Abstract
Following two reports of monkeypox virus infection in individuals who returned from Nigeria to the USA, one who returned to Texas (July 2021) and the other to the Washington, DC area (November 2021), the number of monkeypox infection have dramatically increased. This sounded an alarm of potential for spreading of the virus throughout the USA. During 2022, there was a report of monkeypox virus infection (May 6, 2022) in a British national following a visit to Nigeria who developed readily recognizable signs and symptoms of monkeypox virus infection. Soon following this report, case numbers climbed. By June 10, 2022, more than 1,500 cases were reported in 43 countries, including Europe and North America. While the prevalence of the monkeypox virus is well known in central and western Africa, its presence in the developed world has raised disturbing signs for worldwide spread. While infection was reported during the past half-century, starting in the Democratic Republic of Congo in 1970, in the United States, only sporadic monkeypox cases have been reported. All cases have been linked to international travel or through African animal imports. The monkeypox virus is transmitted through contact with infected skin, body fluids, or respiratory droplets. The virus spreads from oral and nasopharyngeal fluid exchanges or by intradermal injection; then rapidly replicates at the inoculation site with spreads to adjacent lymph nodes. Monkeypox disease begins with constitutional symptoms that include fever, chills, headache, muscle aches, backache, and fatigue. Phylogenetically the virus has two clades. One clade emerged from West Africa and the other in the Congo Basin of Central Africa. During the most recent outbreak, the identity of the reservoir host or the primary carriage remains unknown. African rodents are the suspected intermediate hosts. At the same time, the Centers for Disease Control (CDC) affirmed that there are no specific treatments for the 2022 monkeypox virus infection; existing antivirals shown to be effective against smallpox may slow monkeypox spread. A smallpox vaccine JYNNEOS (Imvamune or Imvanex) may also be used to prevent infection. The World Health Organization (WHO), has warned that the world could be facing a formidable infectious disease challenge in light of the current status of worldwide affairs. These affairs include the SARS-COVID-19 pandemic and the Ukraine-Russia war. In addition, the recent rise in case of numbers worldwide could continue to pose an international threat. With this in mind, strategies to mitigate the spread of monkeypox virus are warranted.
Keywords: Monkeypox outbreak 2022, Viral pathogenesis, Prevention, Antiviral therapies, Transmission
Abbreviations: AIDS, Acquired Immunodeficiency Syndrome; cART, Combination Antiretroviral Therapy; CDC, Centres for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; DNA, Deoxyribose Nucleic Acid; DRC, Democratic Republic of Congo; ELISA, Enzyme Linked Immunosorbent Assay; EMA, European Medicines Agency; EA-IND, Expanded Access Investigational New Drug; FDA, Food and Drug Administration; HIV, Human Immunodeficiency Virus; MPV, Monkeypox Virus; MSM, Men having Sex with Men; MTCT, Mother-to-Child Transmission; MVA, Modified Vaccinia Virus Ankara; PCR, Polymerase Chain Reaction; PLWH, People Living With HIV; PPE, Personal Protective Equipment; RNA, Ribose Nucleic Acid; RT-PCR, Real time Polymerase Chain Reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; STDs, Sexually Transmitted Diseases; TCR, T-cell Receptor; VIGIV, Vaccinia Immune Globulin Intravenous; WHO, World Health Organization
1. Introduction
Concerns about the occurrence of one viral pandemic after another have reached a fever pitch. COVID-19 will soon likely enter an endemic stage. After more than two years of substantive global economic and healthcare impact of COVID-19, unfortunately, we will likely be facing a second new viral outbreak. The second etiological agent is the “monkeypox virus” (MPV). MPV is not new, and it was first discovered in 1958 in Copenhagen. It was isolated from a research facility that housed laboratory animals where monkeys had been shipped from Singapore to Denmark [1]. The name monkeypox was coined for the initial isolate [2]. The first case of animal-to-human zoonotic MPV transmission was reported in 1970 in the Democratic Republic of Congo (DRC) [3]. The MPV belongs to the Orthopoxvirus genus of the Poxviridae family. Variola (smallpox), vaccinia (a research vector tool deployed for the smallpox vaccine), and cowpox viruses belong to the Orthopoxvirus genus. MPV is a double-stranded DNA virus. The poxviruses are known to have a brick-shaped or oval structure measuring 200–400 nm [4].
2. Epidemiology
The first documented MPV case was in a nine-month-old child from DRC in 1970 [5]. MPV outbreaks have risen since 1970 but are primarily contained within the African continent. Notably, there has been limited viral spread to Europe and North America [6]. Up to 48 confirmed cases of monkeypox were reported in six African countries between 1970 to 1979. These included DRC (n = 38), Cameroon (n = 1), Côte d’Ivoire (n = 1), Liberia (n = 4), Nigeria (n = 3), and Sierra Leone (n = 1). By 1986, more than 400 human MPV cases were reported, with mortality approaching 10%. Small viral outbreaks occur routinely in equatorial Central and West Africa [7], including 500 cases in DRC alone between 1991to 1999 [8]. The Congo basin remains endemic in the DRC and includes a high case fatality rate (CFR) [9]. In the DRC, 1000 cases/year were reported.
