Abstract
Background
The use of High-flow nasal cannula (HFNC) is increasing in admitted COPD-patients and could provide a step in between non-invasive ventilation (NIV) and standard oxygen supply. Recent studies demonstrated that HFNC is capable of facilitating secretion removal and reduce the work of breathing. Therefore, it might be of advantage in the treatment of acute exacerbations of COPD (AECOPD). No randomized trials have assessed this for admitted COPD-patients on a regular ward and only limited data from non-randomized studies is available.
Objectives
The aim of our study was to identify the reasons to initiate treatment with HFNC in a group of COPD-patients during an exacerbation, further identify those most likely to benefit from HFNC treatment and to find factors associated with treatment success on the pulmonary ward.
Material and methods
This retrospective study included COPD-patients admitted to the pulmonary ward and treated with HFNC from April 2016 until April 2019. Only patients admitted with severe acute exacerbations were included. Patients who had an indication for NIV-treatment where treated with NIV and were included only if they subsequently needed HFNC, e.g. when they did not tolerate NIV. Known asthma patients were excluded.
Results
A total of 173 patients were included. Stasis of sputum was the indication most reported to initiate HFNC-treatment. Treatment was well tolerated in 83% of the patients. Cardiac and vascular co-morbidities were significantly associated with a smaller chance of successful treatment (Respectively OR = 0.435; p = 0.013 and OR = 0.493;p = 0.035). Clinical assessment judged HFNC-treatment to be successful in 61% of the patients. Furthermore, in-hospital treatment with NIV was associated with a higher chance of HFNC failure afterwards (OR = 0.439; p = 0.045).
Conclusion
This large retrospective study showed that HFNC-treatment in patients with an AECOPD was initiated most often for sputum stasis as primary reason. Factors associated with improved outcomes of HFNC-treatment was the absence of vascular and/or cardiac co-morbidities and no need for in-hospital NIV-treatment.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) patients often suffer from periods of acute worsening of shortness of breath. During severe exacerbations, patients need admission to a hospital [1]. Additional oxygen supply is a cornerstone of the in-hospital treatment of these patients. However, standard oxygen therapy via nasal prongs is limited by a low fraction of inspired oxygen (FiO2) due to a limited flow rate of usually cold and dry air [2–4]. In addition standard oxygen therapy does not influence mucus extraction.
Moreover, in patients with acute acidotic hypercapnic respiratory failure, non-invasive ventilation (NIV) is a proven effective therapy. It reduces the work of breathing, improves gas exchange, reduces the length of hospital stay, decreases the need for endotracheal intubation and improves mortality [5–8]. However, patients may not always tolerate NIV-treatment due to discomfort [8, 9]. Also talking, eating, and especially mucus expectoration, a common problem during COPD-exacerbations, are difficult during NIV-treatment.
If NIV fails, in clinical practice usually two options remain, step-up to invasive ventilation or accept to step-down to standard oxygen therapy. If hypercapnic respiratory failure persists, invasive ventilation is recommended. However, invasive ventilation in COPD-patients is associated with worse outcomes, prolonged hospitalization and mortality as compared to NIV [10]. Next to its limited effect on mucus, standard oxygen therapy will not treat hypercapnic respiratory failure, and may even worsen hypercapnia in some cases.
To address these problems, an alternative treatment option positioned in between NIV and standard oxygen could be of additional value. Potentially, such a treatment could be used to treat COPD-patients with more severe hypoxemic respiratory failure needing more than standard oxygen therapy and patients with a mild to moderate combined hypoxemic/hypercapnic respiratory failure who do not tolerate NIV, especially those with mucus-related problems. Treatment with high-flow nasal cannula (HFNC) could be considered as this step in between standard oxygen and NIV. With HFNC heated and humidified air optionally supplemented with additional oxygen can be delivered at high flow rates up to 60L/min and this may have multiple advantages for the treatment of COPD-exacerbations.
First, the humidification and heating of the delivered air may facilitate secretion removal, reduce airway inflammation and avoid epithelial injury [11], and potentially preventing bronchospasm [12]. Secondly, the high flow provides some positive airway pressure; it increases nasopharyngeal airway pressure that peaks at the end of expiration, which may counteract on the intrinsic positive end-expiratory pressure (PEEPi) and thus may decrease the work of breathing [13–16]. Although the effect of PEEP depends on mouth close of the patient. Furthermore with the high flow of HFNC the generally present higher inspiratory (flow) demand in COPD-patients during exacerbations can be met. Finally, The high flow of oxygen of HFNC reduces dead space. The combination of these mechanisms of HFNC has been shown to reduce PaCO2 [17–19].
While awaiting large randomized controlled trials, HFNC is already being used in clinical practice for COPD-patients. From these clinical practices, valuable information can be obtained with regard to reasons for initiating treatment with HFNC in COPD-patients with an exacerbation and selection of patients that benefit most from treatment. We retrospectively analyzed data of admitted COPD-patients treated with HFNC at a pulmonary ward in order to address these questions. Furthermore, we aimed to describe clinical outcomes with regard to used settings of the HFNC, treatment tolerance and factors associated with successful treatment.
Methods
Study population
We performed a retrospective study including COPD patients (>18 years) admitted with a COPD exacerbation to the pulmonary ward of a Dutch teaching hospital and treated with HFNC in the period of April 2016 until April 2019. The definition of a COPD-exacerbation by the Global initiative for chronic obstructive lung diseases was used [1, 20]. Only patients with exacerbation of COPD as primary reason for admission and treatment were included. Patients who had an indication for NIV-treatment where treated with NIV first and were included only if they subsequently received HFNC. Only ward-based HFNC was analyzed; treatment periods at an Intensive care unit (ICU) were not included, but step down treatments at the pulmonary ward after ICU-treatment however were included. Patients who were admitted and treated with HFNC multiple times were included only once. Patients with asthma, known active lung malignancy, cerebrovascular accident and/or myocardial infarction less than three months ago, neuromuscular disease and hemodialysis were excluded from the analysis.
