Abstract
We explain research gaps on Monkeypox (MPX) virus epidemiology in endemic countries and present hypotheses for the recent increase of MPX cases in West Africa as a possible explanation for the current epidemic in Europe, America, and Australia. The detection of >400 MPX cases in less than a month in May 2022, across many countries underscores the epidemic potential of MPX in humans and demonstrates several important research gaps. First, the true burden of MPX in West and Central Africa is poorly understood, although it is critical for prevention and control of future outbreaks. Second, the diversity and extent of the animal reservoir remain unknown. We hypothesize that the synanthropic rodent population has increased in recent years in Africa leading to more human-rodent interactions and thus increased transmission of MPXV. We further hypothesise that nearly 45 years after the end of routine smallpox vaccination, the larger and more interconnected immune-naïve population has crossed a threshold resulting in more sustainable human-to-human transmission of MPXV. The current epidemic in the Western World is possibly a consequence of increased local transmission of MPXV in Africa. A new estimation of the basic and effective reproduction rate (R0 and Re) in different populations is required. National, regional, and international collaborations are needed to address research gaps related to MPX outbreaks.
Keywords: Monkeypox virus, MPX, outbreaks, West Africa, Nigeria, Reservoirs, smallpox vaccine
Background
Monkeypox virus (MPXV), a member of the Orthopoxvirus genus, is endemic to West and Central Africa and can spillover into human populations from zoonotic hosts. In most cases, however, infection occurs as a result of contact with infected humans (Durski et al. 2018). Following the global eradication of smallpox virus in the 1970s, MPXV is the most widespread and pathogenic Orthopoxvirus in humans (Durski et al. 2018). After the cessation of routine smallpox vaccination, the incidence of monkeypox (MPX) in humans was expected to increase as the loss of cross-immunity from the smallpox vaccine led to a “vacated niche” (Rimoin et al. 2010; Lloyd-Smith 2013). Historically, transmission chains remain small leading to short lasting epidemics (Fine et al. 1988), however, changes in population immunity may lead to sustained epidemics with an initial reproductive rate (R0) >1 (Grant et al. 2020).
MPXV infection shares many clinical features of the smallpox virus, but MPX is significantly less severe (McCollum and Damon 2014). There are two distinct clades of MPXV: the Congo Basin clade and the West African clade. The Congo basin clade is associated with a higher transmission rate and higher case fatality rate (10%). The West African clade is reported as a poor transmitter between humans and has a low case-fatality rate (<1%) (Likos et al. 2005). The effectiveness of smallpox vaccine in preventing monkeypox in humans is estimated to be about 85%, with some evidence towards the efficacy of ring vaccination to prevent transmission chains (Z Jezek and Fenner 1988; Petersen et al. 2019a). Person-to-person transmission occurs via droplet infection and contact with lesions containing replicating viruses. The incidence of MPXV in West Africa has increased at least 20-fold since 1986 through increased human population susceptibility following the end of routine smallpox vaccination (Petersen et al. 2019b).
Mathematical models developed in the 1980’s suggested that MPXV infection would lead to self-terminating epidemics in the human population under conditions of complete absence of vaccine-induced immunity as the estimated reproductive number was less than 1 (R0 = 0.83) (Fine et al. 1988; Lloyd-Smith 2013). However, these assumptions need to be reconsidered for a number of reasons including; i) a larger proportion of people are now naïve to Orthopoxvirus than in the 1980s, ii) both urban and rural human populations are much larger, denser and interconnected, iii) rapid agriculturalization and increasing food storage in villages and towns has led to an increase in synanthropic rodent populations, iv) adaptation of rodents and other animals to urban settings in Africa with increased host interactions (Albery et al. 2022), v) lack of knowledge regarding the definitive reservoir, vi) diagnostics, reporting systems and contact tracing have improved in Africa, allowing detection of more cases and the possibility to derive epidemiological parameters (Kapata et al. 2020; Elton et al. 2021).
A number of countries have recently reported imported MPX cases following recent travel to sub-Saharan Africa (SSA) including the UK in 2018, 2019, 2021, and 2022, Israel in 2018, and Singapore in 2019 (Ng et al. 2019; Mauldin et al., 2020). The CDC also confirmed detection of MPX cases in USA in a patient in July 2021 and November 2021 (CDC 2022). All the above human imported cases were identified as being caused by the West African clade of MPXV with travel links to Nigeria (Alakunle et al. 2020). Our objective was to explain research gaps on MPXV in endemic countries and present hypotheses for the recent increase of MPX cases in West Africa as a possible explanation for current MPXV epidemic in the Western world.