2.1. Viral Re-Emergence
On May 18, 2022, 14, 7, and 13 cases of MPV infection were reported in Portugal, Spain, and Canada, respectively [10]. On May 19, 2022, Belgium, Sweden, and Italy confirmed their first MPV cases. On May 20, Australia reported two cases. One was from Melbourne and a second from Sydney. Both patients recently returned from Europe. France, Germany, and the Netherlands confirmed their first cases on May 20. The Health Secretary of the United Kingdom (UK) reported another eleven cases of MPV on May 20, with a total number of 71 [11]. Belgium became the first country to introduce a 21-day mandatory quarantine for MPV [11]. Switzerland and Israel confirmed their first cases on May 21. Spain reported the first case on May 18, 2022. On June 3, Spain recently reported an increase in number of 20 cases bringing the country's total cases to 186 [12]. On May 23, Denmark reported its first case. This was from an individual that returned from the Canary Islands. In Canada, Quebec announced 15 confirmed cases on May 24, 2022, where at that time, the Czech Republic confirmed its first case. The reported person participated in an international music festival in Belgium. The United Arab Emirates confirmed its first case in late May, a 29-year-old female visitor from West Africa. Slovenia also confirmed its first case. Until May 24, 19 countries have reported MPV cases. However, the source of the ongoing outbreak of MPV remains to be confirmed. The evolving nature of MPV has suggested human-to-human and or animal-to-human transmission of MPV. Infections first took place in a person who travelled from the endemic regions of Africa to North America and Europe and then spread [13] (Table 1 ).
Table 1.
| Country | Confirmed cases | Suspected cases | Total | Date of the first case | Last updated | |
|---|---|---|---|---|---|---|
| 1 | United Kingdom | 366 | 0 | 366 | May 6, 2022 | June 10, 2022 |
| 2 | Spain | 275 | 0 | 275 | May 18, 2022 | June 10, 2022 |
| 3 | Portugal | 209 | 0 | 209 | May 18, 2022 | June 9, 2022 |
| 4 | Germany | 165 | 0 | 165 | May 20, 2022 | June 10, 2022 |
| 5 | Canada | 112 | 23 | 135 | May 19, 2022 | June 10, 2022 |
| 6 | France | 91 | 0 | 91 | May 20, 2022 | June 9, 2022 |
| 7 | Netherlands | 60 | 0 | 60 | May 20, 2022 | June 9, 2022 |
| 8 | USA | 45 | 0 | 45 | May 18, 2022 | June 9, 2022 |
| 9 | Italy | 32 | 0 | 32 | May 19, 2022 | June 10, 2022 |
| 10 | Belgium | 24 | 0 | 24 | May 19, 2022 | June 8, 2022 |
| 11 | Switzerland | 14 | 0 | 14 | May 21, 2022 | June 10, 2022 |
| 12 | UAE | 13 | 0 | 13 | May 24, 2022 | June 7, 2022 |
| 13 | Ireland | 9 | 0 | 9 | May 27, 2022 | June 10, 2022 |
| 14 | Australia | 8 | 0 | 8 | May 20, 2022 | June 9, 2022 |
| 15 | Czech Republic | 6 | 0 | 6 | May 24, 2022 | June 1, 2022 |
| 16 | Slovenia | 6 | 0 | 6 | May 24, 2022 | June 6, 2022 |
| 17 | Sweden | 6 | 0 | 6 | May 19, 2022 | June 10, 2022 |
| 18 | Ghana | 5 | 0 | 5 | June 8, 2022 | June 8, 2022 |
| 19 | Denmark | 4 | 0 | 4 | May 23, 2022 | June 10, 2022 |
| 20 | Israel | 4 | 0 | 4 | May 21, 2022 | June 9, 2022 |
| 21 | Finland | 3 | 0 | 3 | May 27, 2022 | June 9, 2022 |
| 22 | Hungary | 3 | 0 | 3 | May 31, 2022 | June 10, 2022 |
| 23 | Argentina | 2 | 0 | 2 | May 27, 2022 | May 27, 2022 |
| 24 | Mexico | 2 | 0 | 2 | May 28, 2022 | June 8, 2022 |
| 25 | Norway | 2 | 0 | 2 | May 31, 2022 | June 1, 2022 |
| 26 | Latvia | 2 | 0 | 2 | June 3, 2022 | June 8, 2022 |
| 27 | Austria | 1 | 0 | 1 | May 22, 2022 | May 30, 2022 |
| 28 | Malta | 1 | 0 | 1 | May 28, 2022 | May 28, 2022 |
| 29 | Greece | 1 | 0 | 1 | June 8, 2022 | June 8, 2022 |
| 30 | Gibraltar | 1 | 0 | 1 | June 1, 2022 | June 1, 2022 |
| 31 | Morocco | 1 | 0 | 1 | June 2, 2022 | June 2, 2022 |
| 32 | Brazil | 1 | 9 | 10 | June 9, 2022 | June 9, 2022 |
