Supplemental Digital Content is Available in the Text.
Preliminary evidence suggests that physiological variables collected with our low-cost pain meter are correlated with chronic pain, both for individuals and populations.
Keywords: Chronic pain, Physiological data, Pain quantification, Random forest
Abstract
Introduction:
It is unknown if physiological changes associated with chronic pain could be measured with inexpensive physiological sensors. Recently, acute pain and laboratory-induced pain have been quantified with physiological sensors.
Objectives:
To investigate the extent to which chronic pain can be quantified with physiological sensors.
Methods:
Data were collected from chronic pain sufferers who subjectively rated their pain on a 0 to 10 visual analogue scale, using our recently developed pain meter. Physiological variables, including pulse, temperature, and motion signals, were measured at head, neck, wrist, and finger with multiple sensors. To quantify pain, features were first extracted from 10-second windows. Linear models with recursive feature elimination were fit for each subject. A random forest regression model was used for pain score prediction for the population-level model.
Results:
Predictive performance was assessed using leave-one-recording-out cross-validation and nonparametric permutation testing. For individual-level models, 5 of 12 subjects yielded intraclass correlation coefficients between actual and predicted pain scores of 0.46 to 0.75. For the population-level model, the random forest method yielded an intraclass correlation coefficient of 0.58. Bland–Altman analysis shows that our model tends to overestimate the lower end of the pain scores and underestimate the higher end.
Conclusion:
This is the first demonstration that physiological data can be correlated with chronic pain, both for individuals and populations. Further research and more extensive data will be required to assess whether this approach could be used as a “chronic pain meter” to assess the level of chronic pain in patients.
1. Introduction
Chronic pain is a significant public health problem. Recent estimates show that 50 million U.S. adults had chronic pain.22 Chronic pain and pain-related disability in the United States costs $560 to $650 billion dollars, far exceeding the costs of cardiovascular disease, cancer, and diabetes.36 Yet, despite the high cost and the profound social and personal burdens imposed by chronic pain, the clinical means by which we quantify levels of pain are largely relied on subjective self-reports rather than objective measures of pain intensity.18
A large body of work demonstrates that chronic pain causes changes in the brain that cause pain to persist beyond tissue healing. In particular, functional magnetic resonance imaging (fMRI) has been used to demonstrate changes in the brain that are consistent with chronic pain.3–6, 12, 13, 24, 34, 53, 56, 74, 77, 83 One recent randomized controlled study3 found significant changes in fMRI of subjects with chronic back pain before and after psychological pain treatment, showing a possibility of using fMRI in pain measurement. However, fMRI is rather expensive and hard to do for routine use. Less expensive and easy-to-use devices that can also show changes that are consistent with chronic pain are needed.
This research3 along with another randomized controlled study27 demonstrated interventions that not only reduced but in many cases cured chronic pain by retraining the brain. Techniques for retraining the brain away from chronic pain include psychological therapies,1,25,28,29,33,38,42,46,47,51,57,62,68–70,88,89,97 education,61,64,67,79,85 biofeedback,31,76,80 activities,2,17,44,54,55,63,87,91,92,96 and meditation.7,32,43,102 Multimodal combinations of these techniques have been successful.9,19,21,26,35,37,41,50,93
The changes in the brain that can cause chronic pain may also cause physiological changes that can be measured. Recently, there has been significant progress in quantification of pain with physiological sensors.10,14–16,20,30,39,45,58,60,72,73,78,86,98,100,101,103 Sensors that measure physiological signals are usually low-cost and easy-to-use. By measuring physiological changes due to chronic pain, such a device could potentially be useful to diagnose chronic pain more accurately, which can in turn assist in pain reduction. Such a device does not directly help reducing the pain; instead, it assists in pain reduction by better assessing the pain. For example, one can use it to monitor pain before and after an intervention to assess the effectiveness of pain reducing techniques described above. Additionally, quantification of pain is important because there is increasing concern that gender, race, age, and intellectual development disabilities may be involved in diagnostic delays as well as overtreatment or undertreatment of pain.8, 23, 52, 66
A growing body of literature has suggested that self-reported acute pain and experimentally induced acute pain are associated with differences in physiological parameters—for example, heart rate and heart rate variability (HRV); blood pressure; peripheral pulsatile components of the cardiac cycle; and electrodermal activity.20,30,45,78,100,101 Much of this work is consistent with the notion that multivariate assessments are superior to single-parameter models when predicting the subjective experience of pain intensity. For example, in an experimental induction of acute thermal pain, only a linear combination of physiological data from electrocardiograms (ECG), photoplethysmograms (PPG), and galvanic skin response significantly differentiated between no-pain and low-, medium-, and high-pain states—no individual parameter was able to distinguish between no-pain and pain states alone,86 suggesting that information from multiple autonomic physiological signals may indeed offer the most promising avenue for objective pain quantification.58 This modeling approach extended to cases of pure nociception under anesthesia, where the conscious perception of pain was ostensibly impossible: a multivariate approach including HRV, PPG, and pulse transition time accounted for intra-operative clinical measures of noxious intensity of incision.73 Similarly, in another study, heart rate, HRV, PPG, galvanic skin response, and associated temporal/spectral subcomponents of those physiological signals differentiated between noxious and nonnoxious surgical events.10
Of special relevance to the present study, previous research103 has highlighted the promise of machine learning models for pain assessment. Multilayer artificial neural networks using features derived from skin conductance and ECG distinguished between no pain and moderate-to-high levels of experimentally induced thermal pain, with a combination of the 2 features outperforming either measure alone60; in the case of chronic pain resulting from sickle cell anemia, multinomial logistic regression using 6 physiological features significantly predicted pain scores on an 11-point pain scale—both within and between individuals.98 Thus, there is considerable evidence to suggest that multivariate assessments of classical physiological measures can inform our understanding of pain intensity. Critically, however, there is no apparent consensus on the optimal set of features to use: the pioneering work on this question has explored a highly diverse field of potential physiological parameters.14–16,24,39,40,48,49,59,65,81,82,84,90,94,95,99
In this study, we report preliminary pain prediction results using physiological data collected from chronic pain sufferers on our new Pain Meter. We investigated both individual-level models and an overall, population-level model, spanning various combinations of pulse and temperature features. At the individual-level model, our results show that the ability to quantify chronic pain can vary quite considerably, with only half of the participants showing moderately strong predictive accuracies. Aggregating the data into a population-level model, however, significantly predicted pain scores across each recording session, providing preliminary evidence for a generalizable model of chronic pain quantification using physiological parameters.
