Abstract
The novel genes soxFGH were identified, completing the sox gene cluster of Paracoccus pantotrophus coding for enzymes involved in lithotrophic sulfur oxidation. The periplasmic SoxF, SoxG, and SoxH proteins were induced by thiosulfate and purified to homogeneity from the soluble fraction. soxF coded for a protein of 420 amino acids with a signal peptide containing a twin-arginine motif. SoxF was 37% identical to the flavoprotein FccB of flavocytochrome c sulfide dehydrogenase of Allochromatium vinosum. The mature SoxF (42,832 Da) contained 0.74 mol of flavin adenine dinucleotide per mol. soxG coded for a novel protein of 303 amino acids with a signal peptide containing a twin-arginine motif. The mature SoxG (29,657 Da) contained two zinc binding motifs and 0.90 atom of zinc per subunit of the homodimer. soxH coded for a periplasmic protein of 317 amino acids with a double-arginine signal peptide. The mature SoxH (32,317 Da) contained two metal binding motifs and 0.29 atom of zinc and 0.20 atom of copper per subunit of the homodimer. SoxXA, SoxYZ, SoxB, and SoxCD (C. G. Friedrich, A. Quentmeier, F. Bardischewsky, D. Rother, R. Kraft, S. Kostka, and H. Prinz, J. Bacteriol. 182:4476–4487, 2000) reconstitute a system able to perform thiosulfate-, sulfite-, sulfur-, and hydrogen sulfide-dependent cytochrome c reduction, and this system is the first described for oxidizing different inorganic sulfur compounds. SoxF slightly inhibited the rate of hydrogen sulfide oxidation but not the rate of sulfite or thiosulfate oxidation. From use of a homogenote mutant with an in-frame deletion in soxF and complementation analysis, it was evident that the soxFGH gene products were not required for lithotrophic growth with thiosulfate.
The oxidation of hydrogen sulfide or sulfur to sulfuric acid is one of the major reactions of the global sulfur cycle and is mediated by various aerobic lithotrophic and anaerobic phototrophic sulfur-oxidizing bacteria. Paracoccus pantotrophus GB17 is an aerobic, gram-negative, neutrophilic facultatively autotrophic bacterium which grows with thiosulfate or molecular hydrogen as an energy source and heterotrophically with a large variety of carbon sources. P. pantotrophus, isolated as Thiosphaera pantotropha (36) and reclassified as Paracoccus denitrificans (26), was recently renamed (33). The gene region of P. pantotrophus coding for sulfur oxidation (Sox) is located on a 13-kb DNA fragment (29). The region comprises 12 open reading frames, i.e., ORF1, shxVW, and soxXYZABCDEF, with an incomplete soxF′ at the end of the cloned DNA suggesting the extension of the sox gene cluster (18; F. Bardischewsky and C. G. Friedrich, submitted for publication) (Fig. 1).
FIG. 1.
Physical map of the relevant inserts of plasmids pEG12, pSOXF5N, and pVKB9 and of sox-relevant open reading frames and construction of the deletion in soxF.
The thiosulfate-oxidizing enzyme system of P. pantotrophus is located in the periplasm, and four proteins, i.e., SoxXA, SoxYZ, SoxB, and SoxCD, are required for the complete oxidation of thiosulfate (18). SoxXA is a heterodimeric c-type cytochrome, with SoxX (14,216 Da) as a monoheme subunit and SoxA (29,352 Da) as a diheme subunit. The heterodimeric SoxYZ protein is composed of SoxY (10,977 Da) and SoxZ (11,719 Da), neither of which contains a cofactor. The monomeric dimanganese SoxB protein (58,611 Da) exhibits a similarity to bovine 5′-nucleotidase (50). SoxCD is an α2β2 heterotetramer, with SoxC (43,442 Da) as the molybdenum cofactor-containing subunit with a significant similarity to sulfite oxidases. The diheme c-type cytochrome SoxD (37,637 Da) is tightly associated with SoxC (32). SoxCD increases the yield of electrons in the reconstituted thiosulfate-oxidizing system (18), and SoxC is essential for lithotrophic growth with thiosulfate (50).
soxE was predicted to encode a periplasmic diheme c-type cytochrome (23,616 Da) which was thought to be associated with SoxF to form flavocytochrome c (50). The partial soxF gene predicted a periplasmic polypeptide with high identity to FccB, a flavoprotein of the phototrophic purple sulfur bacterium Allochromatium vinosum which is associated with FccA, the cytochrome subunit of flavocytochrome c. FccAB catalyzes hydrogen sulfide oxidation in vitro (51). Disruption of fccA did not affect phototrophic growth of A. vinosum with hydrogen sulfide. Therefore, sulfide-quinone reductase (SQR) was considered essential for growth of A. vinosum with hydrogen sulfide (35). Inactivation of SQR demonstrates its essential role for phototrophic growth of Rhodobacter capsulatus with hydrogen sulfide (41). SoxF is not required for thiosulfate-dependent cytochrome c reduction of P. pantotrophus in vitro. Also, SoxCD, a sulfite dehydrogenase homologue, is not essential for this reaction (17). P. pantotrophus forms a membrane-bound SQR (40). The presence of SQR, the identification of SoxF as a possible subunit of flavocytochrome c-sulfide dehydrogenase, and the identification of the Sox system posed the question of which system was essential for hydrogen sulfide oxidation in this strain (40). Hydrogen sulfide dehydrogenase activity has been assigned to SoxB (39); however, the exclusive involvement of SoxB in hydrogen sulfide oxidation has been questioned (17), which prompted us to reexamine hydrogen sulfide oxidation in this strain.
In this study we report the cloning of novel genes soxFGH. We describe the thiosulfate-induced formation of SoxF, SoxG, and SoxH and characterize the purified proteins. We demonstrate that the Sox system of P. pantotrophus oxidizes different reduced inorganic sulfur compounds. The SoxFGH proteins are not required for sulfur oxidation in vitro and in vivo as evident from biochemical studies, from a mutant with a deletion in soxF, and from complementation analysis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used and constructed in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or phenotypea | Source or reference |
|---|---|---|
| E. coli strains | ||
| S17-1 | recA pro thi hsdS, RP4-tra functions | 43 |
| XL1-Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lacF′[proAB+ lacIqlacZΔM15 Tn10(Tcr)] | Stratagene |
| P. pantotrophus strains | ||
| GB17 | Sox+ Hox+ | L. A. Robertson |
| GB17::pRD123.2 | Sox+ Kmr, cointegrate of pRD123.2 | This study |
| GBsoxFΔ | Sox+soxFΔ | This study |
| Plasmids | ||
| pBluescript SK+ | AprlacZ f1 ori; T7 φ10 promoter | Stratagene |
| pBSK+E1 | 1-kb soxE′F′ EcoRI fragment in pBluescript SK(+) | 50 |
| pSOXF5N | 5-kb NotI wild-type soxFGH fragment in pBluescript SK(+) | This study |
| pEG5 | soxB′CDEF′ in pSUP202 | 49 |
| pUC19 | AprlacZα | 45 |
| pJOE773.2 | AprlacZα′, positive selection vector | 1 |
| pJD1 | KmrlacZ RP4 oriT ColE1ori | 14 |
| pRD119.35 | soxF′GH′ inserted as a 1,991-bp HindIII-SalI fragment (right flank) in pUC19 | This study |
| pRD120.1 | soxD′EF′ inserted as a 1,987-bp HindIII-SalI fragment (left flank) in pUC19 | This study |
| pRD121.1 | Right and left flanks as HindIII-SalI fragments from pRD119.35 and pRD120.1 inserted in SalI site of pJOE773.2 | This study |
| pRD123.2 | 3,978-bp SalI fragment from pRD121.1 soxD′EFΔGH′ in pJD1 | This study |
| pVK101 | Kmr Tcr Tra− Mob+ | 24 |
| pVKB9 | 8,851-bp BamHI fragment orf1′-soxE′ inserted in pVK101 | This study |
Hox, growth with molecular hydrogen; Sox, growth with thiosulfate; Tra, transfer of mobilizable plasmids.