| 33 | Poland | 1 | 0 | 1 | June 10, 2022 | June 10, 2022 |
| 34 | Uganda | 0 | 4 | 4 | June 8, 2022 | |
| 35 | Bolivia | 0 | 3 | 3 | June 3, 2022 | |
| 36 | Mauritius | 0 | 3 | 3 | June 2, 2022 | |
| 37 | Iceland | 0 | 2 | 2 | June 9, 2022 | |
| 38 | Bahamas | 0 | 1 | 1 | June 7, 2022 | |
| 39 | Bangladesh | 0 | 1 | 1 | June 10, 2022 | |
| 40 | Cayman Island | 0 | 1 | 1 | June 2, 2022 | |
| 41 | Kosovo | 0 | 1 | 1 | June 4, 2022 | |
| 42 | Haiti | 0 | 1 | 1 | June 1, 2022 | |
| 43 | Uruguay | 0 | 1 | 1 | June 5, 2022 | |
| Total | 1475 | 50 | 1525 |
2.2. Outbreak in the US
The first confirmed case of MPV was reported in 2003 in the US. Forty-seven known cases of MPV disease were confirmed in six states (Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin). After contacting pet prairie dogs, each of these patients was MPV infected [14]. Imported small mammals infected these pets from Ghana. Animals imported to Texas from Ghana in April 2003 were the likely source of viral spread. This animal transport contained 800 small mammals, including African giant pouched rats, rope squirrels, tree squirrels, brush-tailed porcupines, striped mice, and dormice [15]. The Centers for Disease Control (CDC) confirmed that nine dormice, two giant African pouched rats, and three rope squirrels were infected with MPV. Few infected animals were housed near prairie dogs, an Illinois animal facility. These pets were sold before signs of infection. People got an MPV infection after having contact with the prairie dogs. On November 16, 2021, the CDC and the Maryland Department of Health confirmed that one case of MPV infection is a US resident. This patient had returned from Nigeria to the United States. CDC also reported another case of Monkeypox in July 2021 of Texas, who also traveled from Nigeria to the US. MPV does not occur naturally in the US. Still, there have been cases associated with international travel or contact with imported animals from areas where the disease is more common. On May 18 in Massachusetts, a case was confirmed by the CDC of a person who had recently returned from Canada. CDC has also informed of Monkeypox clusters that have been found in early to mid-May in several countries that do not typically report MPV, including Europe and North America [16]. Until June 10, 49 cases of MPV infection have been confirmed in the US (Table 2 ).
Table 2.
Distribution of US MPV cases [18].
| S.N. | State | Casesa |
|---|---|---|
| 1 | Arizona | 1 |
| 2 | California | 10 |
| 3 | Colorado | 3 |
| 4 | District of Columbia | 2 |
| 5 | Florida | 5 |
| 6 | Georgia | 1 |
| 7 | Hawaii | 3 |
| 8 | Illinois | 4 |
| 9 | Massachusetts | 1 |
| 10 | New York | 11 |
| 11 | Pennsylvania | 1 |
| 12 | Oklahoma | 1 |
| 13 | Rhode Island | 1 |
| 14 | Texas | 1 |
| 15 | Utah | 2 |
| 16 | Virginia | 1 |
| 17 | Washington | 1 |
| Total | 49 |
Last updated on June 10, 2022 (Source: CDC).
3. Signs and symptoms of viral infection
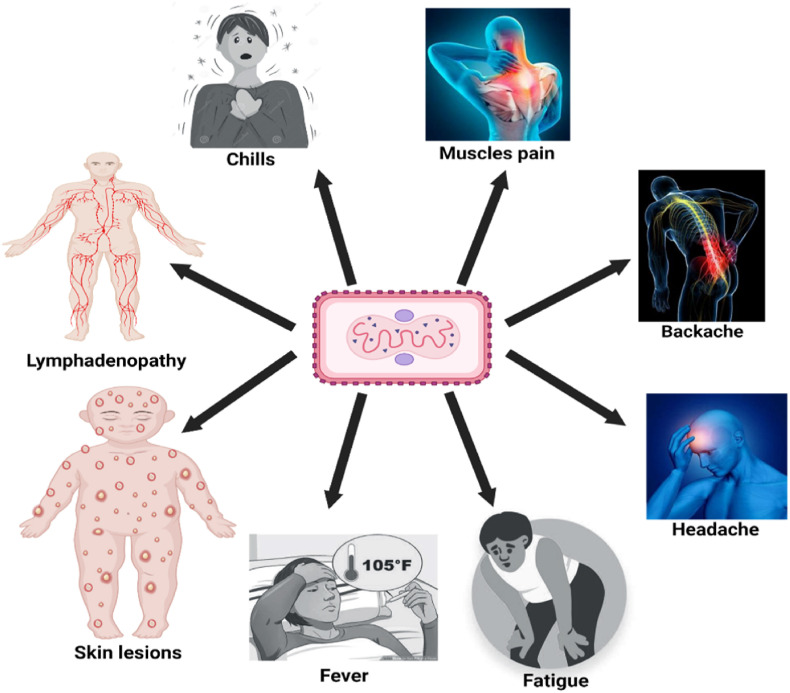
The signs and symptoms of MPV infection reflect a milder form of smallpox [19]. The difference is that MPV infection but not smallpox causes lymphadenopathy. The onset of MPV infection is fever, chills, headache, muscle aches, backache, and fatigue with progression to exhaustion. The incubation period of monkeypox is most commonly 7–14 days but may take up to 21 days. After the appearance of fever, the infected person develops a rash on the face, followed by dissemination to other body parts (Fig. 1 ). Lesions start within the oropharynx and then appear throughout the body. Serum antibodies are detected around 2 weeks post-exposure [20]. The mortality rate ranges from 1 to 10% based on the clade of the infecting MPV strain and the availability of modern healthcare [21].
