in 2019 in adults 18 years of age and older determined to be at high risk for monkeypox infection. This is the only currently approved vaccine for the prevention of monkeypox disease [75].
It was noticed during monkeypox episodes that patients with less severe illnesses had been vaccinated with the smallpox vaccine [76]. Second-generation smallpox vaccines have been shown to protect against monkeypox [77]. In many cases, smallpox vaccines were offered to people who had close contact with human monkeypox cases [78,79]. Secondary transmission is known to have occurred. The U.S. recently distributed 1200 doses of the Jynneos vaccine from its national stockpile across the U.S. for people who have had high-risk exposures to monkeypox. Britain also offers a smallpox vaccine to some healthcare workers and others who may have been exposed, as a handful more cases were confirmed in parts of Europe.
While smallpox vaccination shows immunity against the monkeypox virus, it is reasonable to assume the differences between monkeypox and vaccinia viruses. Proteins that are expressed in monkeypox but not in the vaccinia virus may represent subunits for future vaccines against monkeypox. For instance, it was shown that few proteins are present in the monkeypox virus but are fragmented in the vaccinia strain. This includes COP–B19R (IFN-α/β binding protein), BR-05/BR-226 (TNF binding protein), and BR-207 (apoptosis protein) [80]. It is not clear whether the immune response to the fragment expressed by the vaccinia virus leads to a comparable protective response. Determining the contribution of the individual genes to virulence and genes encoding fragments of proteins with known functions needs more research.
6.2. mRNA-based vaccines
The use of conventional vaccines is a successful approach against various diseases, with many types of vaccines currently available on the market. However, the development of these traditional vaccines might be difficult against certain infectious pathogens, especially those with adaptive immune responses [81]. In addition, in pandemic situations and with the rapid spread of new viral infections, the rapid and large-scale development of these conventional vaccines is not a straightforward option. This is because the development of these types of vaccines could take a long time and requires special types of production instruments. Nucleic acid-based vaccinations are a newer alternative to conventional vaccines used today. It's based on delivering nucleic acids, such as mRNA molecules that encode a viral protein. The translation of the mRNA that is given will drive the immune response of the host and cause it to make antibodies to fight off viral infection.
The first successful report for in vivo application of mRNA molecules was published in 1990 when mRNA molecules were introduced into mice and the translated protein was observed [82]. In 1992, mRNA molecules encoding vasopressin in rats' hypothalamus caused physiological reactions [83]. After these publications, extensive research was done to create nucleic acid vaccines against numerous diseases. This is because nucleic acid vaccines are a promising alternative to traditional immunizations. These mRNA vaccines can alter disease treatment and prevention. Since mRNA vaccines encode a single protein, they are powerful and specific. The development of novel mRNA manufacturing technologies can help produce these molecules at minimal cost. This has led to a lot of preclinical studies and massive amounts of data throughout the years. Vaccinating with mRNA offers various advantages over using a dead or weakened virus. mRNA molecules have no risk of infection, unlike traditional immunizations [84]. The mRNA molecules will break down regularly after translation into the encoded protein, and their half-life can be varied dependent on the manner of administration and kind of alteration [85]. mRNA molecules are effective and specialized because they encode specific proteins. Among nucleic acids, mRNA is the least immunogenic genetic vector, allowing multiple vaccinations [86]. Finally, large-scale production of mRNA vaccines is rapid and inexpensive and can easily be produced on an industrial scale. However, the application of nucleic acid vaccines and, especially mRNA-based types of vaccines, is hampered by some limitations. These are mainly related to the instability of the mRNA molecules, their rapid elimination from the body as a result of the rapid clearance by the nucleus enzymes, and their low level of cellular uptake because of their high negative charge [87].
