Abstract
Background and aim:
Increasing the appropriateness of upper gastrointestinal endoscopy (UGIE) improves the quality of care while containing costs. The aim of this study was to improve the appropriateness of UGIE through a process involving evaluation of prescriptions and the use of a non-invasive alternative.
Materials and methods:
At our Endoscopy Unit of Alto Vicentino Hospital, ULSS 7 Pedemontana, a tertiary center in northeastern Italy, from 2013 to 2015, a senior endoscopist evaluated the appropriateness of all outpatient referrals for UGIE and established the proper timing. Referrals were either accepted and programmed, canceled, or substituted by a non-invasive evaluation of gastric function, determining serum levels of gastrin-17 (G17), Pepsinogen I (PGI) and II (PGII), and antibodies against Helicobacter pylori.
Results:
A total of 5102 requests for UGIE examinations were evaluated; 540 (10.4%) were inappropriate and had been prescribed for: gastroesophageal reflux disease (n=307), surveillance with erroneous timing (n=113), dyspepsia (n=66), other indications (n=20), and absence of written indication (n=34). Gastric function was evaluated in 282/540 patients; findings included normal values in 94 patients without proton-pump inhibitor therapy (PPI) and in 48 on PPI, active H pylori infection in 56, previous H pylori infection in 30, GERD in n=50, and atrophic gastritis in n=4. UGIE was performed in the latter 4 cases. Within 2 years (range 1-22 months) of the initial refusal, 105/504 patients underwent UGIE, with normal endoscopic findings in 71/105 (67.5%), and with no cases of cancer.
Conclusions:
This strategy, based on a strict control of prescriptions, is effective to increase the appropriateness while containing public health costs. The use of gastric function testing improves patient selection for UGIE endoscopy. (www.actabiomedica)
Keywords: upper gastrointestinal endoscopy, appropriateness, non-invasive, gastric function, gastric atrophy, Helicobacter pylori
Introduction
The adequate identification of patients that should undergo upper gastrointestinal endoscopy (UGIE) and their prioritization have always been key clinical and organizational aspects of an Endoscopy Unit. An invaluable exam that allows diagnostic as well as therapeutic procedures to be performed, UGIE is yet invasive and relatively costly. Therefore, it is paramount to ensure appropriateness of its prescription, especially regarding indication and timing. Moreover, UGIE must be adequately placed alongside non-invasive exams as part of an evaluation flowchart that considers multiple clinical and epidemiological factors.
Access to and requests for UGIE in the outpatient setting vary from open systems, in which Endoscopic units program exams prescribed by primary care physicians and/or non-endoscopist medical staff, to closed systems, in which a prior consultation with a specialist in endoscopy is required to decide if UGIE is indicated and with what priority. Although the open access strategy reduces waiting time for the procedure and subsequent diagnosis, concerns have been raised regarding misuse of this system(1), with increasing number of inappropriate referrals and its implications on a public-funded health system.
A rational strategy to optimize healthcare resources, the introduction of the gastric function testing (GastroPanel) has been actively encouraged by our group both in the outpatient scenario, reaching out to general practitioners, as well as for patients who are already being evaluated at our hospital. A non-invasive exam that provides a functional screenshot as well as an indirect picture of the anatomical status of the stomach, testing of gastric function provides the clinician valuable information that may refine the indication for UGIE and in some cases substitute UGIE altogether(2–4).
Gastric function testing, a fasting blood sample-based test, includes the determination of a combination of four parameters which must be interpreted: antibodies against Helicobacter pylori and 3 gastric hormones are dosed: gastrin-17 and pepsinogens I and II (PGI and II). There is a large body of evidence supporting the elevated utility of this combined assay in identifying atrophic gastritis, with sensitivity and specificity of as high as 83% and 95%, respectively, a positive predictive value of 75%, and a negative predictive value of 97%(5–8).
The usefulness of gastric function combined assay as also been demonstrated in patients in whom symptoms are not clearly attributable to acid reflux, either because they are non-responders to therapy with proton-pump inhibitors (PPIs) or because of suspected gastric atrophy, which reduces the risk of reflux symptoms (OR 0.2%, CI 0.00-0.6, in a study correlating histologic findings with reported symptoms)(8). Even stronger than the observed association between H. pylori infection and gastric atrophy with endoscopy-negative reflux disease, a decrease in the serum pepsinogen I/II has shown to be significantly associated with an increased OR for endoscopy-negative reflux disease(9).
Moreover, the Gastropanel test also provides invaluable information on H. pylori status, which aside from being an established carcinogen class I(10,11), is associated with a nine-fold increase in the risk of gastric atrophy(8), while infection with this pathogen and its ensuing gastric atrophy are associated with a reduced risk of esophageal carcinoma, Barrett’s esophagus and reflux esophagitis(8,12).
A recent multicenter Italian study conducted by GIGA-CP (Italian Association of Gastroenterology in Primary Care) in collaboration with SNAMID (National Society of Interdisciplinary Primary Medical Care) and the University of Parma on 227 patients, confirmed the extreme usefulness of this test. Moreover, the use of this combined assay as a screening method for gastric diseases, has reduced general practitioners’ requests for UGIE by 30% in the Parma province(13,14). To our knowledge, however, this is the first large-scale study in which the gastric function assay is employed in the algorithm of patients evaluated for UGIE, either substituting the endoscopic exam or as a functional test that guides a more focused invasive study.
Aim
The aim of the present study was to evaluate the effectiveness of a local protocol including a strict selection of endoscopy prescriptions, the introduction of Gastropanel test, and endoscopic activity reorganization for diagnostic upper endoscopy request prioritization.