2. Methods
2.1. Pain meter
The prototype equipment was built to allow subjects to record at home. For safety, we avoided any sensors that required electrode contact to the subjects' bodies. These in-home subject recordings enable us to capture more natural variation in pain levels for each subject, including high pain levels that would have made travel to our laboratory difficult. The ability to create subject-specific chronic pain models is due to the ability to record in homes.
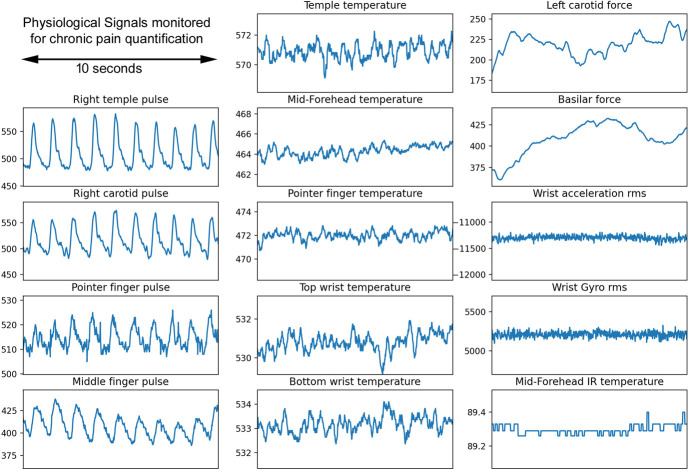
Our Prototype Pain Meter (Fig. 1) represents the seventh iteration of our device. The sensors we chose were inexpensive and available commercially: photoplethysmograms pulse sensors (Pulse Sensor Amplified; http://www.pulsesensor.com); temperature sensors (10K Ohm NTC Thermistors MF52-103); IR temperature sensor (MLX 90614); acceleration/gyroscope sensors (HiLetgo GY-521 MPU-6050 MPU6050 3 Axis Accelerometer Gyroscope Modules); and force sensors (Interlink FSR 402 on the forehead and wrist; and Interlink 406 on the basilar and carotid arteries). An example of the signals recorded from Pain Meter is shown in Figure 2. The Prototype Pain Meter does not apply stimulation to obtain different pain score recordings.
Figure 1.

Pain Meter contains (1) PPG pulse sensors in a headband, in a neck pillow, wristband, and on the fingertip; (2) temperature sensors on the temple, forehead, wrist, and fingertip; (3) 3-axis accelerometers and 3-axis gyros in the headband and wristband; and (4) force sensors on the left carotid and basilar. PPG, photoplethysmograms.
Figure 2.
An example of the signals recorded from Pain Meter.
A Teensy 3.6 microcontroller with a 32-bit 180 MHz ARM Cortex-M4 processor was used to sample all signals at a frequency of 66.67 Hz. Data visualization and acquisition programs for our Pain Meter were written in Python. Data were remotely monitored using the Dropbox cloud storage system.
2.2. Participants
Data were collected from 12 participants (9 women and 3 men; Mage = 38.25, SD = 17.10, range = 21–65), yielding a total of 183 10-minute recordings. Importantly, we did not specify any explicit exclusion criteria for participation: thus, pain etiology/symptomatology, duration, and intensity varied across individuals. For transparency, we summarize these factors in Supplementary Table 1 (available at http://links.lww.com/PR9/A173).
All subjects were recruited via email and provided written informed consent for a protocol approved by the UCSB Human Subjects Committee.
2.3. Data collection
Subjects first opened a computer program on the Pain Meter computer that was supplied to them. They connected to Wi-Fi on the first use. Then they were asked to rate the best representation of their current pain score using a visual analogue scale, which included pictures and short descriptions of pain states from 0 to 10 (eg, the description of state 0 was “no pain” and the description of state 10 was “unimaginable unspeakable”).
Subjects next put on the headband and neck pillow sensors, adjusted their position until they felt comfortable, and secured the sensors with Velcro (headband) and a fastener (neck pillow). Next, they put on a wrist band and 2 finger cuff sensors. Once these were secured, the computer monitored all the pulse signals and gave green lights for all sensors giving good signals (signals with clearly detected peaks) and yellow for signals that were not. If there were any yellow lights, the subject was asked to readjust the sensor and try again.
Once all the sensors were giving good signals, a 10-minute recording session was initiated in which pulse, temperature, force, and motion data were recorded and stored in local memory (at the end of the session, the complete record was automatically uploaded to the cloud).
During this recording, the subject was instructed to relax and to look at a fixation cross on the computer screen. The data being recorded was not visible to the subjects during the recording. After the recording was done, the subjects were asked to report their current pain score again with the same visual analogue scale. The protocol instructed subjects to record for 2 weeks, twice per day.
2.4. Data preprocessing
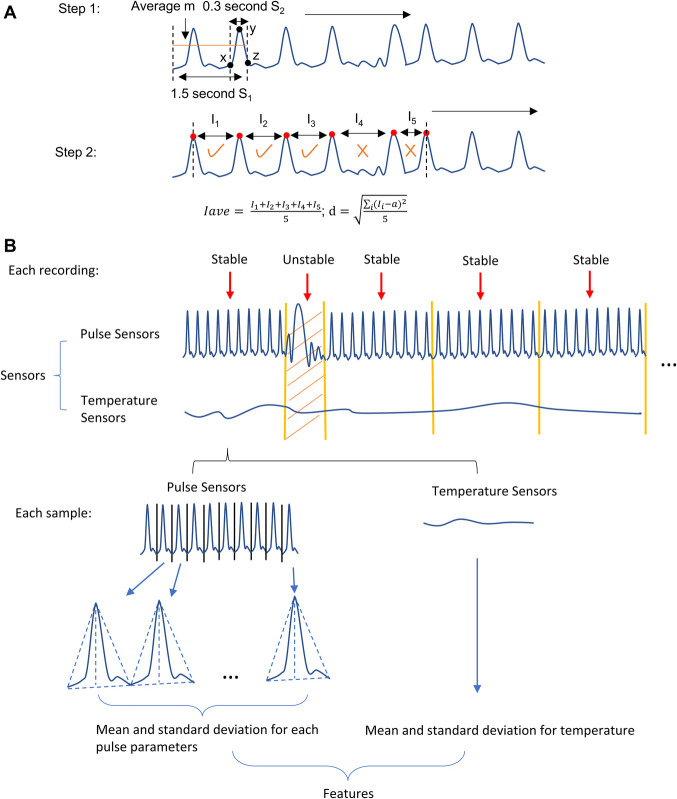
Movements of the subjects during the recording sessions can disturb the pulse signal and add additional noise into the data. Consequently, it can be difficult to extract robust, interpretable features from individual pulses. We use several steps to preprocess the data and reduce the influence of noise in our analysis. After unstable segments are detected (as detailed in Fig. 3), we remove them and split the stable segments into 10-second samples before proceeding to feature extraction. We used 3 pulse sensors in our analysis: temple pulse sensor, carotid pulse sensor, and pointer finger pulse sensor. Note that, because we removed all unstable segments, the number of available recordings and samples differed depending on the combination of sensors—Table 1 shows the number of stable recordings and samples for each combination.