Data recorded
Data were retrieved from the hospital-electronic patient systems. Demographic data such as age, gender, COPD GOLD-stage, presence of co-morbidities (as recorded in medical history), stable state lung function (forced expiratory volume in 1 second (FEV1)) and concomitant treatments were recorded. Recording of co-morbidities was limited to other lung diseases (such as bronchiectasis, lung embolus, interstitial lung disease a.o.) cardiac- (myocardial infarction, heart failure or arrhythmias), vascular- (hypertension, peripheral arterial disease), neurologic co-morbidities and/or diabetes. Also, the number of COPD exacerbations for which treatment with antibiotics and/or corticosteroids was required in the year before admission was recorded, as well as the number of admissions in the previous year.
Vital signs, such as respiratory rate (RR), heart rate (HR) and oxygen saturation (SO2) were retrieved from 3 different time points: before the start of HFNC-treatment (obtained within 24 hours before the start), during HFNC-treatment, and within 24 hours after ending HFNC-treatment. Arterial blood gasses (ABG) values were used for analysis if obtained within 48 hours before and 48 hours after HFNC-treatment. The settings of the HFNC-treatment; (inspired oxygen fraction (FiO2), flow rate and temperature of the humidified air), were recorded at start and during treatment. We assessed the need for NIV or invasive ventilation, length of hospital stay and mortality.
Treatment
The decision to initiate HFNC was made by the treating pulmonologist based on clinical arguments. Every included patient was treated with the same device; (AIRVO® Humidification system; Fisher & Paykel healthcare, Auckland, New Zealand). The HFNC was set according to decision of the treating physician.
Outcome assessment
The judgement whether HFNC was successful or not was made by the attending consultant respiratory medicine based on the clinical examination of the patient, independent of the study team. Treatment was deemed non-successful when patients showed no clinical improvement, or did not tolerate the treatment. Successful treatment was defined as the absence of treatment failure.
Statistical analysis
Descriptive data were expressed as mean ± standard deviation or as percentage (%). The distribution of data was assessed by performing a Shapiro-Wilk test and examining a histogram and Q-Q plot for every independent value. Median and interquartile ranges (Q1 and Q3) were used for non-normal distributed data and percentage (%) was used for categorical data.
A Chi-square or Fisher’s-exact test was used to compare nominal/categorical variables. When comparing values from the same patient a paired T-test was used when the data was normally distributed and a sign-test when the data was non-normally distributed.
For comparison between groups of continuous variables that were normally distributed an unpaired T-test was used. When these values were non-normally distributed a Mann Whitney U-test was used to calculate significance. All tests were two sided and considered significant if the P-value was below 0.05. Analysis was performed using SPSS statistics version 24.
For comparison of factors associated with treatment success a multiple logistic regression was performed. First of a univariate analysis was done for all the variables, next the variables which were shown to have a slight association (p<0.15) were taken into account in the multiple regression analysis. Backward selection was used to further narrow down the variables. The variables were considered statistically significant if the P-value was below 0.05.
Ethics
The Medical Ethics Committee of our institution (RTPO Leeuwarden) confirmed the conduct of this retrospective study and the institutional board approved the execution of the study without the need for consent in accordance with Dutch regulations (ID NMWMO356). All data was handled confidentially and anonymously.
Results
Baseline characteristics
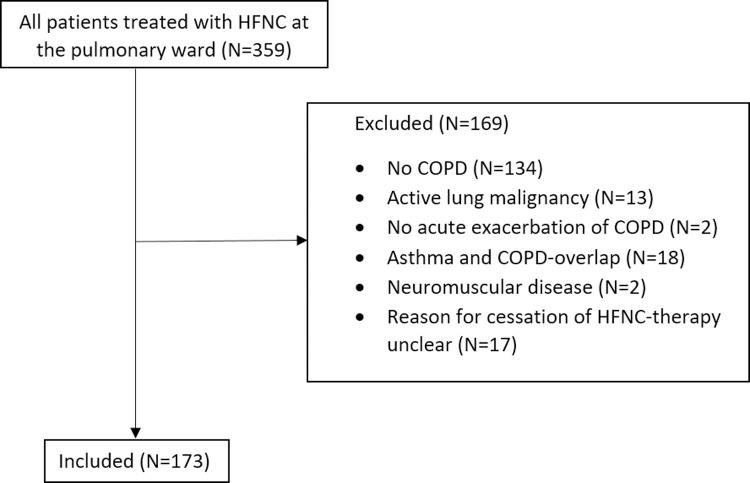
Among all the patients treated with HFNC at the pulmonary ward, 173 patients met the inclusion criteria (Fig 1). The characteristics of the included patients are summarized in Table 1. The mean age of the patients was 71 (± 10) years and 87 of the patients (51%) were male. Most of the patients were former smokers (65%) and had a COPD GOLD classification II (37%) or III (35.8%) with a mean FEV1 of 1.19L (46% of predicted). Almost half of the patients were known with cardiac (48.6%) and/or vascular (54.9%) co-morbidities. The patients had a median exacerbation rate of 1.00 (0–12) exacerbations in the previous year with 0 (0–6) admissions in the previous year.
Fig 1. Flowchart of all included patients.