MPX epidemic in Europe, America, and Australia in 2022
On 7th May 2022, the UK Health Security Agency (UKHSA) reported detection of a human case of MPX in a returning traveller from Nigeria (UKHSA 2022). On 14th May 2022, two additional cases living in the same household in London and unrelated to the case reported on 7th May were confirmed with MPXV. Neither of them had a travel history to West or Central Africa. Subsequently, in the next two weeks UKHSA reported 15 additional cases, totalling to 20 cases. By 24th May 2022, at least 21 other countries reported > 270 MPX cases (178 confirmed and 92 suspected cases) with Spain (n=102), the UK (n=71) and Portugal (n=37) leading current case count. (Global Health 2022). A large proportion of cases identified themselves as gay or bisexual, and other men who have sex with men (GBMSM) (Global Health 2022). No mortality recorded as of 24th May 2022. The sex of 138 cases is known and 137 of them are male, 1 female (Global Health 2022). The ages of 87 individuals are known and 86 of them are below 50 years, with the majority in the age group 20-40 years (Global Health 2022). Sequencing of the patient's first isolate from Portugal collected on 4th May 2022 and subsequently from Germany, the USA and Belgium suggest genetic relatedness of the MXPV isolate to individuals who travelled from Nigeria and is consistent with a West African origin (Nextstrain, 2022; Isidro et al. 2022; Mauldin et al. 2020). There is very little evidence of microevolution in these outbreak clusters, and this warrants detailed investigation to hypothesize any patterns of human-to-human transmission mediated by these identified genomes from the 2022 outbreak (Nextstrain, 2022).
The identification of MPX cases in multiple countries in the absence of direct travel links to an endemic region is suggestive of either multiple exportations from an endemic region with subsequent unrecognised local chains of transmission or a single exported to a non-endemic region where a substantial transmission event occurred that then led to subsequent importation of cases into reporting countries from this transmission event. The Gay Pride Maspalomas festival based in the Canary Island, Spain organized between 5th -15th May 2022 supported the gathering of approximately 80,000 attendants (predominantly GBMSM) from different parts of the world (GÜELL 2022). Mass gatherings, such as these, increase contact rates among individuals who may be infectious with transmissible pathogens such as MPX. High contact rates between individuals could play a role in spreading viruses such as MPX (Zumla et al. 2022). There is currently no independent evidence of transmission within this event and epidemiological and phylogenetic linkage to individual events can be challenging to verify. Nonetheless, the rapid spread of MPX cases were probably favoured by a return to normal social interactions because of easing of lockdown and travel restrictions following relaxation of COVID-19 policies.
MPX case burden in Sub-Saharan Africa: the tip of the iceberg
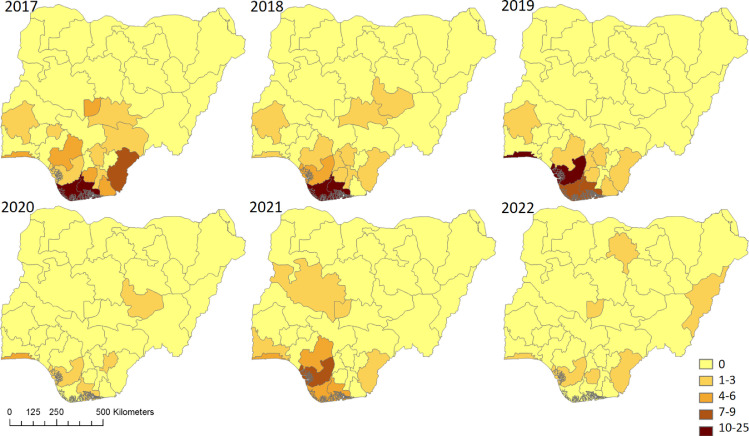
Nigeria reported the first case of MPXV in 1971 with no cases being formally reported between 1978 and 2017. Since 2017 outbreaks of MPX, are reported annually (Fig 1 ). The number of confirmed cases likely represents a small proportion of the true number of infections, due to lack of testing, cases not meeting healthcare services and incomplete reporting practices. Between 1st January 2017 and 30th April 2022, the Nigeria CDC has reported a total of 558 suspected MPX cases; 241 (43%) confirmed cases with 8 deaths giving an estimated case-fatality rate of 3.3% (NCDC 2022). Among confirmed cases, males (69%) and young adults ≤ 40 years old (84%) are over-represented (NCDC 2022).
