Graphical abstract
Keywords: Monkeypox, Medicinal chemistry, Drug repurposing, In silico, Antivirals
Abstract
Monkeypox disease is a zoonosis that has the monkey virus (MPXV) as its etiologic agent, an enveloped double-stranded DNA virus that has caused some severe cases, leading to 3-6% fatality ratio. MPXV exhibits similar symptoms to those observed for Smallpox infection. Human-to-human transmission occurs via droplet respiratory particles and close contact with skin lesions of an infected person or recently contaminated objects. MPXV symptoms include fever, intense headache, back pain, myalgia, severe asthenia, lymphadenopathy, and skin eruption. The 2022 outbreak is growing, spreading over 75 countries/territories. Based on this, the MPXV outbreak was declared a “public health emergency of international concern” by WHO. In 2019, a vaccine-based modified attenuated vaccinia virus (Ankara strain) was approved for MPXV prevention. However, its availability remains limited. Besides, there are no approved or licensed drugs to treat MPXV currently. However, tecovirimat was licensed by the European Medicines Agency (EMA) to treat MPXV infections. Notwithstanding all these aspects and limitations associated with MPXV, How the medicinal chemistry could help us?
Monkeypox infection is caused by monkey virus (MPXV), an enveloped double-stranded DNA virus belonging to the Orthopoxvirus genus from the Poxviridae family, which is a self-limited disease with symptoms lasting from 5 to 21 days.1 However, some severe cases can occur, having a case fatality ratio between 3 and 6 %. MPXV is a zoonosis that mainly has rodents and non-human primates as animal hosts but can also be human-to-human transmitted, exhibiting similar symptoms to those observed in smallpox patients, which is an acute contagious disease caused by Variola virus (VAR), also a member of the Orthopoxvirus genus. Human-to-human transmission occurs via droplet respiratory particles and close contact with skin lesions of an infected person or recently contaminated objects (i.e., bedding). The onset of MPXV symptoms can be divided into two periods: (i) the invasion (0–5 days) that presents fever, intense headache, back pain, myalgia, severe asthenia, and lymphadenopathy. This last symptom is a distinctive feature from the other similar disease (smallpox, measles, and chickenpox); (ii) the skin eruption (which begins within 1–3 days of the appearance of fever), in which the rash evolves from macules to papules, vesicles, pustules, and crusts which dry up and then fall off. In severe cases, these lesions can coalesce until large sections of skin slough off.2
Posteriorly to the smallpox eradication in 1980 (due to the vaccination), MPXV emerged as the most relevant member from its genus for public health. Initially, MPXV was spread in the west and central Africa, typically in proximity to tropical rainforests, which rapidly emerged in urban areas. In this context, MPXV is found in two genetically distinct clades: the west African clade and the central African (Congo Basin) clade.3 Historically, the Congo Basin clade is responsible for the more severe disease. In 1970, Human MPXV infection was firstly reported in the Democratic Republic of the Congo in a child. Since then, this viral disease has been present in the continent, being reported in more than 10 African countries. Nevertheless, the true burden of MPXV remains unknown. Since 2017, Nigeria has experienced a large outbreak, comprising more than 500 suspected cases and over 200 confirmed cases, with a case fatality ratio of ∼ 3 %. In 2003, MPXV was detected outside Africa, which was associated with infected pet prairie dogs. This fact led to over 70 MPXV infection cases in the USA. In 2018, Israel reported the virus in travelers from Nigeria. Still, the United Kingdom also detected MPXV in travelers in September 2018, December 2019, May 2021, and May 2022.2 Since then, the current outbreak is growing, with more than 16,500 infection cases in 75 countries and territories, including five deaths.4 Based on this, the MPXV outbreak spreading globally was declared a “public health emergency of international concern”(PHEIC) by WHO.5 This is the seventh PHEIC highest alarm in the WHO history, which previously occurred for H1N1 (2009), Polio (2014), Ebola (2014), Zika (2016), Ebola again (2019), and recently, COVID-19 (2020).5
Concerning the vaccination against MPXV, the smallpox vaccine was able to prevent about 85 % of MPXV infections due to cross-protection afforded for the immune response to orthopoxviruses. Currently, there are no more first-generation smallpox vaccines for the general public since these are addressed to laboratory or health workers due to their exposure to orthopoxviruses in the workplace. A newer vaccine based on a modified attenuated vaccinia virus (Ankara strain) was approved for MPXV prevention in 2019. However, its availability remains limited. Regarding the pharmacological alternatives, there are no approved or licensed drugs specifically developed to combat MPXV nowadays. However, an antiviral drug used in the treatment of smallpox, known as tecovirimat (ST-246) (Fig. 