Abstract
Monkeypox 2022, a zoonotic virus similar to smallpox, presented as a rapidly escalating human outbreak with community transmission outside endemic regions of Africa. In just over one month of detection, confirmed cases escalated to over 3300, with reports of patients in at least 43 non-African nations. Mechanisms of transmission in animals and the reservoir host remain uncertain; spread from humans to wild or domestic animals risks the creation of new endemic zones. While initial cases were reported in men who have sex with men (MSM), monkeypox is not considered a sexually transmitted infection. Anyone with close contact with an infected person, aerosolized infectious material (e.g., from shaken bedsheets), or contact with fomites or infected animals is at risk. In humans, monkeypox typically presents with a non-specific prodromal phase followed by a classic rash with an incubation period of 5–21 days (usually 6–13 days). The prodrome may be subclinical, and the monkeypox virus may be transmissible from person-to-person before observed symptom onset. Most clinicians are unfamiliar with monkeypox. Information is rapidly evolving, producing an urgent need for immediate access to clear, concise, fact-based, and actionable information for frontline healthcare workers in prehospital, emergency departments/hospitals, and acute care/sexual transmitted infection clinics. This paper provides a novel Identify-Isolate-Inform (3I) Tool for the early detection and management of patients under investigation for monkeypox 2022. Patients are identified as potentially exposed or infected after an initial assessment of risk factors and signs/symptoms. Management of exposed patients includes consideration of quarantine and post-exposure prophylaxis with a smallpox vaccine. For infectious patients, providers must immediately don personal protective equipment and isolate patients. Healthcare workers must report suspected and confirmed cases in humans or animals to public health authorities. This innovative 3I Tool will assist emergency, primary care, and prehospital clinicians in effectively managing persons with suspected or confirmed monkeypox.
Keywords: Monkeypox, Outbreak, Quarantine, Identify-isolate-inform, 3I, Smallpox, Zoonotic virus, Climate change, Orthopox virus, Rash, Prodrome, Transmission, Clinical, Worldwide
Highlights
-
•
The paper reviews the One Health aspect of monkeypox.
-
•
The epidemiology of this virus has evolved from a rare zoonosis to a multinational outbreak.
-
•
This paper describes the classic and new clinical manifestations of monkeypox.
-
•
Transmission, prevention, treatment, sampling, testing, and safe practices are presented.
-
•
The work produced an innovative Identify-Isolate-Inform (3I) Tool for monkeypox 2022.
1. Introduction
On 29 May 2022, the World Health Organization (WHO) described the global public health risk of the multi-country monkeypox outbreak in non-endemic countries as “moderate.” WHO pointed out that “this is the first time that monkeypox cases and clusters are reported concurrently in widely disparate WHO geographical areas and without known epidemiological links to endemic countries" and with new and unusual features [1]. This zoonotic virus is rapidly escalating beyond the previously endemic areas of West and Central Africa, with evidence of widespread human-to-human transmission. While drastically different than the global situation when SARS-CoV-2 virus emerged, there are notable similarities in the plethora of unknowns and the evolving nature of the science surrounding the monkeypox virus. Monkeypox virus is known to have a wide range of hosts, and there is serious concern that, as human-to-human cases increase in other countries, there can be reverse zoonosis into local wildlife that could lead to the establishment of multiple new endemic areas. Moreover, with readily available CRISPR-Cas technology, this spread raises biosecurity issues. In addition to the challenges of public messaging for a situation with rapid changes, healthcare workers are bombarded with information from numerous sources, including fast-moving social media, which is often inaccurate. Therefore, there is an urgent need for a concise, fact-based tool for frontline workers who are likely to encounter patients with a concern for monkeypox infection, particularly since almost all healthcare providers outside of endemic zones/countries will have never previously encountered a case.
Common things being common, most patients are unlikely to have monkeypox. However, it is essential to rapidly identify patients under investigation, ideally via phone or telehealth, so that appropriate personal protective equipment (PPE) is donned immediately, the patient is isolated, and the key public health authorities are notified in a timely fashion. This manuscript describes how monkeypox has shifted from an uncommon zoonotic disease to a major public health emergency. It provides a novel Identify-Isolate-Inform (3I) Tool (Fig. 1) for frontline clinicians working in the prehospital, sexually transmitted infection (STI) and other clinics, and emergency department/hospital settings.
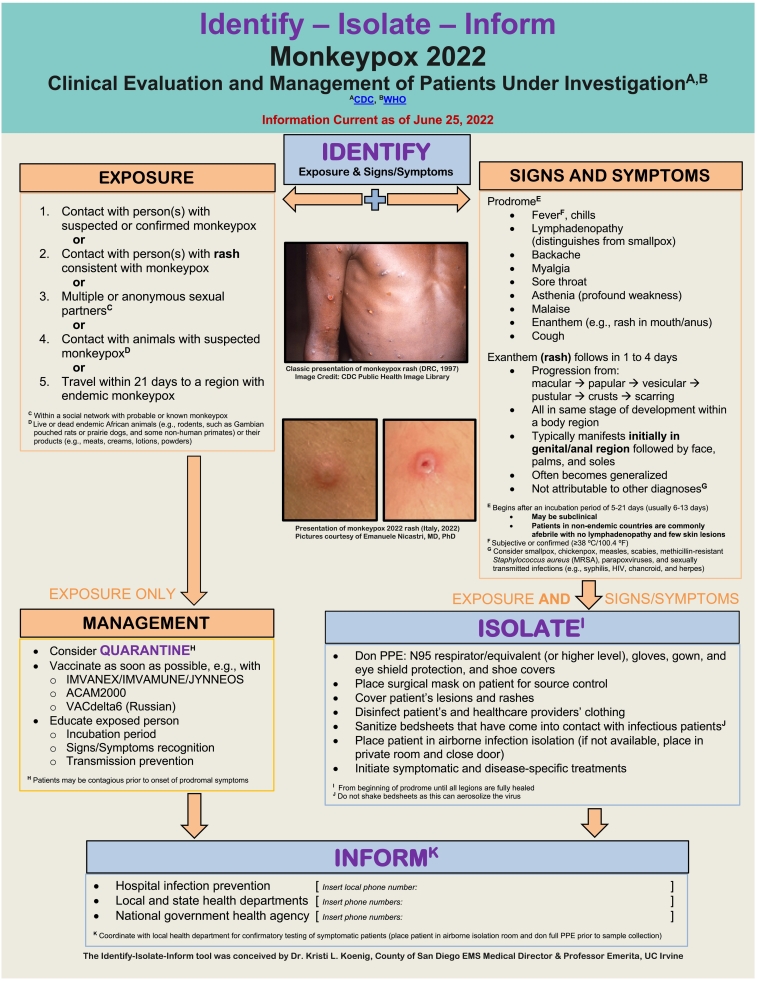
Fig. 1.
Monkeypox 2022 Identify-Isolate-Inform (3I) Tool.
2. Background
On 7 May 2022 [2], the UK confirmed a case of imported human monkeypox. Their report marked the start of detection of this unprecedented rapid global spread of the virus which features human-to-human and community transmission [3]. Approximately one month later, suspected or confirmed human monkeypox cases (US Centers for Disease Control and Prevention; Our World in Data) rose to 3453 in at least 55 non-endemic countries, encompassing every continent other than Antarctica [4]. These data reveal that this is the first large, multi-country person-to-person outbreak of monkeypox that extends outside of Africa. There is concern that continued spread of this internationally significant One Health Threat will facilitate reverse zoonosis and create new endemic zones. A speedy and effective initial global containment strategy can prevent those threats.
Monkeypox is a zoonotic systemic viral exanthema that can have serious consequences. Monkeypox virus can infect and produce clinical disease in a wide range of hosts, including human and non-human primates [5], rodents [6], lagomorphs [7], and some exotic mammals [8], [9]. The close cousin of monkeypox, smallpox, has caused severe human disease since prehistoric times and was a disease of major importance from at least 250 CE until its eradication in 1977 CE [10]. However, monkeypox was largely unknown, and smallpox-like illnesses of monkeys were only sporadically reported [11] until 1958 [12].
After the eradication of smallpox [13], monkeypox became the most significant and most pathogenic Orthopoxvirus of humans, albeit, until the last few years, uncommon. Curiously, the goal of eradicating smallpox contributed to the discovery of monkeypox. Specifically, after Professor Victor Zhdanov [14] proposed, and the World Health Assembly endorsed [15], a smallpox eradication campaign, it became necessary to conduct multiple animal investigations to determine whether smallpox was unique to humans [16]. Two sporadic outbreaks of a non-fatal pox-like illness in two separate shipments from Singapore to Denmark of cynomolgus monkeys led directly to the first definitive identification of monkeypox [12]. Since then, two clades and many strains of monkeypox have been identified and sequenced. The clades are the Congo Basin Clade and the West African Clade. Phylogenetic data reveal that the 1958 and 1961 Macaca fascicularis (cynomolgus) laboratory monkey outbreaks were from the West African Clade [17]. However, it has never been determined why the first eleven known natural outbreaks of monkeypox all originated with animals of Asian origin that were shipped to non-endemic countries from Asian nations [5], nor has the reservoir host [18] been definitively identified [19].
The identification of the first human case of monkeypox was made possible by the intense surveillance for smallpox in an area of the Democratic Republic of the Congo (DRC) where smallpox had been declared eradicated; the first case, a nine-month-old boy detected in 1970 [20], had severe hemorrhagic monkeypox, but recovered. During the 1970s, only 48 confirmed or probable monkeypox cases were reported in six African nations, from Sierra Leone to the DRC. Multiple cases of monkeypox were identified from 1980 to 1985 during an intense surveillance program created to ensure that smallpox was truly eradicated. Those surveillance studies revealed that the smallpox vaccine provided 85% protection against monkeypox and hauntingly predicted the magnitude and duration of monkeypox epidemics would increase as vaccine protection waned [21]. Only 349 confirmed/probable/possible cases of monkeypox were identified between 1980 and 1989 (all in 4 African nations; nearly every case was in the DRC). In the 1990s, there were 520 confirmed/probable/possible human monkeypox cases; all but 9 were in the DRC, with the remainder in Gabon, bringing the total number of human monkeypox cases identified worldwide between 1970 and 1999 to only 917.