Fig 1.
Choropleth maps displaying number of monkeypox cases reported by Nigerian Centre for Diseases Control and Prevention by states (n=37) for years 2017 to 2022 (up to 30th April 2022). Detection of monkeypox cases has expanded from South to North during this period.
Accurate ascertainment of incidence of infection in endemic regions requires targeted surveillance studies which may also provide insight into the likely source of infection. One potential indicator of the scale of under-ascertainment within the endemic area is through observation of exported cases or test-positivity rates (Mauldin et al., 2020; Meslé et al. 2022). MPX cases have been detected in the UK in 2018 (Petersen et al. 2019b), 2019, 2021, and 2022 (UKHSA 2022) in individuals travelling from Nigeria. These exported cases suggest substantial local epidemics, with 49 cases being reported in 2018, 47 cases in 2019, 7 cases in 2021, and 111 as of 8th May in 2022 (NCDC 2022; WHO Africa 2022). Modelling studies of another endemic zoonosis, Lassa fever, which has also been detected as exported cases, predicted that 30% of simulated outbreaks would result in the exportation of one or more cases internationally (Tuite et al. 2019). This suggests that for a case to be exported a substantial outbreak must be ongoing within the origin country.
Several countries in SSA are currently reporting ongoing outbreaks of MPXV to the World Health Organisation (WHO). From the 1st Jan up to 8th May 2022, the Democratic Republic of Congo (DRC) have reported a total of 10,459 suspected MPX cases and 360 deaths (estimated CFR as 3.4%), Cameroon has reported 25 cases and 3 deaths (CFR 8%), Central African Republic reported 6 cases and 2 deaths (CFR 33.3%) (WHO Africa 2022). In 2021 (1st Jan – 31 Dec) 9,175 cases with 308 deaths (CFR: 3.4%) were reported from DRC (WHO Africa 2021). Although details are not known from the weekly bulletin, an unusually high CFR suggests possible missing reporting of cases in the endemic countries.
Animal reservoirs
Acute MPXV infection and positive serology have been detected in several animal species including non-human primates and small mammals (Table 1 ). Despite increasing evidence of acute or prior infection, MPXV has been isolated from wild animals on only two occasions, once from a rope squirrel (Funisciurus. anerythrus) and once from a sooty mangabey (Cercocebus atys) (Khodakevich et al. 1986; Radonić et al. 2014). The squirrel was killed by a local hunter in the Democratic Republic of Congo and was symptomatic with pox-like lesions present, while the sooty mangabey was found dead with pox-like lesions in Tai National Park, Côte d'Ivoire (Khodakevich et al. 1986; Radonić et al. 2014). The definite reservoir species of MPXV remains unknown.
Table 1.
List of animals frequently detected with monkeypox virus from Sub-Saharan African countries
| Animals (Species/ genus) | Scientific name | Country | References |
|---|---|---|---|
| Virus isolation | |||
| Rope squirrel | Funisciurus anerythrus | Democratic Republic of Congo (DRC) | (Khodakevich et al. 1986) |
| Sooty mangabey | Cercocebus atys | Cote d'Ivoire | (Radonić et al. 2014) |
| Antibody detected | |||
| Small mammals | |||
| Rope squirrel | Funisciurus anerythrus | DRC, Ghana, | (Reynolds et al. 2010; Doty et al. 2017; Durski et al. 2018) |
| Giant pouched rats | Cricetomys emini | Ghana, DRC | (Reynolds et al. 2010; Doty et al. 2017) |
| Sun squirrels | Heliociurus spp | Ghana | (Reynolds et al. 2010; Doty et al. 2017) |
| Elephant shrews | Petrodromus tetradactylus | DRC | (Hutin et al. 2001) |
| Domestic pigs | Sus scrofa | DRC | (Hutin et al. 2001) |
| African dormouse | Graphiurus lorraineus | (Doty et al. 2017) | |
| Rusty-nosed rat | Oenomys hypoxanthus | (Doty et al. 2017) | |
| Non-human primates (NHP) | |||
| NHP | Cerocopithecus spp | (Breman et al. 1977) | |
| NHP | Colobus spp | (Breman et al. 1977) | |
The impact of ongoing land-use change in West Africa, and Nigeria in particular, may change the hazard of MPX spillover events into human populations from infected hosts through modification of host distribution and abundance (Nguyen et al. 2021). Identification of competent (synanthropic) host species is therefore vital to understanding the association of urbanisation, and modified human-animal contact matrices on epidemic risk. For example, prior estimates of the reproductive rate of human MPX following spillover are sensitive to the communities in which the events occur (i.e. rural, peri-urban inhabitants) and to local contact-matrices. Limited information on which species are competent hosts of MPXV hinders understanding of the impact of land use and host distribution changes on the hazard of MPXV spillover. It is also feasible that the increasing scale of human MPX epidemics is an artefact of sustained transmission within increasingly susceptible, more connected and growing human populations, with spillover from animals occurring at low but constant rates (Thomassen et al. 2013).