Recent advances in nanotechnology and drug delivery have largely overcome most of the barriers to the development of nucleic acid vaccines, as evidenced by several results for research-based multiple mRNA-based vaccines against various types of infections in both animal and human models [88]. In its most basic form, nanotechnology drug delivery uses a nanocarrier to carry, protect, and deliver therapeutic or vaccination ingredients to target tissues. The nanocarrier is used in the development of mRNA vaccines to protect the loaded mRNA from nucleases and prevent early elimination, deliver these mRNA molecules to their target tissues, and facilitate the uptake and delivery of the loaded mRNA vaccines into the cytoplasm of target cells where they will be translated into the target protein [89,90].
mRNA vaccines against COVID-19 are based on the use of a specific type of nanocarrier to deliver mRNA molecules that encode the viral spike protein of the virus [93,94]. This protein is used by the virus to enter human cells and induce infection. However, the spike protein itself is non-toxic and will not induce any infection agents [95].
Based on the advanced technologies in the development of nanocarriers and the urgent need for the rapid development of effective vaccines, it took only 66 days after the spread of the pandemic to start the first phase I clinical trial of the anti-COVID-19 vaccine in the United States [97]. This trial was based on the delivery of LNPs that encapsulate mRNA molecules that encode the viral spike protein. This led to promising results and the start of further clinical trials, which will continue until the urgent approval of two mRNA-based vaccines against COVID-19 at the end of 2020 [98]. This was considered the first vaccine in history that was developed in a very short period. This very fast development of vaccines means that in the spread of infections, effective mRNA vaccines will be the best option. Several years ago, mRNA-based vaccines were thought to be difficult or even impossible, but the spread of COVID-19 forced pharmaceutical companies and researchers to achieve significant achievements in this area [99]. In addition, the spread of COVID-19 was accompanied by the continuous development of various mutations of the virus, and some of these mutations made the virus more aggressive and highly contagious. However, the two approved mRNA vaccines remain effective against these mutations and were able to successfully limit the level of viral spread and/or the intensity of the infection [100]. Similarly to COVID-19, the development of conventional vaccines based on dead or live attenuated viruses would take a long time and might be effective with multiple side effects [102]. However, based on the acquired experience in the development of mRNA-based vaccines, this might be the best option to combat the increasing spread of the monkeypox virus. In this sense, the same steps that have been taken in the development of the COVID-19 vaccine can be applied here. This will start by determining the exact mRNA sequence of the virus and the surface proteins present in the virus. Studying the exact structure of the virus and the key surface components that help it invade human cells will be the key to the development of mRNA vaccines. With the knowledge of these components, mRNA molecules can be constructed and synthesized to encode these proteins [91].
Anti-COVID mRNA vaccines were based on using LNP to deliver mRNA molecules against the viral spike protein and since these LNP were effective in protecting and delivering the loaded mRNA in the case of CORONA virus, the same LNP could be used in the case of monkeypox viral vaccines but with different mRNA, cargos to induce the immune system against the monkeypox infection. This will be The anti-COVID mRNA vaccines were based on using LNP to deliver mRNA molecules against the viral spike protein. Since these LNP were effective in protecting and delivering the loaded mRNA in the case of the CORONA virus, the same LNP could be used in the case of monkeypox viral vaccines but with different mRNA cargos to induce the immune system against the monkeypox infection. This will be owing to the feasibility of nanotechnology to deliver different types of molecules using the same carrier [103,104].
In the case of monkeypox viral spread, the development of mRNA vaccines would be easier and based solely on the experience gained by the COVID-9 mRNA vaccines and all the lessons learned from that pandemic to avoid the possibility of exposing the world to another life-threatening viral spread and all the other health and socio-economical consequences [105]. The US FDA has only approved one vaccine so far: Modified Vaccinia Ankara-Bavarian Nordic. However, the ACAM2000® smallpox vaccine and the investigational Aventis Pasteur smallpox vaccine will be available in case of an outbreak of monkeypox.