Materials and methods
All outpatient requests for UGIE were prospectively evaluated by a single Senior Endoscopist (G.B.) at our Endoscopy Unit of Alto Vicentino Hospital, ULSS7 Pedemontana, a northeastern tertiary hospital in Italy, considering information on the prescription including: prescribing physician, patient sex, age, indication for UGIE and requested priority. Based on this information, requests were deemed appropriate (Group 1), or inappropriate (Group 2). In the first case, patients proceeded to UGIE according to the requested priority, whereas in the latter, the prescribing physician was contacted and a written recommendation was provided regarding the evaluation for UGIE and advised diagnostic/therapeutic approach. Data collected after the enactment of such an algorithm of appropriateness evaluation and prioritization for UGIE from 2013 to 2015 were analyzed, and data regarding costs were calculated accordingly.
As per protocol in our center, information regarding the following variables is recorded by the endoscopy nurse and/or the endoscopist using our reporting software (Clinical Sphere) upon patient’s arrival at the endoscopy suite, immediately prior to UGIE execution: age, sex, and indication for UGIE. This same program is used to record, amongst others, endoscopic findings, final diagnosis, and adverse events. Internationally validated and standardized classifications and terminology are routinely used in endoscopic reports. As per protocol, all procedures are performed using endoscopist-directed propofol-based sedation and standard biopsies of antrum (2), incisura angularis (1), and stomach corpus (2) according to the Sydney protocol(15) are routinely performed in all patients, except in patients in whom suspension of double antiplatelet therapy or new-oral anticoagulants is not possible. All biopsy samples are reported using the OLGA classification system(16).
Gastropanel results and corresponding patient demographics are prospectively collected, per protocol, at our center since 2013, including patient name, birth date and serum levels of Pepsinogen-I, Pepsinogen-II, Gastrin-17, IgG Antibodies against H. pylori, and Pepsinogen-I/Pepsinogen-II ratio. Gastropanel (GastroPanel®, Biohit, Helsinki, Finland) tests were performed after overnight fasting and following the manufacturer’s instructions. Table 1 describes serologic and clinic profiles according to Gastropanel testing.
Table 1.
Serologic and clinic profile categories according to gastric function combined assay testing. CGA-C, corpus localized chronic atrophic gastritis; G-17, gastrin; GERD, gastroesophageal reflux disease; Hp, Helicobacter pylori; N, normal; PGI, pepsinogen I; PGII, Pepsinogen II, PPI, proton pump inhibitor.
| Parameter (normal range) | Healthy stomach | GERD | Hp infection | Hp eradicated | PPI therapy | CGA-C |
|---|---|---|---|---|---|---|
| G17 (0-10 pm/L) | N | ↓ | N | N | ↑ | ↑↑ |
| PGI (30-165 µg/L) | N | ↑ | N | N | ↑↑ | ↓↓ |
| PGII (2-15 µg/L) | N | N | ↑↑ | N | N or ↑ | N |
| PGI/PGII ratio | N | N | N | N | N | ↓↓ |
| Hp IgG (<30 EIU) | N | N | ↑ | ↓ or N | N | ↑ or N |
For the present study, patient data were matched across the three databases, including the first referral for UGIE, data at endoscopy, and data on Gastropanel results (M.F.). Endoscopic findings were categorized as: (A) clinically significant findings, including erosive esophagitis, benign strictures (such as Schatzki’s ring), esophageal neoplasm, large hiatal hernia, gastric neoplasm, erosive gastritis, atrophic gastritis, gastric ulcer, duodenal ulcer, duodenitis, duodenal neoplasm, and submucosal lesion of the upper gastrointestinal tract (including suspected GIST); or (B) non-clinically significant findings including: small hiatal hernia, erythematous gastritis, and normal esophagogastroduodenal findings. If a patient had more than one endoscopic diagnosis, the most severe one was used for the statistical analysis.
Statistical analysis was performed with the SPSS statistical software program for Windows (version 20.1). Data collected after the enactment of such an algorithm of appropriateness evaluation and prioritization for UGIE for a prolonged period were analyzed, and data regarding costs were calculated accordingly. Data were expressed as mean ± standard deviation (DS) for qualitative variables and as a percentage of the total for quantitative ones. All p values were two-tailed with statistical significance indicated by a value of p< 0.05.
This study was performed in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (Identifier: 92687)
Results
During the study period, a total of 5102 outpatient requests for UGIE were prospectively evaluated, of which 4732 (92.7%) were prescribed by a primary care physician and 370 (7.3%) by a specialist. After evaluation, 4562 (89.4%) requests were deemed appropriate for indication and requested timing/priority, and were programmed accordingly (Group 1), while 540 were deemed inappropriate (Group 2) and either evaluation with combined gastric function assay or clinical follow-up/therapeutic change were advised. Age range (18-96 years) and sex distribution (females, 56.3%) were similar for both group 1 and 2 patients, whereas patients in group 2 were significantly younger (38.8 ± 12.6 years) when compared to group 1 patients 59.2 ± 15.9, respectively (p<0.05). Table 2 reports the indication for UGIE in group 1 patients (n=4562), whereas indications for UGIE in Group 2 (inappropriate UGIE requests) are detailed in Table 3.
Table 2.