Figure 3.
Preprocessing and feature extraction. (A) Step 1: Detect all pulse peaks in the recording by comparing the moving average m of each 100 data-point segment in S1 (S1 is 1.5-second-long) with the last 20 data points in S2 (S2 is 0.3-second-long) of S1. If the first and last data point in S2 is smaller than m and the max value of S2 is larger than m, then a peak is detected. Step 2: Take the first 6 peaks and calculate the mean Iave and SD d of the time between peaks. If Iave is between 0.6 and 1.2 seconds and d is smaller than 0.2 and if the time I between 2 peaks is between 0.9 and 1.1 times Iave, this data segment is classified as stable. Otherwise, this data segment is classified as unstable. Repeat this process for all the data. (B) The data preprocessing and feature extraction steps: (1) Remove unstable segments and divide the rest of the recording into continuous 10-second samples. (2) Inside each sample, extract pulse features such as the mean and SD for each pulse parameters and mean and SD for temperature.
Table 1.
Details of the models trained with different combination of pulse sensors.
| Pulse sensors | # of recordings | # of samples | r | RMSE |
|---|---|---|---|---|
| 1 | 173 | 7755 | 0.60 | 1.52 |
| 2 | 168 | 6671 | 0.44 | 1.78 |
| 3 | 179 | 8900 | 0.60 | 1.52 |
| 1, 2 | 156 | 5446 | 0.45 | 1.69 |
| 1, 3 | 168 | 7033 | 0.57 | 1.56 |
| 2, 3 | 163 | 5978 | 0.49 | 1.66 |
| 1, 2, 3 | 152 | 5044 | 0.41 | 1.71 |
Number of recordings, number of samples, Pearson correlation coefficient (r), and root mean square error (RMSE) are shown. For pulse sensors, 1 represents temple pulse sensor; 2 represents carotid pulse sensor; and 3 represents pointer finger pulse sensor.
Participants provided one pain score at the beginning of each recording and then again at the conclusion. We did not give any instructions to the participants such as telling them to relax or in any way attempt to change their pain. Nevertheless, participants often reported a different pain level at the end of the session. We therefore split the data and assigned the first pain score to samples from the first half of each recording and the second pain score to samples from the second half. This is, of course, only an approximation because we have no information about when the pain level changed, but it seemed preferable to using only the beginning pain score or only the end pain score for the entire recording.
2.5. Feature extraction
Each 10-second sample included stable signals from the pulse sensors and temperature sensors. We defined a series of pulse features analogously to earlier research,44 which suggested that pulse morphology derived from transmitted-light PPG can be used for postoperative pain assessment. Our device, however, uses green reflective-light pulse sensors with highly filtered output (because they are both inexpensive and readily available). We therefore investigated whether our pulse sensor parameters can inform the quantification of chronic pain.
We extracted only pulse parameters that are independent of the height of the pulse because the height of the pulse can be influenced by how tightly the device is worn and its position. Specifically, we extracted pulse width parameters: LR, LF, PPIH, and PPIL (Fig. 4). PPIH is defined as the interval between 2 consecutive high peaks of pulses. PPIL is defined as the interval between 2 consecutive low peaks of pulses. The SD of PPIH is a measure of pulse rate variability, which was used as one of the parameters in our models.
Figure 4.
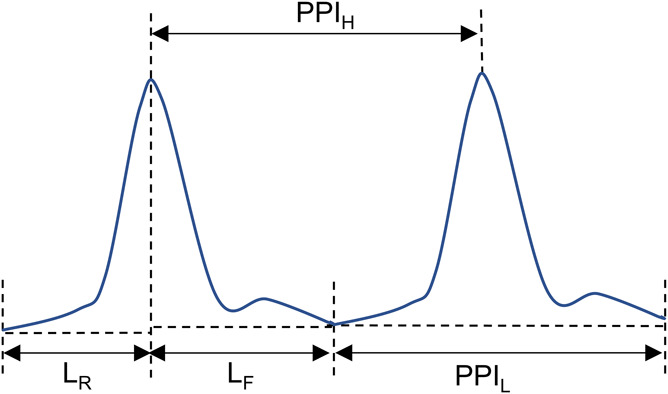
Pulse parameters. LR is the width of the rising part of the pulse; LF is the width of the falling part of the pulse; PPIH is the interval between 2 consecutive highs; and PPIL is the interval between 2 consecutive lows.
From each 10-second sample, after finding the peaks, we segmented the data into individual pulses by finding the local minimum between peaks. Figure 3B illustrates this process.
Thus, the following features were extracted from the samples:
(1) Mean and SD of all pulse sensor parameters.
(2) Mean of temperature at forehead, temple, top of wrist, and bottom of wrist and finger.
Additionally, we computed / , - , / , - , where represents the temple temperature mean for each 10-second sample window, represents the forehead temperature mean and , represents the finger temperature mean. All features were z-scored normalized before further analysis.
2.6. Recursive Feature elimination and random forest regression
Feature selection methods are used to identify a subset of “important” features and simplify a model. These techniques often afford several advantages, such as reducing computational costs, avoiding overfitting, and improving model performance.
One popular method, Recursive Feature Elimination, selects features by training a model with one set of features, ranking them by an importance metric, and removing the least important. This procedure is recursively repeated until the user-specified number of features is reached. We implemented subject-specific linear models for individualized pain prediction, accounting for the fact that relevant physiological parameters could likely vary on a subject-by-subject basis.
At the overall population level, we used random forest regression: an ensemble method that aggregates the predictions from multiple regression trees to make more accurate predictions. Regression trees are decision trees when the outcomes are continuous values, recursively partitioning the data space into smaller regions where simple models can be used to describe the relationships between various model inputs and outcomes. In random forest, each regression tree is created using random subsets of features and random subsets of samples. Accordingly, because random forest uses different subsets from the data and features, it is less likely to overfit and performs well with high-dimensional datasets. Additional advantages include fewer constraints and assumptions about the data and its distribution, as well as robustness to outliers and missing values. After the forest is fit, the predicted pain score of the test set is computed as the mean predicted score of the trees in the forest.
