Abstract
Escherichia coli CAG2242 cells are deficient in the speG gene encoding spermidine acetyltransferase. When these cells were cultured in the presence of 0.5 to 4 mM spermidine, their viability was greatly decreased through the inhibition of protein synthesis by overaccumulation of spermidine. When the cells were cultured with a high concentration of spermidine (4 mM), a revertant strain was obtained. We found that a 55-kDa protein, glycerol kinase, was overexpressed in the revertant and that synthesis of a ribosome modulation factor and the RNA polymerase ς38 subunit, factors important for cell viability, was increased in the revertant. Levels of l-glycerol 3-phosphate also increased in the revertant. Transformation of glpFK, which encodes a glycerol diffusion facilitator (glpF) and glycerol kinase (glpK), to E. coli CAG2242 partially prevented the cell death caused by accumulation of spermidine. It was also found that l-glycerol 3-phosphate inhibited spermidine binding to ribosomes and attenuated the inhibition of protein synthesis caused by high concentrations of spermidine. These results indicate that l-glycerol 3-phosphate reduces the binding of excess amounts of spermidine to ribosomes so that protein synthesis is recovered.
Polyamines (putrescine, spermidine, and spermine) are necessary for normal cell growth, and their proliferative effects are probably due to stimulation of nucleic acid and protein synthesis (3, 24). A decrease in polyamine content causes a decrease in the rate of cell proliferation and protein synthesis (12, 34). Furthermore, overaccumulation of polyamines can inhibit protein synthesis and cell growth (7, 23). Thus, the optimal concentrations of polyamines are necessary for protein synthesis and cell growth. Escherichia coli mutants with disruptions of enzymes in the polyamine synthetic or degradative pathways are useful systems with which to probe the physiological roles of polyamines. One such mutant lacks the speG gene encoding spermidine acetyltransferase, which catalyzes the first step of polyamine degradation in E. coli (5). In the speG mutant, we found that addition of spermidine to the medium reduces cell viability in the late stationary phase due to intracellular accumulation of spermidine (6). The accumulation of spermidine caused a marked decrease in protein synthesis but not in DNA and RNA synthesis in the stationary phase (6).
The synthesis of several kinds of proteins was particularly inhibited by spermidine in the late stationary phase in the speG mutant. These proteins include a ribosome modulation factor (RMF) (6) and the RNA polymerase stationary-phase-specific sigma subunit ςS or ς38 (1). RMF is synthesized in the stationary phase and is uniquely associated with 100S dimer ribosomes (27, 28). A mutant lacking RMF showed decreased cell viability in the stationary phase (32). The ς38 subunit is involved in the transcription of a group of genes for stationary-phase survival and stress response to heat shock or osmotic shock (8, 15, 33).
In this study, we isolated a revertant strain of the speG mutant which can survive during growth in a high concentration of spermidine. Although polyamines were accumulated in the revertant, the synthesis of RMF and the ς38 subunit was increased, apparently due to inhibition of spermidine binding to ribosomes. Inhibition of polyamine binding to ribosomes was due to an increase in l-glycerol 3-phosphate, which interacts with spermidine.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
E. coli CAG2242 (speG supE44 hsdR thi thr leu lacY1 tonA21), a spermidine acetyltransferase-deficient mutant (2), was kindly supplied by E. W. Gerner (University of Arizona Health Sciences Center). The cells were grown in a modified Luria-Bertani (LB) medium (8 g of tryptone per liter, 4 g of yeast extract per liter, and 5 g of NaCl per liter supplemented with 1 mM sodium phosphate, pH 7.4). Where indicated, 2 or 4 mM spermidine was added at the onset of cell growth. Cell growth was monitored by measuring A540. Cell viability was determined by counting colonies in aliquots of the culture grown on an LB medium-containing 1.5% agar plate at 37°C. Thus, the definition of viable cells is cells that are able to grow on an agar plate. When pUC118- or -119-derived plasmids were transformed, 100 μg of ampicillin per ml was added to the medium.
Measurement of polyamines, l-glycerol 3-phosphate, and protein contents.