Indications for UGIE in patients in whom the request for UGIE was deemed appropriate (Group 1). FAP, familial adenomatous polyposis; GERD, gastroesophageal reflux disease; GI, gastrointestinal; MALT, mucosa-associated lymphoid tissue lymphoma; NET, neuroendocrine tumor.
| Indications for UGIE | Overall N,% | Females N,% | Male N,% | Age Mean± SD |
|---|---|---|---|---|
| Main referral indication | 4562 | 2570 | 1992 | |
| Upper GI symptoms: | ||||
| Upper abdominal pain | 1178(25,8) | 732(28,5) | 446(22,4) | 56,1±14,3 |
| Suspected GERD in patients >45 years old | 608(13,3) | 364(14,2) | 244(12,2) | 62,6±9,8 |
| Change in GERD symptoms in patients <45 years old | 144(3,2) | 73(2,8) | 71(3,6) | 38,0±6,8 |
| Dyspepsia in patients >45 years | 361(7,9) | 219(8,5) | 142(7,1) | 59,0±14,4 |
| Suspected GERD unresponsive to therapy | 80(1,8) | 44(1,7) | 36(1,8) | 58,0±15,4 |
| Alarm symptoms/signs: | ||||
| Anemia | 307(6,7) | 207(8,1) | 100(5) | 62,5±17,4 |
| Weight loss | 260(5,7) | 143(5,6) | 117(5,9) | 64,2±18,4 |
| Dysphagia | 318(7) | 159(6,2) | 159(8) | 65,4±15,8 |
| Persistent vomiting of unknown cause | 139(3) | 83(3,2) | 56(2,8) | 56,4±19,6 |
| Active or recent GI bleeding: | ||||
| Acute variceal bleeding (suspected or confirmed) | 32(0,7) | 12(0,5) | 20(1) | 65,0±15,7 |
| Peptic ulcer bleeding (suspected or confirmed) | 160(3,5) | 65(2,5) | 95(4,8) | 63,6±18,8 |
| To confirm healing of esophageal or gastric ulcer | 131(2,9) | 53(2,1) | 78(3,9) | 61,8±15,3 |
| Follow-up: | ||||
| Portal hypertension evaluation (suspected or follow-up) | 195(4,3) | 61(2,4) | 134(6,7) | 62,1±11,7 |
| Suspected or confirmed Celiac Disease | 89(2) | 63(2,5) | 26(1,3) | 39,3±14,9 |
| Obtainment of samples for H. pylori culture* | 30(0,7) | 18(0,7) | 12(0,6) | 61,9±15,2 |
| Surveillance of premalignant conditions: | ||||
| Barrett’s Esophagus | 73(1,6) | 15(0,6) | 58(2,9) | 61,5±12,9 |
| Gastric adenomas and hyperplastic polyps | 25(0,5) | 20(0,8) | 5(0,3) | 63,0±14,3 |
| Atrophic gastritis | 101(2,2) | 80(3,1) | 21(1,1) | 61,5±14,7 |
| FAP | 3(0,1) | 2(0,1) | 1(0,1) | 59,0±10,0 |
| Oncological surveillance: | ||||
| MALT and other lymphomas | 35(0,8) | 18(0,7) | 17(0,9) | 68,7±11,6 |
| GIST and NET | 16(0,4) | 8(0,3) | 8(0,4) | 65,4±13,7 |
| Esophageal malignancy | 22(0,5) | 4(0,2) | 18(0,9) | 65,5±10,9 |
| Gastric malignancy | 38(0,8) | 18(0,7) | 20(1) | 66,8±12,2 |
| Surgery and GI assessment: | ||||
| Preoperative workup (previous to bariatric surgery, cholecystectomy, etc) | 38(0,8) | 27(1,1) | 11(0,6) | 49,8±13,0 |
| Surveillance of patients with previous gastric surgery | 46(1) | 21(0,8) | 25(1,3) | 60,4±13,9 |
| Staging workup for head/neck tumors and other tumors | 49(1,1) | 22(0,9) | 27(1,4) | 66,8±11,7 |
| GI assessment in other medical disordersᵻ | 11(0,2) | 6(0,2) | 5(0,3) | 59,5±16,9 |
| Others | 73(1,6) | 33(1,3) | 40(2) | 48,0±26,1 |
*In cases of failure of two courses of H. pylori eradication therapy, samples for bacterium culture and antibiotic resistance testing are performed. ᵻOther medical disorders include sclerodermia, Crohn’s disease, amongst others. FAP, familial adenomatous polyposis; GI, gastrointestinal; GIST, gastrointestinal stromal tumors; MALT, mucosa-associated lymphoid tissue lymphoma; NET, neuroendocrine tumor.
Table 3.
Indications for UGIE in patients in whom the request for UGIE was judged as inappropriate (Group 2)
| Overall N, (%) |
Females N, (%) |
Males N, (%) |
Age Mean ± SD |
|
|---|---|---|---|---|
| Inappropriate indications for UGIE | 540 | 316 | 224 | 38,6 ± 12,6 |
| Functional symptoms | 66 (12,2) |
38 (12) |
28 (12,6) |
39,6 ± 12,8 |
| Suspected GERD responsive to therapy in patients <45 years | 327 (60,5) |
191 (60,4) |
136 (61,0) |
37,7 ± 12,0 |
| Surveillance of healed benign lesions/ wrong timing of follow up | 113 (20,9) |
68 (21,5) |
45 (19,7) |
45,8 ± 15,6 |
| Missing indication | 34 (6,29) |
19 (6) |
15 (6,7) |
38,2 ± 8,9 |
Almost half of Group 1 patients who underwent UGIE had clinically irrelevant endoscopic findings, including a normal endoscopy, a small hiatal hernia or non-erosive gastritis (Table 4).
Table 4.
