Abstract
Background
Sleep-disordered breathing (SDB) is frequent in stroke patients and negatively affects stroke outcomes. Positive airway pressure (PAP) is the standard first-line treatment for patients with moderate-to-severe SDB. Despite a strong link between PAP adherence and therapeutic response, rates of post-stroke PAP adherence remain underexplored. Our study aimed to determine PAP adherence in patients undergoing comprehensive sleep apnea assessment and in-lab PAP titration in the early subacute phase of stroke.
Methods
In-hospital screening pulse oximetry was performed in consecutive patients with imaging-confirmed acute ischemic stroke. Subjects with desaturation index ≥ 15.3/h were selected as PAP candidates, and polysomnography was recommended. In a sleep laboratory setting, subjects underwent a diagnostic night followed by a titration night, and PAP therapy was initiated in subjects with apnea–hypopnea index ≥ 15/h. Adherence to PAP therapy was assessed at a 6-month follow-up visit.
Results
Of 225 consecutive patients with acute ischemic stroke, 116 were PAP candidates and 52 were able to undergo polysomnography. PAP therapy was initiated in 35 subjects. At a 6-month follow-up visit, out of 34 stroke survivors, PAP adherence (PAP use of > 4 h per night) was present in 47%. Except for the significantly lower minimal nocturnal O2 saturation determined from the polysomnography (74.6 ± 11.7% vs. 81.8 ± 5.2%, p = 0.025), no other significant difference in characteristics of the groups with PAP adherence and PAP non-adherence was found.
Conclusions
Less than half of the stroke subjects remained adherent to PAP therapy at 6 months post-PAP initiation. Special attention to support adaptation and adherence to PAP treatment is needed in this group of patients.
Keywords: Adherence, Ischemic stroke, Polysomnography, Positive airway pressure, Pulse oximetry, Sleep-disordered breathing
Introduction
Sleep-disordered breathing (SDB) is associated with the risk of serious vascular events and belongs to the frequent comorbidities of stroke patients. It is present in up to 71% of stroke subjects [1, 2]. The presence of obstructive sleep apnea can significantly worsen the prognosis of ischemic stroke [3]. In stroke patients, SDB is associated with increased morbidity, mortality, and worse functional outcomes [4, 5]. Positive airway pressure (PAP) is the standard first-line treatment for patients with moderate-to-severe SDB (defined by the apnea–hypopnea index [AHI] ≥ 15/h) and in subjects with good adherence is associated with decreased risk of cerebrovascular events [6]. Significantly better stroke outcomes (improvement in modified Rankin Scale [mRS]) and a nonsignificant decrease in recurrence of vascular events in stroke patients who used PAP treatment were found in a randomized controlled study. Acceptable PAP adherence was present in 62.5% [7]. On the other hand, another randomized controlled study of PAP in patients with SDB following ischemic stroke, with sufficient PAP adherence in 60%, found no benefit of PAP on the reduction of new vascular events [8]. A recent meta-analysis of randomized controlled trials also failed to find any evidence that PAP therapy improves vascular outcomes (including stroke). However, there is a strong link between PAP adherence and therapeutic response in long-term follow-up, so PAP adherence was suggested as one of the factors reducing the strength of the findings [9]. Nevertheless, post-stroke rates of PAP adherence remain unexplored. Colelli et al. revealed 52% adherence with PAP therapy after outpatient titration at a 6-month follow-up visit [10]. Based on the results of our previous study [11], this study aimed to determine PAP adherence in patients undergoing comprehensive sleep apnea assessment and in-lab PAP titration in the early subacute phase of stroke. The main difference between the current study and previously mentioned studies is the prospective selection of possible PAP candidates based on the results of routine sleep apnea assessment in stroke subjects [11].
Material and methods
Study population
As previously described, within the PRESS (pulse oximetric routine examination of sleep apnea in acute stroke) study, 297 consecutive acute stroke subjects hospitalized in the First Department of Neurology, Comenius University, Bratislava, underwent routine sleep apnea screening from March 2019 to February 2020 [11]. A subpopulation with imaging-confirmed acute ischemic stroke (using computerized tomography or magnetic resonance imaging) was chosen for further study. The National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale (mRS) were used to assess the stroke severity, and Barthel index was used for the assessment of functional independence [12, 13]. Additional recorded characteristics of the population included age, sex, and body mass index (BMI).