Polyamine levels in E. coli were determined by high-pressure liquid chromatography as described previously (10) after homogenization and extraction of the polyamines with 5% trichloroacetic acid and centrifugation at 27,000 × g for 15 min at 4°C. Levels of l-glycerol 3-phosphate were measured by the method of Hohorst (9). The reaction mixture (1 ml), consisting of 0.18 M hydrazine, 0.45 M glycine buffer (pH 9.5), 2.5 mM β-NAD, 60 μg of α-glycerophosphate dehydrogenase (Sigma), and 0.2 ml of extract obtained as described above after neutralization with ether, was incubated at 24°C for 30 min, and A334 was measured. Levels of l-glycerol 3-phosphate were calculated from the calibration curve of l-glycerol 3-phosphate. Protein was determined by the method of Lowry et al. (19).
Western blot analysis
Antibody for RMF was made by injecting 1 mg of RMF with Freund's complete adjuvant into a rabbit. Antibody for the RNA polymerase ς38 subunit was prepared as described previously (16). Western blotting was performed by the method of Nielsen et al. (21).
Identification of induced protein in the revertant by protein sequencing.
Induced protein in the revertant was identified by electrophoresis on a sodium dodecyl sulfate (SDS)–10.5% polyacrylamide gel (18). After blotting, the induced protein was analyzed by automated Edman degradation on a protein sequencer (Applied Biosystems model 470A) equipped with a phenylthiohydantoin analyzer (model 120A).
Construction of pUCglpFK, pUCglpFKX, pUCglpF, pUCglpK, and pUCglpX.
PCR was performed by using total chromosomal DNA as a template and 5′-GGCCTGCAGACGTTGCTGCCAGCCGTTCTG-3′ (P1) and 5′-TGGGTCGACGTGTAGCACAGGGGAAGGGAG-3′ as primers to obtain the glpFK gene (22, 30). pUCglpFK was constructed by inserting the 3.2-kb PstI-SalI fragment of the PCR product into the same restriction sites of pUC118 (TaKaRa). PCR was also performed by using total chromosomal DNA as a template and P1 and 5′-CCTGTCGACATTGACTTGCCTTATCTTCGT-3′ (P2) as primers to obtain the glpFKX gene. pUCglpFKX was constructed by inserting the 4.3-kb PstI-SalI fragment of the PCR product into the same restriction sites of pUC118. pUCglpF-1 and pUCglpF-2 (isopropyl-β-d-thiogalactopyranoside [IPTG] inducible) were constructed by deleting the 1.9-kb SmaI-NruI fragment of pUCglpFK and by inserting the 1.4-kb SphI-NruI fragment of pUCglpFK into the SphI-SmaI sites of pUC119 (TaKaRa), respectively. pUCglpK (IPTG inducible) was constructed by inserting the 1.6-kb HindIII-SalI fragment of pUCglpFK into the same restriction sites of pUC119. PCR was performed by using total chromosomal DNA as a template and 5′-GTGCTGCAGTTCGCGCCATTCCTTACTGCT-3′ and P2 as primers to obtain the glpX gene. pUCglpX (IPTG inducible) was constructed by inserting the 1.1-kb PstI-SalI fragment of the PCR product into the same restriction sites of pUC119.
Assays for polyphenylalanine synthesis and spermidine binding to ribosomes.
Salt-washed ribosomes, the 0.25 M NH4Cl fraction of DEAE-cellulose column chromatography of S100, containing aminoacyl-tRNA synthetases and elongation factors, and tRNA mixtures were prepared from E. coli Q13 essentially as described previously (11, 29). Polyphenylalanine synthesis was measured by using 15 μg of salt-washed ribosomes in the presence of 12 mM Mg2+ and 100 mM NH4+ as described previously (27). The reaction mixture (0.1 ml) for spermidine binding to ribosomes contained 50 mM Tris-HCl (pH 7.5), 1 mM magnesium acetate, 30 mM NH4Cl, 150 μg of salt-washed ribosomes, and 0.25 mM [14C]spermidine (1.85 kBq). After incubation for 10 min at 30°C, the reaction mixture was chilled and passed through a cellulose nitrate membrane filter (Advantec). The filter was washed with a buffer containing 50 mM Tris-HCl (pH 7.5), 1 mM magnesium acetate, and 30 mM NH4Cl, and the radioactivity on the filter was counted with a liquid scintillation counter.
RESULTS
Isolation of a revertant viable in the presence of spermidine accumulation.
