Abstract
Objective
Since April 2022, increasing numbers of monkeypox (MPX) cases have been reported outside endemic areas as part of an international outbreak. Our study shows aspects of clinical manifestations as well as epidemiological and virological features impacting transmission, for which only scarce data are available so far.
Methods
We present a descriptive study consisting of epidemiological, clinical and virological data of four patients with confirmed MPX diagnosis. Follow-up examinations included in-depth virological investigations, including MPX virus-specific quantitative PCR and virus isolation.
Results
Between 22 May 2022, and 21 June 2022, four patients with MPX were evaluated. The number of lesions ranged between one and more than 30, with asynchronous eruptions. The periorificial distribution of initial lesions together with the case histories strongly suggest human-to-human transmission during intimate contacts in sexual activities. None of the patients reported about memorable lesions on the skin of potential risk contacts. Virological sampling showed positive MPX virus-specific quantitative PCR results from swabs of the primary lesions (until day 22 after symptom onset), pharyngeal and anal mucosa, urine, seminal fluid, blood and samples of non-affected skin. Virus isolation was positive in 6/14 samples (lesional skin, anal and pharyngeal mucosa). One patient required inpatient treatment for bacterial superinfection; in another patient, three sexually transmitted co-infections were present.
Conclusions
Our report demonstrates asynchronous multiple-site lesions of MPX with prolonged PCR positivity in mucosal swabs, swabs of non-affected skin, urine and seminal fluid. In addition, infectious virus was confirmed on lesional skin and mucosal swabs. The observed virological kinetics together with the suspected pre-symptomatic transmission may lead to effective and sustained human-to-human transmission, particularly in sexual networks. Preventive measures such as vaccination and post-exposure prophylaxis may become important for MPX control in vulnerable groups.
Keywords: MPX, MPXV, Monkeypox, MSM
Introduction
With more than 17 000 confirmed cases in the European region, the current human monkeypox (MPX) outbreak poses the largest outbreak outside endemic areas so far [1]. Cases are predominantly reported among men having sex with men, indicating that major transmission is driven by close or intimate contacts [1,2]. MPX virus (MPXV) belongs to the Orthopoxvirus genus and is closely related to the variola virus. Two distinct MPXV clades are described: the West-African clade, exhibiting a case fatality rate of 3.6%, and the Central-African clade, resulting in more severe disease, with case fatality rates of almost 10% [3]. The clinical presentation of MPX reported so far comprises initial vesicular skin eruptions, with lesions synchronically transforming to pustules and encrusted plaques, accompanied by fever and lymphadenopathy. In endemic countries, the main mode of transmission is by contact with rodents, but human-to-human transmission and transmission in healthcare facilities were also described in approximately 22% to 27% of cases [[4], [5], [6], [7]]. Previous studies indicate protection from MPXV with an efficacy of 85% with smallpox vaccine [5].
Methods
The present study is a detailed description of the clinical and virological findings of four patients with confirmed MPXV infection. Viral DNA was analysed from samples of typical lesions in different stages (vesicle, encrustation, erosion, ulceration), non-lesional skin (forearms/palms), mucosa (pharyngeal/anal), blood (EDTA), urine and seminal fluid. Real-time PCR for MPXV and next-generation sequencing were done as described elsewhere [[8], [9], [10]]. Cycle threshold (Ct) values < 45 were considered positive. Selected samples were subjected to virus isolation on VeroE6 cells.
Ethical review and approval were not required in accordance with the local legislation and institutional requirements. The patients explicitly provided their oral consent to participate in this analysis and the anonymized publication of their clinical data.