Fig. 1.
Signs and symptoms of MPV infection (Created using BioRender program).
4. Viral cell entry and pathogenesis
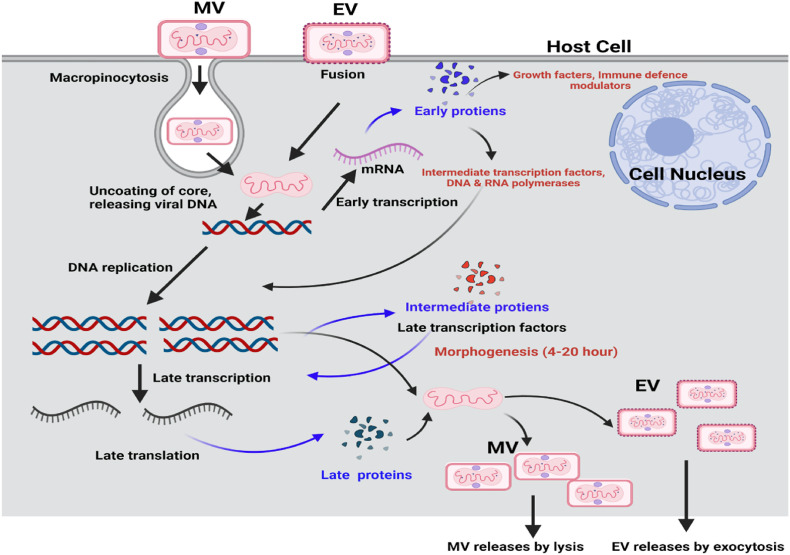
MPV can enter its host through the oropharynx, nasopharynx, or intradermal routes. The virus replicates at the inoculation site and then spreads to regional lymph nodes. Following a period of initial viremia, the virus spreads to other body organs. MPV has a similar morphology to other known orthopoxviruses. MPVs are oval or brick-shaped and are enveloped by a lipoprotein-based outer membrane [22]. The MPV genome is a linear double-stranded DNA (197 kb). Although the MPV is a DNA virus, its life cycle occurs in the cytoplasm. Several proteins are required for viral DNA replication, transcription, and virion assembly [23]. Poxviruses enter the host cells by macropinocytosis endocytosed and by fusion [24] (Fig. 2 ).
Fig. 2.
Cytosolic MPV pathways for the viral life cycle. Enveloped Virion (EV) enters the host cell by fusion and the mature virion (MV) by micropinocytosis or fusion (Created using BioRender program).
Phylogenetically MPV has two clades: the West African and the Congo. The Congo clade originated from Central Africa (Congo Basin) known as the Congo clade. The Congo clade is more pathogenic than the west African clade [25]. Recent sequencing data suggest that the MPV genomic sequence of the present strains detected in Europe (Portugal) matches the West African clade, indicating a milder form of the disease but this needs to be confirmed [26]. In 2003 U S. outbreak in the West African clade suggests that disease severity differs across clades [25]. Generally, West African monkeypox infections show less severity in humans and non-human primates [25,27,28]. However, this 2003 U S. outbreak had several hospitalized patients and severe disease but no fatalities [29]. Congo MPV induces T-cell receptor (TCR) mediated T-cell activation. However, of interest, inflammatory cytokine production is inhibited if the human cells are derived from individuals who had been previously infected with the monkeypox virus. This suggests that monkey poxvirus may produce a modulator that suppresses host T-cell responses [30]. A gene that inhibits complement enzymes is present in Central Africa but is absent in the West African clade. It is a critical immune-modulating factor that potentially increases the virulence of the Central African clade compared to the West African clade [31,32]. Furthermore, it has been reported that the Central African monkeypox clade selectively downregulates host responses compared to the West African clade, as reflected by its ability to specifically modulate apoptosis in the host [33]. A Comparison between the Central African strain (ZAI-96) and three West African strains (SL-V70, COP-58, and WRAIR-61) revealed a 0.55–0.56% nucleotide difference between the Central African strains and the West African strains [34]. This genetic analysis showed that two virus strains clustered separately. The central African strain has 173 unique functional genes, while the West African strain is predicted to have 171 unique genes. There is a difference in virulence between the two strains; therefore, 56 virulence genes were examined, and 53 were shared in both strains. The most significant differences between the two strains are in the orthologs of BR-203, BR-209, and COP–C3L [34].