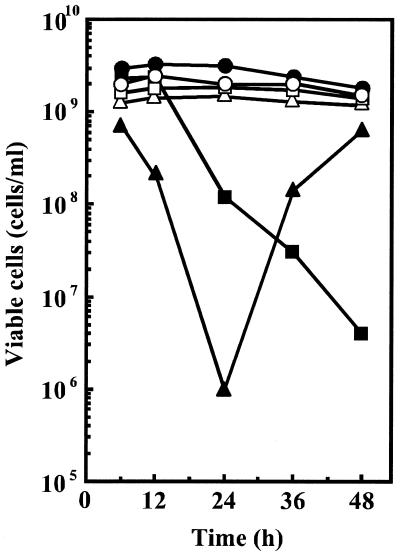
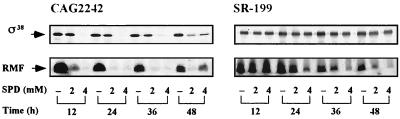
We previously reported that E. coli CAG2242, a speG mutant, showed accumulation of spermidine and a subsequent decrease in cell viability in the late stationary phase of cell growth when cells were cultured in the presence of spermidine (0.5 to 2 mM) (1, 6). When cells were cultured in the presence of a high concentration of spermidine (4 mM), cell viability was drastically decreased until 24 h after the onset of cell growth (Fig. 1). However, viable revertants were obtained at 36 to 48 h after the onset of cell culture (Fig. 1). When these revertants were recultured in the presence of 4 mM spermidine, the revertants were resistant to cell death caused by spermidine accumulation (Fig. 1). We isolated 10 revertant colonies and studied them further. It was first determined whether RMF and the ς38 subunit, factors important for cell viability, can be synthesized by the revertants. All of the revertants could synthesize both RMF and the ς38 subunit until 48 h after the onset of cell culture in the presence of 4 mM spermidine, although synthesis of RMF gradually decreased as cell culture progressed. In Fig. 2, results of experiments done with one of the revertants (E. coli SR-199) are shown.
FIG. 1.
Effect of spermidine on cell viability of E. coli CAG2242 and its revertants. Viable cells were counted at the designated times as described in Materials and Methods. Symbols for E. coli CAG2242: ●, no spermidine; ▪, 2 mM spermidine; ▴, 4 mM spermidine. Revertant cells (▴) were collected after 48 h of culture in the presence of 4 mM spermidine and cultured again under the following conditions: ○, no spermidine; □, 2 mM spermidine; ▵, 4 mM spermidine. Each point is the average of duplicate determinations.
FIG. 2.
Effect of spermidine (SPD) on the synthesis of the ς38 subunit and RMF in E. coli CAG2242 and its revertant SR-199. Western blotting was performed by using 5 μg of protein for ς38 and 25 μg of protein for RMF.
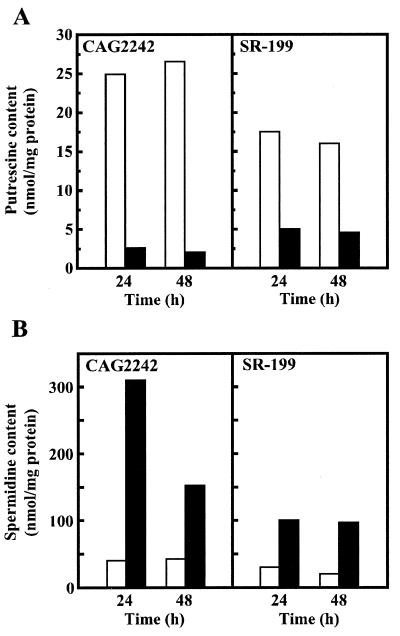
The intracellular polyamine contents of E. coli CAG2242 and the revertant SR-199 were measured after cells were cultured in the presence and absence of 4 mM spermidine. At 24 h after the onset of cell culture in the presence of 4 mM spermidine, the spermidine content of E. coli SR-199 was about one-third of that of E. coli CAG2242 (Fig. 3B) but a significant amount of spermidine still accumulated in E. coli SR-199. At 48 h, the difference in spermidine level between the two strains became about two-thirds due to a decrease in the spermidine level of E. coli CAG2242. (Fig. 3B). When spermidine accumulated in cells, the putrescine content decreased greatly, probably due to inhibition of the synthesis of ornithine decarboxylase (Fig. 3A and B). Similar results were obtained with the other nine revertants.
FIG. 3.
Effect of spermidine on cellular levels of putrescine (A) and spermidine (B). Polyamine contents were measured at the designated times as described in Materials and Methods. E. coli CAG2242 and SR-199 cells were cultured in the absence (□) and presence (▪) of 4 mM spermidine. Each value is the average of duplicate determinations.