Results
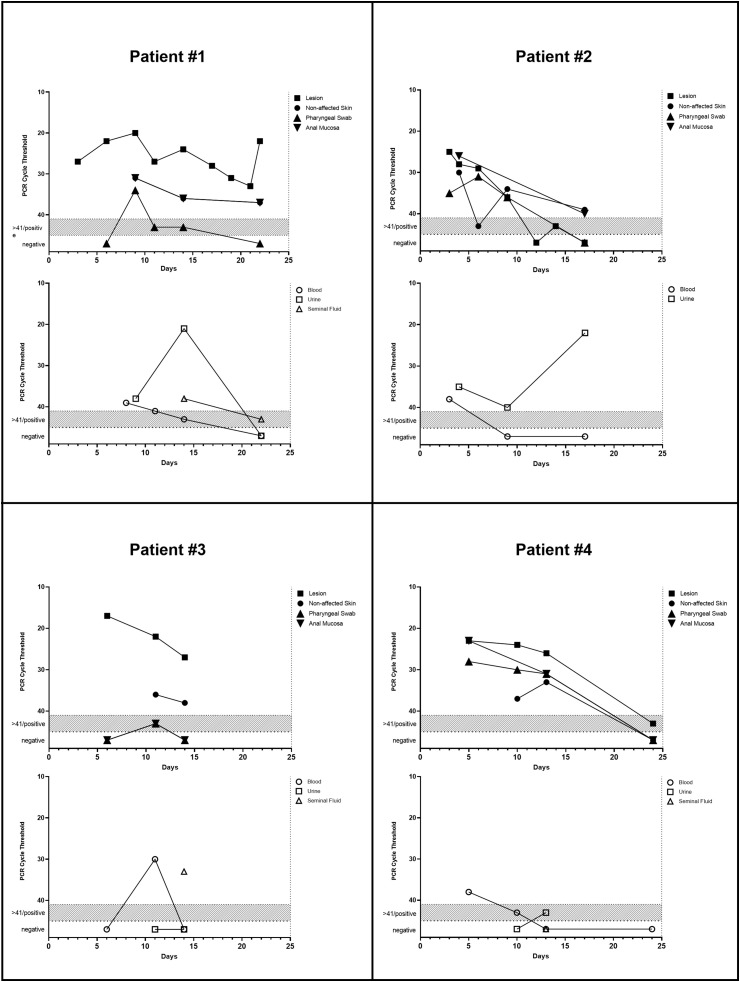
Please see supplementary material for a detailed summary outlining the time courses, clinical manifestations, MPXV detection and virus isolation (Table 1 ) (Fig. 1 ). Whole-genome sequencing showed close clustering within the 2022 outbreak (Western African clade) for all patients with only marginal genomic variations among virus isolates acquired in Spain and Germany [10]. An additional swab, taken from the door handle in a patient's apartment, was positive for MPXV (Ct 31).
Table 1.
Clinical and laboratory features of patients with monkeypox virus infection
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (y) | 29 | 31 | 53 | 38 |
| Sex | Male | Male | Male | Male |
| MSM | Yes | Yes | Yes | Yes |
| Use of PrEP | No | No | Yes | Yes |
| HIV status | Negative | Negative | Negative | Negative |
| Previous smallpox vaccination | No | No | Yes | No |
| Country of acquisition | Spain | Spain | Germany | Germany |
| Use of condoms during risk contact | Yes | Yes | No | No |
| Visible skin lesions in potential risk contacts | No | No | No | No |
| Incubation period (d)a | 4–7 | 9–12 | 13 | <4 |
| Number of primary lesions | approximately 30 | 4 | 1 | 4 |
| Place of primary lesions | Mons pubis | Perioral and perianal | Genital | Perianal, genital and nose |
| Number of subsequently evolving lesions | approximately 5 | approximately 10 | — | 1 |
| Duration until lapse of encrusted lesions (d) | 14 | 12 | 14 | 14–16 |
| Initial fever | Yes | No | No | Yes |
| Lymphadenopathy | Yes | No | No | Yes |
| Hospitalization | Yes | No | No | No |
| Complication or concomitant bacterial infection | Yes (bacterial superinfection) | No | No | Yes (proctitis, chlamydia, gonorrhoea, Mycoplasma) |
| Antibiotic treatment | Yes (piperacillin-tazobactam 4.5 g; 3 times a day for 3 days) | No | No | Yes (ceftriaxone 2 g single dose + doxycycline 100 mg twice a day for 7 days) |
| Supportive medication | Yes (metamizole, pethidine) | No | No | Yes (metamizole) |
| Peak leucocyte (10³/μL) | 11.71 (d14) | 9.6 (d9) | 10.03 (d14) | 6.19 (d10) |
| Nadir lymphocyte (10³/μL) | 1.12 (d14) | 0.76 (d9) | 1.62 (d11) | 0.93 (d10) |
| Peak C-reactive protein (mg/L) | 131 (d6) | 17 (d3) | <3 | 52 (d4) |
| Viraemia | Yes | Yes | Yes | Yes |
HIV: human immunodeficiency Virus; MSM: Men who have sex with men; PrEP: Pre-Exposure Prophylaxis.