Table 1. Baseline characteristics of the included patients.
| Characteristic | N | Mean ± St. dev./Percentage |
|---|---|---|
| Age (years) | 173 | 71 ± 10 |
| Man | 87 | 51% |
| Weight (kg) | 172 | 75.3 ± 20.5 |
| Length (cm) | 172 | 171 ± 9 |
| BMI (kg/m2) | 172 | 25.8 ± 6.4 |
| Smoking | 173 | |
| Current | 51 | 29.5% |
| Former | 113 | 65.3% |
| Never | 9 | 5.2% |
| COPD Gold stage for Airway obstruction | 166 | |
| I | 4 | 2.3% |
| II | 64 | 37.0% |
| III | 62 | 35.8% |
| IV | 36 | 20.8% |
| Comorbidities: | ||
| Other Lung diseases | 40 | 23.3% |
| Cardiac | 84 | 48.6% |
| Vascular | 95 | 54.9% |
| Neurologic | 55 | 31.8% |
| Diabetes | 35 | 20.2% |
| Stable state Lung function: | 162 | |
| FEV1 (L) | 162 | 1.19 ± 0.49 |
| VCmax (L) | 162 | 2.77 ± 0.95 |
| FEV1 (% predicted) | 162 | 46 ± 17 |
| FEV1/VCmax (%) | 162 | 44 ± 13 |
| TLco (% predicted) | 88 | 47 ± 16 |
| Current maintenance treatment at home | ||
| Long-acting β2-agonists | 140 | 80.9% |
| Long-acting muscarinic antagonists | 121 | 69.9% |
| Inhalated corticosteroids | 132 | 76.3% |
| Oral Corticosteroids | 41 | 23.7% |
| Antibiotics | 36 | 20.8% |
| Oxygen treatment at home | 33 | 19.1% |
| Year before treatment: | ||
| Number of exacerbations in which treatment was needed | 173 | 1.00 (0–3) |
| Number of admissions | 173 | 0.00 (0–1) |
| First arterial blood gas values: | ||
| pH | 101 | 7,39 (± 0,07) |
| pCO2 (kPa) | 101 | 6,82 (± 2,01) |
| pO2 (kPa) | 101 | 8,15 (± 2,01) |
Values shown are mean ± standard deviation or percentage (%) for categorical data.
N: number of patients; BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; FEV1 forced expiratory volume in 1 second; L: litre; VCmax: maximal vital capacity; FEV1/VCmax: Tiffeneau index; TLco: diffusing capacity for carbon monoxide.
In addition to the HFNC-treatment, 20.2% of the patients needed treatment with NIV during the hospital admission and 80.3% received treatment with different means of O2-suppletion (Table 2).
Table 2. Concomitant in-hospital treatment.
| Concomitant in-hospital treatment: | N | Percentage |
|---|---|---|
| SABA/SAMA aerosol via nebulizer | 165 | 95.4% |
| Oral corticosteroids | 163 | 94.2% |
| Antibiotics | 133 | 76.9% |
| O2-suppletion | 139 | 80.3% |
| NIV | 35 | 20.2% |
In-hospital treatment in addition to treatment with HFNC for all included patients. Values shown are the number of patients (N) and percentage (%). SABA: short-acting β-agonist; SAMA: short-acting muscarinic antagonist; NIV: Non-invasive ventilation.
HFNC-settings
HFNC-settings were recorded properly in the majority of the treated patients at start of treatment. The median flow rate used was 30 L/min (N = 156, IQR: Q1 30L/min, Q3 40L/min), with a median FiO2 of 25% (N = 164, IQR: Q1 21%, Q3 32%) and a median temperature of 31°C (N = 147, IQR: Q1 31°C, Q3 31°C).
Reasons to start HFNC-treatment
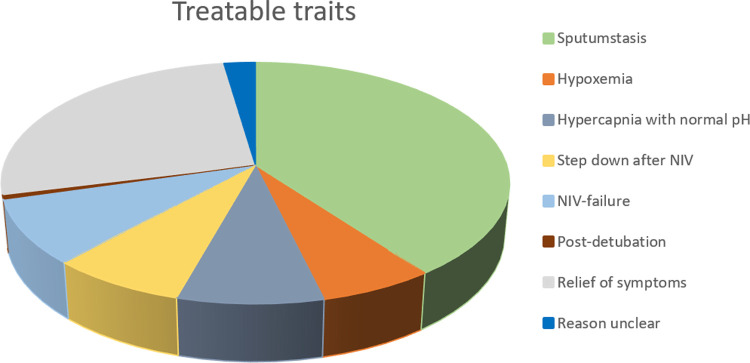
In 69 patients (39.9%), the primary reason for treatment was stasis of sputum. Eleven patients (6.4%) were treated because of hypoxemia despite regular oxygen suppletion and 14 patients (8.1%) because of hypercapnia with normal pH. In 13 patients (7.5%) HFNC-treatment was a step-down after NIV-treatment, in 15 patients (8.7%) HFNC-treatment was initiated after NIV-treatment failed, mostly because NIV-treatment was not tolerated and in only 1 patient (0.6%) HFNC-treatment was initiated after detubation. In 46 patients (26.6%) HFNC was started for relief of symptoms related to dyspnea and in 4 patients (2.3%) the primary reason for starting treatment was not retrievable (Fig 2).
Fig 2. Treatable traits for HFNC in included COPD-patients.
Outcomes
Vital signs
The heart rate decreased after the start of treatment, (Improvement before-during: 4.0/min, p = 0.005) and remained lower after treatment (Improvement before-after: 5.3/min, p = <0.001). SO2 was increased after treatment with HFNC (Improvement before-after: -0.8%, p = 0.033) as well as the respiratory rate (Improvement before-after: 1.3, p = 0.001) (Table 3).
Table 3. Mean values before, during and after treatment with the HFNC.
| Before HFNC-treatment | During HFNC-treatment | After HFNC-treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | NΔ | Improvement Before-During | P-value | N | NΔ | Improvement Before-After | P-value | ||||
| Vital signs | ||||||||||||
| Respiratory rate (/min) | 172 | 21 ± 5 | 142 | 20 ± 4 | 141 | 0.5 (-0.11 to 1.16) | 0.10 | 173 | 19 ± 4 | 172 | 1.3 (0.5 to -0.2) | 0.001 |
| Heart rate (/min) | 172 | 90 ± 19 | 142 | 87 ± 15 | 141 | 4.0 (1.2 to 6.8) | 0.005 | 170 | 85 ± 15 | 169 | 5.3 (2.7–7.8) | <0.001 |
| Saturation (%) | 172 | 92 ± 5 | 145 | 92 ± 4 | 144 | - 0.3 (-1.0 to 0.4) | 0.42 | 173 | 92 ± 4 | 172 | - 0.8 (-1.6 to -0.1) | 0.033 |
Values shown are mean and standard deviation. For Δ-values (Δ Before-During and Δ Before-After) the mean change and 95% confidence interval for the means are shown. A paired T-test was used for comparison between values before and during and before and after.