Significant amounts of spermidine accumulate in E. coli SR-199, but this accumulation is not cytotoxic. This suggests that a substance which disturbs the interaction between ribosomes and polyamines may be induced in the revertant, and subsequently the inhibition of protein synthesis due to overaccumulation of spermidine may be prevented.
Induction of the glpFK operon in the revertant.
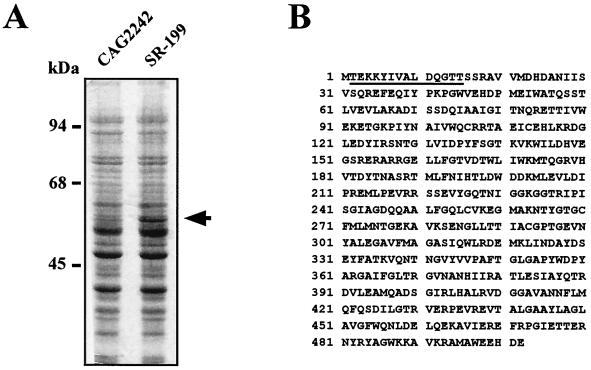
We examined the proteins induced in the revertant E. coli SR-199 by SDS-polyacrylamide gel electrophoresis. As shown in Fig. 4A, a protein of about 55 kDa was strongly induced in the revertant cultured with 4 mM spermidine. A protein of the same molecular mass was also induced in the other nine revertants. The amino acid sequence of the induced protein was determined by Edman degradation, and the NH2-terminal 14 amino acid residues were identified (Fig. 4B). Based on this sequence, the protein was identified as glycerol kinase. Induction of glycerol kinase was also observed in E. coli SR-199 cultured without spermidine (data not shown).
FIG. 4.
SDS-polyacrylamide gel electrophoresis of proteins from E. coli CAG2242 and SR-199 (A) and amino acid sequence of the NH2 terminus of the induced protein in E. coli SR-199 (B). (A) E. coli CAG2242 and SR-199 were cultured in the absence and presence of 4 mM spermidine, respectively, for 24 h. SDS-polyacrylamide gel electrophoresis was performed with 20 μg of protein. The arrow indicates the induced protein. (B) The amino acid sequence determined by Edman degradation is underlined. Accordingly, the induced protein was identified as a glycerol kinase, whose amino acid sequence is shown in panel B.
We next cloned the glpFK operon encoding a glycerol diffusion facilitator and glycerol kinase (22, 31) by PCR and determined whether expression of glpFK could prevent the cytotoxic effect of spermidine accumulation. As shown in Fig. 5, the time course of the decrease in cell viability was greatly changed in E. coli CAG2242 transformed with the vector pUC118 or -119 (Fig. 1). Maximal cell death was observed at 60 h during a 60-h culture; that is, revertants viable during spermidine accumulation were not obtained. However, the mechanism of this effect is unknown. When glpFK was transformed to E. coli CAG2242, cell viability recovered greatly. Either glpF, encoding a glycerol diffusion facilitator, or glpK, encoding glycerol kinase, partially restored cell viability, so the two genes functioned together. In relation to this finding, it has been reported that glycerol kinase is activated by interaction with the glycerol facilitator (26). The level of glycerol kinase in E. coli CAG2242/pUCglpFK or E. coli CAG2242/pUCglpK was slightly higher than that in E. coli SR-199 (data not shown).
FIG. 5.
Effect of glpFKX on E. coli CAG2242 cell viability. Viable cells were counted at the designated times as described in Materials and Methods. Cells transformed with the indicated plasmids were cultured in the absence and presence of 4 mM spermidine. Symbols: ○, pUC119 and no spermidine; ▵, pUCglpFK and no spermidine; ●, pUC119 and 4 mM spermidine; ♦, pUCglpF and 4 mM spermidine; ▪, pUCglpK and 4 mM spermidine; ▾, pUCglpX and 4 mM spermidine; ▴, pUCglpFK and 4 mM spermidine; ×, pUCglpFKX and 4 mM spermidine. When the gene was oriented under the control of the lac promoter, 1 mM IPTG was added at 6 h after the onset of cell culture. In the case of glpF, essentially the same results were obtained with the plasmids with either the glpFK promoter (pUCglpF-1) or the lac promoter (pUCglpF-2). Each point is the average of duplicate determinations.