6.3. Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine
Smallpox has been eradicated since 1980 post the global vaccination using vaccinia virus-based vaccines (VACV) [66]. However, these vaccines were virulent and causes serious adverse reactions [106]. To produce an attenuated safer small vaccine, Chorioallantois vaccinia Virus Ankara (CVA) was successfully attenuated and renamed Modified Vaccinia Ankara (MVA) [107]. Multiple MVA vectors have been investigated to produce several vaccines (e.g., MERS, and HIV). MVA-BN is a live attenuated vaccine that effectively infects human cells that result in transcription of the viral genes without releasing the virus from the cells. MVA-BN cannot replicate in human cell lines which makes it less virulent and safe for immunosuppressed patients [108]. In 2013, the MVA-BN vaccine was approved for smallpox in Europe (Imvanex©) and Canada (Imvamune©) and in 2019, the US FDA approved the MVA-BN vaccine for smallpox and monkeypox under the brand name (Jynneos©) [107]. Currently, Jynneos is the only FDA-approved vaccine for monkeypox in adults 18 years of age and older [44]. MVA-BN is administered by injection subcutaneously and is generally well-tolerated. Like other vaccines, it may cause injection site reactions (eg, pain, redness, swelling) and mild systemic adverse reactions (e.g., nausea, headache, and chills) [109].
6.4. ACAM2000® vaccine
A live vaccinia virus was cloned from the oldest smallpox (Dryvax®) consisting of a mixed pool of vaccinia viruses [110]. It was developed in the 1990s in response to concerns from the US government about the accidental or intentional (i.e. bioterrorism) release of smallpox [111]. The goal was to produce a vaccine from a single purified isolate of vaccinia virus that has an efficacy similar to Dryvax® but with a better safety profile. Also, Dryvax® was manufacturing followed an outdated technique that posed a risk of contamination. ACAM2000® received US FDA approval in 2007 and replaced Dryvax® in 2008 [111]. ACAM2000® has similar immunogenicity to Dryvax®, however, it may cause serious adverse events (e.g., myocarditis, pericarditis, and encephalitis) that warranted a black box warning and limited it is used in the military and for those who are determined to be at high risk for small box infection [110,112]. ACAM2000® has not been investigated or licensed for use for monkeypox prevention, however, evidence suggests that smallpox vaccines can be 85% effective in protecting against monkeypox [112].
In the US, Biomedical Advanced Research and Development Authority (BARDA) supported the development of the Jynneos® vaccine and the Strategic National Stockpile (SNS) supported the development of ACAM2000 [113]. The SNS has over 100 million doses of ACAM2000 and over 36,000 doses of Jynneos® that are immediately available in its inventory. Moreover, BARDA has an agreement with the manufacturer of Jynneos® to provide 16.4 million doses ready upon request from the US government [113]. The SNS holds supply for Tecovirimat, Cidofovir, and Vaccinia Immune Globulin Intravenous as treatment options for monkeypox, and the CDC holds expanded access to Investigational New Drug protocol to use these stockpiled medications and vaccines in case of monkeypox outbreak [109,113]. The SNS has also stockpiled another replication-competent vaccinia virus vaccine called Aventis Pasteur Smallpox Vaccine (APSV). APSV is an investigational vaccine that could potentially be available in a smallpox emergency under the Emergency Use Authorization or Investigational New Drug protocol if currently approved vaccines are depleted, not readily available, or contraindicated for subjects (case by case basis) [66,109].
7. Conclusions
While the world is still dealing with the COVID-19 pandemic, the potential of another breakout raises some major concerns. Interestingly, monkeys are not the primary reservoir for monkeypox viruses. The virus is presumed to thrive mainly in rodents such as rats and squirrels. Whenever a person encounters an infected animal, individual, or contaminated material, the monkeypox virus can be transmitted to and between individuals. In addition to entering the body through broken skin. The key to avoiding monkeypox, like with many contagious pathogens, is to minimize your exposure to the pathogen. In addition, Proper education on the handling of exposure to infectious wildlife, like in places in which the wild game meat trade business is popular, can also help to reduce viral transmission. Monkeypox has no treatment. Treatment approaches are typically centered on relieving symptoms and making patients as comfortable. However, several antiviral therapies may be useful for monkeypox, and the CDC has approved their use in suppressing the monkeypox epidemic. One medication, tecovirimat (TPOXXTM), has been authorized for the treatment of smallpox in the United States, Canada, and Europe from May 2022. It works by distributing the development of mature, enveloped virions by interfering with a viral protein (p37). The recent increase in monkeypox cases has highlighted one major concern: Could monkeypox now have the propensity and the capacity to become pandemic in disposition?