Documented human cases of monkeypox were confined to African nations [9] until 2003 and, except for a small outbreak in the DRC, were mostly isolated cases. However, as anticipated, the incidence of human monkeypox began to rise after 2001, as the percentage of humans immunologically naïve to monkeypox increased substantially [22]. This increase correlated with a lack of vaccination with vaccinia and specific One Health issues such as the expanding encroachment of humans into animal habitats, climate change, and geopolitical conflicts. All of this was heavily influenced by socioeconomic disparities. From 2000 to 2009, there were 10,063 cases in African nations, mainly in the DRC, and 47 in the USA. Cases continued to expand the following decade; thus, from 2010 to 2019, there were 19,065 cases in 7 African nations and 6 cases were exported from Africa to non-endemic countries. Of all nations, the DRC is the only one that has reported human monkeypox every decade since 1970 and overwhelmingly experienced the most cases. Other African nations where monkeypox cases have been considered endemic include Côte d'Ivoire, Liberia, Sierra Leone, Nigeria, Gabon, Central African Republic, and Cameroon, though South Sudan has reported 19 cases [23]. Bunge EM and co-authors produced a comprehensive systematic review of the changing epidemiology of human monkeypox that dramatically illustrates how monkeypox has established itself in humans [24].
The rise in monkeypox cases in Africa since 2016 has been particularly significant, with cases reported and confirmed in the DRC, Nigeria, Republic of the Congo, Central African Republic, Liberia, and Sierra Leone [25]. The largest previous human monkeypox outbreak outside of the DRC was in Nigeria in 2017–2018, involving 122 confirmed or probable human monkeypox cases in 17 states [26]. Cases continued to manifest in Nigeria, so that by November 2021, 218 cases had been confirmed. The nation with the highest numbers, however, has been the DRC, with 2780 cases and 72 deaths between 1 January to 31 October 2021 [33], and with continued reports of new cases. By 8 June 2022, the WHO reported that African nations had 1400 confirmed or suspected cases of human monkeypox and up to 71 deaths, although, as of 23 June 2022, only 1 is a confirmed monkeypox death [27,28].
The first human monkeypox cases outside of Africa resulted from a zoonotic multi-state outbreak in the USA that lasted from May to June 2003. Between May and July, 71 cases were reported to the US Centers for Disease Control and Prevention (CDC) from 6 states; however, only 47 were identified as confirmed (n = 37) or probable (n = 10) [29]. In this 2003 outbreak, all cases were zoonotic, primarily from infected prairie dogs, and while no human-to-human transmission was documented, data did reveal probable infection by indirect contact, possibly by aerosol or fomites [30].
After the 2003 outbreak and before the 2022 multinational outbreak, documented cases of monkeypox outside of endemic areas involved exportation to Southern Sudan in 2005 [31], followed by exportation from Africa by travelers from Nigeria to Israel in September 2018, to the United Kingdom (UK) in September 2018, December 2019, May 2021, and June 2021, to Singapore in May 2019, and to the USA in July [32] and November 2021 [33,34].
The December 2019 importation of monkeypox to the UK led to a nosocomial transmission from contaminated bed linen to a healthcare worker. In June 2021, UK health authorities diagnosed monkeypox in a traveler from Nigeria to the UK, and there were secondary and tertiary infections in the same family during that event [35]. In Africa, by the end of 2019, the number of reported human monkeypox cases had increased ten-fold, and the median age of patients had risen from 4 years old in the 1970s to 21 years old.
In the 2022 multinational outbreak, the UK health security agency reported one case to the World Health Organization on 7 May 2022 of a UK citizen who had recently visited Nigeria [36]. One week later, on 14 May, the UK Health services identified two more cases; both lived in the same household, neither had a history of travel to Africa nor had any known contact with the case reported on 7 May. The outbreak rapidly expanded, and cases were reported from nations in Europe, North and South America, Asia, Oceana, and Africa [37]. Clinical features of the 2022 monkeypox outbreak are similar to prior outbreaks, but some distinctive features are reported. As with any re-emerging virus, next-generation sequencing is critically important to understanding the circulating pathogen. Using these techniques, scientists in multiple nations have confirmed that the predominant virus circulating in the 2022 multi-national outbreak is similar to the monkeypox virus from the 2017–2018 Nigerian outbreak [38].
3. Clinical features and management
3.1. Prevention
In addition to avoiding the risky behaviors described in the Risk Factors for Transmission section, healthcare workers can mitigate monkeypox virus transmission using several specific public health measures. When encountering suspected or confirmed patients with monkeypox, healthcare workers should immediately don PPE and place the patient in an airborne-isolation room, if available. If not feasible, place the patient in a single room, ideally with a private bathroom, and close the door. Avoid aerosolizing activities such as shaking sheets, dry mopping, or vacuuming [39]. Additionally, clinicians should minimize aerosol-generating procedures (AGPs), such as administration of nebulized medications and intubation, as this can aerosolize and spread the virus. If such procedures are clinically unavoidable, healthcare workers should don full PPE prior to proceeding.
In the prehospital setting, providers should also avoid AGPs in the back of the enclosed space of the ambulance, e.g., use an inhaled rather than a nebulized medication. Providers must wear full PPE, including N95 or higher respiratory protection, face shield or goggles, gowns, and shoe covers (if available) if the patient requires an emergency treatment that generates aerosols [39].
As source control, clinicians should place a mask on the patient [40] and cover exposed lesions until they are fully healed. Ideally, only vaccinated healthcare workers (e.g., physicians, nurses, technicians, cleaning staff, and others) should manage known or suspected monkeypox patients. Work clothing, such as hospital scrubs, should never be worn home after caring for these patients as the clothing can act as a fomite and unintentionally transmit the virus to others. Separate and wash all contaminated sheets and clothing in hot water with standard detergent. Provide post-exposure ring vaccination for exposed healthcare workers and close contacts.
3.2. Clinical presentation
3.2.1. Transmission
Illness requires that a sufficient number of viral particles enter the body and replicate at a rate that outpaces the ability of the host immune system to prevent colonization or clinical manifestations. Many monkeypox viral particles can enter via broken skin, the respiratory tract, and mucosal surfaces (e.g., mouth, nose, vagina, anus, eyes) in numbers that can overwhelm the barrier immune system and cause disease. Close physical contact with an infected live or dead person, animal, or fomites provides an opportunity for large numbers of monkeypox particles to enter. Transmission by close contact includes exposure to contaminated body fluids (breastmilk, seminal fluid, blood, respiratory droplets), infected skin lesions, or fomites formed by shed skin (e.g., bed linens, clothing, or towels) [41]. Aerosolization of the monkeypox virus can facilitate transmission, for example, if bed sheets are shaken or when performing AGPs; thus healthcare workers are at risk of contracting the virus if they are not wearing appropriate PPE.
In 2022, the vast majority of early confirmed monkeypox cases have been in persons with multiple or anonymous sexual partners, such as MSM, in social networks with confirmed or suspected infection. It is possible that immunoprivileged sites such as the testes can serve as a ‘genital reservoir’ for monkeypox just as they do for other viral diseases [42]. However, this hypothesis needs further investigation.
3.2.1.1. Avoiding stigma
Despite initial reports during the monkeypox 2022 outbreak that most cases were presenting in MSM, monkeypox is not considered an STI. Transmission during sex is a result of close physical contact. It is critical to avoid labeling this as an infection limited to certain populations as this could lead to unnecessary stigmatization of individuals (as happened during the early days of the HIV/AIDS epidemic). In addition, people outside the stigmatized groups may falsely believe they are not at risk and dismiss public health authority guidance and alerts [105].
3.2.1.2. US CDC Clinical and Laboratory Case Definitions (June 23, 2022) [2]
-
•
Suspected: New onset of characteristic monkeypox rash or meets epidemiological criteria and has initial signs and symptoms consistent with an illness that can be confused with monkeypox.
-
•
Probable: No known or suspected prior exposure to another orthopox virus (e.g., Vaccinia virus in ACAM2000 vaccination) and the demonstrable presence of either Orthopoxvirus DNA by nucleic acid amplification tests (NAATs) of a clinical specimen, or Orthopoxviruses detected via immunohistochemical or electron microscopy testing methods, or detectable levels of anti-orthopoxvirus IgM antibody 4 to 56 days after the onset of rash.
-
•
Confirmed: Demonstration of monkeypox virus DNA by polymerase chain reaction or next-generation sequencing of a clinical specimen or isolation of monkeypox virus in culture from a clinical specimen.
3.2.1.3. Signs and symptoms
The classic signs and symptoms of monkeypox closely resemble those of smallpox with certain unique characteristics (Fig. 2A). Lymphadenopathy in inguinal and cervical regions before rash onset is a key distinguishing feature between monkeypox and smallpox [43].
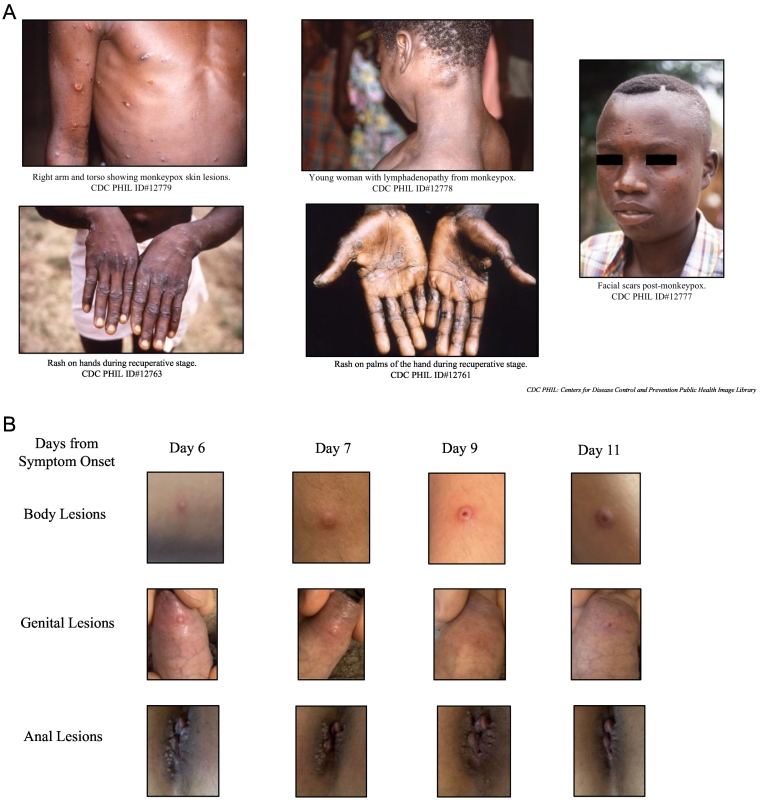
Fig. 2.