1 ), was licensed by the European Medicines Agency (EMA) to also treat MPXV infections, based on studies involving animal and human analyses.2 Besides, some perspectives about the utilization of tecovirimat (ST-246) have been recently published.6 Other antipox compounds have been synthesized and investigating against Vaccinia virus and adenovirus, showing promising results.7, 8 In the Clinical Trials database there are eight active studies involving MPXV, two of them are focused on vaccines (Imvamune9 and MonkeyVax10), while the other two clinical investigations involve tecovirimat (ST-246) drug.11, 12 Additionally, the rest of the clinical studies are focused on biological aspects of this infectious disease. Furthermore, two other antiviral drugs have been in vitro and in vivo investigated, cidofovir (used to treat cytomegalovirus retinitis in AIDS patients) and brincidofovir (used to treat smallpox) (Fig. 1), although data are not available on the effectiveness of these drugs in MPXV-infected persons.13
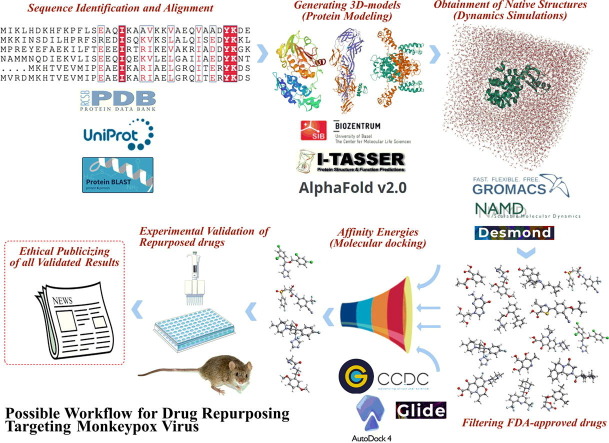
Fig. 1.
Drugs under investigations against Monkeypox and a possible drug repurposing protocol.
Notwithstanding all these aspects and limitations to fight against MPXV, will the global society be hostage to only one drug? Could be possible to quickly develop tecovirimat analogs? If the vaccines to prevent MPXV will not be used in the general population, should we expect the development of a new one? Will MPXV be the next epidemic in Europe? Could MPXV have the potential to become pandemic? Well, many questions remain with no response up to date. As long as these questions are not completely or even satisfactorily answered, medicinal chemistry could save some time and reduce the negative impacts of this emerging viral threat in our society. It has been perfectly worked since the beginning of the COVID-19 pandemic, always investigating new approaches/chemical entities and providing new functions for well-known drugs, by repurposing them. In this context, it is undeniable that the drug repurposing approach boosted several relevant anti-COVID-19 discoveries involving currently commercialized drugs. Again, repurposing old drugs seems to be a good idea against MPXV due to the emergency of the actual situation. With respect to this, the Galaxy Project14 has developed some workflows for the analysis of MPXV sequencing data to enable variant analysis and the generation of consensus sequences. However, there is a very relevant limitation actually for designing drugs – the absence of solved 3D-structures of MPXV macromolecular targets. Currently, there is only one crystal structure deposited (in 2014) in Protein Data Bank, A42R profilin-like protein from MPXV Zaire-96-I-16 (PDB entry: 4QWO).15 Then, the next two questions are: Aren't scientists interested in discovering new targets? How to design/repurpose drugs if there is only one MPXV target? Well, the first question cannot be answered so far. In reply to the second one: Thanks to advances concerning in silico approaches, I strongly believe that is possible to overcome this challenging problem by using different alternatives to build our 3D-models to further investigate them. Initially, some structural detail of MPXV protein sequences can be found in the UniProt database, such as Cell surface-binding protein (entry: Q8V4Y0), E3 ubiquitin-protein ligase p28-like (entry: Q8V571), Poxin-Schlafen (entry: Q8V4S4), mRNA-capping enzyme catalytic subunit (entry: Q5IXS7), Cytokine response-modifying protein B (entry: P0DSV7), Cu-Zn superoxide dismutase-like protein (entry: Q8V4T3), DNA-directed RNA polymerase (entry: Q8V4V3), Thymidine kinase (entry: P0DOM8), among several others. These can be used to build 3D-models by using protein modeling web tools, by using a homology approach or by generating sequence-based 3D-structures. It is essential to build several 3D-structures of different targets since it is not possible to predict the target for every drug investigated. Posteriorly, it is crucial to perform the structural relaxation of these 3D-models by using molecular dynamics simulations, assuming physiological conditions (i.e., ions, water molecules, salts’ concentrations, temperature, pressure, and pH) within a predefined time simulation and under influences of a specific quantum or mechanical force field. Thusly, a robust macromolecular system can be obtained for further investigations. Subsequently, large datasets of ligands can be obtained from the FDA database, and then these can be used in a molecular docking study. This step could be an automatized process (i.e., virtual high-throughput screening – vHTS) or manually performed. In addition, the utilization of more than one searching algorithm is considered a critical point at this step. Then, to improve the chances to identify true-positive drugs, different searching algorithms must be used, in a consensus approach; also, the maximal number of targets must be investigated as potential targets for drugs. Still, more than docking software should be used to predict binding modes for the dataset of ligands. A great is to remove the drugs having dual results (good