Based on reporting of date difference between possible transmission contacts and occurrence of first lesions.
Fig. 1.
Detection of viral DNA with PCR cycle thresholds.
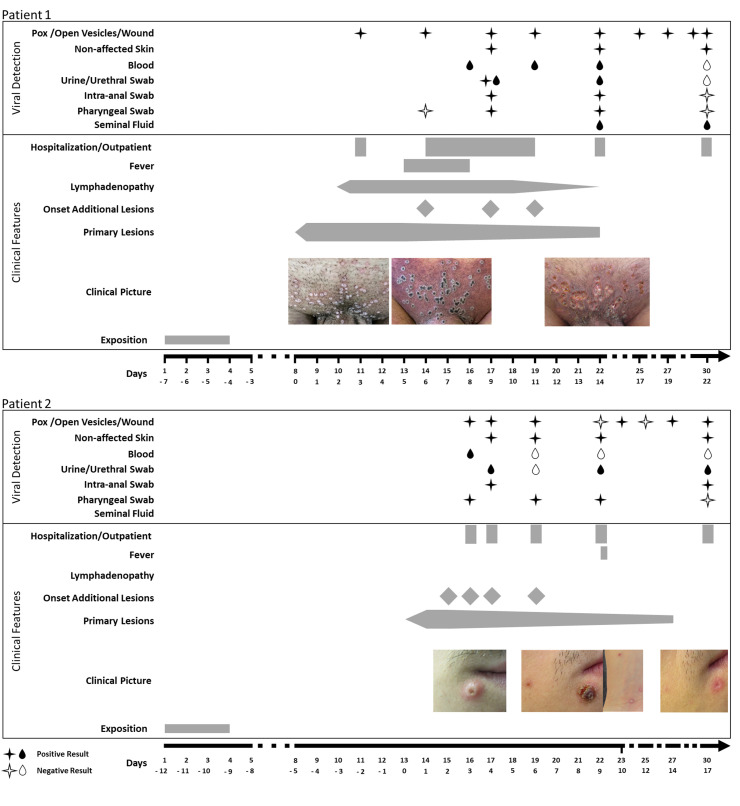
Patient #1 initially sought care at a urologist for multiple vesicular eruptions of the mons pubis 4–7 days after sexual contacts with condom use in Spain. The eruptions aggravated to ulcerous lesions within 3 days after topical treatment against acuminate condylomata. The patient was evaluated at the outpatient clinic of the Department of Dermatology, University Medical Center, Freiburg, on 19 May, 2022, and MPXV was confirmed. After 2 days, the patient developed erythema and swelling of the pubic region, penis and scrotum, with high-grade fever (39.6 °C/103.3 °F), leading to admission to the Infectious Diseases ward at the University Medical Center, Freiburg. Physical examination showed multiple umbilicated pustules and ulcerative skin lesions of the mons pubis with pronounced perilesional erythema and induration as well as prominent inguinal lymphadenopathy. The patient had a slight cough, the chest X-ray investigation findings were normal and examination of the pharyngeal mucosa showed no pathological findings. First laboratory results showed elevated C-reactive protein (CRP) (131 mg/dL) and mild thrombocytopenia (133 × 10³/μL). Supportive therapy with analgesics and antibiotic treatment with piperacillin-tazobactam were initiated for bacterial superinfection. The clinical condition initially improved; however, 9 days after presentation of the initial lesions, newly evolving, disseminated and itchy MPXV-positive lesions and transient dysuria occurred. The primary lesions were encrusting within 8 days after manifestation. At that time, an elevated alanine aminotransferase (481 U/L) and aspartate aminotransferase (332 U/L) were found, whereas a liver ultrasound scan revealed no pathological findings. The patient was discharged 8 days after the admission. Follow-up visits at our outpatient clinic showed a decline of the levels of the elevated liver enzymes and CRP. Lesions were encrusted and crusts fell off within 14 days, revealing sharply demarcated ulcerations.
Patient #2 was the partner of patient #1 and presented on 24 May, 2022, in our outpatient clinic 4 days after he developed itchy, vesicular perioral and perianal lesions that further developed to umbilicated pustules. The patient's history is identical to his partner's history. In this case, the initial lesions presented 9–12 days after the sexual contacts in Spain. Three days after the appearance of the initial lesions, additional lesions appeared in the genital region, ear, temporal scalp, trunk, arms and dorsal side of the hands. Upon presentation, there was no fever, no lymphadenopathy and no visible lesions of the pharyngeal mucosa. Ten days after manifestation of the initial lesions, the patient reported a short, self-limiting episode of increased temperature up to 38.0 °C (100.4 °F) without lymphadenopathy. The primary lesions encrusted and fell off within 12 days after manifestation. Routine laboratory examinations showed no transaminitis but an initially elevated CRP (17 mg/L).