A: Classic Presentation of Monkeypox (DRC, 1997). B: Monkeypox 2022 Clinical Presentation. Daily progression of lesions, which began 1-3 days after the prodrome (Italy, 2022). Pictures used with permission from Emanuele Nicastri, MD, PhD.
Following exposure, the incubation time for the onset of prodromal symptoms can vary from 6-13 days with a mean incubation of 8.5 days and a range of 4.2–21 days [44]. Clinical presentations may vary and may include asymptomatic individuals [30]. Generally, however, the prodrome follows an early period of viremia and commonly includes enanthem, fever, chills, myalgia, headache, back pain, exhaustion, and lymphadenopathy. Patients develop a rash within 1–4 days.
In contrast to the rash of chickenpox (varicella), the monkeypox rash usually spreads centrifugally, a pattern similar to that of smallpox (variola). The centrifugal pattern means the rash presents on the face (may include the eyes), palms, and soles before spreading centrally [45]. In the 2022 monkeypox outbreak, the rash typically initiates in the groin and later may follow the classic pattern (Fig. 2B). The rash lesions progress through stages as macular (1–2 d), papular (1–2 d), vesicular (1–2 d), and pustular (5 d) before scabbing (7–14 d) and healing. Lesions are generally deep-seated, circumscribed, vary in size, may be umbilicated, and cause itch or pain [2]. The entire process can last 2–4 weeks or longer.
In addition to the atypical initial distribution of the rash in the groin, prodromal symptoms such as fever and lymphadenopathy may be absent or subclinical in patients of the monkeypox 2022 outbreak. Furthermore, prodromal symptoms may present simultaneously with the rash. The rash and the enanthem may also present simultaneously [23]. A small sample (n = 17) of patients with monkeypox in May 2022, representing the majority of the 25 cases present in the USA as of 5 June 2022, found only about half presented with lymphadenopathy at any point in the illness (53%) and even fewer reported fever (41%) [43]. Painful mucosal sores (enanthem) in the mouth and rectum remain common features. Many patients have visible perianal vesicular, pustular, or ulcerative skin lesions and proctitis that can produce anorectal pain, tenesmus, or rectal bleeding [46].
Another newly described feature in monkeypox 2022 is that the exanthem may present with fewer, less dramatic skin lesions, which are not always synchronous for a given body region [47]. Patients may have a mild rash or a few skin lesions in isolated areas of the body, and in this 2022 outbreak, the rash most often begins in the anogenital region [47,48]. These presentations can be mistaken for common STIs, such as herpes or syphilis; co-infection with monkeypox is possible. Notably, monkeypox cases in earlier outbreaks were also reported to have lesions originating in the anogenital region, and, just as in the 2022 outbreak, some individuals presented with proctitis. Some of these cases were in persons not suspected of having intimate contact with an infected person [49].
An example of an atypical presentation of monkeypox 2022 is a patient who presented to a Montréal clinic with only one tiny non-painful penile lesion and no other rash/lesion or fever [50]. In these patients, the localized anogenital rash (with vesicular, pustular, or ulcerated lesions) does not always spread to other body regions. In addition, pustules may develop before constitutional symptoms such as fever and manifest with lesions at different stages of development. Both of these features are uncharacteristic of historical monkeypox presentations. The requirement for hospitalization is uncommon and usually reflects a need to treat intractable pain or secondary infections.
3.2.2. Differential diagnosis
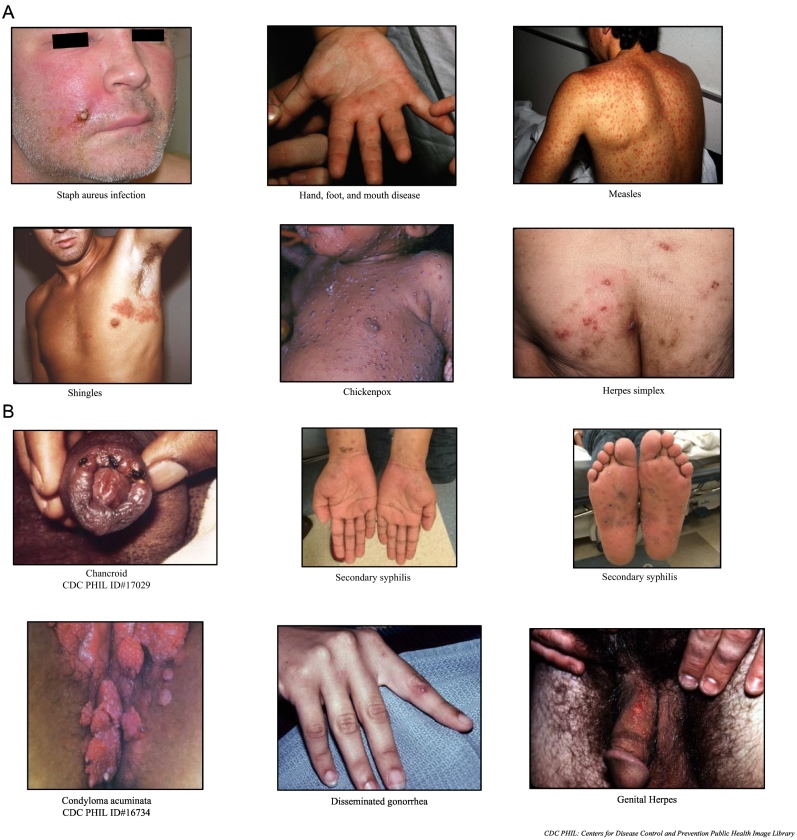
Smallpox and monkeypox classically have similar clinical presentations [34]. Factors, such as prior smallpox vaccination or immunity, disease stage, and virus clade, can alter the clinical presentation. The differential diagnosis of monkeypox is broad (Fig. 3A and B). The non-specific prodrome of influenza-like-illness is common in other diseases; thus, identifying epidemiologic risk factors for potential exposures is critical.
Fig. 3.
A: Sample Infections in Monkeypox Differential Diagnosis. Pictures courtesy of Michael J. Burns, MD. B: Sample Sexually Transmitted Infections in Monkeypox Differential Diagnosis. Pictures courtesy of Michael J. Burns, MD, unless otherwise denoted.
The monkeypox rash may resemble a wide range of acute dermatological conditions, including chickenpox (varicella), impetigo, scabies, Methicillin-resistant Staphylococcus aureus (MRSA), cutaneous anthrax, measles, hand-foot-and-mouth disease, parvovirus B19, rubella, molluscum contagiosum, and typhus. In addition, monkeypox may be mistaken for certain sexually transmitted infections (STIs) that can produce rashes, e.g., syphilis, human immunodeficiency virus (HIV), chancroid, condyloma acuminata, disseminated gonorrhea, and herpes. Co-infections with monkeypox and STIs have been reported. The presence of an STI does not rule out monkeypox [51].
While only present about 70–80% of the time in monkeypox, when present, lymphadenopathy is a useful feature to distinguish it from smallpox. However, clinicians should be aware that this classic feature and the fever may be absent [26]. A detailed history, including comprehensive sexual and travel history, information about close physical contact with anyone with similar symptoms, and the timing of prodromal symptoms and rash progression can help distinguish between these infections.
3.2.2.1. Complications
Monkeypox cases are typically mild and self-resolving after a few weeks in otherwise healthy hosts (who are not at the extremes of age), but skin lesions often produce scars. Less often, it can cause serious complications, including blindness, bronchopneumonia, encephalitis, and sepsis. Similar to smallpox, the most common sequelae of monkeypox are scarring and the appearance of discolored pockmarks, typically in areas with high concentrations of sebaceous glands, such as the face. While death rates in Africa have ranged from 1-10% (depending on the clade), no one died during the first outbreak outside of Africa, the US 2003 outbreak [52]. Similarly, as of 23 June 2022, no confirmed monkeypox deaths have been reported outside of Africa in the 2022 outbreak [53]. However, there have been 72 suspected deaths from monkeypox in African nations in 2022 [54], though only 1 of the 72 is a confirmed death from monkeypox [28,55].
3.2.3. Testing and management of laboratory specimens
3.2.3.1. Testing criteria
Any person who meets the suspected case definition should be offered a test for monkeypox. This includes, but is not limited to, anyone with initial signs and symptoms consistent with an illness that can be confused with monkeypox and those with a rash consistent with monkeypox if the lesions manifested within a few weeks of travel to areas where monkeypox is being reported; after close/physical contact with a person with a similar rash, or who is suspected or known to have monkeypox; after intimate physical or sexual contact, especially any intimate or sexual contact during travel; and intimate contact with an MSM. Use clinical and epidemiological criteria to judge the likelihood of infection to determine testing eligibility. Ideally, an individual who has received a smallpox vaccine should collect samples. On 23 June 2022, the US White House announced that plans were under way to expand the limited testing options and better coordinate with local health departments and the US CDC's national Laboratory Response Network (LRN).
Before collecting samples
-
•
Place the patient in an airborne isolation room (or private room with the door closed if unavailable).
-
•
Don full PPE to include N95 equivalent or higher respiratory protection, eye protection, gown, gloves, and shoe covers.
-
•
Contact the local health department to coordinate types and techniques for sample collection and handling/transportation precautions.
-
•
Consider sending samples for whole genome sequencing (Appendix A).
3.2.3.2. Specimen sampling and collection vary with the stage of the infection
-
•
Viremic stage: Blood (may range from just before the prodrome to just after the rash appears).
-
•
Prodrome stage: Nasopharyngeal, oral swabs. Also, provide blood, urine, and semen samples.
-
•
Exanthem (rash) stage: Skin lesion material, including swabs of exudate from a vesicle, roof from more than one lesion, or crusts from lesions. In addition, provide the same samples as collected during the prior stages.
The viremic stage begins just before the prodrome and extends into the prodrome. However, the viremic stage will likely have passed at the time of rash onset. In the 2022 outbreak, most cases have presented after the viremic stage; neither monkeypox DNA nor antigen is detectable in blood once the viremic stage resolves. Collect blood samples in an ethylenediaminetetraacetic acid (EDTA) tube.