Main endoscopic findings in Group 1 patients.
| Endoscopic finding | Overall n. 4562 N (%) |
Females n. 2570 N (%) |
Males n. 1992 N (%) |
|---|---|---|---|
| Clinically relevant (n.2387) | |||
| Erosive esophagitis | 877 (19,2) | 418 (16,3) | 459(23) |
| Esophageal ulcer | 24 (0,5) | 9 (0,4) | 15(0,8) |
| Barrett’s esophagus | 122(2,7) | 32(1,2) | 90(4,5) |
| Esophageal varices | 171(3,7) | 53(2,1) | 118(5,9) |
| Erosive gastritis | 358(7,8) | 213(8,3) | 145(7,3) |
| Gastric ulcer | 89(2) | 49(1,9) | 40(2) |
| Erosive duodenitis | 79(1,7) | 43(1,7) | 36(1,8) |
| Duodenal ulcer | 81(1,8) | 24(0,9) | 57(2,9) |
| Gastric polyps | 134(2,9) | 103(4) | 31(1,6) |
| Surgery of upper GI tract | 132(2,9) | 52(2) | 80(4) |
| Esophageal/Gastric cancer | 51(1,1) | 17(0,7) | 34(1,7) |
| Atrophic gastritis | 91(2) | 74(2,9) | 17(0,9) |
| Celiac disease | 76(1,7) | 52(2) | 24(1,2) |
| Benign strictures (i.e. Schatzki’s ring) | 92(2) | 49(1,9) | 43(2,2) |
| Actively bleeding lesions | 10(0,2) | 6(0,2) | 4(0,2) |
| Clinically irrelevant (n. 2175) | |||
| Normal | 571(12,5) | 385(15) | 186(9,3) |
| Small hiatal hernia | 157(3,4) | 99(3,9) | 58(2,9) |
| Non-erosive, mild gastritis | 1326(29,1) | 829(32,3 | 497(24,9) |
| OTHERS (including percutaneous endoscopic gastrectomy) | 121(2,7) | 63(2,5) | 58(3) |
Gastric function combined assay was performed in 282 of the 540 patients in Group 2, with the following results: normal values in 94, normal values on PPI therapy in 48, H pylori active infection in 56, H pylori past infection (eradicated) in 30, GERD in 50 and gastric corpus atrophy in 4 patients. In the last 4 cases, UGIE was performed according to gastric atrophy surveillance guidelines(17). Interpreting the combination of studied parameters, six distinct serologic profiles emerged that characterize a clinical picture. Patients who underwent gastric function testing were grouped according to such serological profiling, as illustrated in table 5.
Table 5.
Distribution of patients in the study population according to gastric function assay serologic profile. G-17, gastrin 17; CGA-C, corpus localized chronic atrophic gastritis; F, female; GERD, gastroesophageal reflux disease; Hp, Helicobacter pylori; M, male; PGI, prostaglandin I; PGII prostaglandin II; PPI, proton pump inhibitor.
| Healthy stomach | GERD | Hp infection | Hp eradicated | PPI therapy | CGA-C | |
|---|---|---|---|---|---|---|
| Patients, n. | 94 | 50 | 56 | 30 | 48 | 4 |
| Sex (M/F) | 30/64 | 26/24 | 22/34 | 12/18 | 22/26 | 2/2 |
| Age (yrs) | 36,2±11,3 | 35,8±9,8 | 38,5±10,7 | 50,9±15,8 | 39,3±11,7 | 53,5±22,0 |
| PGI (ug/L) | 81,2±32,0 | 87,7±42,2 | 125,9±64,7 | 122,6±96,1 | 198,0±58,3 | 11,7±7,8 |
| PGII (ug/L) | 6,0±2,4 | 6,9±3,9 | 15,5±10,0 | 10,8±9,8 | 15,6±8,4 | 10.0±8,5 |
| PGI/PGII | 14,0±4,3 | 13,4±4,6 | 8,9±3,4 | 12,2±4,9 | 14,8±6,0 | 1,5±1,3 |
| G-17 (pmol/L) | 3,2±2,7 | 0,7±0,6 | 8,8±8,5 | 9,8±20,1 | 17,5±14,2 | 31,5±28,2 |
| Hp IgG (EIU) | 7,2±6,3 | 5,7±4,1 | 81,8±28,0 | 26,33±12,8 | 9,9±9,1 | 15,7±10,4 |
Within 2 years (range 1-22 months) UGIE was ultimately performed (mostly due to patient’s insistence to perform the exam) in 105 of the 540 patients in whom UGIE had been initially denied as the request was inappropriate, with normal findings in 71 of 105 (67.5%) and no cases of malignancy.
With this strategy, the number of endoscopies decreased from 3724 in the year 2012 to 2768 three years later, as illustrated in figure 1.
Figure 1.
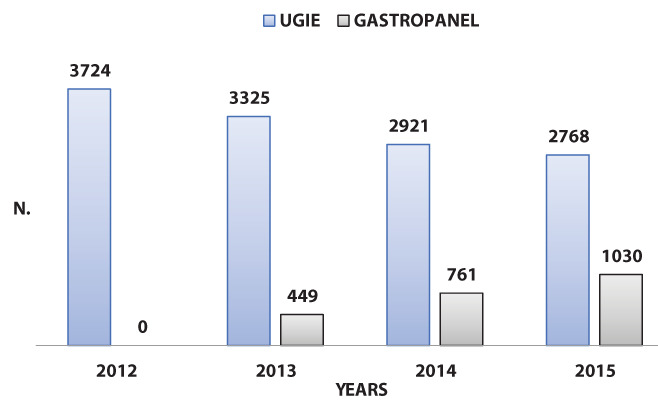
Volume of yearly upper gastrointestinal endoscopies performed at ULSS 7 Pedemontana Endoscopy Unit during the year before (2012) and the three years following the introduction of gastric function testing (2013-2015). UGIE, upper gastrointestinal endoscopies.