Media and growth conditions.
P. pantotrophus GB17 was cultivated aerobically at 30°C. Lithoautotrophically grown cells were cultivated in mineral medium (pH 8.0) with 20 mM thiosulfate (10). Inocula were cultivated mixotrophically in the same mineral medium with 20 mM thiosulfate and 20 mM disodium succinate. Lithotrophic mass cultivation (32) of P. pantotrophus GBsoxFΔ was performed in a 300-liter fermentor with a 220-liter working volume (Bioengineering, Wald, Switzerland). With formate or molecular hydrogen as an energy source, cells were cultivated as previously described (10).
Escherichia coli was cultivated in Luria-Bertani medium (37). Antibiotics were added where appropriate (for P. pantotrophus, 300 μg of kanamycin/ml; for E. coli, 50 μg of kanamycin/ml or 100 μg of ampicillin/ml).
Plasmid transformation, conjugation, and gene replacement.
E. coli was transformed either as described previously (11) or with the aid of RbCl-treated competent E. coli XL1-Blue cells (37). E. coli S17-1 was used to mobilize plasmids into P. pantotrophus GB17 (43). S17-1 was transformed with plasmid pRD123.2. Donor and recipient were cultivated in nutrient broth (Difco). Aliquots of the mating suspensions (109 cells per ml, which contained an initial donor-recipient ratio of 1:1) were plated on nutrient broth plates and incubated at 37°C for 18 h. Cells were scraped off the plates with 36 mM phosphate buffer, pH 7.2. Kanamycin-resistant cointegrates were selected on mineral agar plates containing 10 mM glucose and 300 μg of kanamycin/ml. Cointegrates were resolved after a period of nonselective growth over several days in 10 ml of mineral medium (pH 8.0) with 20 mM succinate. From strain GB17::pDR123.2, kanamycin-sensitive colonies were further analyzed for double-recombinant strains.
DNA techniques.
Standard DNA techniques were applied (37). Plasmids were prepared in small scale from E. coli as described by Kieser (22). For DNA sequencing, plasmid DNA was prepared with a High Pure plasmid isolation kit (Boehringer, Mannheim, Germany). Genomic DNA was isolated according to the protocol of Ausubel et al. (4) Restriction enzymes, T4 DNA ligase, alkaline phosphatase, and Klenow polymerase were obtained from Gibco BRL or Boehringer and used as recommended by the manufacturer. Agarose gel electrophoresis was done in Tris-borate-EDTA buffer (37). DNA fragments were eluted from agarose gels using either the QIAquick gel extraction kit (Qiagen, Düsseldorf, Germany) or an electroelution system. Colony and Southern hybridizations were performed using a digoxigenin (DIG) kit (Boehringer). DNA was sequenced by primer walking with fluorescent primers in an automated DNA sequencer (Li-Cor; MWG-Biotech) by the dideoxy-chain termination method (38) with Taq polymerase and 7-deaza-dGTP from Amersham-Buchler. The DNA sequence of the 5-kb insert of pSOXF5N was analyzed at the facilities of Eurogentec (Seraing, Belgium). Sequence analysis was performed with the BLAST Search Program package (2).
Cloning of soxFGH.
Genomic DNA from P. pantotrophus GB17 (50 μg) was dialyzed twice against 2 liters of 200 mM NaCl, precipitated with ethanol, and resuspended in distilled water. Ten micrograms of this DNA was digested with NotI and separated in a 0.8% agarose gel. DNA fragments of about 5 kb were recovered from the gel and cloned into the NotI-restricted vector pBluescript SK(+). A DIG-labeled probe for colony hybridization was generated by PCR using the primers SK2 (5′-CGACCTGCTTCTTCTCG-3′) and KS1 (5′-GCATCGCCTCGATCAT-3′) and the plasmid pBSK+E1 as a template, producing a 315-bp DNA fragment. DNA from positive clones was isolated for further restriction analysis. One of the analyzed clones was designated pSOXF5N, and the 5-kb insert was sequenced.
Construction of a deletion in soxF.
A 282-bp fragment was deleted from soxF by PCR technology due to the lack of appropriate restriction sites; the strategy is summarized in Fig. 1. PCRs were performed with Taq polymerase essentially as described elsewhere (27). Using plasmid pEG5 as a template, a fragment of 1,987 bp, designated the left flank, was generated by primer 1 (5′-AAAAGTCGACAGGTCGTGCTGCCCAAT-3′), complementary to bp 8443 to 8459, and primer 2 (5′-TTTTAAGCTTCATGGCGAAGACGCCGC-3′), complementary to bp 10423 to 10407. The right flank was produced by primer 3 (5′-AAAAAAGCTTGTGATCCCGGCCCAGAG-3′), matching bp 10707 to 10722, and primer 4 (5′-TTTTGTCGACTCATCATCGACCATTGC-3′), matching bp 12690 to 12674, with plasmid pSOXF5N as a template, leading to a 1,991-bp fragment. These primers introduced restriction sites for HindIII and SalI into the ends of the fragments. To obtain 5′-protruding ends, the PCR fragments were digested with HindIII and SalI (Fig. 1) and were cloned between the HindIII and SalI sites of pUC19 (45), respectively, which resulted in pRD119.35 (right flank) and pRD120.1 (left flank). The left and right flanks were reisolated as HindIII-SalI fragments from pRD119.35 and pRD120.1 and inserted in one step into the SalI-treated positive selection vector pJOE773.2 (1), resulting in the plasmid pRD121.1. In this plasmid the two fragments are fused via the generated HindIII site within soxFΔ. The 3,978-bp SalI fragment carrying the deletion in soxF along with its neighboring genomic regions was cloned into the SalI site of the mobilizable suicide vector pJD1 (14), leading to the plasmid pRD123.1, which was conjugated into P. pantotrophus. Homogenote recombinants with the deletion in soxF were detected by amplification of a DNA fragment reduced by 276 bp compared with the wild-type soxF by using primers 5 (5′-AAAATCTAGAATGGCCAGGGATTCCCA-3′), corresponding to bp 10025 to 10041, and 6 (5′-TTTTGTCGACCCAGCCGAGGCTTTCCT-3′), complementary to bp 11113 to 11097. For quick preparation of genomic DNA, 1 ml of an overnight culture (Luria-Bertani medium) was harvested, resuspended in 100 μl of TE buffer, and boiled for 10 min. Ten microliters of the crude extract was subjected to DNA amplification. PCR products were purified with QIAquick (Qiagen) and analyzed by agarose gel electrophoresis.
Identification of the Sox character.
To identify the Sox character, single colonies were cultivated in thiosulfate mineral medium (5 ml, pH 7.2) supplemented with 20 mM succinate and 10 μg of phenol red/ml on a roller at 30°C overnight. Thiosulfate oxidation caused a drop in pH that was visible as a color change from red to yellow.