This part of the analysis was done with the Python package scikit-learn.71
2.7. Leave-one-recording-out cross-validation
We used leave-one-recording-out cross-validation to assess performance in all models (individual level and population level). For leave-one-recording-out cross-validation, all samples from one recording were iteratively removed from the dataset, the model was trained on the remaining data, and predicted pain scores were obtained for the left-out recording. Pearson correlation coefficient (r), intraclass correlation coefficient, and root mean square error (RMSE) between the predicted pain ratings and the reported values are reported. Intraclass correlation coefficients (ICC(3, 1)) are calculated with R package psych.89
We additionally performed 1000 iterations of nonparametric permutation tests to assess model performance against chance. In this procedure, we constructed a robust empirical null distribution of predictive accuracies expected if pain scores were randomly associated with patterns of physiological parameters. Thus, for each permutation, pain scores were shuffled randomly for all recordings, models were fit using cross-validation as above, and performance was recorded. Significance was determined by the proportion of cases in which null-model accuracy equalled or exceeded the “true” accuracy.
Because Pearson correlation is not an agreement statistic, a high correlation does not necessarily imply a good agreement between the 2 scores. Thus, we also ran a Bland–Altman analysis for our population-level model.
Despite these checks, there are still limitations and potential overfit problems with our methods. Future studies are needed to validate stable models against independent subject samples.
3. Results
We were able to capture a fairly wide range of pain scores (spanning 0–9) through our home recording sessions. However, we note that more extreme ratings (particularly, pain scores 0, 8, and 9) have many fewer samples for analysis relative to the other values. We elected to retain these recordings given the continuous nature of our regression frameworks, but the imbalanced representation of scale endpoints is worth keeping in mind for model assessment.
3.1. Individual-level models
We first trained and cross-validated predictive models on an individualized, within-subject basis. Because of the smaller sample size for individual subjects, we used recursive feature elimination to select 5 features first to avoid overfitting and then fit a linear model to predict the pain scores for each subject. All combinations of pulse sensors were tested for each subject. The models with the combination that gave the best predictive performance (ie, correlations between reported and predicted pain) are reported in Figure 5. As expected, performance was rather variable across individuals. However, pain scores in 5 of 12 subjects were predicted with moderately strong accuracy (0.49 < r < 0.78, P < 0.05). The intraclass correlation coefficients for these 5 subjects are between 0.46 and 0.75. A few features were selected by recursive feature elimination repeatedly in these 5 models. For example, was selected in 5 of 5 models and , and - were selected in 4 of 5 models. However, the relationships between these features with pain score are not consistent across different individuals. For example, and increased with the increase of pain score in 2 of 4 subjects and decreased in the other 2 subjects.
Figure 5.
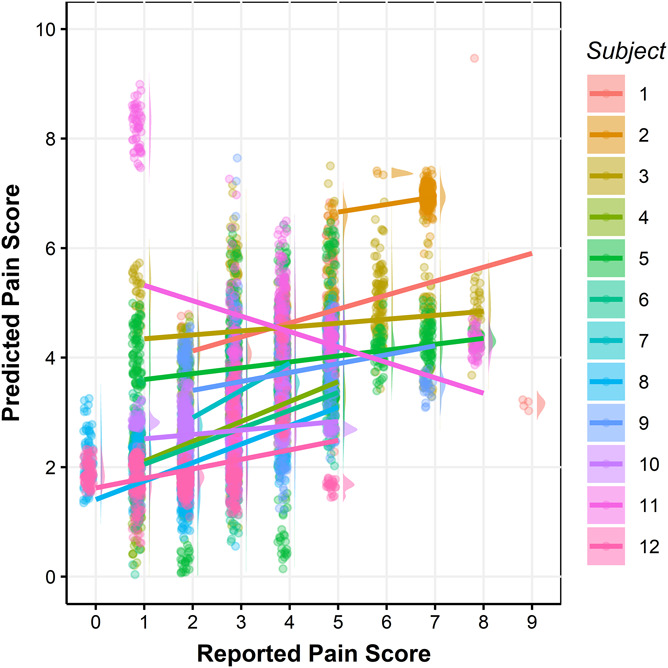
Cross-validated predicted pain scores vs reported pain scores for each subject. Different colors represent different subjects in the raincloud plots. Each dot represents one sample, and each line is the fitted linear regression line for each subject. In total, there are 6982 samples, 99.50% of the data is shown in the figure. The rest were omitted to provide higher resolution for the bulk of the data. The Pearson correlation coefficients between reported and the predicted pain scores are 0.78, 0.29, 0.14, 0.54, 0.19, 0.49, 0.56, 0.50, 0.24, 0.16, −0.31, and 0.35, respectively, for subjects 1 through 12.
3.2. Population-level model
We then aggregated the data from all subjects and fit population-level random forest models using different combinations of pulse sensors. Table 1 shows the results from each possible combination of sensors. In general, modeling more pulse sensors simultaneously leads to a smaller number of potential training samples overall (because a given 10-second window was only used if it was stable for all sensors combined). Models including only the pointer finger sensor or only the temple sensor gave the strongest correspondences between actual and predicted pain (r = 0.60, RMSE = 1.52). The intraclass correlation coefficient was 0.58 for both models, showing a moderate similarity between actual and predicted pain.
However, as aforementioned, predictive accuracy generally suffered the worst at pain scores with fewer available examples (Fig. 6). Because the number of stable recordings and samples varies when using different pulse sensors, there are different number of samples for each pain score. (The number of samples for each pain score are shown in Supplementary Table 2, available at http://links.lww.com/PR9/A173.) The more extreme ends of pain score (8 and 9), in particular, were considerably undershot by the model. Nonetheless, performance on the whole consistently outperformed empirical null models, suggesting that pain prediction was still significantly better than chance (P < 0.001).
Figure 6.
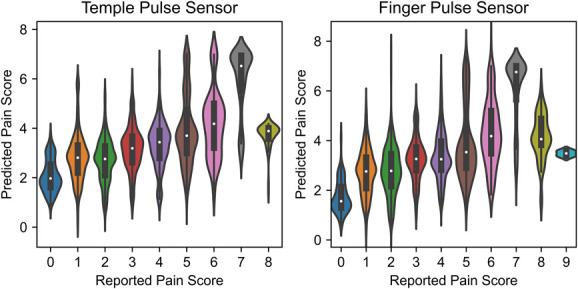
Cross-validated predicted pain scores vs reported pain scores for population-level models. Left panel shows the results from the model using signals from temple pulse sensors, and right panel shows the results from the model using signals from pointer finger pulse sensors. The violin plots for each class shows the distributions of the predicted pain scores.