Disclosure statement
No government, private, or nonprofit organization provided direct funding for this study.
Authors contributions
AAAA, MAO, MBN, AH, and MMT, all authors were responsible for collecting data, and analysis. All authors contributed to the writing and editing of the final manuscript. All authors have read and approved the final version.
CRediT authorship contribution statement
Alaa AA. Aljabali: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Mohammad A. Obeid: Writing – review & editing, Writing – original draft, Conceptualization. Mohammad B. Nusair: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Ali Hmedat: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Murtaza M. Tambuwala: Writing – review & editing, Writing – original draft, Project administration, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol. Rev. 1973;37:1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Looi M.-K. British Medical Journal Publishing Group; 2022. Monkeypox: what We Know about the 2022 Outbreak So Far. [DOI] [PubMed] [Google Scholar]
- 5.Diven D.G. An overview of poxviruses. J. Am. Acad. Dermatol. 2001;44:1–16. doi: 10.1067/mjd.2001.109302. [DOI] [PubMed] [Google Scholar]
- 6.Odom M.R., Hendrickson R.C., Lefkowitz E.J. Poxvirus protein evolution: family wide assessment of possible horizontal gene transfer events. Virus Res. 2009;144:233–249. doi: 10.1016/j.virusres.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harish S., Murugan M., Kannan M., Parthasarathy S., Prabhukarthikeyan S.R., Elango K. Microbial Approaches for Insect Pest Management. Springer; 2021. Entomopathogenic viruses; pp. 1–57. [Google Scholar]
- 8.Shchelkunov S.N., Marennikova S.S., Moyer R.W. Springer Science & Business Media; 2006. Orthopoxviruses Pathogenic for Humans. [Google Scholar]
- 9.Kabuga A.I., El Zowalaty M.E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol. 2019;91:533–540. doi: 10.1002/jmv.25348. [DOI] [PubMed] [Google Scholar]
- 10.Damon I.K. Poxviruses. Man. Clin. Microbiol. 2011:1647–1658. [Google Scholar]
- 11.Essbauer S., Pfeffer M., Meyer H. Zoonotic poxviruses. Vet. Microbiol. 2010;140:229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLange A.M., McFadden G. The role of telomeres in poxvirus DNA replication. Poxviruses. 1990:71–92. doi: 10.1007/978-3-642-75605-4_3. [DOI] [PubMed] [Google Scholar]
- 13.DeMasi J., Du S., Lennon D., Traktman P. Vaccinia virus telomeres: interaction with the viral I1, I6, and K4 proteins. J. Virol. 2001;75:10090–10105. doi: 10.1128/JVI.75.21.10090-10105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dougherty W.G., Semler B.L. Expression of virus-encoded proteinases: functional and structural similarities with cellular enzymes. Microbiol. Rev. 1993;57:781–822. doi: 10.1128/mr.57.4.781-822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wexler P., Anderson B.D., Gad S.C., Hakkinen P.J.B., Kamrin M., De Peyster A., et al. Academic Press; 2005. Encyclopedia of Toxicology. [Google Scholar]
- 16.Lefkowitz E.J., Wang C., Upton C. Poxviruses: past, present and future. Virus Res. 2006;117:105–118. doi: 10.1016/j.virusres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 18.Robinson A.J., Kerr P.J. Poxvirus infections. Infect. Dis. Wild. Mamm. 2001:179–201. [Google Scholar]
- 19.Poranen M.M., Bamford D.H. Assembly of large icosahedral double-stranded RNA viruses. Viral Mol. Machines. 2012:379–402. doi: 10.1007/978-1-4614-0980-9_17. [DOI] [PubMed] [Google Scholar]
- 20.Magnus Pv, Andersen E.K., Petersen K.B., Birch‐Andersen A.J. vol. 46. 1959. pp. 156–176. (A Pox‐like Disease in Cynomolgus Monkeys). [Google Scholar]
- 21.Breman J.G., Kalisa-Ruti M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull. World Health Organ. 1980;58:165. [PMC free article] [PubMed] [Google Scholar]
- 22.Foster S.O., Brink E.W., Hutchins D.L., Pifer J.M., Lourie B., Moser C.R., et al. Human monkeypox. 1972;46:569. [PMC free article] [PubMed] [Google Scholar]