Preparation of cell extracts.
Cell extracts were prepared with a French press (16). To eliminate unspecific cytochrome c reductions from the crude extract, the protein was precipitated by 44 to 65% ammonium sulfate saturation. The precipitate was separated by centrifugation, redissolved in 25 mM sodium-potassium phosphate buffer, and designated the cell extract.
Purification of SoxF, SoxG, and SoxH.
An extract of cells grown with thiosulfate was subjected to differential centrifugation, ammonium sulfate fractionation, and chromatography on Q Sepharose. Proteins were eluted from the Q Sepharose column with a step gradient of 400 ml each at 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, and 0.4 M sodium chloride as detailed previously (18).
(i) SoxF.
SoxF was eluted mainly with 0.1 M sodium chloride and identified by immunoblot analysis as described below. Of this fraction, 70 ml was concentrated to 15 ml by ultrafiltration using an Amicon PM10 membrane. To the concentrate 30 ml of 25 mM sodium potassium phosphate buffer (pH 6.5) containing stabilizing additives (2 mM sodium thiosulfate, 1 mM magnesium sulfate, and 1 μM phenylmethylsulfonyl fluoride [PMSF]) was added and concentrated to 11 ml. This extract was applied to a 6-ml Resource Q column equilibrated against 10 mM bis-Tris buffer (pH 6.0) containing the stabilizing additives specified above. Protein was eluted with a linear gradient of 0 to 500 mM sodium chloride. SoxF-containing fractions were pooled (11.5 ml) and mixed with 90 ml of 10 mM potassium phosphate buffer containing 2 mM sodium thiosulfate, 1 mM magnesium sulfate, and 1 μM PMSF and concentrated to 20 ml. The extract was applied to a hydroxyapatite column (2.6 by 2.5 cm) equilibrated with the same buffer. SoxF was eluted with equilibration buffer, while contaminating proteins remained bound to the column.
(ii) SoxG.
SoxG was identified by immunoblot analysis as described below. SoxG was eluted from the Q Sepharose column at 0.3 M sodium chloride. The pooled fractions (90 ml) were concentrated to 4 ml by Amicon ultrafiltration, and the protein was subjected to gel filtration over a Sephacryl S-200 HR column (1.6 by 60 cm) equilibrated with 25 mM sodium potassium phosphate buffer (pH 6.5) containing 2 mM sodium thiosulfate, 1 mM magnesium sulfate, and 1 μM PMSF. Protein was eluted with the same buffer at a flow rate of 45 ml/h, and fractions of about 1.8 ml were collected. The fractions (1.8 ml) containing SoxG were pooled (9.5 ml) and saturated to 30% with ammonium sulfate. The protein was applied to a phenyl-Sepharose HP column (1.6 by 4 cm) equilibrated against 10 mM potassium phosphate buffer containing 2 mM sodium thiosulfate, 1 mM magnesium sulfate, and 1 μM PMSF that was 30% saturated with ammonium sulfate. Protein was eluted with 240 ml of a linear gradient of 30 to 0% ammonium sulfate at a flow rate of 3 ml/min, and fractions of 4 ml were collected.
(iii) SoxH.
SoxH was identified by immunoblot analysis as described below. SoxH was eluted from the Q Sepharose column with 0.35 M sodium chloride. The pooled fractions (100 ml) were concentrated to 4 ml by Amicon ultrafiltration and subjected to gel filtration over a Sephacryl S-200 HR column (1.6 by 60 cm) equilibrated with potassium phosphate as detailed for SoxF. Protein was eluted with the same buffer at a flow rate of 45 ml/h. The fractions (1.8 ml) containing SoxH were pooled (9.5 ml) and brought to 30% ammonium sulfate saturation, and the protein was applied to a phenyl-Sepharose HP column (1.6 by 4 cm) equilibrated against 25 mM sodium potassium phosphate buffer containing 2 mM sodium thiosulfate, 1 mM magnesium sulfate, and 1 μM PMSF that was 30% saturated with ammonium sulfate. Protein was eluted with 240 ml of a linear gradient of 30 to 0% ammonium sulfate at a flow rate of 3 ml/min, and fractions of 4 ml were collected.
Purification of the sulfur-oxidizing enzyme system.
SoxXA, SoxYZ, SoxB, and SoxCD, which are required to reconstitute thiosulfate-dependent cytochrome c reduction, were purified to homogeneity from cell extracts of thiosulfate-grown cells as described previously (18).
Enzyme assays.
The rate of thiosulfate oxidation from whole cells was determined with an oxygen electrode (Rank Brothers, Bottisham, England). The assay mixture (3 ml) contained 50 μl of cell suspension (optical density at 436 nm = 30) and 100 μmol of K-Na phosphate buffer (pH 8.0). Reactions were started with 30 μl of 0.2 M sodium thiosulfate or 30 μl of 10 M sodium sulfide.
The rates of thiosulfate-, sulfite-, sulfur-, and hydrogen sulfide-dependent reduction of horse heart cytochrome c in cell extracts with the enzyme system reconstituted with 0.5 nmol each of the homogeneous preparations of SoxXA, SoxYZ, SoxB, and SoxCD were monitored at 550 nm with a Shimadzu UV160 A UV-visible spectrophotometer.
One unit of enzyme activity is defined as the reduction of 1 μmol of horse heart cytochrome c (ɛ = 27.8 cm2/μmol) per min at 30°C. Each assay mixture (1 ml) contained 50 μmol of sodium-potassium phosphate buffer (pH 6.5), 35 nmol of cytochrome c, and about 1 to 3 mg of cell extract. Cytochrome c reduction was started by adding either 2 μmol of sodium thiosulfate, 100 nmol of disodium sulfite, 10 nmol of disodium sulfide, or 10 nmol of sulfur. Disodium sulfide was kept as a 1 mM stock solution under argon. Sulfur was dissolved in 1 M sodium hydroxide and added to the assay from a solution of 1 mM S0 in 10 μM sodium hydroxide.
Protein in cell extracts was determined by the method of Bradford (8).
Analytical procedures.
Flavin adenine dinucleotide (FAD) was quantified by spectrophotometric analysis at 450 nm using an extinction coefficient of 11.3 cm2/μmol.
Immunoblot (Western blot) analysis (44) was done by the semidry procedure using the Multiphor electrophoresis system (Pharmacia, Freiburg, Germany) with antibodies raised in rabbits at the facilities of Eurogentec. Antibodies were raised against oligopeptides of highly immunogenic epitopes of SoxE (MRDHSDRPQDIPEAE) (OP-E), SoxF (LDPKDKFSKQALFE) (OP-F), SoxG (PFIGYHLPQGGIGR) (OP-G), and SoxH (ASEDIPDQYPQSVLY) (OP-H).
The molecular masses of native proteins were determined by nondenaturing polyacrylamide gel electrophoresis (PAGE) using a linear gradient of 5 to 27.5% polyacrylamide (3), and those of denatured proteins were determined by sodium dodecyl sulfate (SDS)-PAGE as described by Laemmli (25). Nondenatured or denatured proteins were stained with Coomassie blue as described previously (47). Molecular masses of Sox proteins were determined by nano-electrospray mass spectrometry with a Finnigan LCQ mass spectrometer equipped with a micromanipulator for the correct positioning of the nanospray needle (31).