Bland–Altman plots for both models are shown in Supplementary Figure 1 (available at http://links.lww.com/PR9/A173). Because one of the measurements in this article is the self-reported pain, which we consider as our reference measurement, we used the self-reported pain scores as the x-axis in our Bland–Altman plots. Note that the self-reported pain scores are integers from 0 to 10; thus, all dots in the plots lie on vertical lines with integer x value. The bias and agreement limits are the same for both models. The bias is 0.02, and the range of agreements is −2.95 to 2.99. Our model tends to overestimate the lower end of the pain scores and underestimate the higher end of the pain scores. Note that plotting against reference measurement instead of average measurements might be responsible for the association between difference and pain scores.11
4. Discussion
This is the first demonstration, to our knowledge, that physiological data can be correlated with chronic pain, both for individuals and populations. In our subject-specific models for individualized pain prediction, 5 of 12 subjects yielded Pearson correlation coefficients of 0.49 (P < 0.05) to 0.78 (P < 0.05) and intraclass correlation coefficients between 0.46 and 0.75. In our population-level model, we achieved an intraclass correlation coefficient of 0.58 and a significant Pearson correlation between self-reported and predicted pain (r = 0.60, P < 0.001) in this preliminary study. It is likely that higher correlations will be achieved with better devices in the future. This first demonstration had significant limitations due to the limited amount of data that we could collect shortly before and during COVID-19. Additional research is urgently needed to explore the effects of the type and duration of chronic pain, the age and gender of the subjects, and other factors.
Additional research is also needed to optimize the selection and placement of sensors. As one example of possible future improvements, an optimized pulse sensor would be able to sense the low-frequency variations in the baseline due to breathing, which were filtered out in the commercial pulse sensor that we used. It is also possible (perhaps even probable) that the inclusion of ECG, electromyography, or skin impedance sensors would give better correlations. However, in the interest of subject safety, because these were unsupervised measurements in the subjects' own homes, we did not include any devices with electrodes.
Our long-term goal is the development of a relatively low-cost, easy-to-use system that could be used by patients in their own homes or in physician offices to objectively measure chronic pain. Hopefully, this first demonstration of the possibility of achieving this goal will inspire engineers, physicists, computer scientists, and psychologists to join in doing the work that will be necessary to move from the demonstration of a possibility to a practical system. The current system of subjective self-reported pain levels results in the overprescribing and underprescribing of treatment. Shifting to an objective methodology would provide health care professionals the ability to properly prescribe patients with the appropriate dosage of treatment to alleviate pain. This is especially important with the many risks associated with pain medication, including long-term opioid addiction.
Disclosures
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A173.
Supplementary Material
Acknowledgments
The authors thank Cary Keogh who helped the authors connect with subjects, Celia Vann who collected some of the data, and the subjects who invested their time to supply our data. The authors thank Dr. Kenneth Kosik and his group for helpful suggestions and perspectives. The authors thank the Bill and Melinda Gates Foundation for their support.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Franklin Ly, Email: franklinly8@gmail.com.
Tyler Santander, Email: t.santander@psych.ucsb.edu.
Elyes Turki, Email: elyes@ucsb.edu.
Yun Zhao, Email: yunzhao@ucsb.edu.
Jamie Yoo, Email: jbyoo@ucsb.edu.
Kian Lonergan, Email: kianlonergan@ucsb.edu.
Jordan Gray, Email: jordangray@umail.ucsb.edu.
Christopher H. Li, Email: christopher02px2017@gmail.com.
Henry Yang, Email: henry.yang@ucsb.edu.
Michael Miller, Email: mbmiller@ucsb.edu.
Paul Hansma, Email: phansma108@gmail.com.
Linda Petzold, Email: petzold@ucsb.edu.
References
- [1].Åkerblom S, Perrin S, Fischer MR, McCracken LM. Treatment outcomes in group-based cognitive behavioural therapy for chronic pain: an examination of PTSD symptoms. Eur J Pain 2020;24:807–17. [DOI] [PubMed] [Google Scholar]
- [2].Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol 2015;29:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ashar YK, Gordon A, Schubiner H, Uipi C, Knight K, Anderson Z, Carlisle J, Polisky L, Geuter S, Flood TF, Kragel PA, Dimidjian S, Lumley MA, Wager TD. Effect of pain reprocessing therapy vs placebo and usual care for patients with chronic back pain. JAMA Psychiatry 2022;79:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aytur SA, Ray KL, Meier SK, Campbell J, Gendron B, Waller N, Robin DA. Neural mechanisms of acceptance and commitment therapy for chronic pain: a network-based fMRI approach. Front Hum Neurosci 2021;15. doi: 10.3389/fnhum.2021.587018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian Av. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006;26:12165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 2008;28:1398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ball EF, Nur Shafina Muhammad Sharizan E, Franklin G, Rogozińska E. Does mindfulness meditation improve chronic pain? A systematic review. Curr Opin Obstet Gynecol 2017;29:359–66. [DOI] [PubMed] [Google Scholar]
- [8].Barney CC, Andersen RD, Defrin R, Genik LM, McGuire BE, Symons FJ. Challenges in pain assessment and management among individuals with intellectual and developmental disabilities. PAIN Rep 2020;5:e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Becker N, Sjøgren P, Bech P, Olsen AK, Eriksen J. Treatment outcome of chronic non-malignant pain patients managed in a Danish multidisciplinary pain centre compared to general practice: a randomised controlled trial. PAIN 2000;84:203–11. [DOI] [PubMed] [Google Scholar]
- [10].Ben-Israel N, Kliger M, Zuckerman G, Katz Y, Edry R. Monitoring the nociception level: a multi-parameter approach. J Clin Monit Comput 2013;27:659–68. [DOI] [PubMed] [Google Scholar]
- [11].Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 1995;346:1085–7. [DOI] [PubMed] [Google Scholar]