Recently, the third gene (X) of the glpFK operon was identified as a gene encoding fructose 1,6-bisphosphatase (4). Since fructose 1,6-bisphosphate is a feedback inhibitor of glycerol kinase (25, 35), the viability of E. coli CAG2242 cells cultured with 4 mM spermidine might be more clearly restored if glpFKX were transformed to E. coli CAG2242 instead of glpFK. This possibility was tested, and as shown in Fig. 5, transformation of glpX still significantly restored cell viability but the effect was weaker than that obtained by transformation of glpF or glpK. Furthermore, recovery of cell viability with glpFKX was nearly equal to that obtained with glpFK, suggesting that involvement of glpX in the recovery of cell viability is small.
The levels of l-glycerol 3-phosphate in E. coli CAG2242 and the revertant SR-199 were measured. As shown in Table 1, levels of l-glycerol 3-phosphate were much higher in the revertant SR-199 than in E. coli CAG2242, confirming that expression of glpFK is enhanced in the revertant SR-199. The level of l-glycerol 3-phosphate also increased when E. coli CAG2242 was transformed with pUCglpF, pUCglpK, and pUCglpFK (Table 1). It was maximal with E. coli CAG2242/pUCglpFK, indicating that the level of l-glycerol 3-phosphate correlates with the degree of recovery of cell viability. Addition of 15 mM l-glycerol 3-phosphate also restored cell viability significantly (data not shown).
TABLE 1.
Levels of l-glycerol 3-phosphate in various E. coli strains
| E. coli strain | Culturea time (h) | l-Glycerol 3-phosphate concn (nmol/mg of protein)b |
|---|---|---|
| CAG2242 | 12 | 2.74 ± 0.21 |
| 24 | 2.81 ± 0.14 | |
| SR-199 | 12 | 6.92 ± 0.21 |
| 24 | 7.31 ± 0.18 | |
| CAG2242/pUC118 | 24 | 1.80 ± 0.18 |
| CAG2242/pUCglpF | 24 | 4.52 ± 0.11 |
| CAG2242/pUCglpK | 24 | 4.61 ± 0.13 |
| CAG2242/pUCglpFK | 24 | 5.81 ± 0.14 |
Cells were cultured in the absence of spermidine.
Each value shown is the mean ± the standard deviation of three determinations.
l-Glycerol 3-phosphate reverses inhibition of protein synthesis caused by overaccumulation of spermidine.
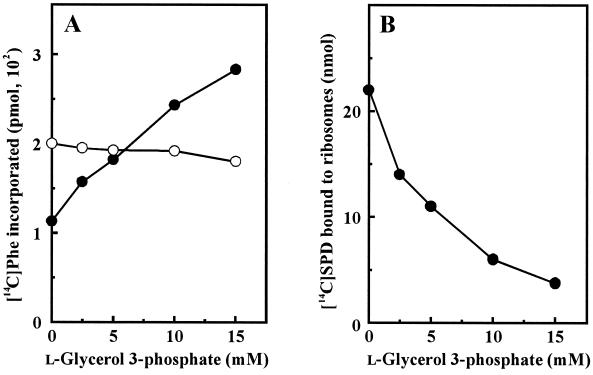
We previously reported that polyamines (putrescine and spermidine) have not only a sparing effect on the Mg2+ requirement for protein synthesis but also a stimulating effect, which cannot be fulfilled by any amount of Mg2+ in the absence of polyamines (14, 29). We also reported that overaccumulation of spermidine inhibits protein synthesis such that cell viability in the stationary phase decreased greatly (6). The inhibition of protein synthesis by overaccumulation of spermidine was mainly due to the inactivation of ribosomes through replacement of Mg2+ at magnesium binding sites by polyamines (7, 13). Thus, we examined whether the inhibition of protein synthesis caused by overaccumulation of spermidine was reversed by l-glycerol 3-phosphate by using a cell-free system. As shown in Fig. 6A, polyphenylalanine synthesis at 12 mM Mg2+ was inhibited by 45% by 4 mM spermidine. Addition of l-glycerol 3-phosphate gradually recovered the inhibition of polyphenylalanine synthesis. The level of polyphenylalanine synthesis in the presence of 15 mM l-glycerol 3-phosphate and 4 mM spermidine was nearly equal to that in the presence of 2 mM spermidine only. On the other hand, polyphenylalanine synthesis in the absence of spermidine was not influenced by l-glycerol 3-phosphate (Fig. 6A). The effect of l-glycerol 3-phosphate on the binding of [14C]spermidine to ribosomes was also examined. As shown in Fig. 6B, spermidine binding to ribosomes was inhibited by l-glycerol 3-phosphate. The results suggest that l-glycerol 3-phosphate interacts with spermidine so that protein synthesis is recovered by disturbing the binding of spermidine to ribosomes.