Hypothesis on possible increase MPX cases
One hypothesis for the recent increase of MPX cases in SSA is associated with increased interaction between rodents and human due to increase of synanthropic rodent population, possible expanding of zoonotic reservoir, adaptation of rodents and other animals to urban settings (Albery et al. 2022). Pathogen sharing between newly sympatric host species, altered species assemblages, and/or increased rodent densities amongst synanthropic rodent populations may have led to a rise in potential spillover events of MPXV into human populations (Ademola et al. 2021) . Changing land-use, urbanisation, agricultural intensification, and increased human density lead to more human-rodent interactions (Morand and Lajaunie 2020) and may have been exacerbated by the COVID-19 pandemic. The World Bank forecasts that an additional 11 million Nigerians will enter poverty as a cause of the COVID-19 pandemic (World Bank 2021). National lockdowns, rising food prices and a prolonged economic downturn has resulted in devastating impact on informal workers, and those living in insecure communities (Human Right Watch 2021). This forced people to look for alternative protein sources, which resulted in increased consumption of hunted and wild animal meat.
Our second hypothesis is that the global population naïve to smallpox infection and/or vaccine has increased significantly since cessation of routine smallpox vaccination in 1978 and a threshold has been crossed where now sustainable human-to-human transmission of MPXV is observed. For example, in Nigeria by 2016, only 10.1% of the total population was vaccinated against smallpox, among which 25.7% had detectable serologic immunity, compared to 2.6% in the overall population (Nguyen et al. 2021). Since the 1980s, the rural and urban population structure has dramatically changed with people living at much higher densities in both rural and urban areas with greater connectivity between communities and increased contact rates locally, regionally, and internationally. For example, compared to 1980, international airflight has increased by 190%, and passengers travelling internationally increased by 460% in 2019 (International Energy Agency 2022). The current epidemic in the Western World is possibly a consequence of increased local transmission of MPXV in Africa.
Concern over sustained human-to-human transmission
The 2022 epidemic of MPX cases detected GBMSM had no direct travel history to MPXV endemic countries (ECDC 2022). Thus, transmission through intimate contact with infectious skin lesions remains the likely mode of transmission among in GBMSM (ECDC 2022). The European clusters are largely naïve to smallpox infection or vaccine as the majority of cases are under 40 (Global Health 2022). The unusually high frequency of human-to-human transmission observed in the young European clusters, and the probable community transmission without any history of traveling to endemic areas raise the possibility of sustained human-to-human transmission among certain groups. However, these need further study as epidemiological and genomic data will be available in near future.
Conclusions
The 2022 European, American and Australian clusters of MPX cases are possibly results of importation of MPXV from West Africa where an increased transmission is facilitated by multiple factors including increased synanthropic rodent population, or species composition, and adaptation of rodents to human settings and declining population immunity from smallpox vaccines. The global spreading was possibly facilitated by the mass gathering of a pride festival celebrated in Europe in early May. The COVID-19 pandemic might have worsened food security forcing many people to depend on hunted meat as a source of protein. While the true contribution of each factor is unknown, these hypotheses need careful examination in order to reduce MPXV transmission. A key gap in our knowledge is the diversity and extent of the animal reservoir. The 2022 epidemic MPX cases among GBMSM raise further concern about sustainable human-to-human transmission among the risk group. A new estimation of the basic reproduction rate (R0) and effective reproduction rate (Re) in different populations is required. Finally, the recent detection of MPX cases outside Africa indicate the importance of One Health research to limit, control and/or eradicate the infectious diseases at it's source. National, regional, and international collaborations are needed to address research gaps associated with MPXV.