5. Novel viral mutations
MPV is a DNA virus and generally does not show many mutations compared to RNA viruses like HIV or SARS-CoV-2 [35]. However, as reported, the virus isolated from the 2022 outbreak of MPV seems to have more mutations. It means the virus is evolving to spread more efficiently. Surprisingly, the isolates from the 2022 outbreak shared 40 mutations that distinguish it from its closest variant. In typical evolutionary timelines, one would expect a virus-like MPV to pick up many mutations that may take over 50 years. However, it looks like MPV has mutated because of its ability to transmit among people. Some mutations do not have any harmful effects on the virus, but some can be harmful, and some mutations can take advantage of other strains. There is not enough evidence on how MPV interacts with the host or how the consequences of these mutations affect the rates of virus replication. In some host's (human) immune systems, enzymes are known to induce mutations in viruses if they encounter [36]. If hosts trigger enough mutations, some of the mutations will be deleterious. Based on available sequences, MPV virus evolution seems to have taken off in 2017. Since 2017, that has been circulating in humans and suggested that MPV has a mutation rate around 10 times higher than the virus's standard mutation rate. Currently, information is lacking on how new mutations in MPV interact with the host (human) or what these individual mutations could do. Nevertheless, many mutations in gene sequences of MPV from the current outbreak are alarming to scientists, and more studies are needed to understand the mechanism of action of these newly evolved mutations [37].
5.1. Multiple viral strains are responsible for the 2022 outbreak
The CDC, Atlanta, GA issued a report (Friday, June 3, 2022) that suggests the existence of at least two distinct clades of MPV in the outbreaks that have occurred outside of Africa. The CDC sequenced 10 different virus isolates from the recent outbreaks within the USA and found that these isolates are different from the viruses that have been sequenced by several countries that are involved in the large outbreak that is spreading in and from Europe. The European outbreak appears to be driven primarily by the gay, bisexual, and MSM community. Out of the 10 US isolates, 3 appeared to be distinct from the remaining 7 isolates. The 3 divergent isolates appear to have a common lineage but also appear to differ from one another [38]. Of interest, these 3 divergent isolates appear to have originated in different geographical locations. One appears to have originated in Nigeria, the second in West Africa and the third in the Middle East or East Africa. As of June 10, 2022, there have been 49 cases reported in the USA occurring in 17 different states (see Table 2).
6. Transmission
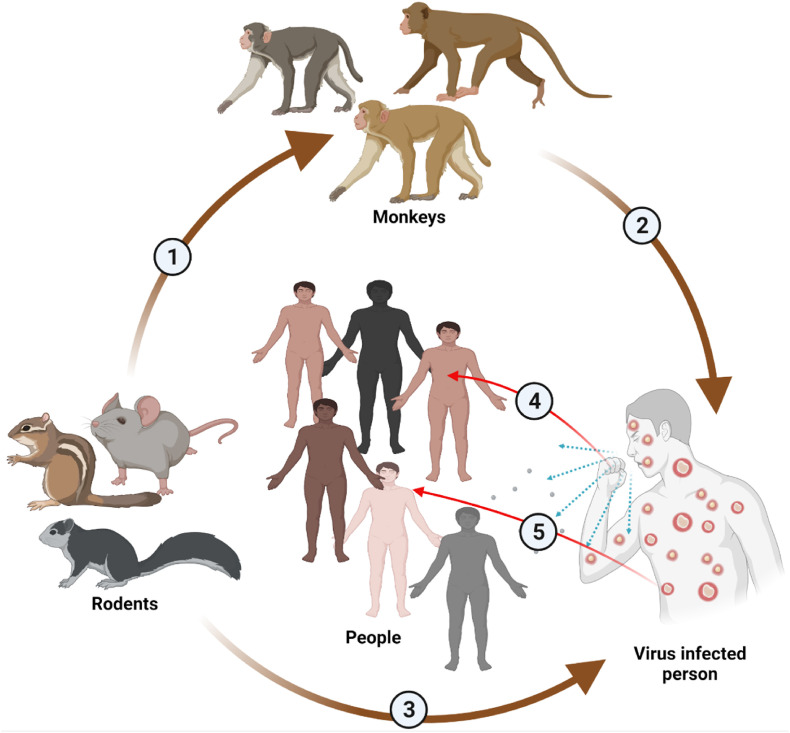
The reservoir host of MPV remains unknown. However, African rodents are suspected of playing a part in the transmission of infection. MPV transmission occurs through contact with skin lesions of the infected animals, body fluids or respiratory droplets [39] (Fig. 3 ). The virus enters the body through the respiratory tract, broken skin, or mucous membranes (eyes, nose, or mouth). Transmission from animal to human may occur through scratch, bite, bush meat preparation, or direct or indirect contact with body fluids or lesion material [40]. Human-to-human transmission occurs through large respiratory droplets, sneezing, coughing, etc. Respiratory droplets do not travel more than a few feet; therefore, prolonged face-to-face contact is necessary for transmission to occur. Other transmission methods from human to human are direct contact with the viral lesion and body fluids and indirect contact with infected materials through clothing or infected linens.
Fig. 3.