Sleep study and PAP titration
Based on a single-night pulse oximetry assessment (WristOx2 device, model 3150, Nonin Medical, Plymouth, USA) in the acute phase of stroke (within 7 days after the stroke onset), patients with acute ischemic stroke were identified as possible PAP candidates. PAP candidate was defined by a desaturation index ≥ 15.3/h (desaturation defined as a decrease of oxygen saturation > 3% lasting > 10 s). We used a cut-off point of 15.3/h that had a 90.5% sensitivity to predict stroke subjects with moderate-to-severe sleep apnea in our previous study [11]. Further diagnostic workup of PAP candidates included standard overnight polysomnography in a sleep laboratory setting. Polysomnography was performed within the early subacute phase of stroke (within 90 days after the stroke onset). Patients were excluded from the polysomnographic assessment in a case of severe disability (mRS 7 days after the stroke onset = 5), agitated confusion, dementia, non-cooperation with the examination, acute exacerbation of medical conditions that could affect the accuracy of polysomnography (exacerbation of cardiovascular, respiratory diseases, infections), or if they refused to participate (see Fig. 1). Alice 6 device (Philips Respironics, USA) was used for polysomnographic assessment in standard sleep laboratory settings. The scorers were blinded for the baseline characteristics of the subjects and standardized criteria were used for the scoring of respiratory events and sleep characteristics [14]. Hypopneas were defined as a reduction of airflow ≥ 30% lasting > 10 s with oxygen desaturation ≥ 3% or arousal and apneas as the reduction of airflow ≥ 90% (or the airflow cessation) lasting > 10 s. Apnea–hypopnea index (AHI) was calculated as an average number of apneas and hypopneas per hour of sleep. Hypopneas/apneas with preserved respiratory effort were considered as obstructive, events with absent respiratory effort as central. In cases, where both, central and obstructive events, were present, sleep apnea was considered as predominantly central if ≥ 50% of events were central [14]. Sleep work-up included also daytime sleepiness evaluation using the Epworth sleepiness scale (ESS), and sleep quality evaluation using the Pittsburgh sleep quality index (PSQI) [15, 16].
Fig. 1.
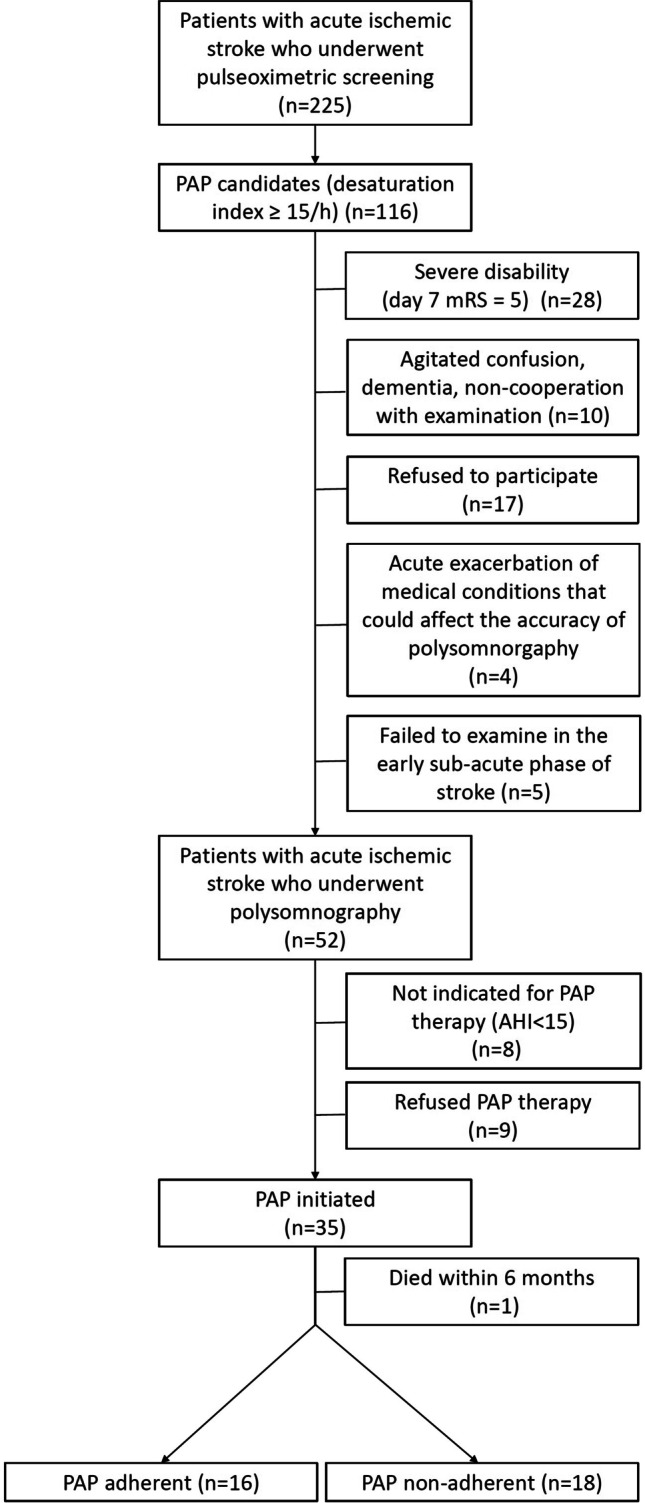
Flowchart: positive airway pressure ordination