Patient #3 first sought care at a local practitioner on 26 May, 2022, for the appearance of a new single vesicle on the penis, which transformed to an indurated painless ulcerative lesion over the next 4 days. Upon presentation at a sexually transmitted infection (STI) clinic on 2 June, 2022, the PCR test result for Treponema pallidum was negative and MPX was confirmed. The patient reported a potential transmission contact (insertive oral sex) without travel history 13 days before development of the lesion and a vaccination against smallpox in childhood. Until presentation in our outpatient clinic on 7 June, 2022, no additional lesions occurred, and the patient did not develop fever or lymphadenopathy. Examination of the pharyngeal mucosa showed no visible lesions. Routine clinical blood evaluation revealed a discrete elevation of the transaminases (alanine aminotransferase 65 U/L, aspartate aminotransferase 59 U/L) with no signs of systemic inflammation. The patient mentioned moderately elevated liver enzymes of unknown origin for several years. A follow-up visit revealed no additional lesion or complication.
Patient #4 sought care at an STI clinic on 2 June 2022, 5 days after presentation of perianal, genital and facial skin lesions. Three days after occurrence of the lesions, the patient developed an additional lesion on the dorsal side of the hand, lymphadenopathy and fever (38.0 °C; 100.4 °F). Medical history revealed potential transmission incidents from unprotected sexual intercourse without travel history, starting few days before the first appearance of the skin eruption. An initial screening for STIs and MPXV was positive for Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and MPXV. Antibiotic treatment with ceftriaxone and doxycycline was initiated. The patient presented in our outpatient clinic 10 days after manifestation of the initial lesions, which were already in an encrusted stage. Fever and lymphadenopathy had ameliorated. Examination of the pharyngeal mucosa showed no visible lesions. The patient experienced intensive pain during defecation in the past 3 days, which responded to intensified analgesic treatment with metamizole. Manual rectal examination revealed no superficial intra-anal lesions. Routine laboratory analysis showed a declining CRP level (17 mg/L, initial external test 52 mg/dL). Follow-up visits revealed amelioration of the lesions without further complications.
Discussion
Our case series of four patients with human MPXV infection demonstrates several important aspects in the current multinational outbreak: (a) distinct features of cutaneous involvement, including asynchronous eruption of lesions; (b) a common pattern of disseminated mucocutaneous viral shedding (without clear correlation to clinical severity) and MPXV isolation from both lesional skin and mucosa without apparent lesions; (c) cases were caused by human-to-human transmission most likely because of intimate contact in sexual activities with absence of visible lesions in potential risk contacts; and (d) co-infections with other sexually transmitted agents need to be considered in patients with MPXV infection.
Until recently, MPXV infections outside endemic areas were rare, with only casuistic human-to-human transmission [11]. Early reports of the current pandemic and findings of our case series point towards predominantly mild clinical manifestations of MPX. The more severe course of patient #1 was considered to be due to the extensively inflamed lesions (which may be in part a result of the erroneously applied topical acuminate condylomata treatment). Fever and lymphadenopathy were present only in two patients with concomitant infections. MPX-associated transaminitis was possibly present in one patient.
In contrast to previous reports of MPX in endemic regions, the cases observed in our clinic showed no palmoplantar involvement; however, additional lesions evolved up to 11 days after the occurrence of the initial lesions [12,13]. The development of these subsequent lesions may be due to haematogenous dissemination, possible asynchronous inoculation or scratch-induced self-inoculation.
Given the prolonged viral shedding of MPX lesions, close physical contacts during sexual encounters enable direct transmission via mucosal surfaces or skin-to-skin contact [14]. Notably, none of our patients reported to have noticed suspicious skin or mucosal lesions during potential risk contacts. In addition, our findings of PCR positivity in non-lesional samples and detection of infectious virus from mucosal samples may indicate a potential transmission in pre-symptomatic stages of MPX or by oligosymptomatic virus carriers. Moreover, the observed PCR positivity on non-viable surfaces and evidence for contamination of surfaces in hospital rooms may allow for indirect transmission, e.g. via sex toys [15]. Critically, Ct values as a proxy for viral DNA concentrations are not well established for MPXV, and virus isolation was not attempted in all samples.