N: number of patients; NΔ: the number of patients with known values before and during or before and after.
Outcome of HFNC-treatment
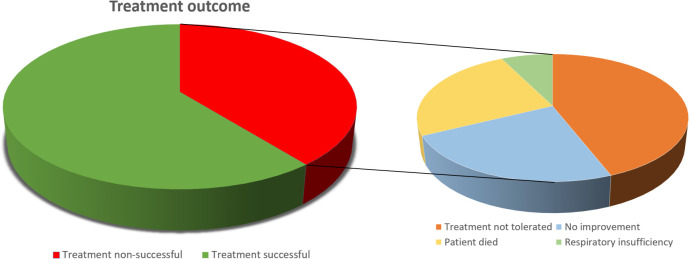
The mean length of hospital stay for all patients was 9.5 ± 6.2 days. Treatment outcome is shown in Fig 3. In 105 patients (61%), treatment was successful and in 68 patients (39%) treatment was non-successful. In 5 patients (3%) treatment was non-successful due to respiratory insufficiency, these patients were initiated on NIV or were intubated. Sixteen patients (9%) showed no improvement, 30 patients (17%) did not tolerate HFNC-treatment and 17 patients (10%) died.
Fig 3. Treatment outcome of HFNC-treatment.
Factors associated with success and failure
Comparing the patients that were treated successfully to those who were not (Table 4), there were no differences in stable state lung function, number of exacerbations and admissions due to exacerbations in the previous year, maintenance treatment at home and in-hospital treatment. Patients with treatment success had significant fewer cardiac and vascular co-morbidities than the patients with treatment failure (cardiac co-morbidities 41% vs. 60%, p = 0.02 and vascular co-morbidities 48% vs. 66%, p = 0.02.
Table 4. Comparison between patients whom had successful treatment or wit treatment failure.
| Patient characteristics | N | Treatment Successful (N = 105) | N | Treatment failure (N = 68) | P-value |
|---|---|---|---|---|---|
| Age, mean (years) | 105 | 70 ± 10 | 68 | 72 ± 10 | 0.45 |
| Gender, man | 105 | 45% | 68 | 59% | 0.072 |
| Weight, mean (kg) | 105 | 74 ± 18 | 67 | 79 ± 23 | 0.13 |
| Length, mean (cm) | 105 | 170 ± 8 | 67 | 172 ± 10 | 0.12 |
| BMI, mean (kg/m2) | 105 | 25.5 ± 6.0 | 67 | 26.4 ± 7.1 | 0.37 |
| Smoking | 0.17 | ||||
| Current | 26 | 24.8% | 25 | 36.8% | |
| Former | 72 | 68.6% | 41 | 60.3% | |
| Never | 7 | 6.7% | 2 | 2.9% | |
| COPD Gold stage for Airway obstruction | 0.81 | ||||
| I | 3 | 2.9% | 1 | 1.5% | |
| II | 36 | 34.3% | 28 | 41.2% | |
| III | 39 | 37.1% | 23 | 33.8% | |
| IV | 22 | 21.0% | 14 | 20.6% | |
| Co-morbidities: | |||||
| Other lung disease | 25 | 23.8% | 21 | 30.9% | 0.38 |
| Cardiac | 43 | 41.0% | 41 | 60.3% | 0.02* |
| Vascular | 50 | 47.6% | 45 | 66.2% | 0.02* |
| Neurologic | 29 | 27.6% | 26 | 38.2% | 0.18 |
| Diabetes | 19 | 18.1% | 16 | 23.5% | 0.44 |
| Year before treatment | |||||
| Number of exacerbations in which treatment was needed | 105 | 1.00 (0–2) | 68 | 1.00 (0–3) | 0.08 |
| Number of admissions | 105 | 0.00 (0–1) | 68 | 0.00 (0–1) | 0.12 |
| Reason for HFNC-treatment | 0.71 | ||||
| Hypoxemia | 9 | 8.6% | 2 | 2.9% | |
| Sputum stasis | 42 | 40.0% | 27 | 39.7% | |
| Hypercapnia with normal pH | 8 | 7.6% | 6 | 8.8% | |
| Step-down after NIV | 7 | 6.7% | 6 | 8.8% | |
| NIV Failure | 7 | 6.7% | 8 | 11.8% | |
| Post-detubation | 1 | 1.0% | 0 | 0% | |
| Relief of Dyspnea | 29 | 27.6% | 17 | 25.0% | |
| Reason unclear | 2 | 1.9% | 2 | 2.9% | |
| Values before HFNC-treatment | |||||
| Respiratory rate, mean (/min) | 105 | 20.4 ± 4.2 | 67 | 20.9 ± 5.0 | 0.48 |
| Heart rate, mean (/min) | 104 | 88.7 ± 18.3 | 68 | 93.2 ± 19.3 | 0.13 |
| Saturation, mean (%) | 104 | 91.6 ± 4.2 | 68 | 91.3 ± 5.2 | 0.70 |
| Values during HFNC-treatment | |||||
| Respiratory rate, mean (/min) | 97 | 19.9 ± 3.5 | 45 | 21.2 ± 3.6 | 0.038* |
| Heart rate, mean (/min) | 97 | 84.9 ± 13.3 | 45 | 90.5 ± 18.0 | 0.038* |
| Saturation, mean (%) | 98 | 92.0 ± 3.2 | 47 | 90.5 ± 4.6 | 0.044* |
| Values after HFNC-treatment | |||||
| Respiratory rate, mean (/min) | 105 | 18.6 ± 2.9 | 68 | 20.3 ± 4.4 | 0.006* |
| Heart rate, mean (/min) | 105 | 81.4 ± 13.1 | 65 | 91.1 ± 16.3 | 0.000* |
| Saturation, mean (%) | 105 | 93.2 ± 3.2 | 68 | 91.1 ± 5.6 | 0.007* |
| Time on the HFNC, median (hours) | 101 | 71 (45–122) | 49 | 26 (13–75) | 0.000* |
| Secondary outcomes | |||||
| Time of Hospital stay, median (days) | 105 | 8 (6–12) | 68 | 7 (5–11) | 0.059 |
| Progression to NIV | 1 | 1.0% | 4 | 5.9% | 0.079 |
| Progression to intubation | 0 | 0% | 2 | 2.9% | 0.15 |
| Progression to O2 at home | 9 | 8.6% | 4 | 5.9% | 0.25 |
| Mortality | 17 | 16.2% | 31 | 45.6% | <0.000* |
| During hos- pital stay | 1 | 1.0% | 18 | 26.5% | <0.000* |
| 90 days | 6 | 5.7% | 20 | 29.4% | 0.066 |
| After 90 days | 11 | 10.5% | 11 | 16.2% | 0.35 |
Mean ± standard deviation are shown for normally distributed data. Median (minimum and maximum) is used for non-normal distributed data and percentage (%) was used for categorical data. For every independent variable the number of patients are shown (N).