MPV transmission. From rodents to monkeys (1). From monkey to human (2). From rodents to humans (3). From infected person to healthy people through cough droplets (4). From infected person to healthy people through direct skin contact (5). (Created using BioRender program).
Mother-to-child transmission (MTCT) may also occur via the placenta (congenital Monkeypox), via close contact during and after birth. Although close physical contact is needed for the transmission of Monkeypox, it is not clear whether the monkeypox virus can be transmitted through sexual routes. Further research studies with well-controlled animal models are needed to understand better whether the virus transmits through the sexual route. In 2020, based on mathematical modeling and comparison with smallpox viruses, it was shown that the reproduction number (R) for MPV is > 1, which indicates that it has an epidemic potential [40]. The quarantine of exposed and infected persons can prevent viral spread [41]. Therefore, identifying infected individuals and quarantining the potentially infected individual for an extended period, such as up to 3 weeks, is critical to controlling the viral spread.
7. Diagnosis
Diagnostic assays are essential to confirm MPV infection and need to be correlated with clinical and epidemiological information. Diagnosis of MPV infection is based on history, clinical symptoms, and laboratory tests. The latter include PCR, ELISA, western blot, and immunohistochemistry. Confirmatory diagnosis is critical to rule out other possible infectious diseases like smallpox [42]. Lesion exudate or crust is collected on a swab to isolate viral nucleic acids for diagnosis. Viral DNA is then used for the MPV genome-specific real-time polymerase chain reaction (RT-PCR) test. On the other hand, MPV proteins are used for western blot analysis to confirm the monkeypox virus infection [21]. As per WHO, the RT-PCR test is the preferred test for diagnosing monkey poxvirus during acute infection [43].
8. Prevention
Some preventative measures can be taken to avoid MPV infection. This includes but is not limited to (1) avoiding direct contact with animals that are suspected of harboring MPV, especially in geographical locations where monkeypox disease is prevalent, (2) isolation of infected patients in a negative pressure room to prevent human to human spread of the virus, (3) isolate and euthanize the animals suspected to be the reservoirs of the virus, (4) avoid contact with any material that has been in contact with a sick animal or human, (5) Front-line workers taking care of MPV infected patients and other high-risk individuals who are expected to come in contact with the infected persons should wear proper personal protective equipment (PPE) that are capable of preventing air-borne infectious agents which includes N-95 mask, entire body covered water resistance gowns, double-layered gloves among others.
Due to their genetic similarities, the smallpox vaccine is expected to provide some protection against MPV infection. According to the United States CDC, prevention of MPV is expected if the vaccine is administered within four days of exposure to MPV due to the virus's long incubation period. It is reasoned that such vaccination should provide complete protection against the disease [13]. To limit the current outbreak of MPV, health departments have implemented policies to administer smallpox vaccines to front-line workers taking care of the infected patients in many countries. On May 24, 2022, the United States CDC decided to release some of their JYNNEOS vaccine (a live vaccinia vaccine originally approved for smallpox virus in 2019) from their National stockpile for persons at high risk of coming in contact with MPV [44]. However, the use of this vaccine is not recommended for the general public. On May 25, the German government had a press release outlining a plan to buy 40,000 smallpox vaccine produced by Bavarian Nordic. On May 26, the United Kingdom Health Security Agency announced that it had already produced 20,000 doses of the smallpox vaccine to combat the rise of MPV cases. Modified Vaccinia virus Ankara (MVA) is a third-generation vaccine against smallpox [45]. USA and Canada have licensed MVA to use against monkeypox. Bavarian Nordic is in contact with the European Medicines Agency (EMA) for the approval of the MVA vaccine [46].
9. Treatment
Monkeypox disease usually induces mild symptoms, and most patients recover without therapy. Per the CDC guidelines, there is currently no specific treatment for monkeypox virus infections. However, antiviral drugs approved to treat smallpox may be used to treat monkeypox disease. Cidofovir (Vistide) is an antiviral medication that inhibits the viral DNA polymerase and effectiveagainst poxviruses in in-vitro and preclinical studies [42]. As per the current CDC guidelines, this drug may be used to treat severely ill monkeypox patients, but the clinical outcome remains unknown. Tecovirimat (ST-246) is an antiviral medication used to treat human smallpox disease in adults and pediatric patients. This antiviral drug is approved by the FDA and can be used to treat Monkeypox during an outbreak. Tecovirimat is given orally (200 mg capsule) as an injectable formulation. Vaccinia Immune Globulin Intravenous (VIGIV) is used to treat complications due to vaccinia vaccination, including eczema vaccinium, severe generalized vaccinia, and infections induced by vaccinia virus. VIGIV can be used for the treatment of Monkeypox during an outbreak. Brincidofovir (Tembexa) is an antiviral drug that the FDA approved to treat human smallpox disease in adult and pediatric patients. CDC is currently developing an Expanded Access Investigational New Drug (EA-IND) to use Brincidofovir as a treatment strategy for Monkeypox.