Amino acid sequences were determined from pure proteins by automated Edman degradation using a protein sequencer system (model 494A/190A; Applied Biosystems, Foster City, Calif.) as described previously (16). Peptides of trypsin-digested SoxF were determined by matrix-assisted laser desorption ionization at the facilities of the Max-Delbrück-Centrum für Molekulare Medizin, Berlin-Buch, Germany.
The metal content of proteins was analyzed by total-reflection X-ray fluorescence analysis (23).
RESULTS
Cloning of soxFGH
NotI-restricted genomic DNA of P. pantotrophus hybridized with the 5′ region of soxF using a DIG-labeled probe of the known partial soxF sequence. The signal indicated a fragment with a size of about 5 kb (data not shown). The nucleotide sequence completed soxF and uncovered two additional open reading frames, soxG and soxH.
soxF
The soxF gene extended from bp 9884 to 11146. The intervening sequence contained a short inverted repeat with the potential to form a hairpin structure and a ribosome binding site 6 nucleotides 5′ of the start codon (50). soxF was predicted to encode a polypeptide of 420 amino acids with a molecular mass of 44,614 Da. A putative signal peptide of 26 amino acids and 2,611 Da exhibited a twin-arginine motif diagnostic for periplasmic proteins which are transported to the periplasm via the Tat system (5, 7, 48). For the mature protein an isoelectric point (pI) of 5.15 was calculated. The deduced amino acid sequence of SoxF was 37% identical and 50% similar to that of the flavoprotein FccB of flavocytochrome c of A. vinosum and exhibited a flavin binding site at cysteine-70 as suggested for FccB (15). Hydrogen sulfide-dependent cytochrome c reduction, designated sulfide dehydrogenase activity, has been demonstrated for FccAB (19). Also, the primary sequence of SoxF is 25% identical and 44% similar to that of DHSU, the predicted flavoprotein of flavocytochrome c sulfide dehydrogenase of Aquifex aeolicus (13).
soxG
The soxG gene was located 17 bp downstream of soxF and extended from bp 11164 to 12075, with a putative ribosome binding site 5 bp upstream of the start codon. soxG was predicted to encode a protein of 302 amino acids and 32,401 Da, including a putative signal peptide of 25 amino acids and 2,744 Da with a twin-arginine motif (SRRHFLA) which is indicative of periplasmic proteins with redox centers. From the mature protein, a pI of 4.34 and two zinc binding motifs (THGHPDH and TPGHTPGH) were predicted (28). The protein was partially identical to hypothetical proteins from Streptomyces coelicolor A3 (34) and to beta-lactamase-like proteins from Mycobacterium strains (National Center for Biotechnology Information accession numbers AAD31327 and AAD38170).
soxH
The soxH gene extended from bp 12075 to 13028, and the start codon overlapped with the stop codon of soxG. A putative ribosome binding site is located 5 bp upstream of the start codon. From soxH a polypeptide of 317 amino acids and 34,401 Da was deduced, with a leader peptide of 20 amino acids (2,084 Da) containing a double arginine and a pI of 4.56 for the mature protein. The deduced SoxH sequence exhibited identities to a putative beta-lactamase precursor from A. aeolicus (13) and cyclases from different Streptomyces strains (26%) (6, 9) and suggested binding sites for heavy metals (CxxC) and zinc (PGHGHPT).
Sequence analysis of the sox gene region.
Reexamination of the sox gene region (18) corrected the former ORF1 as a putative ArsR transcriptional regulator of 149 amino acids (16,470 Da) and uncovered a new ORF2 predicted to encode a periplasmic thioredoxin of 130 amino acids with a putative signal peptide of 26 amino acids and a molecular mass of 11,077 Da for the mature protein, with a predicted pI of 4.29 (Fig. 1). Both genes are also present in Rhodopseudomonas palustris and are part of the sox gene cluster of this strain. They are 47 and 41% identical to those of P. pantotrophus (IGwit database accession numbers RRPA00685 and RRPA00708). Also, both genes are directed inversely to the sox genes in P. pantotrophus and R. palustris.
Induction of SoxE, SoxF, SoxG, and SoxH.
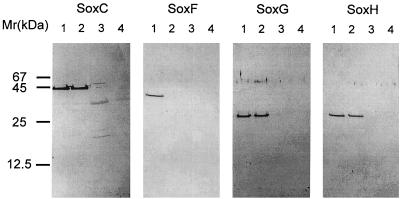
SoxE, SoxF, SoxG, and SoxH were specifically detected in cell extracts by immunoblot analysis as specified in Materials and Methods. After SDS-PAGE of extracts from thiosulfate-grown cells, OP-E antibodies detected a 24-kDa antigen (data not shown) whose size matched the predicted size of the mature soxE gene product (23,616 Da). OP-F antibodies determined an antigen of 42 kDa similar to the deduced mature SoxF (42,003 Da). OP-G and OP-H antibodies detected antigens of 30 and 32 kDa, respectively (Fig. 2), similar to those deduced for the mature SoxG and SoxH (31,657 and 32,317 Da). These antigens were detected neither in cells grown lithoautotrophically with molecular hydrogen or autotrophically with formate (Fig. 2) nor in cells grown heterotrophically with glucose (data not shown).
FIG. 2.
Western blot analysis of cell extracts from P. pantotrophus GB17 and GBsoxFΔ. Proteins of cell extracts (10 μg of protein per well) were separated by SDS-PAGE. Antibodies raised against OP-C, OP-F, OP-G, and OP-H were used. The wild-type GB17 was cultivated with thiosulfate (lanes 1), with molecular hydrogen (lanes 3), or with formate (lanes 4); GBsoxFΔ was cultivated with thiosulfate (lanes 2).
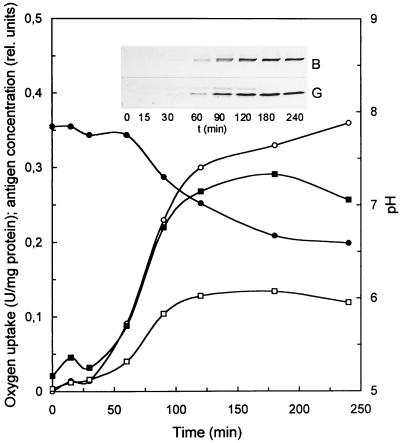
The induction of SoxFGH by thiosulfate was examined with SoxB as representative of the sox structural gene products and SoxG as representative of the novel sox gene products. After addition of thiosulfate, the thiosulfate-dependent oxygen uptake rate increased after 30 min, and uptake appeared to be completed after 2 h. Also, the concentrations of SoxB and SoxG increased similarly with time as evident from quantification of Western blots of the respective antigens (Fig. 3). These results demonstrated that expression of soxFGH was specifically induced by thiosulfate and that these genes were subject to the regulation of the sox gene cluster. For characterization, the novel Sox proteins were purified by the procedures detailed in Materials and Methods, using the immunochemical reaction for their identification.
FIG. 3.
Thiosulfate-induced formation of SoxB and SoxG. P. pantotrophus was cultivated in mineral medium (400 ml) with 10 mM sodium succinate plus 10 mM glucose in a 1-liter baffled Erlenmeyer flask. After the cells reached the stationary phase at an optical density at 436 nm of 6.35, sodium thiosulfate (20 mM) was added. Cells (0.7 ml) were collected by centrifugation, resuspended with 150 μl of SDS sample buffer, and incubated at 60 and 100°C for 5 min each. Cell debris was removed by centrifugation, and the supernatant was diluted with four aliquots of sample buffer. From this preparation, 20 μg of protein was subjected to SDS-PAGE. Immunoblotting was performed as described in Materials and Methods. Signal intensity was quantified using the Scion Image software. ○, oxygen uptake rate; ●, pH; ▪, SoxG; □, SoxB.