N: number of patients; BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; AECOPD: acute exacerbation of COPD; NIV: non-invasive ventilation.
Vital signs at the start of HFNC-treatment did not differ significantly. During and after HFNC-treatment, patients treated successfully showed significant lower respiratory rates (During; 20/min vs. 21/min, p = 0.038, After; 19/min vs. 20/min, p = 0.006) and a significant higher SO2 (During; 92% vs. 91%, p = 0.044, After; 93 vs. 91, p = 0.007).
No significant difference between the two groups was observed in PaCO2, PaO2 and pH before, during or after HFNC-treatment. Patients treated successfully were treated longer on average than the non-successful treatment group (median 71 hours vs. 26 hours, p<0.001). There was no difference observed between patients treated non-successfully and patients treated successfully in intubation rate or progression to O2-treatment at home, although numbers for analysis were small (Table 4).
In the multivariate logistic regression analysis, the presence of cardiac and vascular co-morbidities and in-hospital treatment with NIV were independently associated with a lower HFNC success rate (Respectively OR = 0,435, OR = 0,493 and OR = 0,439, see Table 5).
Table 5. Multivariate logistic regression analysis.
| Patient characteristics | N | Odds Ratio | P-value | Lower bound (95%) | Upper bound (95%) |
|---|---|---|---|---|---|
| Co-morbidities: | |||||
| Cardiac | 84 | 0,435 | 0,013* | 0,226 | 0,840 |
| Vascular | 95 | 0,493 | 0,035* | 0,256 | 0,952 |
| Year before treatment | |||||
| Number of exacerbations in which treatment was needed | 173 | 0,847 | 0,064 | 0,711 | 1,010 |
| In-hospital treatment | |||||
| NIV | 35 | 0,439 | 0,045* | 0,196 | 0,983 |
N: number of patients; NIV: non-invasive ventilation.
The data used for this study was made publicly available at https://www.ebi.ac.uk/biostudies/studies/S-BSST870
Discussion
In this retrospective study, we aimed to describe the clinical practice of HFNC-treatment in patients admitted with an AECOPD and to identify factors associated with success or treatment failure.
We observed that HFNC-treatment in patients with an AECOPD was initiated most frequently for sputum stasis (N = 69, 39.9% of all treated patients) and to relief symptoms of dyspnea (N = 46, 27% of all treated patients) as primary goal. In 61% of the patients treated the treatment was successful suggesting a possible role for HFNC-treatment in COPD-patients. HFNC-treatment was tolerated well, in only 17% of the treated patients treatment was stopped because it was not tolerated. This 17% is lower than the reported incidence of discomfort in treatment with NIV; 30–50% [8, 9]. This suggests that HFNC might be more easy to tolerate than NIV. This is supported by Jing et al. who reported that treatment with HFNC was better tolerated than treatment with NIV before [21].
To our knowledge this is one of the first large real-life studies investigating HFNC-treatment in a population with severe exacerbations of COPD. This study was also performed on the regular ward rather than on the ICU. In our study clinicians choose for HFNC most often because of sputum clearance problems. In most COPD-patients chronic sputum production as well as decrease in mucociliary clearance is a challenging problem [22–25]. By delivering warm and humidified air, the HFNC can facilitate secretion removal through optimal function of mucosa and increased water content in mucous [26, 27]. In a study by Hasani et al. treatment with humidified high flow resulted in a significant increase in mucociliary clearance in patients with bronchiectasis [26]. Which indicates that HFNC-treatment could play a role in treatment of COPD-patients and/or bronchiectasis with complaints of sputum stasis. Trials assessing sputum extraction however are difficult due to the limited availability of strong endpoints.
In our cohort, the presence of vascular and/or cardiac co-morbidities and treatment with NIV were independently associated with HFNC-treatment failure. We hypothesize that both factors are more common in more vulnerable or severely ill patients with an AECOPD influencing HFNC success rates.
Perhaps due to the fact that these co-morbidities often remain untreated. Heart failure for example can mimic or present itself concomitantly with an AECOPD [28], which makes proper treatment more difficult. Like multiple other studies our study suggests an urgent need for better assessment and treatment of co-morbidities in COPD-patients [29–31]. Next the HFNC itself can have a negative outcome on the co-morbidities, e.g. in the occurrence of cardiac arrhythmias [32].