Other antiviral therapeutic drugs have also shown some effects against Orthopoxviruses species as antiviral therapeutics. These include CMX-001, which is a modified cidofovir drug. It lacks the extent of nephrotoxicity seen with cidofovir and has demonstrated antiviral activity against Orthopoxvirus species, including Monkeypox[42,47] ST-246 (Tecovirimat, also known as TPOXX) is another promising antiviral effect against a variety of Orthopoxviruses species. It blocks the release of the intracellular virus from the cell [42,47]. The use of these drugs in endemic areas to treat MPV infections can be considered, and physicians are allowed to make these decisions depending on the status of the infected persons.
10. Conclusions and future prospectives
The WHO has issued a warning that the world may yet face another major challenge after having met the challenges of the COVID-19 pandemic in the form of the Monkeypox outbreak in the backdrop of the catastrophic Ukraine-Russian war. Until June 10, 2022, 1,475 cases have been confirmed worldwide. The UK itself has reported 366 - cases of Monkeypox. In other countries like Spain (n = 275), Portugal (n = 209), Canada (n = 112), and the USA (n = 49), the number of cases has grown substantially. Therefore, this MPV outbreak is becoming a concern for the whole world at present. The Monkeypox outbreak has been a focus of attention to scientists, epidemiologists, clinicians, and policy leaders. The UK health department has issued an advisory on self-isolation for monkeypox patients. Belgium is the first country to announce a quarantine of three weeks for monkeypox patients. One can control this disease by vaccinations, good hygiene practices, and self-isolation or quarantine for the patients and their contacts. It is important to note that many cases have been detected in the Men having Sex with Men (MSM) population, and this population is at a higher risk of other Sexually Transmitted Diseases (STDs) like Human Immunodeficiency Virus/ Acquired Immunodeficieny Syndrome (HIV/AIDS). The virus exploits this particular group of people for transmission, which is unique to the current outbreak and was not reported previously. Nevertheless, whether MPV is transmitted by sexual route or not remains an enigma for clinical virologists. There are reports from Spain of spreading the MPV among People living with HIV (PLWH) who are on combination antiretroviral therapy (cART) and have entirely suppressed viremia [48]. Therefore, the pathophysiologyof MPV and HIV coinfection needs to be closely monitored. People living with HIV have a compromised immune system; accordingly, how the immune system of PLWH will react against MPV remains to be explored. Other comorbidities, including coinfection of MPV and other STDs like hepatitis B or C infection, will require close monitoring in the coming days. This will also be a formidable task and a big challenge.
The need of the hour is to plan aggressively and put into place an active contract tracing program, quarantine the exposed and infected persons with MPV, and use post-exposure vaccines that may prevent the further spread of the virus. The world is currently facing economic challenges due to the COVID-19 pandemic. The current rise in monkeypox cases in the USA and the world will immediately threaten economic growth prospects. The economic challenges will become more challenging if this monkeypox disease is not brought under control quickly. Social distancing and social stigma are also other challenges for people. People are already facing this situation for the last two years. The awareness of the disease dynamics remains poorly defined, even among the wealthier and more educated parts of the population. People have resisted being screened for the disease and flouted quarantines with impunity. Cough hygiene is mainly absent. Hand hygiene is equally suspect in developing countries. Therefore, better public health strategies are urgently needed, including controlled studies in animal models and others, to prevent the spread of the virus. Special attention needs to be given to children, the elderly, and pregnant women since they are more susceptible to transmission.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This work was partially supported by the National Institute of Allergy and Infectious Diseases grants R01 AI129745, R01 AI113883, and National Institute on Drug Abuse DA052845 to S.N.B. Furthermore, S.N.B. acknowledges independent research and development (IRAD) funding from the National Strategic Research Institute (NSRI) at the University of Nebraska. Further, this work is partially supported by National Institutes of Health grants R01 NS034239; T32 NS105594; R01 NS036126; R01 MH115860; R01 NS126089; R01 AI145542; R01 AI158160; R01 MH121402 and University of Nebraska Foundation to HEG (donations from the Carol Swarts, M.D. Emerging Neuroscience Research Laboratory; the Margaret R. Larson Professorship; and the Frances and Louie Blumkin, and Harriet Singer Endowments).
Data availability
No data was used for the research described in the article.