At our center, the rough estimated cost for a diagnostic UGIE with biopsies is €130 and the cost of the gastric function combined assay is €38. Directly performing UGIE in all 5192 requested cases would have represented a cost of approximately €674.940. By filtering and evaluating these referrals, 540 UGEI, corresponding to €70.200, were substituted by 282 Gastropanel tests for a total of €10.7016. With this strategy, including performance of UGIE in 4 patients in whom Gastropanel test was diagnostic for atrophic gastritis, costs amounted to €615.986, representing savings for a total of €58.954. However, an extra €13.650 were spent due to the fact that 105 patients underwent UGIE in spite the request had been judged as inappropriate, driven by patient will.
Adverse events were observed in 21 (0.5%) cases, including 19 cases of self-limiting hypoxia and 2 cases of self-limiting bradycardia. All events responded to conservative therapy within minutes and patients were uneventfully discharged shortly thereafter.
Discussion
With an elevated prevalence and significant morbidity in the general population, upper gastrointestinal (GI) disorders are responsible for an important disease burden leading to impairment in quality of life and considerable healthcare costs(18,19). Frequently, GI symptomatology is nonspecific and poorly correlates with objective findings; benign pathology is frequently associated with bothersome symptoms, while clinically significant conditions, including malignancies may go clinically unnoticed. Fear of malignant disease or another severe condition oftentimes prompts the patient to seek medical assistance and the general practitioner to prescribe an invasive exam such as an UGIE.
Although gastric nationwide cancer screening programs are justified and ongoing in high-incidence countries such as South Korea and Japan(20,21) the relatively low incidence of this tumor in the Western World, including Italy, renders a screening program not appropriate in the setting of the general population(22).
Unfortunately, alarm signs and symptoms, including dysphagia and gastrointestinal bleeding, are typical only in the advanced stages of cancer. Therefore, there is still a need to identify patients who should undergo UGIE.
In a UK study evaluating the clinical efficacy and cost-effectiveness of direct access endoscopy, Broe and coworkers analyzed data on 4262 patients referred by primary care physicians for UGIE; upper gastrointestinal cancer was diagnosed in 30 patients (diagnostic yield of 0.8%), while Barrett’s esophagus and peptic ulcer disease were identified in 148 (3.5%) and 185 (4.34%) patients, respectively. Of note, 3734 patients (87.6%) who had open access to UGIE had normal endoscopic findings, representing a cost of 2,296,410 Euro. Moreover, the authors determined that in patients under 40 years of age the diagnostic yield for upper gastrointestinal neoplasm was 0.14%, and endoscopy was normal in 92.2% of patients less than 40 years of age, suggesting that this subset of patients may not benefit from immediate UGIE through a direct access program(23).
The open-access strategy, although favoring overall cost containment associated with reduction in number of specialist consultations, translates into increased demand in terms of number of requested UGIE. Moreover, while reducing waiting times in a significant manner, endoscopic diagnoses do not differ significantly between UGIE performed following a prescription from a referring specialist/hospital vs the open-access system(24).
At this point, clinical decision-making, whether in the hands of primary care physicians in an open-access system or in the hands of the specialist, relies on established guidelines, which aim at identifying patients who will benefit from UGIE. Some studies suggest that most of the cancers are diagnosed in patients having appropriate indications(25), whereas others have found a substantial proportion of cancers among examinations deemed inappropriate(26,27).
In a study evaluating the appropriateness of UGIE in over 1000 patients who underwent endoscopic examination in New Zealand, 58% of referrals were judged to be appropriate and 42% inappropriate, with no cases of cancer being found in inappropriately requested UGIE. However, Barrett’s disease, a premalignant condition, was diagnosed more frequently among patients with inappropriate indications. In fact, a third of the patients with an inappropriate indication had a significant finding, including erosive esophagitis, erosive gastritis, duodenal ulcer, erosive duodenitis, and esophageal stenosis. Therefore, although UGIE endoscopies judged appropriate yielded significantly more relevant lesions than those judged to be inappropriate [65% vs 32%; odds ratio 3.94, 99% confidence interval (CI) 2.78, 5.57; p < 0.01], significant pathology was present also in patients without indication to perform UGIE according to ASGE guidelines(28). As the author suggests, such negative predictive values, not higher than 70%, make the use of these guidelines in the care of individual patients more uncertain.
Importantly, a large nine-year audit of open-access UGIE procedures regarding over 20,000 patients found significant endoscopic lesions including gastric, esophageal and duodenal cancer, in 4.9% of patients in whom UGIE was performed for non-ASGE indications(29). Moreover, Hassan et al (2007) reported more malignancies in the group without an appropriate ASGE indication(30).
These relevant data emphasizes the usefulness of a combined approach in the decision-making process and evaluation of patients for UGIE; while adherence to ASGE guidelines is associated with a positive yield, a case-by-case evaluation of clinical factors is fundamental. The fact that neoplasms have been found in subjects with unremarkable or “negative” endoscopic findings as early as three years before UGIE with tumor diagnosis(31) warrants further caution in the decision-making process of whether to accept or deny the request for a new UGIE. While endoscopy with a proper biopsy follow-up remains the standard for early detection of cancer and related premalignant lesions, non-invasive exams, in particular the Gastropanel, allow for identification of a subset of patients who are at high risk of premalignant conditions and warrant UGIE evaluation or, on the contrary, display normal gastric function and therefore a low probability of organic disease.