Moderate-to-severe sleep apnea patients (AHI ≥ 15) were chosen to undergo PAP therapy, consistent with prior literature [17]. After the first (diagnostic) night, these subjects underwent titration night using automatic bilevel positive airway pressure (BiPAP) device. In predominantly central sleep apnea subjects with post-titration residual AHI ≥ 10, additional titration with adaptive support ventilation was performed. Principles of the PAP therapy were explained to the patients in detail by sleep technicians, and patients received fitting masks and appropriate PAP devices (CPAP with constant inspiratory positive airway pressure, or adaptive support ventilation in selected subjects with predominantly central sleep apnea) for home therapy.
Follow-up and outcome measures
Therapy adherence was checked 6 months after the PAP initiation. PAP adherence was determined from the data collected from PAP devices. Average usage over 6 months was assessed. The average usage of PAP higher than 4 h per night from therapy initiation to follow-up was considered as therapy adherence. The study aimed to search for predictors of PAP adherence at 6 months post-PAP initiation. Patient characteristics and demographic data including age, gender, and BMI were considered. The assessment included stroke characteristics (NIHSS, mRS, Barthel index) [12, 13], sleep apnea characteristics (type of sleep apnea, AHI, average, and minimal nocturnal O2 saturation), PAP settings, and therapy adherence data (type of PAP device, values of inspiratory/expiratory PAP on titration [IPAP/EPAP], residual AHI, time from stroke onset to PAP initiation) as well as other sleep characteristics (ESS, PSQI) [15, 16]. Social status (living alone/non-living alone) was also considered. During the period of follow-up, the sleep technician was available on call for the cases of adherence issues or PAP technical troubleshooting.
Statistics
Continuous variables were expressed as means ± standard deviation, or median, interquartile range, and range. Categorical variables were expressed as numbers and proportions (%). Chi-squared test, Student’s t test, and Mann–Whitney test were used for comparison of particular variables between groups with PAP adherence and PAP non-adherence. In multivariable binary logistic regression analysis, the odds ratios (OR) with the 95% CI were reported to declare the statistical significance and strength of association between PAP adherence and the independent variables. The model fitness was assessed by the Hosmer–Lemeshow test.