Furthermore, patients who did not tolerate NIV (n = 15), often also not tolerated HFNC-treatment (N = 8) potentially due to the severity of respiratory failure. Moreover NIV-treatment is often needed in patients who are severely ill and are in need of reducing their work of breathing. As in NIV, HFNC is able to reduce work of breathing by reducing minute ventilation [16, 33]. Furthermore HFNC generates a slight positive end-expiratory pressure (PEEP), but far less than NIV. This PEEP has been shown to improve V/Q matching by improving recruitment of alveoli [34]. Multiple studies have assessed HFNC in comparison to NIV. These studies have shown that HFNC is non-inferior in reducing PaCO2 and that there was no difference in 30-day mortality, intubation-rate or treatment failure compared to NIV-treatment [35–38]. The difference in established PEEP between the two devices could be a factor in why HFNC-treatment also fails in patients in which NIV has been unsuccessful. Moreover due to the only slight generation of PEEP inspiratory demands of COPD-exacerbated patients may not be met In HFNC. Spoletini et al. stated that in patients with a high breathing workload and NIV might be more suitable than HFNC and HFNC might be best situated in between standard oxygen and NIV to threat hypoxemia and respiratory failure [39].
Our study suggests that HFNC-treatment might be less suitable for patients who received NIV-treatment as is seen in the multivariate logistic regression analysis. However no differences were seen between patients treated successfully and non-successfully when looking at step-down after NIV-treatment or NIV-failure as reason for HFNC-treatment (see Table 4). Only a slight difference was seen in progression to NIV (1 vs. 4), although numbers were small. Furthermore patients treated successfully were treated longer on average than patients with treatment failure. Although this was not an independent risk factor for treatment success.
The stable state lung function was not associated with a better or worse outcome of HFNC-treatment. FEV1 has been known to show high variability among individuals and therefore FEV1 might not be such a good predictor for outcomes during exacerbations [40, 41]. Potentially this might be different when hyperinflation is present during exacerbations, however data assessing hyperinflation were not available [42, 43].
In our study COPD-patients showed a significant reduction in respiratory rate and improved saturation after HFNC-treatment. This is consistent with a study by Jeong et al. in which an improved respiratory rate and saturation in hypercapnic patients (33 patients with AECOPD) was seen after HFNC-treatment [44]. The reduction of respiratory rate also suggests a reduction in work of breathing, as is also seen in other studies in COPD-patients [45, 46]. Successful treatment in this COPD-population seems depended on the improvement of these respiratory parameters, this is shown by the significant difference in respiratory rate and saturation between patient treated successfully and non-successfully during and after treatment (see Table 4).
Our study suggests that HFNC could also play a role in the treatment of AECOPD in patients in which O2-suppletion alone is not enough. As is also stated in a recent published practical guideline for the use of HFNC [47]. The guideline was not yet published when this study started, but recommends HFNC over conventional oxygen therapy in hypoxemic acute respiratory failure. A recommendation that this study supports considering the success rate of 61% in our patient population of COPD patients with respiratory failure. Although our study did not investigate the difference between treatment with HFNC versus conventional oxygen therapy.
Moreover, our study implies a possible role for HFNC in COPD-patients with sputum-related problems. As was stated before by Crimi et al. whom showed that HFNC was capable of increasing mucus production in patients with AECOPD and coexisting bronchiectasis [48]. NIV and other O2-suppletion are often less suitable for this indication due to the fact that the O2- or NIV-mask needs to be removed to clear sputum. Resolving sputum stasis might reduce the work of breathing as well; as it can reduce dyspnea and lower the respiratory rate and thereby work of breathing, but further research is needed to confirm this notion.
Our study has some limitations. This study is a retrospective study, performed in a single center without a control group. Therefore, it is difficult to completely value data about clinical efficacy. Furthermore, the group of patients was mixed as HFNC was used for several indications and aims. However, there were no differences in reasons for HFNC-treatment between the success and non-success group. Third, the definition of successful treatment could be debated as this was decided on judgement of the clinicians without strict criteria. Since this is a retrospective study a standard operating procedure was not available, we defined successful treatment as the absence of treatment failure combined with the clinical aim of treatment and whether this goal was achieved based on the judgement of the treating clinical physician to obtain the most objective outcome. The main advantage of such a clinical endpoint is however that it reflects actual care for this severely exacerbated COPD-patients. For future prospective studies we would recommend to predefine such criteria. Another weakness of this study is that arterial blood gasses analysis data is not available. During clinical practice these are not routinely performed during HFNC-treatment. This reflects the aims and position of the HFNC-treatment, since HFNC was not used as treatment for respiratory acidosis.
This large retrospective study showed that real world HFNC-treatment in patients with an AECOPD was initiated most often for sputum stasis as primary reason (N = 69, 40%), 61% of all patients ended the treatment successfully based on clinical judgement, and were treated longer. Factors associated with improved outcomes of HFNC-treatment was the absence of vascular and/or cardiac co-morbidities and a longer duration of HFNC-treatment. In clinical practice HFNC-treatment reduces respiratory rate and improves saturation in patients with a COPD-exacerbation. Based on the now reported results, future trials should consider to add mucus related outcomes to their designs, to address the apparent position of HFNC use in the clinic.
Data Availability
The data used for this study were made publicly available at https://www.ebi.ac.uk/biostudies/studies/S-BSST870.
Funding Statement
MD reports grants from Fisher and Paykel LtD (High-TeC trial funded by a public-private partnership of Health-Holland/Dutch Lung Foundation, with co-funding of Fisher and Paykel LtD, and Vivisol BV; registered at ClinicalTrials.gov NCT03564236); grants and personal fees from Vivisol BV (High-TeC trial funded by a public-private partnership of Health-Holland/Dutch Lung Foundation, with co-funding of Fisher and Paykel LtD, and Vivisol BV; registered at ClinicalTrials.gov NCT03564236), grants and personal fees from RESMED LtD (RECAPTURE trial, ClinicalTrials.gov Identifier: NCT03053973), grants and personal fees from Philips BV (RECONSIDER trial funded by the Dutch Lung Foundation with in-kind co-funding of Philips (Longfonds grant nr 5.2.15.057JO, registered at ClinicalTrials.gov NCT02652559), outside the submitted work. The funders did not play any role in study design, data collection and analysis."