The role of specific stomach plasma biomarkers in diagnosis and screening for atrophic gastritis is emerging as a useful tool that provides information on gastric mucosal function. As this permits detection of atrophic gastritis, its use allows for identification of patients at a higher risk for gastric cancer, in whom endoscopic evaluation and follow-up is mandatory. The Gastropanel allows to distinguish normal mucosal function and excessive acid production from atrophic gastritis and H. pylori-related gastritis; these findings have been confirmed by results from our group(32), in which histological alterations and PCA positivity were found. According to ESGE guidelines, low pepsinogen I serum levels or/and low pepsinogen I/II ratio identify patients with advanced stages of atrophic gastritis and endoscopy is recommended for these patients, particularly if H. pylori serology is negative (moderate quality evidence, strong recommendation)(17). In contrast, serum PGI and PGI/PGII ratio lack sensitivity and specificity for the detection of advanced OLGA/OLGIM stages in H. pylori- positive subjects, underlying the fact that gastric function testing represents a valuable screening tool for atrophic gastritis, and that patients such identified are best served by prompt endoscopic evaluation with histologic assessment(33).
The use of gastrin, pepsinogens and H. pylori antibodies in gastric cancer screening, both in high-incidence as well as intermediate-incidence regions has demonstrated to be cost-effective(34,35) and has been incorporated in nationwide screening programs alongside periodic endoscopic surveillance of patients with established atrophy-metaplasia(36–38). A 2015 meta-analysis on pepsinogen tests in gastric cancer and atrophic gastritis suggested a good correlation between decreased pepsinogen serum levels and atrophy (35). In this meta-analysis, the summary sensitivity and summary specificity for gastric cancer diagnosis were 0.69 (95%CI 0.60 – 0.76) and 0.73 (95 %CI 0.62 – 0.82), respectively. Corresponding values for atrophic gastritis diagnosis were 0.69 (95%CI 0.55 – 0.80) and 0.88 (95%CI 0.77 – 0.94), respectively. The AUC for gastric cancer diagnosis was 0.76 (95 %CI 0.72 – 0.80) and for atrophic gastritis it was 0.85 (95 %CI 0.82 – 0.88).
Of note, functional testing is a valid tool in the evaluation of PPI non-responders, a group that represents a relevant proportion of UGIE requests. Low levels of gastrin and low levels of pepsinogens while on PPIs suggests non-compliance or low efficacy of the employed drug, which in turn may give the clinician the opportunity to repeat testing after switching drugs or after reinforcing adequate drug assumption. Moreover, the combined gastric function assay allows for patients with active H. pylori infection to be identified and subsequently treated. In addition, gastric function testing of refluxers, characterized by low gastrin levels in the presence of elevated pepsinogen I, might give further support even to the clinician’s hypothesis of GERD and his/her decision for a symptom-driven therapeutic approach to start the patient on PPIS(39).
As far as the economic analysis is concerned, in the Western world, healthcare costs continue to rise, with a significant proportion of gastroenterology-related healthcare costs attributed to endoscopy(40). The annual cost of UGIE in the United States amounts to 12.3 billion US dollars(41). Reported data vary whether the considered costs refer to the requested refund fee vs the cost reported internally (institutional fee). In Canada, Roth and Adams reported a mean (± SD) national fee for gastroscopy of $114.19±$31.47 per procedure, with a range of $52.50 to $156.10 (cost expressed in Canadian dollars, CAD), whereas international data showing fees of AUS $159.95 (CAD $143.41) for gastroscopies in Australia, €96.00 (CAD $152.93) per procedure for gastroscopy in France, €188.00 (CAD $288.91) for gastroscopy in Italy, and Medicare values in the United States of US $130.73 (CAD $155.04) in Michigan vs US $125.22 (CAD $148.54) in New York State(42). Using the Ontario Ministry of Health billing codes and institutional fees to determine the cost of biopsies performed at UGIE, Teriaky and coworkers reported the gastroenterology biopsy billing code was $15.10 Canadian dollars (CAD), while the pathology billing code per site biopsied consisted of a technical component of $16.54 CAD and a professional component of $48.65 CAD, and the institutional fee consisted of the cost of the biopsy forceps of $11.50 CAD. On average, the mean cost per esophageal biopsy was $78.22 CAD, $80.34 CAD for gastric biopsy, and $79.83 CAD for duodenal biopsy(43). Thus, the costs of performing an UGIE in Northeast Italy, in the public health system, are in line with those reported in Europe.
With respect to cost-effectiveness, Yah and collaborators report that pepsinogen testing resulted in a gain in QALYs, dominating serology and endoscopy for the detection of premalignant gastric lesions; in this Western (US) study, pepsinogen assay yielded a sensitivity of 71% and a specificity of 98%, with a cost of US$ 40, and US$ 105,400/QALY, as compared to the cost of US$980 for every endoscopy(44). Moreover, the ABC method, which is used in several Asian countries and employs a combination of assessing the presence of H. pylori antibodies and measuring PG concentrations and scheduling endoscopies accordingly, costs less than annual endoscopic screening (64,489 vs. 64,074 USD) and saved more lives (18.16 vs. 18.30 quality-adjusted life years)(36).
Conclusions
To the best of our knowledge, this is the first study that employs the non-invasive assessment of gastric function combined testing to prioritize the access to endoscopy services and to improve the appropriateness of prescriptions for UGIE. This strategy allows to reduce health costs by two mechanisms. The first one implies direct sparing of unnecessary endoscopic examinations; the second one implies indirect savings in terms of neoplasm-free years, quality of life and health expenditure by identifying precancerous lesions especially chronic atrophic gastritis with a low-cost, non-invasive exam. In this study we have demonstrated the usefulness of evaluating gastric function in evaluating patients without alarm symptoms, prioritizing access to endoscopy services in cases of abnormal results and guiding adequate diagnostic/therapeutic decisions such as eradication of H. pylori infection and identification of patients with chronic atrophic gastritis. This strategy has not only represented savings in terms of economic burden but has also reduced UGIE waiting list. Not only is Gastropanel useful for specialists in prioritizing access to UGIE, but also Primary Care Physicians can largely benefit from a readily available, low-cost and non-invasive exam that guides decision-making, improving the quality of care while containing costs.