- 23.Sklenovská N. Springer; 2020. Monkeypox Virus. Animal-Origin Viral Zoonoses; pp. 39–68. [Google Scholar]
- 24.Ogoina D., Izibewule J.H., Ogunleye A., Ederiane E., Anebonam U., Neni A., et al. The 2017 human monkeypox outbreak in Nigeria—report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haller S.L., Peng C., McFadden G., Rothenburg S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014;21:15–40. doi: 10.1016/j.meegid.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen E., Abubakar I., Ihekweazu C., Heymann D., Ntoumi F., Blumberg L., et al. Monkeypox - enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int. J. Infect. Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faye O., Pratt C.B., Faye M., Fall G., Chitty J.A., Diagne M.M., et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect. Dis. 2018;18:246. doi: 10.1016/S1473-3099(18)30043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okanume O.B. 2018. Perception of Warri Metropolitan Residents on Online Newspaper Reportage of the Monkey Pox Virus Vaccination Hoax. [Google Scholar]
- 29.Control ECfDPa . European Centre for Disease Prevention and Control; 2022. Epidemiological Update: Monkeypox Outbreak. [Google Scholar]
- 30.Fine P.E.M., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan A., Aarons E., Astbury J., Brooks T., Chand M., Flegg P., et al. vol. 26. 2020. p. 782. (Human-to-human Transmission of Monkeypox Virus). United Kingdom, October 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khodakevich L., Ježek Z., Messinger D. Monkeypox virus: ecology and public health significance. Bull. World Health Organ. 1988;66:747. [PMC free article] [PubMed] [Google Scholar]
- 33.Parker S., Nuara A., Buller R.M.L., Schultz D.A. 2007. Human Monkeypox: an Emerging Zoonotic Disease. [DOI] [PubMed] [Google Scholar]
- 34.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Neglected Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moss B. vol. 74. 2001. pp. 2905–2945. (Fields Virology). Chapter. [Google Scholar]
- 37.Henderson D.A., Inglesby T.V., Bartlett J.G., Ascher M.S., Eitzen E., Jahrling P.B., et al. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999;281:2127–2137. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 38.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., et al. The Lancet Infectious Diseases; 2022. Clinical Features and Management of Human Monkeypox: a Retrospective Observational Study in the UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.22.2200421. May 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutson C.L., Kondas A.V., Mauldin M.R., Doty J.B., Grossi I.M., Morgan C.N., et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. mSphere. 2021;6 doi: 10.1128/mSphere.00927-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosenbach D.W., Honeychurch K., Rose E.A., Chinsangaram J., Frimm A., Maiti B., et al. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 2018;379:44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosenbach D.W., Jordan R., Hruby D.E. Development of the small-molecule antiviral ST-246 as a smallpox therapeutic. Future Virol. 2011;6:653–671. doi: 10.2217/fvl.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wharton M., Strikas R.A., Harpaz R., Rotz L.D., Schwartz B., Casey C.G., et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the advisory committee on immunization Practices (ACIP) and the healthcare infection Control Practices advisory committee (HICPAC) MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2003;52:1–16. [PubMed] [Google Scholar]
- 44.Administration USFaD. 2022. FDA Approves the First Drug with an Indication for Treatment of Smallpox. [Google Scholar]
- 45.Technologies S. 2022. SIGA Announces Health Canada Regulatory Approval of Oral TPOXX. [Google Scholar]
- 46.Agency E.M., Tecovirimat SIGA (2022). https://www.ema.europa.eu/en/medicines/human/EPAR/tecovirimat-siga.