FIG. 6.
Effect of l-glycerol 3-phosphate on protein synthesis (A) and spermidine binding to ribosomes (B). Experiments were performed as described in Materials and Methods. (A) Polyphenylalanine (Phe) synthesis at 12 mM Mg2+ (○) and at 12 mM Mg2+ and 4 mM spermidine (●). (B) Spermidine binding to ribosomes was measured in the presence of 1 mM Mg2+ and 0.25 mM spermidine. Each point is the average of duplicate determinations.
DISCUSSION
The results of this study show that l-glycerol 3-phosphate negates the toxicity of spermidine. It presumably does this by disturbing spermidine binding to ribosomes, because it has been already reported that ribosomes are inactivated by an increase in the level of spermidine bound to ribosomes (13, 30). Inactivation of the ribosomes occurred when more than 40% of the Mg2+ originally bound to ribosomes was replaced with spermidine (13). One simple explanation is that lower-molecular-mass phosphate compounds, such as l-glycerol 3-phosphate, directly interact with polyamines and thereby modulate their functions. We also reported recently that the function of Mg2+-ATP is modulated by the formation of an Mg2+-ATP-polyamine (spermidine or spermine) complex (20). The ATPase activity of PotA (17) was greatly enhanced by spermine, and the activity of protein kinase A was also stimulated about twofold by spermine (20). However, direct evidence for the interaction between spermidine and l-glycerol 3-phosphate has not been obtained thus far. Thus, another function(s) of l-glycerol 3-phosphate in the recovery of cell viability may also exist.
We have shown that spermidine has not only a sparing effect on the Mg2+ requirement for polyphenylalanine synthesis but also a stimulating effect, which cannot be fulfilled by any amount of Mg2+ in the absence of spermidine (14). Polyphenylalanine synthesis in the presence of 12 mM Mg2+ and 100 mM NH4+ was stimulated by 50% by 2 mM spermidine and was inhibited by 45% by 4 mM spermidine (Fig. 6A). Polyphenylalanine synthesis at 4 mM spermidine gradually increased with the increase in l-glycerol 3-phosphate and finally reached the level obtained by 2 mM spermidine. The results support the idea that l-glycerol 3-phosphate disturbs the binding of spermidine to ribosomes, and this is confirmed by the data shown in Fig. 6B. If l-glycerol 3-phosphate similarly functions in the logarithmic phase, it is expected that protein synthesis would be inhibited. However, expression of the glpK gene in the logarithmic phase was much weaker than that in the stationary phase (data not shown).
In the revertants, the glpFK operon was induced in cells cultured with or without spermidine. This suggests that a mutation may occur in the promoter region of the glpFK operon or in a regulatory protein of the operon. We isolated 10 revertant colonies, and the properties of the 10 colonies were indistinguishable, suggesting that they were derived from the same origin. Furthermore, an unidentified membrane protein was induced only in the cells cultured with spermidine (data not shown). This protein may be involved in the decrease in spermidine at 24 h after the onset of cell culture. The activity of spermidine uptake was almost the same in E. coli CAG2242 and the revertant SR-199. Thus, we expect that the protein is involved in the excretion of spermidine from the cells. Since expression of the glpFK gene could not restore cell viability completely, the unidentified membrane protein is also important for complete recovery of cell viability. Experiments intended to identify this protein are in progress.
ACKNOWLEDGMENTS
We thank A. J. Michael, C. Hanfrey, and K. Williams for kind suggestions and help in preparing the manuscript. Thanks are also due to E. W. Gerner for kindly supplying E. coli CAG2242.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan, and a grant-in-aid from the Tokyo Biochemical Research Foundation.