There are no skin lesions in the prodromal stage, but viral DNA is detectable in the nasal, oral, and pharyngeal passages. Enanthem (e.g., oral lesions) have ample viral particles. These lesions are painful, so care in the collection process is important. Use a DACRON® or polyester-flocked swab with or without viral transport media (VTM) to collect the sample. Also, provide a urine sample in a universal sterile container and a blood sample in an EDTA tube.
The skin lesions of the exanthem stage have very high viral concentrations and are the best specimens to collect for testing for monkeypox virus during this stage. Thus, skin materials are the recommended specimen type for laboratory confirmation of monkeypox. Use a dry swab or a swab soaked in VTM to rub the lesion vigorously. Collect samples from two distinct lesions (ideally with different morphologies) from two different body locations and place them into the same tube. Send two tubes (each with two specimens) to the laboratory for testing if possible. In addition to skin samples, collect and submit an oropharyngeal sample [56], provide a urine sample in a universal sterile container, a blood sample in an EDTA tube, and consider submitting a semen sample in a sterile collection tube. Rectal or genital swabs can be collected using DACRON® or polyester-flocked swabs with or without VTM.
3.2.4. Treatment
As of 23 June 2022, no specific US Food and Drug Administration (FDA)-approved treatments exist for monkeypox patients, but the US CDC recommends treating monkeypox 2022 patients with FDA-approved smallpox antivirals [57].
Symptomatic treatment is the mainstay of therapy during the prodromal phase. Standard antiemetic and antifebrile medications can mitigate nausea, myalgias, and fever; however, limiting non-steroidal anti-inflammatory drugs (NSAIDs) is reasonable due to the concern for developing hemorrhagic lesions. Mucosal lesions (e.g., mouth, eyes, and rectal area) can be painful and warrant the use of appropriate analgesics. Trifluridine ophthalmic, a medication approved for herpes keratoconjunctivitis/keratitis, may benefit monkeypox patients with eye lesions [105].
The rash lesions are frequently extremely painful and are a source of secondary infection. Lesions also facilitate person-to-person viral transmission. A clean, moist microenvironment can mitigate transmission potential by covering infectious sores and promoting the re-epithelization of the damaged exanthem. Before applying dressings, clinicians should wash sores with soap and water or dilute povidone‑iodine solution. Bandages should be changed, and lesions washed as often as necessary to maintain a hygienic microenvironment. Oral and topical analgesics or acetaminophen/paracetamol can be administered to attenuate pain.
Patients manifesting secondary bacterial infections should be treated with appropriate antibiotics. These infections can include pneumonia, sepsis, and superinfection of skin lesions. Ophthalmic antivirals can also be administered to mitigate the effect of eye infections [105]. Patients who present with severe immunoreactions to monkeypox can be prescribed immunomodulators. Immunotherapeutics such as tyrosine kinase inhibitors show promise for patients with monkeypox and other Orthopoxvirus infections [58]. Blocking viral proteins that interfere with NF-κB transcription functions may help the body fight off orthopox viruses [59]. Experimental evidence shows that cidofovir combined with selected small interfering RNA (siRNAs) [60] or select siRNA alone [61] can inhibit the replication of orthopox viruses.
Antiviral medications approved for smallpox can be prescribed for those with monkeypox. Tecovirimat (formerly ST-246, with the trade name TPOXX®) is an orthopox antiviral that inhibits the highly conserved p37 Orthopoxvirus protein responsible for the development of virions. The drug has demonstrated efficacy against smallpox in both human and animal models [62]. Tecovirimat was originally approved in 2018 in oral form; in 2022, the drug was approved as an IV formulation [63]. A more available alternative, the nucleotide analog cidofovir, can inhibit the progression of both smallpox and monkeypox [64]. As of 6 June 2022, cidofovir is not US FDA-approved for the treatment of monkeypox. Since this is an outbreak, cidofovir can be used with appropriate regulatory authority, e.g., under emergency use authorization (EUA) or within an investigational new drug (IND) protocol. Alternatively, patients can be prescribed brincidofovir, another US FDA-approved medication for smallpox. Both tecovirimat and brincidofovir are available in the US Strategic National Stockpile. Clinicians should consider the risks and benefits of initiating a specific treatment based on the clinical presentation and other available diagnostic information [65].
The US CDC has an expanded access IND that allows the use of intravenous vaccinia immune globulin (VIG) for treating Orthopoxviruses (including monkeypox) in an outbreak. The US FDA licenses intravenous VIG for the treatment of severe complications from vaccinia [57].
3.2.5. Vaccines
Historical data show that vaccines against smallpox have about 85% efficacy against monkeypox [21]. The earliest prophylactic to protect from smallpox was variolation. During the late 18th century, largely thanks to Jenner's work, the switch was made to vaccination with less dangerous orthopox viruses. The early smallpox vaccines used material from infected cows or horses or substances from the lesions of a human who had been inoculated with material from cows, horses, or a previously vaccinated person.
Vaccinia virus developed from the natural evolution of cowpox, horsepox virus combinations, and human passage of these viruses. This iatrogenic creation of vaccinia led to the first-generation smallpox vaccine, a vaccinia preparation produced by propagating the virus on animal skin, usually the skin of a calf. In the twentieth-century mammalian cell cultures of vaccinia strains produce material referred to as second-generation smallpox vaccines [66]. The ACAM2000 is a purified clone of a second-generation vaccine [67].
These first- and second-generation smallpox vaccines have a significant risk of adverse side effects and, thus, are of limited use [68]. ACAM2000, a purified version of a second-generation vaccine, has the serious potential to replicate uncontrollably in immunocompromised persons and those with a history of atopic dermatitis [69].
Third-generation vaccines use multiple passaging of a vaccinia strain in the cell culture of a heterologous host, which attenuates the vaccinia virus, thereby reducing risks of adverse effects. These viruses do not replicate in mammalian cells (replication-defective) and have lost the immune evasion and virulence factors of the original vaccinia virus while retaining immunogenic properties.
-
•
Bavarian Nordic passaged the Ankara strain of vaccinia multiple times in a chick fibroblast culture which produced numerous mutations and long deletions in the genome. The result was the development of modified vaccinia Ankara (MVA) as a third-generation smallpox vaccine [70]. MVA-BN cannot replicate in most mammalian cells, including human cells. After phase 1, 2, and 3 clinical trials, the vaccine based on the MVA (IMVANEX/IMVAMUNE/JYNNEOS™) stain has been licensed in multiple countries for protection from smallpox or monkeypox. MVA-BN vaccine produces a neutralizing antibody profile similar to conventional first-generation vaccines and protects laboratory animals against zoonotic orthopoxviruses. In non-human primate studies, 2 doses of MVA-BN provided 100% protection against a lethal challenge of aerosolized monkeypox [71]. While pre-exposure vaccination data are excellent, is not known if MVA-BN can provide post-exposure protection from clinical disease.
-
•
KM Biologics (previously Kaketsuken) developed a third-generation smallpox vaccine, LC16m8, licensed in Japan. LC16m8 was produced from the Lister vaccinia strain using multiple passages in a primary rabbit kidney tubular cell culture at a decreased temperature (30 °C). The attenuation of the LC16m8 vaccine results mainly from a critical mutation (single nucleotide deletion) in the B5R gene that encodes a protein essential for extracellular enveloped virion formation. LC16m8 protects laboratory animals against zoonotic Orthopoxvirus, and clinical trials show it is safe and effective in humans [72].
A fourth-generation, genetically-engineered vaccine was produced in Russia [73]. A vaccinia virus with six targeted deletions, VACdelta6 (VACΔ6), was created by engineering targeted deletions/insertions into vaccinia variants that already had impaired genes [74]. Russian scientists successively introduced targeted deletions/insertions into five individual genes of the LIVP vaccinia strain [75]. Then, they introduced targeted deletion into the hemagglutinin A35R gene, which yielded the highly immunogenic attenuated strain, VACΔ6 [76]. Clinical trials in humans and animals have shown excellent safety and efficacy. The WHO predicts the complete registration and licensing of VACΔ6 in 2022 [77].
In the USA, the currently approved vaccines include ACAM2000, the purified clone of the Dryvax vaccine by Sanofi, and the modified vaccinia Ankara by Bavarian Nordic (JYNNEOS™ in the USA, IMVANEX™ in Europe, IMVAMUNE™ in Canada). JYNNEOS™ is FDA-approved for both smallpox and monkeypox, and the US Strategic National Stockpile contains both JYNNEOS™ and ACAM2000. The immunocompromised and those with atopic dermatitis should not be vaccinated with a second-generation vaccine such as ACAM2000, but can receive a third-generation vaccine such as JYNNEOS™/IMVANEX/IMVAMUNE or LC16m8. People with allergies to vaccine components should not be vaccinated. Intravenous VIG by Cangene Corp., a US FDA-approved product in the strategic national stockpile can be administered to individuals who are ineligible for a vaccine. Vaccination is not indicated in symptomatic patients.
3.2.5.1. Pre-Exposure Prophylaxis (PrEP)
As of 23 June 2022, the US CDC does not recommend routine smallpox vaccinations, but does encourage laboratory staff handling orthopox viruses to be vaccinated and receive a booster every three years. Conversely, some countries have started offering vaccines to other high-risk groups, such as MSM.
3.2.5.2. Post-Exposure Prophylaxis (PEP)
Ring vaccination with an approved smallpox vaccine is indicated for post-exposure prophylaxis (PEP). Vaccination for PEP must be initiated as soon as possible, ideally within four days of exposure and up to 14 days after exposure. Once a patient becomes symptomatic, vaccination is no longer beneficial. Fortunately, persons vaccinated before the discontinuation of routine immunization do have some protection against smallpox [78], but there is insufficient data to determine if this residual immunity protects against monkeypox. While it is reasonable to consider that the efficacy of third- or fourth-generation vaccines for PEP is similar to that of second-generation vaccines, as of 23 June 2022, there are no definitive studies.