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2019. report. [Google Scholar]
- 2.Sim MAB, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008. Sep;63(9):938–40. doi: 10.1111/j.1365-2044.2008.05536.x [DOI] [PubMed] [Google Scholar]
- 3.Aubier M, Murciano D, Milic-Emili J. Effects of administration of O2 on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. Am Rev Respir Dis. 1980. Nov;122(5):747–54. [DOI] [PubMed] [Google Scholar]
- 4.Robinson TD, Freiberg DB, Regnis JA, Young IH. The role of hypoventilation and ventilation-perfusion redistribution in oxygen-induced hypercapnia during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000. May;161(5):1524–9. doi: 10.1164/ajrccm.161.5.9904119 [DOI] [PubMed] [Google Scholar]
- 5.Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2004;(1):CD004104 doi: 10.1002/14651858.CD004104.pub2 [DOI] [PubMed] [Google Scholar]
- 6.Appendini L, Patessio A, Zanaboni, Carone M, Gukov B, Donner CF et al. Physiologic effects of positive end-expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994. May;149(5):1069–76. doi: 10.1164/ajrccm.149.5.8173743 [DOI] [PubMed] [Google Scholar]
- 7.Demoule A, Girou E, Richard JC, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32(11):1756. doi: 10.1007/s00134-006-0324-1 [DOI] [PubMed] [Google Scholar]
- 8.Peter JV, Moran JL, Phillips-Hughes J, Warn D. Noninvasive ventilation in acute respiratory failure a meta-analysis update. Crit Care Med. 2002;30(3):555–62. doi: 10.1097/00003246-200203000-00010 [DOI] [PubMed] [Google Scholar]
- 9.Carron M, Freo U, Bahammam AS, Dellweg D, Guarracino F, Cosentini R et al. Complications of non-invasive ventilation techniques: A comprehensive qualitative review of randomized trials. British Journal of Anaesthesia. 2013. Jun;110(6):896–914. doi: 10.1093/bja/aet070 [DOI] [PubMed] [Google Scholar]
- 10.Crisafulli E, Ielpo A, Barbeta E, Ceccato A, Huerta A, Gabarrus A et al. Clinical variables predicting the risk of a hospital stay for longer than 7 days in patients with severe acute exacerbations of chronic obstructive pulmonary disease: a prospective study. Respir Res. 2018. Dec 27;19(1):261. doi: 10.1186/s12931-018-0951-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chidekel A, Zhu Y, Wang J, Mosko JJ, Rodriguez E, Shaffer TH. The effects of gas humidification with High-flow nasal cannula on cultured human airway epithelial cells. Pulm Med. 2012;2012:380686. doi: 10.1155/2012/380686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.On LS, Boonyongsunchai P, Webb S, Davies L, Calverley PMA, Costello RW. Function of pulmonary neuronal M2 muscarinic receptors in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001. May;163(6):1320–5. doi: 10.1164/ajrccm.163.6.2002129 [DOI] [PubMed] [Google Scholar]
- 13.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009. Dec;103(6):886–90. doi: 10.1093/bja/aep280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisani L, Fasano L, Corcione N, Comellini V, Assunta Musti M, Brandao M et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. 2017. Apr;72(4):373–375. doi: 10.1136/thoraxjnl-2016-209673 [DOI] [PubMed] [Google Scholar]
- 15.Bräunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth HJ, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013;85(4):319–25. doi: 10.1159/000342027 [DOI] [PubMed] [Google Scholar]
- 16.Longhini F, Pisani L, Lungu R, Comellini V, Bruni A, Garofalo E et al. High-Flow Oxygen Therapy After Noninvasive Ventilation Interruption in Patients Recovering From Hypercapnic Acute Respiratory Failure: A Physiological Crossover Trial. Crit Care Med. 2019. Jun;47(6):e506–e511. doi: 10.1097/CCM.0000000000003740 [DOI] [PubMed] [Google Scholar]
- 17.Fricke K, Tatkov S, Domanski U, Franke JK, Nilius G, Schneider H. Nasal high flow reduces hypercapnia by clearance of anatomical dead space in a COPD patient. Respir Med Case Rep. 2016. Aug 26;19:115–7. doi: 10.1016/j.rmcr.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A et al. High-flow nasal cannula: Impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46(1):67–74. doi: 10.1002/ppul.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Möller W, Celik G, Feng S, Bartenstein P, Meyer G, Eickelberg O et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol. 2015. Jun 15;118(12):1525–32. doi: 10.1152/japplphysiol.00934.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease (2020. report). [Google Scholar]
- 21.Jing G, Li J, Hao D, Wang T, Sun Y, Tian H et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: A pilot randomized controlled trial. Res Nurs Heal. 2019. Jun;42(3):217–225. doi: 10.1002/nur.21942 [DOI] [PubMed] [Google Scholar]
- 22.Vastag E, Matthys H, Kohler D. Mucociliary clearance and airways obstruction in smokers, ex smokers and normal subjects who never smoked. Eur J Respir Dis. 1985;139:93–100. [PubMed] [Google Scholar]
- 23.Scheuch G, Kohlhäufl M, Möller W, Brand P, Meyer T, Haussinger K et al. Particle clearance from the airways of subjects with bronchial hyperresponsiveness and with chronic obstructive pulmonary disease. Exp Lung Res. 2008. Feb 15;177(4):426–32. doi: 10.1080/01902140802341710 [DOI] [PubMed] [Google Scholar]
- 24.Isawa T, Teshima T, Hirano T, Ebina A, Motomiya M, Konno K. Lung clearance mechanisms in obstructive airways disease. J Nucl Med. 1984. Apr;25(4):447–54. [PubMed] [Google Scholar]