- [12].Borsook D, Becerra LR. Breaking down the barriers: fMRI applications in pain, analgesia and analgesics. Mol Pain 2006;2:1744–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buffington ALH, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. PAIN 2005;113:172–84. [DOI] [PubMed] [Google Scholar]
- [14].Campbell E, Phinyomark A, Scheme E. Feature extraction and selection for pain recognition using peripheral physiological signals. Front Neurosci 2019;13. doi: 10.3389/fnins.2019.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chu Y, Zhao X, Han J, Su Y. Physiological signal-based method for measurement of pain intensity. Front Neurosci 2017;11. doi: 10.3389/fnins.2017.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chu† Y, Zhao X, Yao† J, Zhao Y, Wu Z. Physiological signals based quantitative evaluation method of the pain. IFAC Proc Volumes 2014;47:2981–6. [Google Scholar]
- [17].Coleman JF. Spring forest qigong and chronic pain. J Holist Nurs 2011;29:118–28. [DOI] [PubMed] [Google Scholar]
- [18].Cook KF, Dunn W, Griffith JW, Morrison MT, Tanquary J, Sabata D, Victorson D, Carey LM, MacDermid JC, Dudgeon BJ, Gershon RC. Pain assessment using the NIH Toolbox. Neurology 2013;80:S49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Corry A, Linssen G, Spinhoven P. Multimodal treatment programmes for chronic pain: a quantitative analysis of existing research data. J Psychosomatic Res 1992;36:275–86. [DOI] [PubMed] [Google Scholar]
- [20].Cowen R, Stasiowska MK, Laycock H, Bantel C. Assessing pain objectively: the use of physiological markers. Anaesthesia 2015;70:828–47. [DOI] [PubMed] [Google Scholar]
- [21].Cutler RB, Fishbain DA, Rosomoff HL, Abdel-Moty E, Khalil TM, Rosomoff RS. Does nonsurgical pain center treatment of chronic pain return patients to work? Spine 1994;19:643–52. [DOI] [PubMed] [Google Scholar]
- [22].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morbidity Mortality Weekly Rep 2018;67:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Daoust R, Paquet J, Piette É, Sanogo K, Bailey B, Chauny JM. Impact of age on pain perception for typical painful diagnoses in the emergency department. J Emerg Med 2016;50:14–20. [DOI] [PubMed] [Google Scholar]
- [24].Davis KD, Flor H, Greely HT, Iannetti GD, Mackey S, Ploner M, Pustilnik A, Tracey I, Treede R-D, Wager TD. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol 2017;13:624–38. [DOI] [PubMed] [Google Scholar]
- [25].Day MA, Ward LC, Ehde DM, Thorn BE, Burns J, Barnier A, Mattingley JB, Jensen MP. A pilot randomized controlled trial comparing mindfulness meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain. Pain Med 2019;20:2134–48. [DOI] [PubMed] [Google Scholar]
- [26].Deardorff WW, Rubin HS, Scott DW. Comprehensive multidisciplinary treatment of chronic pain: a follow-up study of treated and non-treated groups. PAIN 1991;45:35–43. [DOI] [PubMed] [Google Scholar]
- [27].Donnino MW, Thompson GS, Mehta S, Paschali M, Howard P, Antonsen SB, Balaji L, Bertisch SM, Edwards R, Ngo LH, Grossestreuer Av. Psychophysiologic symptom relief therapy for chronic back pain: a pilot randomized controlled trial. PAIN Rep 2021;6:e959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eccleston C, Hearn L, Williams ACdeC. Psychological therapies for the management of chronic neuropathic pain in adults. Cochrane Database Syst Rev 2015. doi: 10.1002/14651858.CD011259.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eccleston C, Palermo TM, Williams ACdeC, Lewandowski Holley A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2014. doi: 10.1002/14651858.CD003968.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eriksson M, Storm H, Fremming A, Schollin J. Skin conductance compared to a combined behavioural and physiological pain measure in newborn infants. Acta Paediatr 2007;97:27–30. [DOI] [PubMed] [Google Scholar]
- [31].Fahrenkamp A, Sim L, Roers L, Canny M, Harrison T, Harbeck-Weber C. An innovative and accessible biofeedback intervention for improving self-regulatory skills in pediatric chronic pain: a pilot study. J Altern Complement Med 2020;26:212–18. [DOI] [PubMed] [Google Scholar]
- [32].Finn SB, Perry BN, Clasing JE, Walters LS, Jarzombek SL, Curran S, Rouhanian M, Keszler MS, Hussey-Andersen LK, Weeks SR, Pasquina PF, Tsao JW. A randomized, controlled trial of mirror therapy for upper extremity phantom limb pain in male amputees. Front Neurol 2017;8. doi: 10.3389/fneur.2017.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fisher E, Law E, Dudeney J, Eccleston C, Palermo TM. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2019. doi: 10.1002/14651858.CD011118.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flodin P, Martinsen S, Altawil R, Waldheim E, Lampa J, Kosek E, Fransson P. Intrinsic brain connectivity in chronic pain: a resting-state fMRI study in patients with rheumatoid arthritis. Front Hum Neurosci 2016;10. doi: 10.3389/fnhum.2016.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. PAIN 1992;49:221–30. [DOI] [PubMed] [Google Scholar]
- [36].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13:715–24. [DOI] [PubMed] [Google Scholar]
- [37].Gatchel RJ, Okifuji A. Evidence-Based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J Pain 2006;7:779–93. [DOI] [PubMed] [Google Scholar]
- [38].Gilpin HR, Keyes A, Stahl DR, Greig R, McCracken LM. Predictors of treatment outcome in contextual cognitive and behavioral therapies for chronic pain: a systematic review. J Pain 2017;18:1153–64. [DOI] [PubMed] [Google Scholar]
- [39].Gruss S, Geiger M, Werner P, Wilhelm O, Traue HC, Al-Hamadi A, Walter S. Multi-modal signals for analyzing pain responses to thermal and electrical stimuli. J Visualized Experiments 2019. doi: 10.3791/59057 [DOI] [PubMed] [Google Scholar]
- [40].Gruss S, Treister R, Werner P, Traue HC, Crawcour S, Andrade A, Walter S. Pain intensity recognition rates via biopotential feature patterns with support vector machines. PLoS One 2015;10:e0140330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Guzman J. Multidisciplinary rehabilitation for chronic low back pain: systematic review. BMJ 2001;322:1511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Henschke N, Ostelo RW, van Tulder MW, Vlaeyen JW, Morley S, Assendelft WJ, Main CJ. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev 2010. doi: 10.1002/14651858.CD002014.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hilton L, Hempel S, Ewing BA, Apaydin E, Xenakis L, Newberry S, Colaiaco B, Maher AR, Shanman RM, Sorbero ME, Maglione MA. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med 2017;51:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Holmberg C, Rappenecker J, Karner J, Witt CM. The perspectives of older women with chronic neck pain on perceived effects of qigong and exercise therapy on aging: a qualitative interview study. Clin Interventions Aging 2014;9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Holper L, Gross A, Scholkmann F, Humphreys BK, Meier ML, Wolf U, Wolf M, Hotz-Boendermaker S. Physiological effects of mechanical pain stimulation at the lower back measured by functional near-infrared spectroscopy and capnography. J Integr Neurosci 2014;13:121–42. [DOI] [PubMed] [Google Scholar]