Conflict of interest
The authors declare that they have no conflict of interest
Acknowledgments
Acknowledgments
All authors have a specialist interest in emerging and re-emerging pathogens. NH, JG, DS, DS, RA, IH, TPV, FN, RK and AZ are members of the Pan-African Network on Emerging and Re-emerging Infections (PANDORA-ID-NET - https://www.unza-uclms.org/pandora-id-net) funded by the European and Developing Countries Clinical Trials Partnership the EU Horizon 2020 Framework Programme for Research and Innovation. AZ is a National Institutes of Health Research senior investigator. TPV is a member of the PAN-ASEAN Coalition for Epidemic and Outbreak Preparedness (PACE-UP; DAAD Project ID: 57592343).
Ethical approval
This study does not include any individual-level data and thus does not require any ethical approval.
Data sharing statement
All the data presented in this manuscript are publicly available in scientific manuscript or on the website of Nigeria CDC. However, the corresponding author can be reached to specific queries on the data sources.
Funding Source
There was no funding for this research.
Author contribution
NH, RK and AZ originally planned the study. NH, JG, DS collected and analysed the data. NH prepared the first draft manuscript. JG, DS, DA, RA, IH, TPV, FN, SRV, EP, RK, AZ reviewed the draft manuscripts. All authors approved the final version manuscript for journal submission and subsequent publication.
References
- Ademola OJ, Vanden Broecke B, Leirs H, Mulungu LS, Massawe AW, Makundi RH. Effects of forest disturbance on the fitness of an endemic rodent in a biodiversity hotspot. Ecol Evol. 2021;11(5):2391–2401. doi: 10.1002/ece3.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020;12(11):1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albery GF, Carlson CJ, Cohen LE, Eskew EA, Gibb R, Ryan SJ, et al. Urban-adapted mammal species have more known pathogens. Nat Ecol Evol. 2022 doi: 10.1038/s41559-022-01723-0. [DOI] [PubMed] [Google Scholar]
- Breman JG, Bernadou J, Nakano JH. Poxvirus in West African nonhuman primates: Serological survey results. Bull World Health Organ. 1977 [PMC free article] [PubMed] [Google Scholar]
- CDC. Monkeypox in the United States. 2022.
- Doty JB, Malekani JM, Kalemba LN, Stanley WT, Monroe BP, Nakazawa YU, et al. Assessing monkeypox virus prevalence in small mammals at the human–animal interface in the democratic republic of the congo. Viruses. 2017 doi: 10.3390/v9100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durski KN, McCollum AM, Nakazawa Y, Petersen BW, Reynolds MG, Briand S, et al. Emergence of Monkeypox — West and Central Africa, 1970–2017. MMWR Morb Mortal Wkly Rep. 2018 doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC. Monkeypox cases reported in UK and Portugal. 2022.
- Elton L, Haider N, Kock R, Thomason MJ, Tembo J, Arruda LB, et al. Zoonotic disease preparedness in sub-Saharan African countries. One Heal Outlook. 2021;3(1):5. doi: 10.1186/s42522-021-00037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine PEM, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- Global Health . The Global Health; 2022. Global.health: Monkeypox: Confirmed and suspected cases. [Google Scholar]
- Grant R, Nguyen L-BL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98(9):638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÜELL O. A massive party in Gran Canaria, the second major focus of the monkeypox outbreak in Spain. EL PAIS. 2022 [Google Scholar]
- Nextstrain Genomic epidemiology of monkeypox virus [Internet] Nextstrain team. 2022 https://nextstrain.org/monkeypox [cited 2022 May 24]. Available from. [Google Scholar]
- Human Right Watch . 2021. Between Hunger and the Virus: The Impact of the Covid-19 Pandemic on People Living in Poverty in Lagos. Nigeria. [Google Scholar]
- Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001 doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Energy Agency. World air passenger traffic evolution, 1980-2020. 2022.
- Isidro J, Borges V, Pinto M, Ferreira R, Sobral D, Nunes A, et al. First draft genome sequence of Monkeypox virus associated with the suspected multi-country outbreak, May 2022 (confirmed case in Portugal). 2022.