Vaccination against smallpox poses a promising tool for the control of MPX in vulnerable groups. However, kinetics concerning viraemia and viral shedding of samples from the patient who received a smallpox vaccination in childhood were not divergent from those of the non-vaccinated patients. Condom use, the importance of which is unquestionable, prevents transmission of MPXV by mucosal contacts and/or genital secretions. Nevertheless, direct skin-to-skin contact occurs and may also lead to transmission. Whether concurrent infection by other sexually transmitted pathogens, particularly those causing genital ulcer disease, may enhance MPXV transmission deserves further investigations.
Author contributions
S.R., M.P. and D.H. conceived the study and its design, had full access to the data, and take responsibility for the integrity of the data. T.D., D. Hu., S.B., V.F., G.K., L.J. and J.F. were responsible for the analysis and interpretation of virological investigations. V.G., M. Mu., S.U., A.L. and M.Mo. participated in collecting and analysing data. M.P. and S.R. have equal contribution in the writing of this manuscript. All authors critically revised the drafted manuscript and approved its submission.
Transparency declaration
S.R. reports personal fees from Pfizer, Med Update GmbH and Falk Foundation.
D.H. reports personal fees from Akademie für Infektionsmedizin e.V. and Thieme Publishing Group.
S.U. reports personal fees from Gilead Sciences, Merck & Co (MSD) and ViiV Health Care.
M.P. reports personal fees from Siemens Healthineers.
D.Hu. reports personal fees from Siemens Healthineers and Euroimmun AG.
M.Mo. reports personal fees from Boehringer/Ingelheim, Sanofi-Aventis, Pfizer, UpToDate – Dermatology and Paul-Ehrlich-Institut.
All other authors declare no competing interests.
Funding
None.
Acknowledgements
The authors gratefully acknowledge the patients for providing consent to publish their clinical and laboratory data.
Editor: R. Chemaly
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.09.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.European Centre for Disease Prevention and Control Monkeypox multi-country outbreak. https://www.ecdc.europa.eu/en/monkeypox-outbreak [Accessed 29 August 2022]. Available from:
- 2.Minhaj F.S., Ogale Y.P., Whitehill F., Schultz J., Foote M., Davidson W., et al. Monkeypox outbreak — nine states, may 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jezek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 5.Fine P.E.M., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 6.Nakoune E., Lampaert E., Ndjapou S.G., Janssens C., Zuniga I., Van Herp M., et al. A nosocomial outbreak of human monkeypox in the Central African Republic. Open Forum Infect Dis. 2017;4:ofx168. doi: 10.1093/ofid/ofx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurth A., Achenbach J., Miller L., Mackay I.M., Pauli G., Nitsche A. Orthopoxvirus detection in environmental specimens during suspected bioterror attacks: inhibitory influences of common household products. Appl Environ Microbiol. 2008;74:32–37. doi: 10.1128/AEM.01501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs J., Nekrtenko A., Kohl A.K., Technau-Hafsi K., Hornuss D., Rieg S., et al. 2022. Travel-associated monkeypox virus genomes from two German patients and of a derived virus isolate all closely related to a US sequence, 2022. Monkeypox/Genome Reports. Virological.https://virological.org/t/travel-associated-monkeypox-virus-genomes-from-two-german-patients-and-of-a-derived-virus-isolate-all-closely-related-to-a-us-sequence-2022/844 [Google Scholar]
- 11.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00228-6/fulltext [Accessed 5 June 2022]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erez N., Achdout H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . 2022. Clinical Recognition. Key characteristics for identifying monkeypox.https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html [Accessed 12 June 2022]. Available from: [Google Scholar]
- 14.Endo A., Murayama H., Abbott S., Ratnayake R., Pearson C.A.B., Edmunds W.J., et al. 2022. Heavy-tailed sexual contact networks and the epidemiology of monkeypox outbreak in non-endemic regions.http://medrxiv.org/lookup/doi/10.1101/2022.06.13.22276353 Epidemiology. 2022 [Accessed 30 June 2022]. Available from: [DOI] [PubMed] [Google Scholar]
- 15.Nörz D., Pfefferle S., Brehm T.T., Franke G., Grewe I., Knobling B., et al. Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.26.2200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.