3.2.6. Special considerations in pregnancy
Pregnant and breastfeeding women may be at high risk of severe disease. The virus can be passed from mother to child via the placenta or contact with the perineum during delivery. Data regarding clinical management of pregnant and postpartum women exposed to monkeypox are limited [79]. Considerations include:
-
1.
administer PEP vaccination using a third- or fourth-generation non-replicating smallpox vaccine, e.g., MVA-BN (JYNNEOS™/IMVANEX/IMVAMUNE)
-
2.
perform C-section if the mother has active lesions
-
3.
isolate the mother from her child during active disease
-
4.
avoid breastfeeding during active disease (milk should be expressed so breastfeeding can resume once lesions heal)
If the woman is severely ill, consider using cidofovir, a US FDA Category C drug. Do not administer tecovirimat (also known as TPOXX®), which is unapproved in pregnancy [80]. There are no data on the use of VIG in pregnant women.
4. Investigative product (results) & discussion
4.1. Identify-Isolate-Inform (3I) Tool
As a result of an intensive review and synthesis of the evidence surrounding the monkeypox 2022 outbreak, an Identify-Isolate-Inform (3I) Tool for monkeypox is provided. This innovative tool is based on the original 3I concept that was first conceived during the US Ebola outbreak in 2014 [81,82]. In subsequent years, the 3I concept has been adapted to create individualized tools for measles [83], Middle East respiratory syndrome (MERS) [84], mumps [85], Zika virus disease [86], hepatitis A [87], pertussis [88], scabies [89], and coronavirus disease 2019 (COVID-19) [90]. The monkeypox 2022 iteration of the Tool (Fig. 1) is designed to provide frontline clinicians with an accurate, actionable algorithm to apply to patients under investigation for the virus. Before proceeding with the algorithm, clinicians must observe the Vital Sign Zero concept [91], which uses a preliminary assessment to determine the need to don appropriate PPE before physically contacting a patient, thus minimizing the risk of clinician exposure to a contagious disease. Precautions to prevent the spread of COVID-19 have undoubtedly strengthened the awareness of Vital Sign Zero, even if not by name.
According to the Monkeypox 2022 3I Tool, providers should first identify a patient's monkeypox exposure risk and then assess for signs and symptoms. Patients may meet the criteria of exposure, signs/symptoms, or both. In the case of exposure, consider quarantine in consultation with local public health authorities. If a patient has a confirmed risk factor, then PEP and education are crucial to reducing the risk of illness in the individual and for transmission to others.
The second action for the 3I Tool is to isolate symptomatic patients. Patients can present with a combination of some or all of the symptoms listed in the 3I Tool, and non-specific prodromal symptoms can be subclinical. Similar to 2003 monkeypox cases, 2022 cases range in their presentations despite having key features: lymphadenopathy, fever, and a characteristic exanthem as described above. Healthcare workers should place a surgical mask on patients who have signs/symptoms, and patients should be isolated immediately in an airborne infection isolation room or a private room with the door closed if no isolation room is available. In addition, any exposed lesions and rashes should be covered. Fomites, particularly on bed sheets and clothing, pose a high risk to others. Avoid shaking bed sheets or dry mopping to prevent aerosolizing virus from skin sheddings. Healthcare workers and other close contacts should sanitize clothing following contact with a patient.
Following the identification and isolation stages of the algorithm, providers should inform the appropriate public health authorities of clinically suspected or confirmed monkeypox. Human monkeypox is not endemic outside Africa; therefore, even a single case in a non-endemic region represents an outbreak. Local and state health departments play vital roles in monkeypox laboratory confirmation, reporting results, and managing infectious persons. The Emergency Operations Center of the government health agency should also be directly contacted to report suspected or confirmed cases of monkeypox. By following the general framework of the 3I Tool, clinicians can optimize the management of patients, protect themselves and others from contracting infectious diseases, initiate the correct patient treatments, and effectively notify public health authorities promptly. Appendix B provides a version of the 3I Tool that allows users to insert their unique healthcare facility and local, state, and national health agency phone numbers, as applicable. The 3I Tool can be integrated into electronic health records, printed for frontline and triage personnel, and displayed as a poster.
4.2. Public health policy considerations
The global monkeypox 2022 outbreak highlights several key One Health challenges of critical concern for public health and emergency preparedness. These include:
-
•
lack of a definitively known reservoir host
-
•
capacity to infect many hosts creates the potential for reverse zoonosis that introduces a serious concern that the 2022 outbreak could lead to the formation of new endemic zones
-
•
likely economic impact on health care with spinoff consequences to the general economy
-
•
potential for psychosocial disruptions
-
•
initial lack of easily available decentralized testing resources to enable rapid and early identification of cases to facilitate outbreak containment
-
•
higher viral circulation can lead to further adaptations and produce variants with greater human-to-human transmission
-
•
limited access to rapid genomic sequencing to identify circulating variants
-
•human factors, including:
-
odistrust of validated government information sources
-
ohuman behavior and the need to plan for what people will do rather than for what we want them to do
-
o
The lack of generalized vaccine protection in the population likely influenced the 2022 monkeypox outbreak. Climate change, human encroachment on previous wilderness areas, trade and travel, and human behavior – all of which are One Health issues – undoubtedly also contributed.
4.2.1. Public health actions
Public health interventions in an environment with rapidly evolving knowledge are extremely difficult to orchestrate and must abide by the basic principles of crisis and emergency risk communication [92,93]. Healthcare provider education and early clear public messaging are essential. Concurrent with developing and disseminating education and training resources, public health must rapidly act, as for any outbreak [94], to include activities such as contact tracing.
A particular challenge for decision-makers is to determine whether quarantine would be a valuable public health measure to employ for persons exposed to monkeypox.
4.2.2. Quarantine and isolation
Quarantine and isolation are public health tools that require physical separation and confinement of individuals to prevent disease transmission and protect public health [95]. When used appropriately, they deter disease spread within a population but do not directly benefit the individual. Quarantine of healthy (asymptomatic) individuals after exposure to a contagious illness is reasonable only if there is potential for them to be a source of human-to-human or human-to-animal transmission [[96], [97], [98], [99]]. Conversely, isolation is applied to infected (symptomatic) people and must remain in place during the entire infectious period. Patients are considered contagious until all skin lesions have healed, crusts have separated, and a fresh layer of skin has formed; this can take several weeks.
While isolating symptomatic patients with monkeypox is accepted practice, the question of whether exposed persons should be quarantined, and for how long, is more challenging. Information about transmission in the 2022 monkeypox outbreak is evolving, including the possibility of fomite and aerosol transmission rather than direct contact or inoculation [30]. Older studies considered human-to-human transmissions [100] as unsustainable and rare [44], but it has been clear since the 2003 outbreak in the Republic of the Congo that at least six serial transmissions can evolve in a short time and that transmission is not related to the severity of infection [101]. More recent studies document longer and longer chains of human-to-human transmissions [102]. Animal studies show that monkeypox is transmissible during the prodromal stage [103]. Moreover, transmission before a skin rash appears, is consistent with the epidemiology and virological characteristics of some patients of the 2022 outbreak [47]. Transmission before the appearance of the exanthem raises important public health policy questions regarding quarantine for contacts of monkeypox patients.
Public health experts should contemplate implementing a quarantine for up to 21 days (the outer incubation timeframe) for people exposed to the monkeypox virus; however, there are several details to consider:
-
•What is the goal of the quarantine?
-
oCan the outbreak be contained? Or has it progressed to a phase where mitigation is the only option?
-
o
-
•What is the risk to public health?
-
oDo individuals have contact with populations at high risk from infection (e.g., persons at the extremes of age or immunocompromised) or have close contact with persons who interact with high-risk populations (e.g., nursery school or nursing home workers)
-
o
-
•
Was the exposure in a person wearing full PPE, or was it an unprotected exposure?
-
•
Would other types of public health monitoring, including active symptom detection (with twice-daily temperature monitoring), be adequate?
-
•
What is the socioeconomic impact of quarantine of an exposed healthcare worker (or any essential worker) during a period of staffing shortages? Would the removal of this individual from work for 21 days contribute to disruptions in critical healthcare or other infrastructures?
-
•
Does quarantine authority exist? If so, will people be likely to comply with the restrictions, and are they enforceable?
These complex analyses regarding the implementation of quarantine may have unintended consequences, particularly if public messaging is suboptimal. Experience from the COVID-19 pandemic, including negative effects on the educational system, economy, and mental health, will likely limit the use of quarantine for the monkeypox 2022 outbreak. However, quarantine may be considered to protect vulnerable populations at greater risk for negative outcomes and death if the outbreak expands to those populations. Vulnerable populations include immunocompromised persons, young children, pregnant women, and the elderly.
5. Conclusion
Monkeypox 2022, a close cousin of smallpox, is a re-emerging zoonotic disease that has spread rapidly around the globe and demonstrated uncharacteristic reports of human-to-human and community transmissions. While initial cases were reported in MSM, anyone in close contact with infected persons, animals, or fomites has an exposure risk lasting up to 21 days. The classic prodrome may be subclinical, and the rash may first appear in the genital or anal regions and can be subtle. Persons may be infectious before symptom onset.
Healthcare providers working in STI/urgent care clinics, emergency medical services systems, and emergency departments are likely to encounter patients needing evaluation for monkeypox. This paper reviews the known One Health aspects of monkeypox and delivers a novel, concise, fact-based, Identify-Isolate-Inform (3I) Tool for frontline clinicians in the early detection and management of patients under investigation for monkeypox 2022.
Funding disclosure
One of the investigators received a small grant from Strategic Solutions Emerging Infectious Diseases to support this research.
Human participant protection
This project did not require institutional review board approval.
Research data related to this submission
There are no linked research data sets for this submission.
The following reason is given: No data set was used for the research described in the article.
Disclaimer
Due to the rapidly evolving nature of this outbreak, and in the interests of rapid dissemination of reliable, actionable information, this paper went through expedited peer review. Additionally, information should be considered current only at publication and may evolve as the science develops.
Declaration of Competing Interest
The authors report no conflicts of interest. The views expressed in this paper are those of the authors and do not necessarily represent the views of the institutions with which they are affiliated.
Acknowledgments
The authors thank Michael J. Burns, MD, and Emanuele Nicastri, MD, PhD, for their assistance with providing clinical images for this publication.
We thank the Editor and Reviewers for their thorough, rapid, and comprehensive review of our manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100410.