REFERENCES
- 1.Apirakaramwong A, Kashiwagi K, Raj V S, Sakata K, Kakinuma Y, Ishihama A, Igarashi K. Involvement of ppGpp, ribosome modulation factor, and stationary phase-specific sigma factor ςS in the decrease in cell viability caused by spermidine. Biochem Biophys Res Commun. 1999;264:643–647. doi: 10.1006/bbrc.1999.1556. [DOI] [PubMed] [Google Scholar]
- 2.Carper S W, Willis D G, Manning K A, Gerner E W. Spermidine acetylation in response to a variety of stresses in Escherichia coli. J Biol Chem. 1991;266:12439–12441. [PubMed] [Google Scholar]
- 3.Cohen S S. A guide to the polyamines. Oxford, United Kingdom: Oxford University Press; 1998. pp. 1–543. [Google Scholar]
- 4.Donahue J L, Bownas J L, Niehaus W G, Larson T J. Purification and characterization of glpX-encoded fructose 1,6-bisphosphatase, a new enzyme of the glycerol 3-phosphate regulon of Escherichia coli. J Bacteriol. 2000;182:5624–5627. doi: 10.1128/jb.182.19.5624-5627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuchi J, Kashiwagi K, Takio K, Igarashi K. Properties and structure of spermidine acetyltransferase in Escherichia coli. J Biol Chem. 1994;269:22581–22585. [PubMed] [Google Scholar]
- 6.Fukuchi J, Kashiwagi K, Yamagishi M, Ishihama A, Igarashi K. Decrease in cell viability due to the accumulation of spermidine in spermidine acetyltransferase-deficient mutant of Escherichia coli. J Biol Chem. 1995;270:18831–18835. doi: 10.1074/jbc.270.32.18831. [DOI] [PubMed] [Google Scholar]
- 7.He Y, Kashiwagi K, Fukuchi J, Terao K, Shirahata A, Igarashi K. Correlation between the inhibition of cell growth by accumulated polyamines and the decrease of magnesium and ATP. Eur J Biochem. 1993;217:89–96. doi: 10.1111/j.1432-1033.1993.tb18222.x. [DOI] [PubMed] [Google Scholar]
- 8.Hengge-Aronis R, Lange R, Henneberg N, Fischer D. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–265. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohorst H-J. l-(−)-Glycerol-1-phosphate. Determination with glycerol-1-phosphate dehydrogenase. In: Bergmeyer H-U, editor. Methods of enzymatic analysis. New York, N.Y: Academic Press, Inc.; 1963. pp. 215–219. [Google Scholar]
- 10.Igarashi K, Kashiwagi K, Hamasaki H, Miura A, Kakegawa T, Hirose S, Matsuzaki S. Formation of a compensatory polyamine by Escherichia coli polyamine-requiring mutants during growth in the absence of polyamines. J Bacteriol. 1986;166:128–134. doi: 10.1128/jb.166.1.128-134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi K, Kashiwagi K, Kishida K, Watanabe Y, Kogo A, Hirose S. Defect in the split proteins of 30-S ribosomal subunits and under-methylation of 16-S ribosomal RNA in a polyamine-requiring mutant of Escherichia coli grown in the absence of polyamines. Eur J Biochem. 1979;93:345–353. doi: 10.1111/j.1432-1033.1979.tb12829.x. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi K, Koga K, He Y, Shimogori T, Ekimoto H, Kashiwagi K, Shirahata A. Inhibition of the growth of various human and mouse tumor cells by 1,15-bis(ethylamino)-4,8,12-triazapentadecane. Cancer Res. 1995;55:2615–2619. [PubMed] [Google Scholar]
- 13.Igarashi K, Sugawara K, Hirose S. Effects on ribosomes of substitution of spermidine or divalent cations for magnesium ions. J Biochem (Tokyo) 1975;77:753–759. doi: 10.1093/oxfordjournals.jbchem.a130779. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi K, Sugawara K, Izumi I, Nagayama C, Hirose S. Effect of polyamines on polyphenylalanine synthesis by Escherichia coli and rat-liver ribosomes. Eur J Biochem. 1974;48:495–502. doi: 10.1111/j.1432-1033.1974.tb03790.x. [DOI] [PubMed] [Google Scholar]
- 15.Ishihama A. Adaptation of gene expression in stationary phase bacteria. Curr Opin Genet Dev. 1997;7:582–588. doi: 10.1016/s0959-437x(97)80003-2. [DOI] [PubMed] [Google Scholar]