- 47.Technologies S. 2022. SIGA Receives Approval from the FDA for Intravenous (IV) Formulation of TPOXX® (Tecovirimat) [Google Scholar]
- 48.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Clercq E., Holý A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 50.Lea A.P., Bryson H.M. Cidofovir. Drugs. 1996;52:225–230. doi: 10.2165/00003495-199652020-00006. ; discussion 31. [DOI] [PubMed] [Google Scholar]
- 51.Plosker G.L., Noble S. Cidofovir: a review of its use in cytomegalovirus retinitis in patients with AIDS. Drugs. 1999;58:325–345. doi: 10.2165/00003495-199958020-00015. [DOI] [PubMed] [Google Scholar]
- 52.Baker R.O., Bray M., Huggins J.W. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 2003;57:13–23. doi: 10.1016/S0166-3542(02)00196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Clercq E. Cidofovir in the treatment of poxvirus infections. Antivir. Res. 2002;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kern E.R. In vitro activity of potential anti-poxvirus agents. Antivir. Res. 2003;57:35–40. doi: 10.1016/S0166-3542(02)00198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrei G., Snoeck R. Cidofovir activity against poxvirus infections. Viruses. 2010;2:2803–2830. doi: 10.3390/v2122803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huggins M.S. Cidofovir (HPMPC) treatment of monkeypox. Antivir. Res. 1998;37:A73. [Google Scholar]
- 57.Stittelaar K.J., Neyts J., Naesens L., van Amerongen G., van Lavieren R.F., Holý A., et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 58.Wei H., Huang D., Fortman J., Wang R., Shao L., Chen Z.W. Coadministration of cidofovir and smallpox vaccine reduced vaccination side effects but interfered with vaccine-elicited immune responses and immunity to monkeypox. J. Virol. 2009;83:1115–1125. doi: 10.1128/JVI.00984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Clercq E. Cidofovir in the therapy and short-term prophylaxis of poxvirus infections. Trends Pharmacol. Sci. 2002;23:456–458. doi: 10.1016/S0165-6147(02)02091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin. Microbiol. Rev. 2003;16:569–596. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolf D.L., Rodríguez C.A., Mucci M., Ingrosso A., Duncan B.A., Nickens D.J. Pharmacokinetics and renal effects of cidofovir with a reduced dose of probenecid in HIV-infected patients with cytomegalovirus retinitis. J. Clin. Pharmacol. 2003;43:43–51. doi: 10.1177/0091270002239705. [DOI] [PubMed] [Google Scholar]
- 62.Hostetler K.Y., Beadle J.R., Trahan J., Aldern K.A., Owens G., Schriewer J., et al. Oral 1-O-octadecyl-2-O-benzyl-sn-glycero-3-cidofovir targets the lung and is effective against a lethal respiratory challenge with ectromelia virus in mice. Antivir. Res. 2007;73:212–218. doi: 10.1016/j.antiviral.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tippin T.K., Morrison M.E., Brundage T.M., Momméja-Marin H. Brincidofovir is not a substrate for the human organic anion transporter 1: a mechanistic explanation for the lack of nephrotoxicity observed in clinical studies. Ther. Drug Monit. 2016;38:777–786. doi: 10.1097/FTD.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quenelle D.C., Collins D.J., Wan W.B., Beadle J.R., Hostetler K.Y., Kern E.R. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 2004;48:404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crump R., Korom M., Buller R.M., Parker S. Buccal viral DNA as a trigger for brincidofovir therapy in the mousepox model of smallpox. Antivir. Res. 2017;139:112–116. doi: 10.1016/j.antiviral.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fenner F. Smallpox: emergence, global spread, and eradication. Hist. Philos. Life Sci. 1993;15:397–420. [PubMed] [Google Scholar]