The dependent variable was PAP adherence and the independent variables were sociodemographic factors (age, sex, BMI, living alone status), stroke characteristics (NIHSS, mRS at baseline, on day 7, day 90, and Barthel index on day 7, and day 90), sleep apnea characteristics (AHI, moderate to severe sleep apnea, central/obstructive apnea, average nocturnal O2 saturation, and minimal nocturnal O2 saturation determined from the polysomnography), other sleep characteristics (ESS, PSQI), and PAP characteristics (device type, residual AHI, time from stroke onset to PAP initiation, IPAP, and EPAP on titration). P values < 0.05 were considered statistically significant. SPSS (version 18, SPSS Inc., Chicago, USA) was used for statistical analysis.
Ethics
The study was approved by the Ethics Committee of the Faculty of Medicine, Comenius University, and the University Hospital in Bratislava (Old Town Hospital). Informed consent was provided by all participants (or their next of kin) before the enrollment.
Results
Out of 225 patients with acute ischemic stroke, 116 were identified as possible PAP candidates according to pulse oximetric screening in the acute phase of stroke. In this population, 52 patients underwent overnight polysomnography in the early subacute phase of stroke. According to the polysomnographic findings, PAP therapy was initiated in 35 subjects. PAP adherence was checked 6 months after the PAP initiation in 34 stroke survivors. PAP adherence was observed in 47.1%, and PAP non-adherence in 52.9% of these subjects.
During the period of follow-up, adherence issues/PAP technical troubleshooting was reported in 4 out of 18 PAP non-adherent subjects. Pressure adjustment was performed in two subjects, mask exchange was required in one subject, and a repeated explanation of PAP therapy principles was needed in one subject. The discomfort was the most frequently reported reason for PAP non-adherence (6 subjects), followed by negative attitude/ “PAP uselessness” (5 subjects), psychological causes (2 subjects), and shift-work (1 subject). Four subjects could not specify the reason for non-adherence.
Except for the significantly lower minimal nocturnal O2 saturation (74.6 ± 11.7% vs. 81.8 ± 5.2%, p = 0.025), we failed to find any other significant difference in demographic, clinical, or sleep characteristics between the groups with PAP adherence and PAP non-adherence (see Table 1). In multivariable binary logistic regression analysis, minimal nocturnal O2 saturation determined from the polysomnography in the early subacute phase of stroke was the only significant factor associated with PAP adherence (OR: 0.873, 95% CI: 0.765–0.998, p = 0.046, R2 = 0.229; Hosmer–Lemeshow test: p = 0.569).
Table 1.
Characteristics of the population with PAP adherence vs. PAP non-adherence
| PAP adherent | PAP non-adherent | P | |
|---|---|---|---|
| N | 16 (47%) | 18 (53%) | |
| Age (years) | 69.0 ± 9.3 | 67.1 ± 11.3 | 0.601 |
| Males/females | 10/6 | 13/5 | 0.545 |
| BMI (kg/m2) | 30.9 ± 4.9 | 28.6 ± 4.2 | 0.157 |
| NIHSS baseline | 4.0, 5.5 (1.0–18.0) | 3.5, 4.5 (1.0–19.0) | 0.670 |
| NIHSS day 7 | 1.0, 2.75 (0–13.0) | 1.0, 1.5 (0–4.0) | 0.721 |
| NIHSS day 90 | 0, 1.0 (0–7.0) | 0, 1.25 (0–3) | 0.746 |
| mRS baseline | 2.5, 2.0 (1.0–5.0) | 2.0, 2.25 (1.0–5.0) | 0.175 |
| mRS day 7 | 1.5, 2.75 (0–4.0) | 1.0, 0.25 (0–3.0) | 0.597 |