- [46].Hsu MC, Schubiner H, Lumley MA, Stracks JS, Clauw DJ, Williams DA. Sustained pain reduction through affective self-awareness in fibromyalgia: a randomized controlled trial. J Gen Intern Med 2010;25:1064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hughes JM, Seemann EA, George JM, Willis KD. The effects of pre-treatment depressive symptoms on quality of life across cognitive behavioral therapy for chronic pain. J Clin Psychol Med Settings 2019;26:97–105. [DOI] [PubMed] [Google Scholar]
- [48].Jiang M, Mieronkoski R, Syrjälä E, Anzanpour A, Terävä V, Rahmani AM, Salanterä S, Aantaa R, Hagelberg N, Liljeberg P. Acute pain intensity monitoring with the classification of multiple physiological parameters. J Clin Monit Comput 2019;33:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kachele M, Thiam P, Amirian M, Schwenker F, Palm G. Methods for person-centered continuous pain intensity assessment from bio-physiological channels. IEEE J Selected Top Signal Process 2016;10:854–64. [Google Scholar]
- [50].Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJEM, Ostelo RW, Guzman J, van Tulder MW. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev 2014. doi: 10.1002/14651858.CD000963.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Karjalainen KA, Malmivaara A, van Tulder MW, Roine R, Jauhiainen M, Hurri H, Koes BW. Multidisciplinary rehabilitation for fibromyalgia and musculoskeletal pain in working age adults. Cochrane Database Syst Rev 1999;2010. doi: 10.1002/14651858.CD001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kennel J, Withers E, Parsons N, Woo H. Racial/ethnic disparities in pain treatment. Med Care 2019;57:924–9. [DOI] [PubMed] [Google Scholar]
- [53].Keszthelyi D, Aziz Q, Ruffle JK, O'Daly O, Sanders D, Krause K, Williams SCR, Howard MA. Delineation between different components of chronic pain using dimension reduction - an ASL fMRI study in hand osteoarthritis. Eur J Pain 2018;22:1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kong LJ, Lauche R, Klose P, Bu JH, Yang XC, Guo CQ, Dobos G, Cheng YW. Tai chi for chronic pain conditions: a systematic review and meta-analysis of randomized controlled trials. Scientific Rep 2016;6:25325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee MS, Pittler MH, Ernst E. External qigong for pain conditions: a systematic review of randomized clinical trials. J Pain 2007;8:827–31. [DOI] [PubMed] [Google Scholar]
- [56].Li T, Zhang S, Ikeda E, Kobinata H. Functional connectivity modulations during offset analgesia in chronic pain patients: an fMRI study. Brain Imaging Behav 2022. doi: 10.1007/s11682-022-00652-7 [DOI] [PubMed] [Google Scholar]
- [57].Lim JA, Choi S-H, Lee WJ, Jang JH, Moon JY, Kim YC, Kang DH. Cognitive-behavioral therapy for patients with chronic pain. Medicine 2018;97:e10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Loggia ML, Napadow V. Multi-parameter autonomic-based pain assessment: more is more? PAIN 2012;153:1779–80. [DOI] [PubMed] [Google Scholar]
- [59].Lopez-Martinez D, Picard R. Continuous pain intensity estimation from autonomic signals with recurrent neural networks. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 2018. pp. 5624–7. doi: 10.1109/EMBC.2018.8513575 [DOI] [PubMed] [Google Scholar]
- [60].Lopez-Martinez D, Picard R. Multi-task neural networks for personalized pain recognition from physiological signals. 2017 Seventh International Conference on Affective Computing and Intelligent Interaction Workshops and Demos (ACIIW). IEEE, 2017. pp. 181–4. doi: 10.1109/ACIIW.2017.8272611 [DOI] [Google Scholar]
- [61].Louw A, Zimney K, Puentedura EJ, Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature. Physiother Theor Pract 2016;32:332–55. [DOI] [PubMed] [Google Scholar]
- [62].Lumley MA, Schubiner H, Lockhart NA, Kidwell KM, Harte SE, Clauw DJ, Williams DA. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. Pain 2017;158:2354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lynch M, Sawynok J, Hiew C, Marcon D. A randomized controlled trial of qigong for fibromyalgia. Arthritis Res Ther 2012;14:R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Malfliet A, Kregel J, Coppieters I, de Pauw R, Meeus M, Roussel N, Cagnie B, Danneels L, Nijs J. Effect of pain neuroscience education combined with cognition-targeted motor control training on chronic spinal pain. JAMA Neurol 2018;75:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mamontov D, Polonskaia I, Skorokhod A, Semenkin E, Kessler V, Schwenker F. Evolutionary algorithms for the design of neural network classifiers for the classification of pain intensity, 2019; 84–100. doi: 10.1007/978-3-030-20984-1_8 [DOI] [Google Scholar]
- [66].Miller AJ. Gender disparities in diagnosis and pain management. Temple University, 2018. [Google Scholar]
- [67].Mittinty MM, Vanlint S, Stocks N, Mittinty MN, Moseley GL. Exploring effect of pain education on chronic pain patients' expectation of recovery and pain intensity. Scand J Pain 2018;18:211–19. [DOI] [PubMed] [Google Scholar]
- [68].Monticone M, Cedraschi C, Ambrosini E, Rocca B, Fiorentini R, Restelli M, Gianola S, Ferrante S, Zanoli G, Moja L. Cognitive-behavioural treatment for subacute and chronic neck pain. Cochrane Database Syst Rev 2015;2016. doi: 10.1002/14651858.CD010664.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. PAIN 1999;80:1–13. [DOI] [PubMed] [Google Scholar]
- [70].Palermo TM, Law EF, Fales J, Bromberg MH, Jessen-Fiddick T, Tai G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents. PAIN 2016;157:174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay E. Scikit-learn: machine learning in Python. J Machine Learn Res 2011;12:2825–30. [Google Scholar]
- [72].Prichep LS, Shah J, Merkin H, Hiesiger EM. Exploration of the pathophysiology of chronic pain using quantitative EEG source localization. Clin EEG Neurosci 2018;49:103–13. [DOI] [PubMed] [Google Scholar]