- 67.Shchelkunov S.N., Totmenin A.V., Babkin I.V., Safronov P.F., Ryazankina O.I., Petrov N.A., et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tognotti E. The eradication of smallpox, a success story for modern medicine and public health: what lessons for the future? J. Infect. Devel Countries. 2010;4:264–266. doi: 10.3855/jidc.1204. [DOI] [PubMed] [Google Scholar]
- 69.Jezek Z., Khodakevich L.N., Wickett J.F. Smallpox and its post-eradication surveillance. Bull. World Health Organ. 1987;65:425. [PMC free article] [PubMed] [Google Scholar]
- 70.World Population Review. 2020. [Google Scholar]
- 71.Lloyd-Smith J.O. Vacated niches, competitive release and the community ecology of pathogen eradication. Phil. Trans. Biol. Sci. 2013;368 doi: 10.1098/rstb.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization Practices—United States. MMWR (Morb. Mortal. Wkly. Rep.) 2022;71:734. doi: 10.15585/mmwr.mm7122e1. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonville C., Suryadevara M. Springer; 2021. Smallpox. Vaccines; pp. 333–342. [Google Scholar]
- 74.Bonville C., Domachowske J., Suryadevara M. Vaccines: A Clinical Overview and Practical Guide; 2020. Rotavirus Infection Etiology; p. 313. [Google Scholar]
- 75.WHO Organization . World Health Organization; 2022. Monkeypox. [Google Scholar]
- 76.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J. Infect. Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 77.Nordic B. BAVARIAN NORDIC ANNOUNCES; U.S.: 2022. FDA APPROVAL OF JYNNEOS™ (SMALLPOX AND MONKEYPOX VACCINE, LIVE, NON-REPLICATING) FOR PREVENTION OF SMALLPOX AND MONKEYPOX DISEASE IN ADULTS. [Google Scholar]
- 78.Erez N., Achdout H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., et al. Diagnosis of imported monkeypox, Israel. Emerg. Infect. Dis. 2019;25:980. doi: 10.3201/eid2505.190076. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.England P.H. 2022. Monkeypox: Information for Primary Care. [Google Scholar]
- 80.Ng O.T., Lee V., Marimuthu K., Vasoo S., Chan G., Lin R.T.P., et al. A case of imported Monkeypox in Singapore. Lancet Infect. Dis. 2019;19:1166. doi: 10.1016/S1473-3099(19)30537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 83.Jirikowski G.F., Sanna P.P., Maciejewski-Lenoir D., Bloom F.E. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255:996–998. doi: 10.1126/science.1546298. [DOI] [PubMed] [Google Scholar]
- 84.Kauffman K.J., Webber M.J., Anderson D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Contr. Release. 2016;240:227–234. doi: 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 85.Kim J., Eygeris Y., Gupta M., Sahay G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021;170:83–112. doi: 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597:318–324. doi: 10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- 87.Obeid M.A., Dufès C., Somani S., Mullen A.B., Tate R.J., Ferro V.A. Proof of concept studies for siRNA delivery by nonionic surfactant vesicles: in vitro and in vivo evaluation of protein knockdown. J. Liposome Res. 2019;29:229–238. doi: 10.1080/08982104.2018.1531424. [DOI] [PubMed] [Google Scholar]
- 88.Guan S., Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24:133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 89.Obeid M.A., Alyamani H., Amawi H., Aljabali A.A., Rezigue M., Abdeljaber S.N., et al. Melanoma. Springer; 2021. Sirna delivery to melanoma cells with cationic niosomes; pp. 621–634. [DOI] [PubMed] [Google Scholar]
- 90.Alyamani H., Obeid M.A., Tate R.J., Ferro V.A. Exosomes: fighting cancer with cancer. Ther. Deliv. 2019;10:37–61. doi: 10.4155/tde-2018-0051. [DOI] [PubMed] [Google Scholar]