Abbreviations:
CGA-C, corpus localized chronic atrophic gastritis; FAP, familial adenomatous polyposis; G-17, gastrin 17; GI, gastrointestinal; GERD, gastroesophageal reflux disease; GI, gastrointestinal; GIST, gastrointestinal stromal tumors; Hp, Helicobacter pylori; MALT, mucosa-associated lymphoid tissue lymphoma; NET, neuroendocrine tumor; PGI, pepsinogen I; PGII, pepsinogen II; PPI, proton pump inhibitor; UGIE, upper gastrointestinal endoscopy.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- García-Alonso FJ, Hernández Tejero M, Rubio Benito E, et al. Implementation and evaluation of early gastroscopy for patients with dyspepsia and warning signs in Primary Care. Gastroenterol Hepatol. 2017 May 1;40(5):331–8. doi: 10.1016/j.gastrohep.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Goni E, Riccò M, Franceschi M, et al. Gastrin 17 as non invasive marker of reflux disease. United Eur Gastroenterol J. 2015;3(5(S)):A653. [Google Scholar]
- Franceschi M, Masella A, Panozzo M, et al. GastroPanel serum markers as a non-invasive method for the diagnosis of H. pylori-related Gastritis. Helicobacter. 2015;(20(Suppl 1)):86. [Google Scholar]
- Di Mario F, Curlo M, Cavestro G, et al. “GastroPanel Test” in the clinical outcome of GERD. Gastroenterology. 2009;136(5):A-293 (S1921). [Google Scholar]
- Väänänen H, Vauhkonen M, Helske T, et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: a multicentre study. Eur J Gastroenterol Hepatol. 2003 Aug;15(8):885–891. doi: 10.1097/00042737-200308000-00009. [DOI] [PubMed] [Google Scholar]
- Kazumasa M. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006 Nov;9(4):245–53. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- Iijima K, Abe Y, Kikuchi R, et al. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009 Feb 21;15(7):853–9. doi: 10.3748/wjg.15.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LA, Murphy SJ, Johnston BT, et al. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: Results from the FINBAR case-control study. Gut. 2008 Jun;57(6):734–9. doi: 10.1136/gut.2007.132662. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Higuchi K, Shiba M, et al. Differences in clinical characteristics between patients with endoscopy-negative reflux disease and erosive esophagitis in Japan. Am J Gastroenterol. 2005 Apr;100(4):754–8. doi: 10.1111/j.1572-0241.2005.40966.x. [DOI] [PubMed] [Google Scholar]
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61(1):1–241. [PMC free article] [PubMed] [Google Scholar]
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002 Oct 10;347(15):1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- Nordenstedt H, Nilsson M, Johnsen R, Lagergren J, Hveem K. Helicobacter pylori infection and gastroesophageal reflux in a population-based study (The HUNT Study) Helicobacter. 2007 Feb;12(1):16–22. doi: 10.1111/j.1523-5378.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- De Bastiani R, Bacchin P, Bortot M, et al. Il Gastropanel: un test di diagnostica gastrica prezioso e poco conosciuto in medicina generale. Il Caduceo. 2014;16(2) [Google Scholar]
- Di Mario F, Cavallaro LG, Moussa AM, Franze A. Serological diagnosis of atrophic gastritis. G Ital di Endosc Dig. 2003;26(4) [Google Scholar]
- Nieuwenburg SAV, Waddingham WW, Graham D, et al. Accuracy of endoscopic staging and targeted biopsies for routine gastric intestinal metaplasia and gastric atrophy evaluation study protocol of a prospective, cohort study: the estimate study. BMJ Open. 2019 Sep 1;9(9) doi: 10.1136/bmjopen-2019-032013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: The OLGA staging system. Gut. 2007;56(5):631–6. doi: 10.1136/gut.2006.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel-Nunes PD, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Port. Endoscopy. 2019;51(4):365–88. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- Kurata JH, Nogawa AN, Everhart JE. A prospective study of dyspepsia in primary care. Dig Dis Sci. 2002;47(4):797–803. doi: 10.1023/a:1014748202229. [DOI] [PubMed] [Google Scholar]
- Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut. 2005;54:710–7. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38(4):259–67. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- Choi KS, Jun JK, Lee HY, et al. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci. 2011;102(8):1559–64. doi: 10.1111/j.1349-7006.2011.01982.x. [DOI] [PubMed] [Google Scholar]
- Gupta N, Bansal A, Wani SB, Gaddam S, Rastogi A, Sharma P. Endoscopy for upper GI cancer screening in the general population: A cost-utility analysis. Gastrointest Endosc. 2011;74(3) doi: 10.1016/j.gie.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Broe M, Barry M, Patchett S, Hill ADK. Evaluating the clinical efficacy and cost effectiveness of direct access endoscopy. Surgeon. 2013;11(6):304–8. doi: 10.1016/j.surge.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Johnston SD, Kirby J, Mackle E, Robinson TJ. A comparison of open access endoscopy and hospital-referred endoscopy in a district general hospital. Ulster Med J. 1999;68(2):73–8. [PMC free article] [PubMed] [Google Scholar]