and bad affinities in distinct docking runs) for the same targets, in order to reduce the false-positives. Finally, the most promising well-known drugs (10–30 entries) must be screened. Herein, another question emerges: Should these drugs be biologically evaluated on assays involving the isolated target (protein or enzyme)? The answer to that is simply no! Since not addressed drugs for MPXV have been repurposed to it, there is no way to know their “new” mechanism of action. So, an ideal situation is to test all of them upon MPXV-infected cells to validate all in silico protocol and findings. It is known that compounds with a specific mechanism of action can target the same conserved target in a family or even genus. However, it is a good alternative to look at the results always saying “maybe” instead of “for sure”. Fig. 1 summarizes all steps to “identify” or repurpose drugs against MPXV. It is expected that some research teams could be able to use these steps described here and boost the race to identify new-old drugs to stop the monkeypox outbreaks, as soon as possible. Finally, it is only a matter of time prior the first huge drug repurposing studies begin to be published. Sincerely, I hope the authors have a lot of responsibility and scientific ethics in publicizing their repositioned drugs, to prevent what happened at the beginning of the COVID-19 pandemic, in which there was a real rush to be the pioneer on the hot-topic; making publicity for false anti-covid-19 pills. This often meant that manuscripts related to COVID-19 were published anyway (without in-depth investigations). Actually, it is a great responsibility to publicize the results of “pharmacological promises”, so we must hope that scientific ethics prevail.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Kumar N., Acharya A., Gendelman H.E., Byrareddy S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131 doi: 10.1016/j.jaut.2022.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Monkeypox - key facts. World Health Organization. Published 2022. Accessed August 12, 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox.
- 3.WHO. Monkeypox - Overview. World Health Organization. Published 2022. Accessed August 12, 2022. https://www.who.int/health-topics/monkeypox#tab=tab_1.
- 4.WHO. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox - 23 July 2022. World Health Organization. Published 2022. Accessed August 8, 2022. https://www.who.int/news-room/speeches/item/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022.
- 5.Kozlov M. Monkeypox declared a global emergency: will it help contain the outbreaks? Nature. Published online July 25, 2022. doi:10.1038/d41586-022-02054-7. [DOI] [PubMed]
- 6.Sherwat A, Brooks JT, Birnkrant D, Kim P. Tecovirimat and the treatment of monkeypox — past, present, and future considerations. N Engl J Med. Published online August 3, 2022:1-3. doi:10.1056/NEJMp2210125. [DOI] [PubMed]
- 7.Kang D., Zhang H., Zhou Z., et al. First discovery of novel 3-hydroxy-quinazoline-2,4(1H,3H)-diones as specific anti-vaccinia and adenovirus agents via ‘privileged scaffold’ refining approach. Bioorg Med Chem Lett. 2016;26:5182–5186. doi: 10.1016/j.bmcl.2016.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., Liu Q., Xie H., et al. Screening and evaluation of potential inhibitors against vaccinia virus from 767 approved drugs. J Med Virol. 2019;91:2016–2024. doi: 10.1002/jmv.25544. [DOI] [PubMed] [Google Scholar]
- 9.Petersen B, Kaba KD. IMVAMUNE® smallpox vaccine in adult healthcare personnel at risk for monkeypox in the democratic Republic of the Congo. Clinical Trials. Accessed August 12, 2022. https://clinicaltrials.gov/ct2/show/NCT02977715?term=Monkey+pox&cond=Monkeypox&draw=2&rank=3.
- 10.Nguyen L binh luong. Follow-up of contact at risk of monkeypox infection: a prospective cohort study (MonkeyVax). Clinical Trials. Published 2022. Accessed August 12, 2022. https://clinicaltrials.gov/ct2/show/NCT05438953?term=Monkey+pox&cond=Monkeypox&draw=2&rank=5.
- 11.Devlin FK, Karaszkiewicz JW. Tecovirimat (ST-246) treatment for orthopox virus exposure. Clinical Trials. Published 2022. Accessed August 12, 2022. https://clinicaltrials.gov/ct2/show/NCT02080767?term=Monkey+pox&cond=Monkeypox&draw=2&rank=8.
- 12.Marbury TC. Phase I trial of an investigational small pox medication. Clinical Trials. Published 2022. Accessed August 12, 2022. https://clinicaltrials.gov/ct2/show/NCT00728689?term=Monkey+pox&cond=Monkeypox&draw=2&rank=7.
- 13.CDC. Monkeypox - treatment information for healthcare professionals. Center for Diseases Control and Prevention. Published 2022. Accessed August 13, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#:∼:text=Data is not available on the effectiveness of,use of Brincidofovir as a treatment for monkeypox.
- 14.Afgan E., Baker D., Batut B., et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minasov G, Shuvalova L, Dubrovska I, et al. 1.52 Angstrom crystal structure of A42R profilin-like protein from monkeypox virus zaire-96-I-16. Protein Data Bank. Published 2014. Accessed August 13, 2022. https://www.rcsb.org/structure/4QWO.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.