- 91.Hussain A., Yang H., Zhang M., Liu Q., Alotaibi G., Irfan M., et al. mRNA vaccines for COVID-19 and diverse diseases. J. Contr. Release. 2022;345:314–333. doi: 10.1016/j.jconrel.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sangboonruang S., Semakul N., Obeid M.A., Ruano M., Kitidee K., Anukool U., et al. Potentiality of melittin-loaded niosomal vesicles against vancomycin-intermediate Staphylococcus aureus and Staphylococcal skin infection. Int. J. Nanomed. 2021;16:7639. doi: 10.2147/IJN.S325901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hussain A., Hasan A., Babadaei M.M.N., Bloukh S.H., Chowdhury M.E., Sharifi M., et al. Targeting SARS-CoV2 spike protein receptor binding domain by therapeutic antibodies. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abbasi J. COVID-19 and mRNA vaccines—first large test for a new approach. JAMA. 2020;324:1125–1127. doi: 10.1001/jama.2020.16866. [DOI] [PubMed] [Google Scholar]
- 99.Hogan M.J., Pardi N. mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 2022;73:17–39. doi: 10.1146/annurev-med-042420-112725. [DOI] [PubMed] [Google Scholar]
- 100.Rubin E.J., Longo D.L. Mass Medical Soc; 2022. Covid-19 mRNA Vaccines—Six of One, Half a Dozen of the Other; pp. 183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otu A., Ebenso B., Walley J., Barceló J.M., Ochu C.L. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. The Lancet Microbe. 2022;3(8):E554–E555. doi: 10.1016/S2666-5247(22)00153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gebril A., Obeid M.A., Bennett E.M., Pujol A., Chovel M.L., Mahy T., et al. Mucosal and systemic immune responses following mucosal immunisation of tetanus toxoid entrapped in lipid nanoparticles prepared by microwave reactor. Eur. J. Pharm. Biopharm. 2022;171:111–118. doi: 10.1016/j.ejpb.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 104.Obeid M.A., Aljabali A.A., Rezigue M., Amawi H., Alyamani H., Abdeljaber S.N., et al. Use of nanoparticles in delivery of nucleic acids for melanoma treatment. Methods Mol. Biol. 2021;2265:591–620. doi: 10.1007/978-1-0716-1205-7_41. [DOI] [PubMed] [Google Scholar]
- 105.Adesokan A., Obeid M.A., Lawal A.F. SARS-CoV-2: vaccinology and emerging therapeutics; challenges and future developments. Ther. Deliv. 2022;13:187–203. doi: 10.4155/tde-2021-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kennedy J.S., Greenberg R.N. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev. Vaccines. 2009;8:13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volkmann A., Williamson A.L., Weidenthaler H., Meyer T.P.H., Robertson J.S., Excler J.L., et al. The Brighton Collaboration standardized template for collection of key information for risk/benefit assessment of a Modified Vaccinia Ankara (MVA) vaccine platform. Vaccine. 2021;39:3067–3080. doi: 10.1016/j.vaccine.2020.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stittelaar K.J., Kuiken T., de Swart R.L., van Amerongen G., Vos H.W., Niesters H.G., et al. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine. 2001;19:3700–3709. doi: 10.1016/s0264-410x(01)00075-5. [DOI] [PubMed] [Google Scholar]
- 109.Adminstration UfaD., JYNNEOS (2022). https://www.fda.gov/media/160858/download.
- 110.Tack D.M., Karem K.L., Montgomery J.R., Collins L., Bryant-Genevier M.G., Tiernan R., et al. Unintentional transfer of vaccinia virus associated with smallpox vaccines: ACAM2000(®) compared with Dryvax(®) Hum. Vaccines Immunother. 2013;9:1489–1496. doi: 10.4161/hv.24319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nalca A., Zumbrun E.E. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010;4:71–79. doi: 10.2147/dddt.s3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 113.Mahase E. British Medical Journal Publishing Group; 2022. Monkeypox: what Do We Know about the Outbreaks in Europe and North America? [DOI] [PubMed] [Google Scholar]