- Minoli G, Prada A, Gambetta G, et al. The ASGE guidelines for the appropriate use of upper gastrointestinal endoscopy in an open access system. Gastrointest Endosc. 1995;42(5):387–9. doi: 10.1016/s0016-5107(95)70036-6. [DOI] [PubMed] [Google Scholar]
- Gonvers JJ, Burnand B, Froehlich F, et al. Appropriateness and diagnostic yield of upper gastrointestinal endoscopy in an open-access endoscopy unit. In: Endoscopy. 1996:661–6. doi: 10.1055/s-2007-1005573. [DOI] [PubMed] [Google Scholar]
- Rossi A, Bersani G, Ricci G, et al. ASGE guidelines for the appropriate use of upper endoscopy: Association with endoscopic findings. Gastrointest Endosc. 2002 Nov 1;56(5):714–9. doi: 10.1067/mge.2002.129222. [DOI] [PubMed] [Google Scholar]
- Tahir M. Appropriateness of Upper Gastrointestinal Endoscopy: Will the Diagnostic Yield Improve by the use of American Society of Gastroenterology Guidelines? Euroasian J hepato-gastroenterology. 2016;6(2):143–8. doi: 10.5005/jp-journals-10018-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D, Rainis T, Stermer E, Lavy A. A nine-year audit of open-access upper gastrointestinal endoscopic procedures: Results and experience of a single centre. Can J Gastroenterol. 2011;25(2):83–8. doi: 10.1155/2011/379014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan C, Bersani G, Buri L, et al. Appropriateness of upper-GI endoscopy: an Italian survey on behalf of the Italian Society of Digestive Endoscopy. Gastrointest Endosc. 2007;65(6):767–74. doi: 10.1016/j.gie.2006.12.058. [DOI] [PubMed] [Google Scholar]
- Voutilainen M, Kunnamo I. A survey of open-access endoscopy in primary health care centres: Outcome of gastric carcinoma patients diagnosed by general practitioners compared with hospital-referred endoscopy. Dig Liver Dis. 2005;37(2):119–23. doi: 10.1016/j.dld.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Antico A, Panozzo M, et al. Serological Diagnosis of Upper GI Diseases in Primary Care Setting. 2015;148(4):S-322. [Google Scholar]
- Coelho MCF, Ribeiro HG, Gomes CGDO, Marinho FP, Barbosa AJA, Coelho LGV. Helicobacter pylori chronic gastritis on patients with premalignant conditions: Olga and olgim evaluation and serum biomarkers performance. Arq Gastroenterol. 2021;58(1):39–47. doi: 10.1590/S0004-2803.202100000-08. [DOI] [PubMed] [Google Scholar]
- Enríquez-Sánchez LB, Gallegos-Portillo LG, Camarillo-Cisneros J, et al. Cost-benefit of serum pepsinogen screening for gastric adenocarcinoma in the Mexican population. Rev Gastroenterol Mex. 2021;Nov 15;(21):S2255–543X. doi: 10.1016/j.rgmxen.2021.11.002. [DOI] [PubMed] [Google Scholar]
- Tu H, Sun L, Dong X, et al. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Am J Gastroenterol. 2017 May 1;112(5):704–15. doi: 10.1038/ajg.2017.55. [DOI] [PubMed] [Google Scholar]
- Saito S, Azumi M, Muneoka Y, et al. Cost-effectiveness of combined serum anti-Helicobacter pylori IgG antibody and serum pepsinogen concentrations for screening for gastric cancer risk in Japan. Eur J Heal Econ. 2018 May 1;19(4):545–55. doi: 10.1007/s10198-017-0901-y. [DOI] [PubMed] [Google Scholar]
- Park CH, Kim EH, Jung DH, et al. The new modified ABCD method for gastric neoplasm screening. Gastric Cancer. 2016 Jan 1;19(1):128–35. doi: 10.1007/s10120-015-0473-4. [DOI] [PubMed] [Google Scholar]
- Kazumasa M. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels-“ABC method”. Vol. 87, Proceedings of the Japan Academy Series B: Physical and Biological Sciences. Proc Jpn Acad Ser B Phys Biol Sci. 2011:405–14. doi: 10.2183/pjab.87.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mario F, Crafa P, Franceschi M, et al. Low Levels of Gastrin 17 are Related with Endoscopic Findings of Esophagitis and Typical Symptoms of GERD. J Gastrointestin Liver Dis. 2021 Feb 12;30(1):25–9. doi: 10.15403/jgld-2952. [DOI] [PubMed] [Google Scholar]
- Harewood GC. Economic comparison of current endoscopic practices: Barrett’s surveillance vs. ulcerative colitis surveillance vs. biopsy for sprue vs. biopsy for microscopic colitis. Dig Dis Sci. 2004;49(11–12):1808–14. doi: 10.1007/s10620-004-9575-2. [DOI] [PubMed] [Google Scholar]
- Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5) doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth LS, Adams PC. Variation in physician reimbursement for endoscopy across Canada. Can J Gastroenterol. 2009;23(7):503–5. doi: 10.1155/2009/345719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teriaky A, Alnasser A, Mclean C, Gregor J, Yan B. The Utility of Endoscopic Biopsies in Patients with Normal Upper Endoscopy. Can J Gastroenterol Hepatol. 2016;2016(3026563):1–7. doi: 10.1155/2016/3026563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JM, Hur C, Ward Z, Schrag D, Goldie SJ. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: A cost-effectiveness analysis. Gut. 2016 Apr 1;65(4):563–74. doi: 10.1136/gutjnl-2014-308588. [DOI] [PMC free article] [PubMed] [Google Scholar]