SoxF.
To identify the soxF gene product, SoxF was subjected to amino acid sequence analysis. The N terminus of SoxF was blocked, and the chemical nature of this block could not be resolved. After tryptic digestion of SoxF, the amino acid sequences of two peptides (DFESLQHGYDK and LVLSPGJEFKPDSVPGXSLE) identified SoxF as the soxF gene product. The molecular mass of SoxF as determined by SDS-PAGE was 42 kDa (Fig. 4A), identical to that deduced from the nucleotide sequence of the mature protein of 42,003 Da. Size exclusion chromatography by native gradient PAGE revealed a mass of 45,000 Da, indicating that SoxF was monomeric (Fig. 4B). Determination of the molecular mass by electrospray ionization mass spectroscopy revealed two masses of 42,832 and 42,481 Da, indicating the presence of a prosthetic group (data not shown). Spectrophotometric analysis of SoxF (1.04 mg/ml) showed two peaks of absorption at 450 and 460 nm, characteristic of FAD (ɛ450 nm = 11.3 cm2/μmol), and the absorbance at 450 nm of 0.062 revealed 0.71 mol of FAD per mol of SoxF. No metals were detected in SoxF. Also, SoxF was not associated with a cytochrome, while flavocytochromes c from Chlorobium limicola (42), A. vinosum (51), and Thiobacillus strain W5 (46) are associated with a cytochrome c.
FIG. 4.
Determination of molecular masses of SoxF, SoxG, and SoxH by native gradient PAGE (A) and denaturing SDS-PAGE (B) using 1.5 μg of each protein. Lanes: 1, SoxF; 2, SoxG; 3, SoxH.
SoxG.
SoxG was verified as the soxG gene product by analysis of the N terminus (ATTVAMGPVRIDRLXDGHLE), whose amino acid sequence is identical to that deduced from soxG, also demonstrating the specificity of the immunochemical detection (Fig. 2). SoxG appeared as a single band of 31 kDa after SDS-PAGE (Fig. 4B). The molecular mass determined by native gradient PAGE was 64 kDa (Fig. 4A), indicating that SoxG was homodimeric. Molecular mass determination by electrospray ionization mass spectroscopy revealed a single mass of 29,656 Da, which was identical to that deduced from the nucleotide sequence of the mature SoxG of 29,657 Da (data not shown). In the SoxG preparation 0.90 mol of zinc, 0.13 mol of copper, and 0.13 mol of iron per mol of SoxG were present, while only trace amounts of other metals were detected (data not shown).
SoxH.
SoxH was identified as the soxH gene product by the N-terminal amino acid sequence (SEDIPDQYPQSVLYS), which was identical to that deduced from soxH. Purified SoxH appeared as single bands of 31 kDa in SDS-PAGE (Fig. 4B) and of 54 kDa in native gradient PAGE (Fig. 4A), indicating its homodimeric structure. SoxH as isolated contained 0.28 mol of zinc, 0.20 mol of copper, and 0.11 mol of cobalt per mol of subunit. Other metals were present in negligible amounts (data not shown). The molecular mass of 32,315 Da as determined by electrospray ionization mass spectroscopy was almost identical to that deduced from the nucleotide sequence for the mature SoxH (32,317 Da) (data not shown).
Sulfur-dependent cytochrome c reduction.
The specific rate of hydrogen sulfide-dependent horse heart cytochrome c reduction was 0.018 U/mg of protein in cell extracts of thiosulfate-grown P. pantotrophus (data not shown). This activity was reconstituted from fractions of proteins of the cell extract eluted from Q Sepharose at 0.05, 0.25, 0.30, and 0.35 M sodium chloride (data not shown). The pure Sox proteins SoxXA, SoxYZ, SoxB, and SoxCD mediated thiosulfate- and sulfite-dependent cytochrome c reduction (18). This system also mediated hydrogen sulfide- and sulfur-dependent cytochrome c reduction (Table 2). The ratio of activities with hydrogen sulfide, sulfur, thiosulfate, and sulfite as electron donors was 1:0.63:0.39:0.17. Residual sulfur- and hydrogen sulfide-dependent activities of about 3 and 11% were observed when either SoxB or SoxYZ was omitted from the assay (Table 2). Thus, the Sox system of P. pantotrophus represents a new type of hydrogen sulfide dehydrogenase. Moreover, since it oxidized different reduced inorganic sulfur compounds, it was considered to be a general sulfur-oxidizing system.
TABLE 2.
Sox proteins required for thiosulfate-, hydrogen sulfide-, sulfite-, and sulfur-dependent reduction of horse heart cytochrome c
| Sox proteins in assay
mixturea
|
Cytochrome c
reduction (nmol/min) with the following substrate:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SoxF | SoxG | SoxH | SoxXA | SoxYZ | SoxB | SoxCD | H2S | S0 | Thiosulfate | Sulfite |
| − | − | − | + | + | + | + | 23.2 | 14.6 | 9.0 | 4.0 |
| − | − | − | + | − | + | + | 2.0 | 0.4 | 0 | 0 |
| − | − | − | + | + | − | + | 2.7 | 0.4 | 0 | 0 |
| − | − | − | + | + | + | − | 4.4 | 3.7 | 2.7 | 2.4 |
| − | − | − | − | + | + | + | 3.9 | 2.4 | 0.72 | 0 |
| + | − | − | + | + | + | + | 18.9 | 14.0 | 9.0 | 3.9 |
| − | + | − | + | + | + | + | 22.0 | 14.6 | 10.2 | 5.1 |
| − | − | + | + | + | + | + | 22.0 | 12.9 | 9.4 | 3.3 |
| + | + | + | + | + | + | + | 17.7 | 12.5 | 8.7 | 4.2 |
| + | + | + | − | − | − | − | 0 | 0 | 0 | 0 |
The Sox proteins were added to the assay mixtures at a concentration of 0.5 nmol/ml.
No sulfur-oxidizing activity was detected for SoxF, SoxG, or SoxH, either as single proteins or in combination with each other (Table 2). Addition of equimolar concentrations of SoxF to the reconstituted system reduced the hydrogen sulfide oxidation rate by 18.5%, while the sulfur, thiosulfate, and sulfite oxidation rates were not affected by SoxF (Table 2). Addition of SoxFGH revealed a similar result, while SoxG and SoxH alone had little or no effect on the activity (Table 2). This indicated that SoxF slightly inhibited the hydrogen sulfide-oxidizing activity of the reconstituted Sox system of P. pantotrophus.
Homogenous SoxF combined with SoxE present in the 0.10 M NaCl Q Sepharose fraction did not reconstitute hydrogen sulfide-oxidizing activity with horse heart cytochrome c as an electron acceptor (data not shown).