| mRS day 90 | 0, 1.0 (0–4.0) | 0, 1.0 (0–2.0) | 0.905 |
| Barthel index day 7 | 100.0, 16.3 (40.0–100.0) | 95.0, 6.3 (80.0–100.0) | 0.881 |
| Barthel index day 90 | 100.0, 5.0 (85.0–100.0) | 100.0, 5.0 (90.0–100.0) | 0.636 |
| AHI (n/h) | 34.5, 22.0 (16.6–82.6) | 29.2, 17.2 (15.3–45.0) | 0.075 |
| Moderate SAS (15 ≤ AHI˂30) | 5 (31.25%) | 9 (50%) | 0.268 |
| Severe SAS (AHI ≥ 30) | 11 (68.75%) | 9 (50%) | 0.268 |
| Obstructive/central apnea | 12/4 (75/25%) | 9/9 (50/50%) | 0.134 |
| Stroke onset to PAP initiation (days) | 70.5 ± 17.2 | 64.9 ± 20.4 | 0.400 |
| CPAP/ASV | 12/4 (75/25%) | 14/4 (78/22%) | 0.849 |
| Residual AHI (n/h) | 5.6, 5.1 (1.4–45.1) | 5.8, 10.4 (0.5–36.7) | 0.726 |
| IPAP on titration (cmH2O) | 12.8 ± 2.4 | 11.6 ± 2.9 | 0.186 |
| EPAP on titration (cmH2O) | 9.0 ± 2.6 | 8.0 ± 2.7 | 0.295 |
| Average O2 saturation—polysomnography (%) | 87.6 ± 4.6 | 89.5 ± 2.5 | 0.130 |
| Minimal O2 saturation—polysomnography (%) | 74.6 ± 11.7 | 81.8 ± 5.2 | 0.025* |
| ESS | 3.0, 5.25 (0–9.0) | 4.0, 4.0 (0–10.0) | 0.384 |
| PSQI | 7.0, 6.0 (1.0–14.0) | 6.0, 5.0 (3.0–14.0) | 0.305 |
| Living alone | 4 (25%) | 5 (28%) | 0.855 |
PAP, positive airway pressure; BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; AHI, apnea–hypopnea index; SAS, sleep apnea syndrome; CPAP, continuous positive airway pressure; ASV, adaptive support ventilation; IPAP, inspiratory positive airway pressure; EPAP, expiratory positive airway pressure; ESS, Epworth sleepiness scale, *p ˂ 0.05
Additionally, pulse oximetry data were compared between PAP adherent and PAP non-adherent group. No significant differences were found in desaturation index (median: 28.4, range: 15.1–67.8 vs. median: 25.1, range: 15.2–65.1; p = 0.863), baseline oxygen saturation (92.1 ± 1.8% vs. 92.4 ± 1.6%; p = 0.606), and minimal oxygen saturation (76.0 ± 13.8% vs. 76.7 ± 10.3%; p = 0.873) between PAP adherent and PAP non-adherent subjects.
When comparing minimal oxygen saturation in pulse oximetry and polysomnography, a nonsignificant decrease was observed in the PAP adherent group 76.0 ± 13.8% vs. 74.6 ± 11.7%; p = 0.763). Contrary, nonsignificant increase was found in PAP non-adherent group (76.7 ± 10.3% vs. 81.8 ± 5.2%; p = 0.066).
Discussion
In a current study, out of 116 possible PAP candidates, selected by screening pulse oximetry, polysomnography was feasible in less than half (52 patients) of the population, and PAP therapy was initiated in 35 subjects with AHI ≥ 15. Despite early comprehensive sleep apnea work-up, detailed patient selection, and explanation of the PAP therapy principles by the sleep specialist, only 47% of patients remained adherent to PAP therapy at the 6-month post-PAP initiation (with average PAP use of > 4 h per night). Minimal nocturnal O2 saturation determined from the polysomnography (performed within the early subacute phase of stroke) was the only significant factor associated with PAP adherence in binary logistic regression analysis. Approaches applied to enhance PAP therapy adherence in the current study seem insufficient, and even greater effort could be needed to support acclimation and adherence to PAP treatment in this group of patients. Another possible reason for PAP non-adherence is the spontaneous improvement of sleep apnea in post-stroke patients. Future studies should consider reassessment of sleep apnea in PAP non-adherent post-stroke subjects.