Appendix A and B. Supplementary data
Appendix A: Laboratory Testing for Monkeypox
Appendix B: Identify-Isolate-Inform (3I) Tool - Fillable Version
References
- 1.Multi-country monkeypox outbreak in non-endemic countries. World Health Organization. May 29, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON388 Accessed June 6, 2022. [Google Scholar]
- 2.Minhaj F.S., Ogale Y.P., Whitehill F., et al. Monkeypox outbreak — nine states, may 2022. MMWR Morb Mortal Wkly Rep. 2022 doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monkeypox cases confirmed in England – latest updates. UK Health Security Agency. May 14, 2022. https://www.gov.uk/government/news/monkeypox-cases-confirmed-in-england-latest-updates Accessed June 6, 2022. [Google Scholar]
- 4.Mathieu E., Dattani S., Ritchie H., et al. Our World in Data. Updated June 19, 2022. Monkeypox.https://ourworldindata.org/monkeypox Accessed June 20, 2022. [Google Scholar]
- 5.Arita I., Henderson D.A. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 1968;39(2):277–283. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2554549/?page=7 [PMC free article] [PubMed] [Google Scholar]
- 6.Monkeypox. World Organization for Animal Health. Updated May 30, 2022. https://www.woah.org/en/disease/monkeypox/ Accessed June 6, 2022. [Google Scholar]
- 7.Prier J.E., Sauer R.M. A pox disease of monkeys. Ann. N. Y. Acad. Sci. 1960;85:951–959. doi: 10.1111/j.1749-6632.1960.tb50015.x. https://www.cabdirect.org/cabdirect/abstract/19612201105 [DOI] [PubMed] [Google Scholar]
- 8.Peters J.C. Eine “Monkey-Pox” Enzootie im Affenhaus des Tiergartens “Blijdorp”[ A “monkey-pox” enzootic in the monkey house of the “Blijdorp” zoo] Kleintierpraxis. 1966;11:65–71. [Google Scholar]
- 9.Reynolds M.G., Doty J.B., McCollum A.M., Olson V.A., Nakazawa Y. Monkeypox re-emergence in Africa: a call to expand the concept and practice of one health. Expert Rev. Anti-Infect. Ther. 2019;17(2):129–139. doi: 10.1080/14787210.2019.1567330. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6438170/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.History of Smallpox. Centers for Disease Control and Prevention. Reviewed February 20, 2021. https://www.cdc.gov/smallpox/history/history.html Accessed June 6, 2021. [Google Scholar]
- 11.Bleyer J.C. Ueber Auftreten von Variola unter Affen der genera Mycetes und Cebus bei Vordringen einer Pockenepidemie im Urwaldgebiete an den Nebenflüssen des Alto Uruguay in Südbrasilien [On the Occurrence of Smallpox among Monkeys of the Genera Mycetes and Cebus, resulting from an Epidemic in the Primeval Forest on the Tributaries of the Upper Uruguay River, Brazil.] Münchener Medizinische Wochenschrift. 1922;69(27):1009–1010. https://www.cabdirect.org/cabdirect/abstract/19232901380 [Google Scholar]
- 12.von Magnus P., Anderson E.K., Petersen K.B., Birch-Anderson A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. doi: 10.1111/j.1699-0463.1959.tb00328.x. [DOI] [Google Scholar]
- 13.Executive Board 65. World Health Organization Institutional Repository for Information Sharing. Executive Board 65. EB65.R17 Global Smallpox eradication. https://apps.who.int/iris/handle/10665/154647
- 14.Marennikova S.S. TSERIS; Novosibirsk: 2018. How it was: The Global smallpox eradication program in reminiscences of its participants. English Translation.http://www.zero-pox.info/other_docs/ussr-hist.pdf [Google Scholar]
- 15.World Health Organization Notes Resolution WHA11.54 adopted by the Eleventh World Health Assembly on this matter. https://apps.who.int/iris/handle/10665/105431
- 16.Breman J.G. World Health Organization. Human Monkeypox: Update 1978. SME/78.15 Global Commission WP/78.47. https://apps.who.int/iris/handle/10665/68294
- 17.Nakazawa Y., Emerson G.L., Carroll D.S., Zhao H., Li Y., Reynolds M.G., Karem K.L., Olson V.A., Lash R.R., Davidson W.B., Smith S.K., Levine R.S., Regnery R.L., Sammons S.A., Frace M.A., Mutasim E.M., Karsani M.E., Muntasir M.O., Babiker A.A., Opoka L., Chowdhary V., Damon I.K. Phylogenetic and ecologic perspectives of a monkeypox outbreak, southern Sudan, 2005. Emerg. Infect. Dis. 2013;19(2):237–245. doi: 10.3201/eid1902.121220. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3559062/ PMID: 23347770; PMCID: PMC3559062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiee M.S., Harrigan R.J., Thomassen H.A., Smith T.B. Ghosts of infections past: using archival samples to understand a century of monkeypox virus prevalence among host communities across space and time. R. Soc. Open Sci. 2018;5(1) doi: 10.1098/rsos.171089. doi: 10.1098/rsos.171089. PMID: 29410823; PMCID: PMC5792900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NIO Silva, de Oliveira J.S., Kroon E.G., Trindade G.S., Drumond B.P. Here, there, and everywhere: the wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses. 2020;13(1):43. doi: 10.3390/v13010043. Published 2020 Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593–597. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2480792/ PMID: 4340218; PMCID: PMC2480792. [PMC free article] [PubMed] [Google Scholar]
- 21.Fine P.E., Ježek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. https://academic.oup.com/ije/article-abstract/17/3/643/729853?redirectedFrom=fulltext&login=true PMID: 2850277. [DOI] [PubMed] [Google Scholar]
- 22.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., Blumberg S., Thomassen H.A., Pike B.L., Fair J.N., Wolfe N.D., Shongo R.L., Graham B.S., Formenty P., Okitolonda E., Hensley L.E., Meyer H., Wright L.L., Muyembe J.J., et al. Proc Natl Acad Sci U S A. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. doi: 10.1073/pnas.1005769107. Epub 2010 Aug 30. PMID: 20805472; PMCID: PMC2941342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multi-country monkeypox outbreak in non-endemic countries. World Health Organization. May 21, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 Accessed June 6, 2022. [Google Scholar]
- 24.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S., Djingarey M.H., Olson V., Damon I.K., Khalakdina A. Emergence of Monkeypox - west and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67(10):306–310. doi: 10.15585/mmwr.mm6710a5. https://www.cdc.gov/mmwr/volumes/67/wr/mm6710a5.htm?s_cid=mm6710a5_w (Erratum in: MMWR Morb. Mortal. Wkly Rep. 2018 Apr 27;67(16):479. PMID: 29543790; PMCID: PMC5857192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCollum A., Disu Y., Mamadu I., Akinpelu A., Ahmad A., Burga J., Ndoreraho A., Nkunzimana E., Manneh L., Mohammed A., Adeoye O., Tom-Aba D., Silenou B., Ipadeola O., Saleh M., Adeyemo A., Nwadiutor I., Aworabhi N., Uke P., John D., Wakama P., Reynolds M., Mauldin M.R., Doty J., Wilkins K., Musa J., Khalakdina A., Adedeji A., Mba N., Ojo O., Krause G., Ihekweazu C., CDC Monkeypox Outbreak Team Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. https://www.sciencedirect.com/science/article/pii/S1473309919302944?via%3Dihub Epub 2019 Jul 5. PMID: 31285143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Director-General’s opening remarks at the COVID-19 media briefing – 8 June 2022. World Health Organization; June 8, 2022. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-covid-19-media-briefing---8-june-2022 Accessed June 8, 2022. [Google Scholar]
- 28.WHO Director-General’s opening remarks at International Health Regulations (2005) Emergency Committee regarding the multi-country monkeypox outbreak – 23 June 2022. World Health Organization; 2022. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-international-health-regulations-(2005)-emergency-committee-regarding-the-multi-country-monkeypox-outbreak---23-june-2022 [Google Scholar]
- 29.Reynolds M.G., Yorita K.L., Kuehnert M.J., Davidson W.B., Huhn G.D., Holman R.C., Damon I.K. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 2006;194(6):773–780. doi: 10.1086/505880. https://academic.oup.com/jid/article/194/6/773/864712?login=true Epub 2006 Aug 8. PMID: 16941343. [DOI] [PubMed] [Google Scholar]
- 30.Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I., Wong S.W., Yoshihara P., Hanifin J.M., Slifka M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005 Sep;11(9):1005–1011. doi: 10.1038/nm1273. https://www.nature.com/articles/nm1273 Epub 2005 Aug 7. PMID: 16086024. [DOI] [PubMed] [Google Scholar]
- 31.Damon I.K., Roth C.E., Chowdhary V. Discovery of monkeypox in Sudan. N. Engl. J. Med. 2006;355(9):962–963. doi: 10.1056/NEJMc060792. doi: 10.1056/NEJMc060792. PMID: 16943415. [DOI] [PubMed] [Google Scholar]
- 32.Monkeypox - United States of America. World Health Organization. July 27, 2021. https://www.who.int/emergencies/disease-outbreak-news/item/monkeypox---the-united-states-of-america Accessed June 7, 2022. [Google Scholar]
- 33.Monkeypox - United States of America. World Health Organization. November 25, 2021. https://www.who.int/emergencies/disease-outbreak-news/item/2021-DON344 Accessed June 7, 2022. [Google Scholar]
- 34.Monkeypox. World Health Organization. May 19, 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox Accessed June 7, 2022. [Google Scholar]
- 35.Monkeypox - United Kingdom of Great Britain and Northern Ireland. World Health Organization. July 8, 2021. https://www.who.int/emergencies/disease-outbreak-news/item/monkeypox---united-kingdom-of-great-britain-and-northern-ireland Accessed June 7, 2022. [Google Scholar]
- 36.Vol. 16. May 16, 2022. Monkeypox - United Kingdom of Great Britain and Northern Ireland. World Health Organization.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON381 [Google Scholar]
- 37.World Health Organization . Vol. 22. Technical Document; 2022. Surveillance, case investigation and contact tracing for Monkeypox.https://www.who.int/publications/i/item/WHO-MPX-surveillance-2022.1 [Google Scholar]
- 38.Genomic epidemiology of monkeypox virus. Next Strain; Updated June 17, 2022. https://nextstrain.org/monkeypox/hmpxv1 Accessed June 20, 2022. [Google Scholar]