The sulfur-oxidizing enzyme system reconstituted from SoxXA, SoxYZ, SoxB, and SoxCD released 8 mol of electrons per mol of thiosulfate (Table 3). Omission of SoxCD from the system reduced the rate of thiosulfate oxidation by 70% (Table 2) and the electron yield to 2 mol of electrons per mol of thiosulfate (Table 3). SoxCD was also required for the maximum rates of hydrogen sulfide-, sulfur-, and sulfite-dependent cytochrome c reduction, which were 18, 25, and 60% of the rate with SoxCD included, respectively (Table 2). From the complete assay mixture about 4 mol of electrons per mol was released from hydrogen sulfide, and without SoxCD only 2 mol of electrons per mol was released. The same result was obtained with sulfur as an electron donor (Table 3). The use of anaerobic conditions for the assays did not alter the electron yield (data not shown).
TABLE 3.
Thiosulfate-, hydrogen sulfide-, and soluble sulfur-dependent degree of cytochrome c reduction mediated by the sulfur-oxidizing enzyme system
| Electron donor | Conc (μM) | Sox proteins in assay
mixturea
|
Cytochrome c reduced (nmol) | Yield of electrons (mol of cytochrome c reduced/mol of electron donor) | ||||
|---|---|---|---|---|---|---|---|---|
| SoxFGH | SoxXA | SoxYZ | SoxB | SoxCD | ||||
| Thiosulfate | 5 | + | + | + | + | + | 43.5 | 8.7 |
| 5 | − | + | + | + | + | 40.5 | 8.1 | |
| 5 | − | + | + | + | − | 11.5 | 2.3 | |
| Hydrogen sulfide | 5 | + | + | + | + | + | 19.0 | 3.8 |
| 5 | − | + | + | + | + | 21.5 | 4.3 | |
| 5 | − | + | + | + | − | 9.5 | 1.9 | |
| Sulfur | 10 | − | + | + | + | + | 40.1 | 4.0 |
| 10 | − | + | + | + | − | 19.0 | 1.9 | |
The Sox proteins were added to the assay mixtures at a concentration of 0.5 nmol/ml.
Construction of GBsoxFΔ.
SoxF was not required for the oxidation of reduced sulfur compounds in vitro (Table 2). Therefore, a deletion in soxF was designed to examine its function in vivo. Within soxF, bp 10424 to 10705, coding for 94 amino acids including three cysteine residues, were deleted by PCR technology. The deletion was verified by the reduction of the size of the mutant PCR fragment. A 1,109-bp fragment was generated from the wild type, and an 833-bp fragment was generated from the mutated genome of GBsoxFΔ (Fig. 5). The construction of the deletion generated a HindIII restriction site by combining the left and right genomic flanks, and two additional amino acids, Lys and Leu, were created in the altered SoxFΔ. The correct construction was demonstrated from the HindIII restriction site within the 833-bp PCR fragment amplified from mutant DNA (data not shown).
FIG. 5.
PCR analysis of the deletion in soxF of GBsoxFΔ. Genomic DNA was used as a template for PCR with primers 5 and 6 binding within soxF. Purified PCR assay mixtures (4 μl) were applied to each well. Lanes: 1, HindIII-digested λ DNA; 2, plasmid control pRD123.2; 3, mutant GBsoxFΔ; 4, cointegrate GB17::pRD123.2; 5, GB17.
Characterization of GBsoxFΔ.
Cell extracts from autotrophically grown P. pantotrophus and the mutant GBsoxFΔ were analyzed for SoxF antigens using Western blot analysis. With antibodies against OP-F, a band of 42 kDa was detected for GB17 (Fig. 2), as for pure SoxF (Fig. 4A). As a result of the deletion no signal was detected in extracts of GBsoxFΔ (Fig. 2), as the immunogenic epitope was located within the deleted middle part of the SoxFΔ protein (32.2 kDa). Strain GBsoxFΔ grew lithotrophically with thiosulfate, and no significant differences were detected with respect to specific growth rate, cellular yield, and rates of oxidation of hydrogen sulfide and thiosulfate compared to the wild type (data not shown).
The lack of an obvious phenotype of strain GBsoxFΔ prompted us to examine a possible gene duplication of soxF. Southern hybridizations were performed using the truncated soxF gene present in the 720-bp PvuI-EcoRI fragment of the wild type (Fig. 1) as a probe for NotI-digested genomic DNAs of GBsoxFΔ and the wild type under nonstringent conditions. With this probe, single signals of 5 and 4.7 kb were detected in the wild type and in the mutant GBsoxFΔ, respectively, indicating that soxF was not duplicated in P. pantotrophus (data not shown).
Complementation of the Sox system.
Using OP-B and OP-C antibodies, the respective antigens were not detected in extracts of the Tn5 insertional mutant strain TP19 when cultivated mixotrophically with succinate and thiosulfate (data not shown). Thus, a functional Sox system could not be reconstituted. Therefore, the structural genes required for sulfur oxidation in vitro were complemented with pVKB9, which does not contain the soxFGH genes (Fig. 1). P. pantotrophus TP19(pVKB9) grew lithotrophically with thiosulfate at a rate and extent similar to those of the wild type, and SoxB and SoxC antigens were detected by Western blot analysis in this strain. SoxG antigens were not detected in strain TP19(pVKB9) (data not shown). This result indicated that the soxFGH gene products were not essential for lithotrophic growth with thiosulfate.
DISCUSSION
In the present study the sequence of the sox gene cluster of P. pantotrophus was completed, and three genes, soxFGH, and their products were characterized. The respective proteins were predicted to be located in the periplasm, and their formation was induced by thiosulfate. However, the soxFGH gene products were not required for growth with thiosulfate. Homogenous SoxF, SoxG, and SoxH did not exhibit sulfur-oxidizing activity and were not required for sulfur oxidation in vitro. Instead, the Sox system reconstituted from SoxXA, SoxYZ, SoxB, and SoxCD was found to generally oxidize inorganic reduced sulfur compounds, namely, thiosulfate, sulfite, sulfur, and hydrogen sulfide.
SoxF is a flavoprotein, as evident from the identification of FAD. The molecular mass (42 kDa) of SoxF of P. pantotrophus was similar to those of flavoproteins (40 to 47 kDa) from other sources (17). SoxF was not associated with a c-type cytochrome as described for flavocytochromes c of different strains of the genus Chlorobium, of A. vinosum, and of Thiobacillus strain W5, which exhibit sulfide dehydrogenase activity in vitro (46, 51). No catalytic activity was detected for SoxF alone or in combination with a Q Sepharose fraction containing SoxE. However, SoxF inhibited hydrogen sulfide oxidation significantly, by about 20%. This mode of action is so far unexplained. Also, a deletion within soxF and complementation analysis did not indicate a function for growth with thiosulfate. No catalytic activity was detected for the homodimeric zinc-containing protein SoxG and the homodimeric SoxH protein, which possibly contains zinc and copper. No possible function could be deduced from alignments of SoxG and SoxH; thus, their specific functions are presently unknown.
SoxF has high identity to the flavoprotein FccB of flavocytochrome c sulfide dehydrogenase of A. vinosum. Flavocytochrome c of A. vinosum also binds sulfite, thiosulfate, and mercaptans, and its function in vivo as a sulfide dehydrogenase has been questioned (12, 15). There is no experimental support for a crucial role of flavocytochrome c in sulfur metabolism, although the ability to grow with thiosulfate is seemingly correlated with its presence in different sulfur-oxidizing bacteria (17). Disruption of the gene for the cytochrome subunit of flavocytochrome c of A. vinosum did not affect phototrophic growth with hydrogen sulfide, and SQR was suggested to be responsible for this metabolism (35). In fact, deletion of the sqr gene of R. capsulatus demonstrated that SQR is essential for phototrophic growth with hydrogen sulfide (41), the only sulfur compound that this strain is able to utilize for this metabolism. SQR is also present in membranes of P. pantotrophus, and its physiological function in this strain is not known (40).