- 16.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of ς70 and ς32. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashiwagi K, Endo H, Kobayashi H, Takio K, Igarashi K. Spermidine-preferential uptake system in Escherichia coli. ATP hydrolysis by PotA protein and its association with membranes. J Biol Chem. 1995;270:25377–25382. doi: 10.1074/jbc.270.43.25377. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Meksuriyen D, Fukuchi-Shimogori T, Tomitori H, Kashiwagi K, Toida T, Imanari T, Kawai G, Igarashi K. Formation of a complex containing ATP, Mg2+, and spermine. Structural evidence and biological significance. J Biol Chem. 1998;273:30939–30944. doi: 10.1074/jbc.273.47.30939. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen P J, Manchester K L, Towbin H, Gordon J, Thomas G. The phosphorylation of ribosomal S6 in rat tissues following cycloheximide injection, in diabetes, and after denervation of diaphragm. A simple immunological determination of the extent of S6 phosphorylation on protein blots. J Biol Chem. 1982;257:12316–12321. [PubMed] [Google Scholar]
- 22.Pettigrew D W, Ma D-P, Conrad C A, Johnson J R. Escherichia coli glycerol kinase. Cloning and sequencing of the glpK gene and the primary structure of the enzyme. J Biol Chem. 1988;263:135–139. [PubMed] [Google Scholar]
- 23.Suzuki T, He Y, Kashiwagi K, Murakami Y, Hayashi S, Igarashi K. Antizyme protects against abnormal accumulation and toxicity of polyamines in ornithine decarboxylase-overproducing cells. Proc Natl Acad Sci USA. 1994;91:8930–8934. doi: 10.1073/pnas.91.19.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabor C W, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 25.Thorner J W, Paulus H. Catalytic and allosteric properties of glycerol kinase from Escherichia coli. J Biol Chem. 1973;248:3922–3932. [PubMed] [Google Scholar]
- 26.Voegele R T, Sweet G D, Boos W. Glycerol kinase of Escherichia coli is activated by interaction with glycerol facilitator. J Bacteriol. 1993;175:1087–1094. doi: 10.1128/jb.175.4.1087-1094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada A, Igarashi K, Yoshimura S, Aimoto S, Ishihama A. Ribosome modulation factor: stationary growth phase-specific inhibitor of ribosome functions from Escherichia coli. Biochem Biophys Res Commun. 1995;214:410–417. doi: 10.1006/bbrc.1995.2302. [DOI] [PubMed] [Google Scholar]
- 28.Wada A, Yamazaki Y, Fujita N, Ishihama A. Structure and probable genetic location of a “ribosome modulation factor” associated with 100S ribosomes in stationary-phase Escherichia coli cells. Proc Natl Acad Sci USA. 1990;87:2657–2661. doi: 10.1073/pnas.87.7.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe Y, Igarashi K, Hirose S. Differential stimulation by polyamines of phage RNA-directed synthesis of proteins. Biochim Biophys Acta. 1981;656:134–139. doi: 10.1016/0005-2787(81)90078-2. [DOI] [PubMed] [Google Scholar]
- 30.Weiss R L, Morris D R. Cations and ribosome structure. I. Effects on the 30S subunit of substituting polyamines for magnesium ion. Biochemistry. 1973;12:435–441. doi: 10.1021/bi00727a012. [DOI] [PubMed] [Google Scholar]
- 31.Weissenborn D L, Wittekindt N, Larson T J. Structure and regulation of the glpFK operon encoding glycerol diffusion facilitator and glycerol kinase of Escherichia coli K-12. J Biol Chem. 1992;267:6122–6131. [PubMed] [Google Scholar]
- 32.Yamagishi M, Matsushima H, Wada A, Sakagami M, Fujita N, Ishihama A. Regulation of the Escherichia coli rmf gene encoding the ribosome modulation factor: growth phase- and growth rate-dependent control. EMBO J. 1993;12:625–630. doi: 10.1002/j.1460-2075.1993.tb05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashino T, Ueguchi C, Mizuno T. Quantitative control of the stationary-specific sigma factor, ςS, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO J. 1995;14:594–602. doi: 10.1002/j.1460-2075.1995.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida M, Meksuriyen D, Kashiwagi K, Kawai G, Igarashi K. Polyamine stimulation of the synthesis of oligopeptide-binding protein (OppA). Involvement of a structural change of the Shine-Dalgarno sequence and the initiation codon AUG in OppA mRNA. J Biol Chem. 1999;274:22723–22728. doi: 10.1074/jbc.274.32.22723. [DOI] [PubMed] [Google Scholar]
- 35.Zwaig N, Lin E C C. Feedback inhibition of glycerol kinase, a catalytic enzyme in Escherichia coli. Science. 1966;153:755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]