- 39.Monkeypox: Infection Control: Healthcare Settings. Centers for Disease Control and Prevention. Updated May 22, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html Accessed June 7, 2022. [Google Scholar]
- 40.N95 Respirators, Surgical Masks, Face Masks, and Barrier Face Coverings. Food and Drug Administration. Updated September 15, 2021. https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-surgical-masks-face-masks-and-barrier-face-coverings Accessed June 7, 2022. [Google Scholar]
- 41.Vaughan A., Aarons E., Astbury J., Brooks T., Chand M., Flegg P., Hardman A., Harper N., Jarvis R., Mawdsley S., McGivern M., Morgan D., Morris G., Nixon G., O’Connor C., Palmer R., Phin N., Price D.A., Russell K., Said B., Schmid M.L., Vivancos R., Walsh A., Welfare W., Wilburn J., Dunning J., et al. Emerg Infect Dis. 2020;26(4):782–785. doi: 10.3201/eid2604.191164. https://wwwnc.cdc.gov/eid/article/26/4/19-1164_article Epub 2020 Apr 17. PMID: 32023204; PMCID: PMC7101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Routy J.P., Dupuy F.P., Lin J., Isnard S. More than a gender issue: testis as a distinctive HIV reservoir and its implication for viral eradication. Methods Mol. Biol. 2022;2407:173–186. doi: 10.1007/978-1-0716-1871-4_13. [DOI] [PubMed] [Google Scholar]
- 43.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. doi: 10.1016/s1473-3099(03)00856-9. https://www.sciencedirect.com/science/article/pii/S1473309903008569?via%3Dihub Erratum in: Lancet Infect. Dis. 2004 Apr;4(4):251. PMID: 14720564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miura F., van Ewijk C.E., Backer J.A., Xiridou M., Franz E., Op de Coul E., Brandwagt D., van Cleef B., van Rijckevorsel G., Swaan C., van den Hof S., Wallinga J. The incubation period for monkeypox cases confirmed in the Netherlands. medRxiv. 2022 doi: 10.1101/2022.06.09.22276068. 2022.06.09.22276068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ježek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J. Infect. Dis. 1987;156(2):293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 46.Updated Case-finding Guidance: Monkeypox Outbreak—United States, 2022 June 14, 2022, 5:00 PM EST CDCHAN-00468. CDC Health Alert Network. June 14, 2022. https://emergency.cdc.gov/han/2022/han00468.asp Accessed June 14, 2022. [Google Scholar]
- 47.Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., D’Abramo A., Cicalini S., Lapa D., Pittalis S., Puro V., Rivano Capparuccia M., Giombini E., CEM Gruber, Garbuglia A.R., Marani A., Vairo F., Girardi E., Vaia F., Nicastri E., INMI Monkeypox Group Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. PMID: 35656836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patrocinio-Jesus R., Peruzzu F. Monkeypox genital lesions [published online ahead of print, 2022 Jun 15] N. Engl. J. Med. 2022 doi: 10.1056/NEJMicm2206893. [DOI] [PubMed] [Google Scholar]
- 49.Petersen E., Kantele A., Koopmans M., Asogun D., Yinka-Ogunleye A., Ihekweazu C., Zumla A. Human Monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. N. Am. 2019 Dec;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0891552019300170 Epub 2019 Apr 11. PMID: 30981594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doucleff M. Monkeypox can look different than what doctors thought. Here’s what they’re learning. NPR/WLRN. June 3, 2022 https://www.npr.org/sections/goatsandsoda/2022/06/03/1102945017/monkeypox-can-look-different-than-what-doctors-thought-heres-what-theyre-learnin [Google Scholar]
- 51.Clinician FAQ . Updated June 10, 2022. Centers for Disease Control and Prevention.https://www.cdc.gov/poxvirus/monkeypox/clinicians/faq.html Accessed June 20, 2022. [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC) Update: multistate outbreak of monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(27):642–646. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5227a5.htm PMID: 12855947. [PubMed] [Google Scholar]
- 53.Asadu C., Kamale J.-K. Monkeypox Kills 9 in Congo; First Death in Nigeria in. 2022. https://www.washingtonpost.com/world/first-monkeypox-death-in-nigeria-in-2022-21-cases-confirmed/2022/05/30/dfb7f0d2-e017-11ec-ae64-6b23e5155b62_story.html
- 54.Multi-Country monkeypox outbreak: Situation Update. World Health Organization. June 10, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON392#:~:text=Since%20the%20beginning%20of%202022,same%20period%20(Table%202) Accessed June 10, 2022. [Google Scholar]
- 55.Multi-country monkeypox outbreak: situation update. World Health Organization. June 17, 2022. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393 Accessed June 17, 2022. [Google Scholar]
- 56.Laboratory Testing for the monkeypox virus. World Health Organization. May 23, 2022. https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 June 7, 2022. [Google Scholar]
- 57.Interim Clinical Guidance for the Treatment of Monkeypox: Medical Countermeasures Available for the Treatment of Monkeypox. Centers for Disease Control and Prevention. Updated June 17, 2022. 2022. https://www.cdc.gov/poxvirus/monkeypox/treatment.html Accessed June 18, 2022. [Google Scholar]
- 58.Yang H., Kim S.K., Kim M., Reche P.A., Morehead T.J., Damon I.K., Welsh R.M., Reinherz E.L. Antiviral chemotherapy facilitates control of poxvirus infections through inhibition of cellular signal transduction. J. Clin. Invest. 2005;115(2):379–387. doi: 10.1172/JCI23220. (PMID: 15690085; PMCID: PMC546427. https://www.jci.org/articles/view/23220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albarnaz J.D., Ren H., Torres A.A., Shmeleva E.V., Melo C.A., Bannister A.J., Brember M.P., Chung B.Y., Smith G.L. Molecular mimicry of NF-κB by vaccinia virus protein enables selective inhibition of antiviral responses. Nat. Microbiol. 2022;7(1):154–168. doi: 10.1038/s41564-021-01004-9. https://www.nature.com/articles/s41564-021-01004-9 Epub 2021 Dec 23. PMID: 34949827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vigne S., Duraffour S., Andrei G., Snoeck R., Garin D., Crance J.M. Inhibition of vaccinia virus replication by two small interfering RNAs targeting B1R and G7L genes and their synergistic combination with cidofovir. Antimicrob Agents Chemother. 2009;53(6):2579–2588. doi: 10.1128/AAC.01626-08. doi: 10.1128/AAC.01626-08. Epub 2009 Mar 23. PMID: 19307376; PMCID: PMC2687203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vigne S., Germi R., Duraffour S., Larrat S., Andrei G., Snoeck R., Garin D., Crance J.M. Specific inhibition of orthopoxvirus replication by a small interfering RNA targeting the D5R gene. Antivir. Ther. 2008;13(3):357–368. doi: 10.1177/135965350801300307. PMID: 18572748. [DOI] [PubMed] [Google Scholar]
- 62.Grosenbach D.W., Honeychurch K., Rose E.A., Chinsangaram J., Frimm A., Maiti B., Lovejoy C., Meara I., Long P., Hruby D.E. Oral Tecovirimat for the treatment of smallpox. N. Engl. J. Med. 2018;379(1):44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Highlights of Prescribing Information (TPOXX). Food and Drug Administration. Updated May 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214518s000lbl.pdf Accessed June 6, 2022. [Google Scholar]
- 64.Huggins J.W., Zwiers S.H., Baker R.O., et al. Cidofovir treatment of variola (smallpox) in the hemorrhagic smallpox primate model and the IV monkeypox primate model. World Health Organization. Published July. 2015;24 https://www.who.int/publications/m/item/cidofovir-treatment-of-variola-(smallpox)-in-the-hemorrhagic-smallpox-primate-model-and-the-iv-monkeypox-primate-model [Google Scholar]
- 65.Smallpox Treatment. Centers for Disease Control and Prevention. October 18, 2021. https://www.cdc.gov/smallpox/clinicians/treatment.html#:~:text=Cidofovir,had%20diseases%20similar%20to%20smallpox Accessed June 7, 2022. [Google Scholar]
- 66.Sánchez-Sampedro L., Perdiguero B., Mejías-Pérez E., García-Arriaza J., Di Pilato M., Esteban M. The evolution of poxvirus vaccines. Viruses. 2015;7(4):1726–1803. doi: 10.3390/v7041726. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4411676/ PMID: 25853483; PMCID: PMC4411676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nalca A., Zumbrun E.E. ACAM2000: the new smallpox vaccine for United States strategic national stockpile. Drug Des Devel Ther. 2010;25(4):71–79. doi: 10.2147/dddt.s3687. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2880337/ PMID: 20531961; PMCID: PMC2880337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kretzschmar M., Wallinga J., Teunis P., Xing S., Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 2006;3(8) doi: 10.1371/journal.pmed.0030272. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1551910/ Erratum in: PLoS Med. 2006 Oct;3(10):e429. PMID: 16933957; PMCID: PMC1551910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frey S.E. New smallpox vaccines for an ancient scourge. Mo. Med. 2014;111(4):332–336. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6179474/ [PMC free article] [PubMed] [Google Scholar]
- 70.Greenberg R.N., Overton E.T., Haas D.W., Frank I., Goldman M., von Krempelhuber A., Virgin G., Bädeker N., Vollmar J., Chaplin P. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis. 2013;207(5):749–758. doi: 10.1093/infdis/jis753. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3611764/ Epub 2012 Dec 7. PMID: 23225902; PMCID: PMC3611764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatch G.J., Graham V.A., Bewley K.R., et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 2013;87(14):7805–7815. doi: 10.1128/JVI.03481-12. doi: 10.1128/JVI.03481-12. Epub 2013 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishiyama Y., Fujii T., Kanatani Y., Shinmura Y., Yokote H., Hashizume S. Freeze-dried live attenuated smallpox vaccine prepared in cell culture “LC16-KAKETSUKEN”: post-marketing surveillance study on safety and efficacy compliant with good clinical practice. Vaccine. 2015 Nov 9;33(45):6120–6127. doi: 10.1016/j.vaccine.2015.09.067. https://www.sciencedirect.com/science/article/pii/S0264410X15013432 Epub 2015 Oct 9. PMID: 26455406. [DOI] [PubMed] [Google Scholar]