- Jezek Z, Fenner F. In: Monographs in virology. Melnick JL, editor. Karger; Basel: 1988. Human monkeypox. [Google Scholar]
- Kapata N, Ihekweazu C, Ntoumi F, Raji T, Chanda-Kapata P, Mwaba P, et al. Is Africa prepared for tackling the COVID-19 (SARS-CoV-2) epidemic. Lessons from past outbreaks, ongoing pan-African public health efforts, and implications for the future. Int J Infect Dis. 2020;93:233–236. doi: 10.1016/j.ijid.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakevich L, Jezek Z, Kinzanzka K. ISOLATION OF MONKEYPOX VIRUS FROM WILD SQUIRREL INFECTED IN NATURE. The Lancet. 1986 doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith JO. Vacated niches, competitive release and the community ecology of pathogen eradication. Philos Trans R Soc Lond B Biol Sci. 2013;368(1623) doi: 10.1098/rstb.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin MR, McCollum AM, Nakazawa YJ, Mandra A, Whitehouse ER, Davidson W, et al. Exportation of Monkeypox Virus From the African Continent. J Infect Dis. 2020;225(8):1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum AM, Damon IK. Human Monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- Meslé MMI, Vivancos R, Hall IM, Christley RM, Leach S, Read JM. Estimating the potential for global dissemination of pandemic pathogens using the global airline network and healthcare development indices. Sci Rep. 2022;12(1):3070. doi: 10.1038/s41598-022-06932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand S, Lajaunie C. Outbreaks of Vector-Borne and Zoonotic Diseases Are Associated With Changes in Forest Cover and Oil Palm Expansion at Global Scale. SSRN Electron J. 2020;8(March):1–11. doi: 10.3389/fvets.2021.661063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCDC. Situation report: Monkeypox (MPX) in Nigeria. NCDC Situation report. 2022.
- Ng OT, Lee V, Marimuthu K, Vasoo S, Chan G, Lin RTP, et al. A case of imported Monkeypox in Singapore. Lancet Infect Dis. 2019;19(11):1166. doi: 10.1016/S1473-3099(19)30537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P-Y, Ajisegiri WS, Costantino V, Chughtai AA, MacIntyre CR. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017-2020. Emerg Infect Dis. 2021;27(4):1007–1014. doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BW, Kabamba J, McCollum AM, Lushima RS, Wemakoy EO, Muyembe Tamfum J-J, et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res. 2019;162:171–177. doi: 10.1016/j.antiviral.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Abubakar I, Ihekweazu C, Heymann D, Ntoumi F, Blumberg L, et al. Monkeypox - Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonić A, Metzger S, Dabrowski PW, Couacy-Hymann E, Schuenadel L, Kurth A, et al. Fatal monkeypox in wild-living sooty mangabey, Côte d'Ivoire, 2012. Emerg Infect Dis. 2014 doi: 10.3201/eid2006.131329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MG, Carroll DS, Olson VA, Hughes C, Galley J, Likos A, et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: Evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg. 2010 doi: 10.4269/ajtmh.2010.09-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen HA, Fuller T, Asefi-Najafabady S, Shiplacoff JAG, Mulembakani PM, Blumberg S, et al. Pathogen-Host Associations and Predicted Range Shifts of Human Monkeypox in Response to Climate Change in Central Africa. Khudyakov YE, editor. PLoS One. 2013;8(7):e66071. doi: 10.1371/journal.pone.0066071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite AR, Watts AG, Kraemer MUG, Khan K, Bogoch II. Potential for seasonal lassa fever case exportation from Nigeria. Am J Trop Med Hyg. 2019;100(3):647–651. doi: 10.4269/ajtmh.18-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKHSA. Monkeypox case confirmed in England [Internet]. UKHSA. 2022. Available from: https://www.gov.uk/government/news/monkeypox-case-confirmed-in-england-1
- WHO Africa. Weekly bulletin on outbreaks and other emergencies : Week 52, 2021. 2021.
- WHO Africa. Weekly bulletin on Outbreaks and other emergencies. 2022.
- World Bank. Using data to combat the ongoing crisis, and the next, in Nigeria. 2021.
- Zumla A, Valdoleiros SR, Haider N, Asogun D, Ntoumi F, Petersen E, et al. Monkeypox outbreaks outside endemic regions – scientific and social priorities. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]