References
- 1.Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol. Rev. 1973;37:1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.About Monkeypox. CDC; 2022. [Google Scholar]
- 3.Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull. World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 4.Louten J. Essential Human Virology; 2016. Virus Structure and Classification; pp. 19–29. [Google Scholar]
- 5.Marennikova S.S., Seluhina E.M., Mal'ceva N.N., Cimiskjan K.L., Macevic G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull. World Health Organ. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 6.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Neglected Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer H., Perrichot M., Stemmler M., Emmerich P., Schmitz H., Varaine F., et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymann D.L., Szczeniowski M., Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 9.Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S., et al. Emergence of monkeypox - west and central Africa, 1970-2017. MMWR Morb. Mortal. Wkly. Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.B. NEWS. Monkeypox . 2022. Two More Confirmed Cases of Viral Infection. [Google Scholar]
- 11.Monkeypox Cases Confirmed in England – Latest Updates. UK Health Security Agency (UKHSA); 2022. [Google Scholar]
- 12.REUTERS. Factbox . 2022. Monkeypox Cases Around the World. [Google Scholar]
- 13.Kozlov M. Monkeypox outbreaks: 4 key questions researchers have. Nature. 2022;606(7913):238–239. doi: 10.1038/d41586-022-01493-6. [DOI] [PubMed] [Google Scholar]
- 14.Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 15.Martin S. Tennessean; 2022. Rare Case of Monkeypox Confirmed in England, but Risk to the General Public Is Low. [Google Scholar]
- 16.Monkeypox in the United States. Centers for Disease Control and Prevention (CDC); 2022. [Google Scholar]
- 17.2022 Monkeypox Outbreak. WIKIPEDIA; 2022. [Google Scholar]
- 18.Monkeypox U.S. Centers for Disease Control and Prevention (CDC); 2022. 2022: Situation Summary. [Google Scholar]
- 19.U.S. CDC; 2022. Monkeypox Investigation. [Google Scholar]
- 20.Monkeypox M. Moore. vol. 22. National Library of Medicine; Monkeypox: 2022, May. (Publishing ISITIFS). [Google Scholar]
- 21.Adalja A., Inglesby T. Ann Intern Med; 2022. A Novel International Monkeypox Outbreak. [DOI] [PubMed] [Google Scholar]
- 22.Alakunle E., Moens U., Nchinda G., Okeke M.I. vol. 12. Viruses; 2020. (Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kugelman J.R., Johnston S.C., Mulembakani P.M., Kisalu N., Lee M.S., Koroleva G., et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014;20:232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt F.I., Bleck C.K., Mercer J. Poxvirus host cell entry. Curr Opin Virol. 2012;2:20–27. doi: 10.1016/j.coviro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 26.Velavan T.P., Meyer C.G. Trop Med Int Health; 2022. Monkeypox 2022 Outbreak: an Update. [DOI] [PubMed] [Google Scholar]
- 27.Damon I.K. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(Suppl 4):D54–D59. doi: 10.1016/j.vaccine.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in nonhuman primates. J. Gen. Virol. 2009;90:2266–2271. doi: 10.1099/vir.0.010207-0. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds M.G., Yorita K.L., Kuehnert M.J., Davidson W.B., Huhn G.D., Holman R.C., et al. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 2006;194:773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 30.Hammarlund E., Dasgupta A., Pinilla C., Norori P., Fruh K., Slifka M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estep R.D., Messaoudi I., O'Connor M.A., Li H., Sprague J., Barron A., et al. Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J. Virol. 2011;85:9527–9542. doi: 10.1128/JVI.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepers A., Shaw L., Schneckenburger P., Cacan R., Verbert A., Schauer R. A study on the regulation of N-glycoloylneuraminic acid biosynthesis and utilization in rat and mouse liver. Eur. J. Biochem. 1990;193:715–723. doi: 10.1111/j.1432-1033.1990.tb19391.x. [DOI] [PubMed] [Google Scholar]
- 33.Kindrachuk J., Arsenault R., Kusalik A., Kindrachuk K.N., Trost B., Napper S., et al. Systems kinomics demonstrates Congo Basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.M111.015701. M111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanjuan R., Domingo-Calap P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016;73:4433–4448. doi: 10.1007/s00018-016-2299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siggs O.M. Dissecting mammalian immunity through mutation. Immunol. Cell Biol. 2014;92:392–399. doi: 10.1038/icb.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph A. 2022. What the Surprising Mutations in the Monkeypox Virus Could Indicate about the New Outbreak. [Google Scholar]
- 38.Branswell H. STAT NEWS; 2022. Genetic Data Indicate at Least Two Separate Monkeypox Outbreaks Underway, Suggesting Wider Spread. [Google Scholar]
- 39.Angelo K.M., Petersen B.W., Hamer D.H., Schwartz E., Brunette G. Monkeypox transmission among international travellers-serious monkey business? J. Trav. Med. 2019;26 doi: 10.1093/jtm/taz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant R., Nguyen L.L., Breban R. Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 2020;98:638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peter O.J., Kumar S., Kumari N., Oguntolu F.A., Oshinubi K., Musa R. Transmission dynamics of Monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2021:1–12. doi: 10.1007/s40808-021-01313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macneil A., Reynolds M.G., Braden Z., Carroll D.S., Bostik V., Karem K., et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin. Infect. Dis. 2009;48:e6–8. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monkeypox. WHO; 2022. [Google Scholar]
- 44.Harris E. JAMA; 2022. What to Know about Monkeypox. [DOI] [PubMed] [Google Scholar]
- 45.Volz A., Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv. Virus Res. 2017;97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kupferschmidt K. Monkeypox vaccination plans take shape amid questions. Science. 2022;376:1142–1143. doi: 10.1126/science.add3743. [DOI] [PubMed] [Google Scholar]
- 47.Parker S., Handley L., Buller R.M. Therapeutic and prophylactic drugs to treat orthopoxvirus infections. Future Virol. 2008;3:595–612. doi: 10.2217/17460794.3.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen J. Monkeypox outbreak questions intensify as cases soar. Science. 2022;376:902–903. doi: 10.1126/science.add1583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.