- 25.Pavia D, Sutton PP, Agnew JE. Measurement of bronchial mucociliary clearance. Eur J of Resp Dis. 1983;127:41–56. [PubMed] [Google Scholar]
- 26.Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5(2):81–6. doi: 10.1177/1479972307087190 [DOI] [PubMed] [Google Scholar]
- 27.Williams R, Rankin N, Smith T, Galler D, Seakins P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med. 1996. Nov;24(11):1920–9. doi: 10.1097/00003246-199611000-00025 [DOI] [PubMed] [Google Scholar]
- 28.Rutten FH, Cramer MJM, Grobbee DE, Sachs APE, Kirkels JH, Lammers JW et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005. Sep;26(18):1887–94. doi: 10.1093/eurheartj/ehi291 [DOI] [PubMed] [Google Scholar]
- 29.Vespasiani-Gentilucci U, Pedone C, Muley-Vilamu M, Antonelli-Incalzi R. The pharmacological treatment of chronic comorbidities in COPD: mind the gap! Pulmonary Pharmacology and Therapeutics. 2018. Jun, 51:48–58. doi: 10.1016/j.pupt.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 30.Menn P, Heinrich J, Huber RM, Jorres RA, John J, Karrasch S, et al. Direct medical costs of COPD—an excess cost approach based on two population-based studies. Respir Med. 2012. Apr;106(4):540–8. doi: 10.1016/j.rmed.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 31.Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology. 2015. Nov;20(8):1160–71. doi: 10.1111/resp.12642 [DOI] [PubMed] [Google Scholar]
- 32.Meijer PM, Oudman KWE, van der Leest S, Wempe JB, Coster JE, Wijkstra PJ et al. Nasal high flow therapy in heart failure patients with central sleep apnea: a report of disproportional occurrence of cardiac arrhythmias. Sleep Medicine. 2021;119–121. doi: 10.1016/j.sleep.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 33.Longhini F, Pisani L, Lungu R, Comellini V, Bruni A, Garofalo E et al. High-Flow Oxygen Therapy After Noninvasive Ventilation Interruption in Patients Recovering From Hypercapnic Acute Respiratory Failure: A Physiological Crossover Trial. Crit Care Med. 2019. Jun;47(6):e506–e511. doi: 10.1097/CCM.0000000000003740 [DOI] [PubMed] [Google Scholar]
- 34.Gattinoni L, Pelosi P, Crotti S, Valenza F. Effects of positive end expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med 1995;151(6):1807–1814. doi: 10.1164/ajrccm.151.6.7767524 [DOI] [PubMed] [Google Scholar]
- 35.Kim ES, Lee H, Kim SJ, Park J, Lee YJ, Park JS et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018. Feb;10(2):882–888. doi: 10.21037/jtd.2018.01.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Li Y, Ling B, Zhu Q, Hu Y, Tan D et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019. Jun 5;14:1229–1237. doi: 10.2147/COPD.S206567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortegiani A, Longhini F, Madotto F, Groff P, Scala R, Crimi C et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020. Dec 14;24(1):692. doi: 10.1186/s13054-020-03409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim SH et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018. Jun;12(6):2046–2056. doi: 10.1111/crj.12772 [DOI] [PubMed] [Google Scholar]
- 39.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated Humidified High-Flow Nasal Oxygen in Adults. Chest. 2015. Jul;148(1):253–261. [DOI] [PubMed] [Google Scholar]
- 40.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Peter PB et al. Changes in Forced Expiratory Volume in 1 Second over Time in COPD. N Engl J Med. 2011. Sep 29;365(13):1184–92. doi: 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 41.Traver A.U., Cline BB M.G. Predictors of mortality in chronic obstructive pulmonary disease. A 15-year follow-up study. Am Rev Respir Dis. 1979;119:895. doi: 10.1164/arrd.1979.119.6.895 [DOI] [PubMed] [Google Scholar]
- 42.van Geffen W.H., Kerstjens H.A.M. Static and Dynamic Hyperinflation During Severe Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2018. Apr 18;13:1269–1277. doi: 10.2147/COPD.S154878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Geffen W.H., Slebos D.J., Kerstjens H.A.M. Hyperinflation in COPD Exacerbations. Lancet Respir Med. 2015. Dec;3(12):e43–4. doi: 10.1016/S2213-2600(15)00459-2 [DOI] [PubMed] [Google Scholar]
- 44.Jeong JH, Kim DH, Kim SC, Kang C, Lee SH, Kang TS et al. Changes in arterial blood gases after use of high-flow nasal cannula therapy in the ED. Am J Emerg Med. 2015. Oct;33(10):1344–9. doi: 10.1016/j.ajem.2015.07.060 [DOI] [PubMed] [Google Scholar]
- 45.Bräunlich J, Köhler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016. May 25;11:1077–85. doi: 10.2147/COPD.S104616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pisani L, Fasano L, Corcione N, Comellini V, Musti MA, Brandao M et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. 2017. Apr;72(4):373–375. doi: 10.1136/thoraxjnl-2016-209673 [DOI] [PubMed] [Google Scholar]
- 47.Oczkowski S, Ergan B, Bos L, Chatwin M, Ferrer M, Gregoretti C et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022. Apr 14;59(4):2101574. doi: 10.1183/13993003.01574-2021 [DOI] [PubMed] [Google Scholar]
- 48.Crimi C, Noto A, Cortegiani A, Campisi R, Heffler E, Gregoretti C et al. High Flow Nasal Therapy Use in Patients with Acute Exacerbation of COPD and Bronchiectasis: A Feasibility Study. COPD. 2020. Apr;17(2):184–190. doi: 10.1080/15412555.2020.1728736 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study were made publicly available at https://www.ebi.ac.uk/biostudies/studies/S-BSST870.