- 73.Shchelkunova G.A., Schelkunov S.N. 40 years without smallpox. Acta Nat. 2017;9(4):4–12. doi: 10.32607/20758251-2017-9-4-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maksyutov R.A., Yakubitskyi S.N., Kolosova I.V., Shchelkunov S.N. Comparing new-generation candidate vaccines against human Orthopoxvirus infections. Acta Nat. 2017;9(2):88–93. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5509005/ [PMC free article] [PubMed] [Google Scholar]
- 75.Shchelkunov S.N., Yakubitskiy S.N., Sergeev A.A., Kabanov A.S., Bauer T.V., Bulychev L.E., Pyankov S.A. Effect of the route of Administration of the Vaccinia Virus Strain LIVP to mice on its virulence and immunogenicity. Viruses. 2020;12(8):795. doi: 10.3390/v12080795. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7472337/ PMID: 32722032; PMCID: PMC7472337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yakubitskiy S.N., Kolosova I.V., Maksyutov R.A., Shchelkunov S.N. Attenuation of Vaccinia Virus. Acta Naturae. 2015;7(4):113–121. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4717256/ PMID: 26798498; PMCID: PMC4717256. [PMC free article] [PubMed] [Google Scholar]
- 77.WHO Advisory Committee on Variola Virus Research. Report of the twenty-first meeting, Geneva 30 October-1 November 2019. November 1, 2019. https://www.who.int/publications-detail-redirect/9789240012998 Accessed June 8, 2022. [Google Scholar]
- 78.Taub D.D., Ershler W.B., Janowski M., Artz A., Key M.L., McKelvey J., Muller D., Moss B., Ferrucci L., Duffey P.L., Longo D.L. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am. J. Med. 2008;121(12):1058–1064. doi: 10.1016/j.amjmed.2008.08.019. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2610468/ PMID: 19028201; PMCID: PMC2610468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khalil A., Samara A., O’Brien P., Morris E., Draycott T., Lees C., Ladhani S. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet. Gynecol. 2022 Jun 2 doi: 10.1002/uog.24968. 35652380 [DOI] [PubMed] [Google Scholar]
- 80.Assessment report: Tecovirimat SIGA. European Medicines Agency. November 11, 2021. https://www.ema.europa.eu/en/documents/assessment-report/tecovirimat-siga-epar-public-assessment-report_en.pdf Accessed June 7, 2021. [Google Scholar]
- 81.Koenig K.L. Identify, isolate, inform: a 3-pronged approach to Management of Public Health Emergencies. Disaster Med Public Health Prep. 2015;9(1):86–87. doi: 10.1017/dmp.2014.125. [DOI] [PubMed] [Google Scholar]
- 82.Koenig K.L., Majestic C., Burns M.J. Ebola virus disease: essential public health principles for clinicians. West. J. Emerg. Med. 2014;15(7):728–731. doi: 10.5811/westjem.2014.9.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koenig K.L., Alassaf W., Burns M.J. Identify-isolate-inform: a tool for initial detection and Management of Measles patients in the emergency department. West. J. Emerg. Med. 2015;16(2):212–219. doi: 10.5811/westjem.2015.3.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koenig K.L. Identify-isolate-inform: a modified tool for initial detection and management of middle east respiratory syndrome patients in the emergency department. West. J. Emerg. Med. 2015;16(5):619–624. doi: 10.5811/westjem.2015.7.27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koenig K.L., Shastry S., Mzahim B., et al. Mumps virus: modification of the identify-isolate-inform tool for frontline healthcare providers. West. J. Emerg. Med. 2016;17(5):490–496. doi: 10.5811/westjem.2016.6.30793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koenig K.L., Almadhyan A., Burns M.J. Identify-isolate-inform: a tool for initial detection and Management of Zika Virus Patients in the emergency department. West. J. Emerg. Med. 2016;17(3):238–244. doi: 10.5811/westjem.2016.3.30188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koenig K.L., Shastry S., Burns M.J. Hepatitis a virus: essential knowledge and a novel identify-isolate-inform tool for frontline. Healthcare Providers. West J Emerg Med. 2017;18(6):1000–1007. doi: 10.5811/westjem.2017.10.35983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koenig K.L., Farah J., Thihalolipavan S., et al. Pertussis: the identify, isolate, inform tool applied to a re-emerging respiratory illness. West. J. Emerg. Med. 2019;20(2):191–197. doi: 10.5811/westjem.2018.11.40023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng T., Mzahim B., Koenig K.L., et al. Scabies: application of the novel identify-isolate-inform tool for detection and management. West. J. Emerg. Med. 2020;21(2):191–198. doi: 10.5811/westjem.2020.1.46120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koenig K.L., Beÿ C.K., McDonald E.C. nCoV: the identify-isolate-inform (3I) tool applied to a novel emerging coronavirus. West J Emerg Med. 2019;21(2):184–190. doi: 10.5811/westjem.2020.1.46760. Published 2020 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koenig K.L. Ebola Triage Screening and Public Health: The New “Vital Sign Zero.”. Disaster Med Public Health Prep. 2015;9(1):57–58. doi: 10.1017/dmp.2014.120. [DOI] [PubMed] [Google Scholar]
- 92.Marty A.M. Chapter 5: command, control & communication (C3) chapter 5 in World Health Organization and Public Health England. Public health for mass gatherings: key considerations. 2015:51–58. https://www.who.int/publications/i/item/public-health-for-mass-gatherings-key-considerations [Google Scholar]
- 93.Reynolds B.J., Shenhar G., Crisis and Emergency Risk Communication . 2nd edition. 2016. Chapter 25 In Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices.https://scholar.google.com/scholar_lookup?title=Chapter%2025%20In%20Koenig%20and%20Schultz%27s%20Disaster%20Medicine%3A%20Comprehensive%20Principles%20and%20Practices&author=B.J.%20Reynolds&publication_year=2016 (ISBN: 9781107040755) [Google Scholar]
- 94.Gamage S.D., Kralovic S.M. 2nd edition. 2016. Roselle GA. Emerging Infectious Diseases. Chapter 8 In Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices.https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.660.8353&rep=rep1&type=pdf ISBN: 9781107040755. [Google Scholar]
- 95.Barbisch D., Koenig K.L., Shih F.Y. Is there a case for quarantine? Perspectives from SARS to Ebola. Disaster Med Public Health Prep. 2015 Oct;9(5):547–553. doi: 10.1017/dmp.2015.38. https://www.cambridge.org/core/journals/disaster-medicine-and-public-health-preparedness/article/is-there-a-case-for-quarantine-perspectives-from-sars-to-ebola/451C41BD5A980A45FFA9F9AE8670CC85 Epub 2015 Mar 23. PMID: 25797363. [DOI] [PubMed] [Google Scholar]
- 96.Koenig K.L. Health care worker quarantine for Ebola: to eradicate the virus or alleviate fear? Ann. Emerg. Med. 2015;65(3):330–331. doi: 10.1016/j.annemergmed.2014.12.003. https://www.annemergmed.com/article/S0196-0644(14)01571-6/fulltext [DOI] [PubMed] [Google Scholar]
- 97.Koenig K.L. Quarantine for Zika virus? Where is the science? Disaster Med Public Health Prep. 2016 Oct;10(5):704–706. doi: 10.1017/dmp.2016.56. https://www.cambridge.org/core/journals/disaster-medicine-and-public-health-preparedness/article/quarantine-for-zika-virus-where-is-the-science/A81ED950444E743D6347E19BE604B2E0 Epub 2016 Apr 1. PMID: 27032458. [DOI] [PubMed] [Google Scholar]
- 98.Koenig K.L. The quarantine conundrum: perspectives for the humanitarian community. Blog for Evidence Aid. March 14, 2022 https://evidenceaid.org/the-quarantine-conundrum-perspectives-for-the-humanitarian-community/ [Google Scholar]
- 99.American College of Emergency Physicians Ebola Expert Panel Consensus Statement on Restrictive Movement including Quarantine of Health Care Workers. American College of Emergency Physicians. November 13, 2014. https://www.acep.org/globalassets/uploads/uploaded-files/acep/clinical-and-practice-management/resources/publichealth/acep-ebola-expert-panel-consensus-statement.111314.pdf Accessed June 8, 2022. [Google Scholar]
- 100.Ježek Z., Arita I., Mutombo M., Dunn C., Nakano J.H., Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am. J. Epidemiol. 1986;123(6):1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- 101.Learned L.A., Reynolds M.G., Wassa D.W., Li Y., Olson V.A., Karem K., Stempora L.L., Braden Z.H., Kline R., Likos A., Libama F., Moudzeo H., Bolanda J.D., Tarangonia P., Boumandoki P., Formenty P., Harvey J.M., Damon I.K. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005 Aug;73(2):428–434. https://www.ajtmh.org/view/journals/tpmd/73/2/article-p428.xml PMID: 16103616. [PubMed] [Google Scholar]
- 102.Nolen L.D., Osadebe L., Katomba J., Likofata J., Mukadi D., Monroe B., Doty J., Hughes C.M., Kabamba J., Malekani J., Bomponda P.L., Lokota J.I., Balilo M.P., Likafi T., Lushima R.S., Ilunga B.K., Nkawa F., Pukuta E., Karhemere S., Tamfum J.J., Nguete B., Wemakoy E.O., McCollum A.M., Reynolds M.G. Extended human-to-human transmission during a monkeypox outbreak in the democratic republic of the congo. Emerg Infect Dis. 2016;22(6):1014–1021. doi: 10.3201/eid2206.150579. https://wwwnc.cdc.gov/eid/article/22/6/15-0579_article Erratum in: Emerg. Infect. Dis. 2016 Oct;22(10): PMID: 27191380; PMCID: PMC4880088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hutson C.L., Carroll D.S., Gallardo-Romero N., Weiss S., Clemmons C., Hughes C.M., Salzer J.S., Olson V.A., Abel J., Karem K.L., Damon I.K. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS One. 2011;6(12):e28295. doi: 10.1371/journal.pone.0028295. doi: 10.1371/journal.pone.0028295. Epub 2011 Dec 2. PMID: 22164263; PMCID: PMC3229555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reynolds M.G., McCollum A.M., Nguete B., et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox Biodefense Research. Virus. 2017;9(12):380. doi: 10.3390/v9120380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daskalakis D., McClung R.P., Mena L., et al. Monkeypox: avoiding the mistakes of past infectious disease epidemics. Ann. Intern. Med. 2022 doi: 10.7326/M22-1748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A: Laboratory Testing for Monkeypox
Appendix B: Identify-Isolate-Inform (3I) Tool - Fillable Version