The reconstituted sulfur-oxidizing enzyme system of P. pantotrophus performs sulfite-, thiosulfate-, sulfur-, and hydrogen sulfide-dependent cytochrome c reduction. The highest activity was observed with hydrogen sulfide. This substrate versatility characterizes the enzyme complex as the first to generally oxidize reduced inorganic sulfur compounds and corrects a previous report suggesting SoxB to act as a sulfide dehydrogenase (39). The biochemical data are supported by previous reports of a soxB::Tn5 insertional mutant unable to oxidize thiosulfate and hydrogen sulfide (10, 49).
Using the reconstituted Sox system, 1 mol of thiosulfate yielded about 8 mol of electrons, which was in accordance with the theoretical yield. However, with hydrogen sulfide or sulfur as substrate, a yield of only about 4 mol of electrons per mol of substrate was observed. Autoxidation of hydrogen sulfide was excluded by anaerobic performance of the assays. Thus, the low electron yield is presently unexplained. The material balance of sulfur substrates and products is hampered by the interference of proteins present in the assays for quantitative analysis. Omission of SoxCD from assays with sulfur or hydrogen sulfide as an electron donor resulted in a yield of 2 mol of electrons, which was in accordance with a previous finding with sulfite and thiosulfate as substrates (18) and supported the finding that anaerobic conditions did not alter the yield of electrons. The enhancement of the yield of electrons by SoxCD points to a function different from sulfite dehydrogenase and suggests for SoxCD the novel function of a sulfur dehydrogenase.
The Sox system described for P. pantotrophus appears to be also present in polythionate-oxidizing sulfur-oxidizing bacteria. Using the system for insertional mutagenesis previously applied for P. pantotrophus (10), a soxA gene was inactivated in the polythionate-utilizing facultatively lithotrophic thiobacterium KCT001, resulting in a Sox− phenotype (30). Analysis of the partial nucleotide sequence of strain KCT001 indicated the presence of a soxZ′AB′ gene sequence. The deduced partial SoxZ is 64% identical in a 33-amino-acid overlap to SoxZ of P. pantotrophus, the diheme SoxA is 45% identical, and the twin-arginine motif of the putative leader peptide of SoxB is identified. The order of sox genes in strain KCT001 suggested a gene cluster similar to that of P. pantotrophus. Also, the order of the soxB′CD′ genes has been detected in the thiosulfate- and tetrathionate-oxidizing bacterium Starkeya novella (20). Moreover, genes homologous to the sox genes of P. pantotrophus have also been detected in phototrophic bacteria like R. palustris (IGwit database accession number RRPA00660) and Chlorobium tepidum (IGwit database accession number RCL01710). Thus, the sox gene cluster of P. pantotrophus may represent a general Sox system also present in different sulfur-oxidizing bacteria, including polythionate-utilizing strains. Polythionate hydrolysis yields sulfate and sulfane monosulfonic acids. These compounds or spontaneous decomposition products may be further metabolized via such a common sulfur-oxidizing system. In light of the recent genetic data (18, 30) and the biochemical data reported here, the distinction between polythionate-utilizing and non-polythionate-utilizing sulfur-oxidizing bacteria (17, 21) is not applicable with respect to the system that actually oxidizes sulfur.
ACKNOWLEDGMENTS
We thank R. Kraft and S. Kostka for determination of the amino acid sequences, A. Bohlen for help in metal analysis, and B. Heller and R. Ringk for excellent technical assistance.
This work was supported by grant Fr 318/6-3 of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Altenbuchner J, Viell P, Pelletier I. Positive selection vector based on palindromic DNA sequences. Methods Enzymol. 1992;216:457–566. doi: 10.1016/0076-6879(92)16042-i. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson L O, Borg H, Mikaelsson M. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 1972;20:199–202. doi: 10.1016/0014-5793(72)80793-2. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1994. [Google Scholar]
- 5.Berks B C. A common export pathway for proteins binding complex redox cofactors. Mol Microbiol. 1996;22:393–404. doi: 10.1046/j.1365-2958.1996.00114.x. [DOI] [PubMed] [Google Scholar]
- 6.Bibb M J, Sherman D H, Omura S, Hopwood D A. Cloning, sequencing and deduced functions of a cluster of Streptomycesgenes probably encoding biosynthesis of the polyketide antibiotic frenolicin. Gene. 1994;142:31–39. doi: 10.1016/0378-1119(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 7.Bogsch E G, Sargent F, Stanley N R, Berks B C, Robinson C, Palmer T. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J Biol Chem. 1998;273:18003–18006. doi: 10.1074/jbc.273.29.18003. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Bruenker P, McKinney K, Sterner O, Minas W, Bailey J E. Isolation and characterization of the naphthocyclinone gene cluster from Streptomyces arenaeDSM 40737 and heterologous expression of the polyketide synthase genes. Gene. 1999;227:125–135. doi: 10.1016/s0378-1119(98)00618-0. [DOI] [PubMed] [Google Scholar]
- 10.Chandra T S, Friedrich C G. Tn5-induced mutations affecting sulfur-oxidizing ability (Sox) of Thiosphaera pantotropha. J Bacteriol. 1986;166:446–452. doi: 10.1128/jb.166.2.446-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cusanovich M A, Meyer T E, Bartsch R G. Flavocytochrome c. In: Muller F, editor. Chemistry and biochemistry of flavoenzymes. Vol. 2. Boca Raton, Fla: CRC Press; 1991. pp. 377–399. [Google Scholar]
- 13.Deckert G, Warren P V, Gaasterland T, Young W G, Lenox A L, Graham D E, Overbeek R, Snead M A, Keller M, Aujay M, Huber R, Feldman R A, Short J M, Olson G J, Swanson R V. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 14.Dernedde J, Eitinger T, Patenge N, Friedrich B. hyp gene products in Alcaligenes eutrophusare part of a hydrogenase-maturation system. Eur J Biochem. 1996;235:351–358. doi: 10.1111/j.1432-1033.1996.00351.x. [DOI] [PubMed] [Google Scholar]
- 15.Dolata M M, Van Beeumen J J, Ambler R P, Meyer T E, Cusanovich M A. Nucleotide sequence of the heme subunit of flavocytochrome cfrom the purple phototrophic bacterium Chromatium vinosum. A 2.6-kilobase pair DNA fragment contains two multiheme cytochromes, a flavoprotein, and a homolog of human ankyrin. J Biol Chem. 1993;268:14426–14431. [PubMed] [Google Scholar]
- 16.Fischer J, Quentmeier A, Kostka S, Kraft R, Friedrich C G. Purification and characterization of the hydrogenase from Thiobacillus ferrooxidans. Arch Microbiol. 1996;165:289–296. doi: 10.1007/s002030050329. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich C G. Physiology and genetics of sulfur-oxidizing bacteria. Adv Microb Physiol. 1998;39:235–289. doi: 10.1016/s0065-2911(08)60018-1. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich C G, Quentmeier A, Bardischewsky F, Rother D, Kraft R, Kostka S, Prinz H. Novel genes coding for lithotrophic sulfur oxidation of Paracoccus pantotrophusGB17. J Bacteriol. 2000;182:4677–4687. doi: 10.1128/jb.182.17.4677-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukumori Y P, Yamanaka I. Flavocytochrome c of Chromatium vinosum. Some enzymatic properties and subunit structure. J Biochem (Tokyo) 1979;85:1405–1414. doi: 10.1093/oxfordjournals.jbchem.a132467. [DOI] [PubMed] [Google Scholar]