- [73].Rantanen M, Yli-Hankala A, van Gils M, Yppa¨rila¨-Wolters H, Takala P, Huiku M, Kyma¨la¨inen M, Seitsonen E, Korhonen I. Novel multiparameter approach for measurement of nociception at skin incision during general anaesthesia† ‡. Br J Anaesth 2006;96:367–76. [DOI] [PubMed] [Google Scholar]
- [74].Reddan MC, Wager TD. Brain systems at the intersection of chronic pain and self-regulation. Neurosci Lett 2019;702:24–33. [DOI] [PubMed] [Google Scholar]
- [75].Revelle WR. psych: Procedures for psychological, psychometric, and personality research, 2022. Available: https://cran.r-project.org/package=psych. [Google Scholar]
- [76].Rosenthal S. Watch the screen”: biofeedback can improve mindfulness for chronic pain. Biofeedback 2018;46:15–20. [Google Scholar]
- [77].Santana AN, Cifre I, de Santana CN, Montoya P. Using deep learning and resting-state fMRI to classify chronic pain conditions. Front Neurosci 2019;13. doi: 10.3389/fnins.2019.01313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Seok HS, Choi B-M, Noh GJ, Shin H. Postoperative pain assessment model based on pulse contour characteristics analysis. IEEE J Biomed Health Inform 2019;23:2317–24. [DOI] [PubMed] [Google Scholar]
- [79].Shala R, Roussel N, Lorimer Moseley G, Osinski T, Puentedura EJ. Can we just talk our patients out of pain? Should pain neuroscience education be our only tool? J Man Manipulative Ther 2021;29:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sielski R, Rief W, Glombiewski JA. Efficacy of biofeedback in chronic back pain: a meta-analysis. Int J Behav Med 2017;24:25–41. [DOI] [PubMed] [Google Scholar]
- [81].Singh SK, Rastogi V, Singh SK. Pain assessment using intelligent computing systems. Proc Natl Acad Sci India Section A: Phys Sci 2016;86:285–95. [Google Scholar]
- [82].Susam BT, Akcakaya M, Nezamfar H, Diaz D, Xu X, de Sa VR, Craig KD, Huang JS, Goodwin MS. Automated pain assessment using electrodermal activity data and machine learning. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 2018. pp. 372–5. doi: 10.1109/EMBC.2018.8512389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tanasescu R, Cottam WJ, Condon L, Tench CR, Auer DP. Functional reorganisation in chronic pain and neural correlates of pain sensitisation: a coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci Biobehavioral Rev 2016;68:120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Teichmann D, Klopp J, Hallmann A, Schuett K, Wolfart S, Teichmann M. Detection of acute periodontal pain from physiological signals. Physiol Meas 2018;39:095007. [DOI] [PubMed] [Google Scholar]
- [85].Traeger AC, Lee H, Hübscher M, Skinner IW, Moseley GL, Nicholas MK, Henschke N, Refshauge KM, Blyth FM, Main CJ, Hush JM, Lo S, McAuley JH. Effect of intensive patient education vs placebo patient education on outcomes in patients with acute low back pain. JAMA Neurol 2019;76:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Treister R, Kliger M, Zuckerman G, Aryeh IG, Eisenberg E. Differentiating between heat pain intensities: the combined effect of multiple autonomic parameters. PAIN 2012;153:1807–14. [DOI] [PubMed] [Google Scholar]
- [87].von Trott P, Wiedemann AM, Lüdtke R, Reißhauer A, Willich SN, Witt CM. Qigong and exercise therapy for elderly patients with chronic neck pain (QIBANE): a randomized controlled study. J Pain 2009;10:501–8. [DOI] [PubMed] [Google Scholar]
- [88].Turner JA, Anderson ML, Balderson BH, Cook AJ, Sherman KJ, Cherkin DC. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. PAIN 2016;157:2434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive–behavioral therapy for chronic pain. PAIN 2007;127:276–86. [DOI] [PubMed] [Google Scholar]
- [90].Walter S, Gruss S, Kächele M, Schwenker F, Werner P, Al-Hamadi A, Andrade A, Moreira G, Traue H. Data fusion for automated pain recognition. Proceedings of the 9th International Conference on Pervasive Computing Technologies for Healthcare. ICST, 2015. doi: 10.4108/icst.pervasivehealth.2015.259166 [DOI] [Google Scholar]
- [91].Wang C, Schmid CH, Hibberd PL, Kalish R, Roubenoff R, Rones R, McAlindon T. Tai Chi is effective in treating knee osteoarthritis: a randomized controlled trial. Arthritis Rheum 2009;61:1545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, Lee Y, McAlindon T. A randomized trial of tai chi for fibromyalgia. New Engl J Med 2010;363:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Waterschoot FPC, Dijkstra PU, Hollak N, de Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. PAIN 2014;155:179–89. [DOI] [PubMed] [Google Scholar]
- [94].Waxman JA, Pillai Riddell RR, Tablon P, Schmidt LA, Pinhasov A. Development of cardiovascular indices of acute pain responding in infants: a systematic review. Pain Res Manag 2016:1–15. doi: 10.1155/2016/8458696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Werner P, Lopez-Martinez D, Walter S, Al-Hamadi A, Gruss S, Picard R. Automatic recognition methods supporting pain assessment: a survey. IEEE Trans Affective Comput 2019:1. doi: 10.1109/TAFFC.2019.2946774 [DOI] [Google Scholar]
- [96].Wieland LS, Skoetz N, Pilkington K, Vempati R, D'Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev 2017;2017. doi: 10.1002/14651858.CD010671.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Williams ACdeC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012. doi: 10.1002/14651858.CD007407.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yang F, Banerjee T, Narine K, Shah N. Improving pain management in patients with sickle cell disease from physiological measures using machine learning techniques. Smart Health 2018;7:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yang L, Wang S, Jiang X, Cheng S, Kim HE. PATTERN: pain Assessment for paTients who can't TEll using Restricted Boltzmann machiNe. BMC Med Inform Decis Making 2016;16:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yang Yla, Seok HS, Noh G-J, Choi B-M, Shin H. Postoperative pain assessment indices based on photoplethysmography waveform analysis. Front Physiol 2018;9. doi: 10.3389/fphys.2018.01199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zaproudina N, Ming Z, Hänninen OOP. Plantar infrared thermography measurements and low back pain intensity. J Manipulative Physiol Ther 2006;29:219–23. [DOI] [PubMed] [Google Scholar]
- [102].Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Ann N Y Acad Sci 2016;1373:114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhao Y, Ly F, Hong Q, Cheng Z, Santander T, Yang HT, Hansma PK, Petzold L. How much does it hurt: A deep learning framework for chronic pain score assessment. 2020 international conference on data mining workshops (ICDMW). IEEE, 2020; 651–60. doi: 10.1109/ICDMW51313.2020.00092 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A173.