- 20.Kappler U, Friedrich C G, Trüper H G, Dahl C. Evidence for two pathways of thiosulfate oxidation in Starkeya novella (formerly Thiobacillus novellus) Arch Microbiol. 2001;175:102–111. doi: 10.1007/s002030000241. [DOI] [PubMed] [Google Scholar]
- 21.Kelly D P, Shergill J K, Lu W-P, Wood A P. Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie Leeuwenhoek. 1997;71:95–107. doi: 10.1023/a:1000135707181. [DOI] [PubMed] [Google Scholar]
- 22.Kieser T. Factors affecting the isolation of ccc DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984;12:19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 23.Klockenkämper R, von Bohlen A. Elemental analysis of environmental samples by TXRF: a review. X-Ray Spectrom. 1996;25:156–162. [Google Scholar]
- 24.Knauf V C, Nester E W. Wide host range cloning vectors: a cosmic cloning bank of AgrobacteriumTi plasmid. Plasmid. 1982;8:45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig W, Mittenhuber G, Friedrich C G. Transfer of Thiosphaera pantotropha to Paracoccus denitrificans. Int J Syst Bacteriol. 1993;43:363–367. doi: 10.1099/00207713-43-2-363. [DOI] [PubMed] [Google Scholar]
- 27.McPherson M J, Quirke P, Taylor G R. PCR—a practical approach. New York, N.Y: Oxford University Press; 1992. [Google Scholar]
- 28.Melino S, Capo C, Dragani B, Aceto A, Petrucelli R. A zinc-binding motif conserved in glyoxylase II, β-lactamase and arylsulfatases. Trends Biochem Sci. 1998;23:381–382. doi: 10.1016/s0968-0004(98)01264-x. [DOI] [PubMed] [Google Scholar]
- 29.Mittenhuber G, Sonomoto K, Egert M, Friedrich C G. Identification of the DNA region responsible for sulfur-oxidizing ability of Thiosphaera pantotropha. J Bacteriol. 1991;173:7340–7344. doi: 10.1128/jb.173.22.7340-7344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhopadhyaya P N, Deb C, Lahiri C, Roy P. A soxA gene, encoding a diheme cytochrome c, and a soxlocus, essential for sulfur oxidation in a new sulfur lithotrophic bacterium. J Bacteriol. 2000;182:4278–4287. doi: 10.1128/jb.182.15.4278-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prinz H, Lavie A, Scheidig A, Spangenberg O, Konrad M. Binding of nucleotides to guanylate kinase, ras p21 and to nucleoside diphosphate kinase studied by nano-electrospray mass spectrometry. J Biol Chem. 1999;274:35337–35342. doi: 10.1074/jbc.274.50.35337. [DOI] [PubMed] [Google Scholar]
- 32.Quentmeier A, Kraft R, Kostka S, Klockenkämper R, Friedrich C G. Characterization of a new type of sulfite dehydrogenase from Paracoccus pantotrophusGB17. Arch Microbiol. 2000;173:117–125. doi: 10.1007/s002039900118. [DOI] [PubMed] [Google Scholar]
- 33.Rainey F A, Kelly D P, Stackebrandt E, Burghardt J, Hiraishi A, Katayama Y, Wood A P. A re-evaluation of the taxonomy of Paracoccus denitrificans and a proposal for the combination Paracoccus pantotrophuscomb. nov. Int J Syst Bacteriol. 1999;49:645–651. doi: 10.1099/00207713-49-2-645. [DOI] [PubMed] [Google Scholar]
- 34.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolorA3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 35.Reinartz M, Tschäpe J, Brüser T, Trüper H G, Dahl C. Sulfide oxidation in the phototrophic sulfur bacterium Chromatium vinosum. Arch Microbiol. 1998;170:59–68. doi: 10.1007/s002030050615. [DOI] [PubMed] [Google Scholar]
- 36.Robertson L A, Kuenen J G. Thiosphaera pantotrophagen. nov. sp. nov., a facultatively anaerobic, facultative autotrophic sulphur bacterium. J Gen Microbiol. 1983;129:2847–2855. [Google Scholar]
- 37.Sambrook J, Maniatis T, Fritsch E F. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider A, Friedrich C. Sulfide dehydrogenase is identical with the SoxB protein of the thiosulfate-oxidizing enzyme system of Paracoccus denitrificansGB17. FEBS Lett. 1994;350:61–65. doi: 10.1016/0014-5793(94)00732-2. [DOI] [PubMed] [Google Scholar]
- 40.Schütz M, Klughammer C, Griesbeck C, Quentmeier A, Friedrich C G, Hauska G. Sulfide-quinone reductase activity in membranes of the chemotrophic bacterium Paracoccus denitrificansGB17. Arch Microbiol. 1998;170:353–360. [Google Scholar]
- 41.Schütz M, Maldener I, Griesbeck C, Hauska G. Sulfide-quinone reductase from Rhodobacter capsulatus: requirement for growth, periplasmic location, and extension of gene sequence analysis. J Bacteriol. 1999;181:6516–6523. doi: 10.1128/jb.181.20.6516-6523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahak Y, Arieli B, Padan E, Hauska G. Sulfide-quinone reductase (SQR) activity in Chlorobium. FEBS Lett. 1992;299:127–130. doi: 10.1016/0014-5793(92)80230-e. [DOI] [PubMed] [Google Scholar]
- 43.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivogenetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 44.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:6762–6766. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira J, Messing J. The pUC plasmids: an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 46.Visser J M, de Jong G A H, Robertson L A, Kuenen J G. A novel membrane-bound flavocytochrome c sulfide dehydrogenase from the colorless sulfur bacterium Thiobacillussp.W5. Arch Microbiol. 1997;167:295–301. doi: 10.1007/s002030050447. [DOI] [PubMed] [Google Scholar]
- 47.Weber K, Pringle J R, Osborn M. Measurements of molecular weights by electrophoresis on SDS polyacrylamide gels. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- 48.Weiner J H, Bilous P T, Shaw G M, Lubitz S P, Frost L, Thomas G H, Cole J A, Turner R J. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell. 1998;93:93–101. doi: 10.1016/s0092-8674(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 49.Wodara C, Kostka S, Egert M, Kelly D P, Friedrich C G. Identification and sequence analysis of the soxB gene essential for sulfur oxidation of Paracoccus denitrificansGB17. J Bacteriol. 1994;176:6188–6191. doi: 10.1128/jb.176.20.6188-6191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wodara C, Bardischewsky F, Friedrich C G. Cloning and characterization of sulfite dehydrogenase, two c-type cytochromes, and a flavoprotein of Paracoccus denitrificansGB17: essential role of sulfite dehydrogenase in lithotrophic sulfur oxidation. J Bacteriol. 1997;179:5014–5023. doi: 10.1128/jb.179.16.5014-5023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamanaka T, Fukumori Y, Okonuki K. Preparation of subunits of flavocytochromes c derived from Chlorobium limicola f. thiosulfatophilum and Chromatium vinosum. Anal Biochem. 1979;95:209–213. doi: 10.1016/0003-2697(79)90207-0. [DOI] [PubMed] [Google Scholar]