Similar findings have been reported previously. In a recent study, 52% out of 88 post-stroke/transitory ischemic attack patients with obstructive sleep apnea were adherent to PAP therapy at the 6-month visit. The same definition of PAP adherence was used. Contrary to our study, either Apnea-Link or polysomnography was used for SDB assessment and outpatient PAP titration with Auto-CPAP was used. Better adherence was associated with less daytime fatigue and greater functional capacity [10]. In another study, PAP adherence, defined as PAP use ≥ 75% of nights for ≥ 4 h/night, was observed in 63% of stroke patients at 12-month follow-up. Diagnostic workup included polysomnography, and titration polysomnography was used before PAP initiation [7]. Despite the absence of head-to-head comparison, these findings suggest similar PAP adherence in subjects undergoing in-lab PAP titration and outpatient PAP titration with Auto-CPAP. Additionally, the use of less costly, technically and staff demanding diagnostic approaches, e.g., type III diagnostic devices, could help to select more patients feasible for PAP therapy. We supposed that the initial selection of possible PAP candidates based on the results of routine sleep apnea assessment will result in the selection of more severe stroke patients. However, stroke severity was similar in the study of Colelli et. al (median NIHSS: 1.0 on PAP initiation vs. median NIHSS: 1.0 7 days after stroke onset in our study) [10]. Comparison with other PAP adherence studies is limited by the absence of stroke severity assessment [7, 18]. Nevertheless, future prospective studies focusing on this topic are warranted. Future studies should also evaluate the optimal definition of PAP adherence because the “historical benchmark “ of 4 h of PAP use per night does not necessarily ameliorate SDB-related functional outcomes [19].
Our findings suggest that strategies to augment adherence to PAP therapy are needed in stroke patients. Despite good adherence of the patients with acute stroke to the pulse oximetric screening, reported in our previous study [11], a relatively high proportion of the PAP candidates (17 out of 116) refused to undergo further diagnostic and therapeutic workup. Similarly, a high proportion of the patients who underwent polysomnography and were indicated for PAP therapy (9 out of 45) refused PAP therapy immediately after titration polysomnography despite a detailed explanation of untreated SDB risks and therapy principles by a sleep specialist. Future strategies should consider predictors of PAP adherence. Minimal nocturnal O2 saturation determined from the polysomnography was the only significant factor associated with PAP adherence in this study. Another recent study revealed two significant predictors of PAP adherence: better functional status and not endorsing daytime tiredness post-stroke [10]. Matsuura et al. reported in stroke patients good adherence to PAP therapy after discharge when PAP was successfully introduced during hospitalization [18]. Similar to our results, the association of PAP adherence with more severe SDB has been described previously. This fact could be explained by a more significant amount of daytime symptoms and subsequent more significant benefits from the use of PAP resulting in the patient’s perception of the cost–benefit ratio [20]. The prognostic value of minimal nocturnal O2 saturation is questionable. Despite significantly lower values in PAP adherent vs. PAP non-adherent subjects within the early subacute phase of stroke (74.6 ± 11.7% vs. 81.8 ± 5.2%; p = 0.025), no significant difference was found in the acute phase of stroke (76.0 ± 13.8% vs. 76.7 ± 10.3%; p = 0.873). The optimal timing of the sleep apnea assessment should be elucidated by future prospective studies.
We are aware of the multiple limitations of our study. The open study design is the most important limitation of the current study, and our results must be confirmed by the results of future randomized controlled trials. The small sample size is another important limitation. We suppose that approaches applied to enhance PAP therapy in our patients were probably insufficient. Physicians and sleep technicians provided a detailed explanation of PAP therapy principles to the patients. Patients received an appropriate PAP device, a fitting mask, and troubleshooting of the most common issues related to the PAP therapy was performed on PAP therapy initiation. However, only the sleep technician was available on call for the cases of PAP-related issues during the period of follow-up, which could probably not provide the support that some patients needed. It is also necessary to admit that a follow-up visit was done 6 months post-PAP initiation only. This decision was made due to epidemiological reasons after the COVID-19 outbreak (most of the follow-up visits were done in 2020). We suppose that results might be different if there was a more intensive PAP education and follow-up. Consistent with prior literature, moderate-to-severe sleep apnea patients were chosen to undergo PAP therapy [17]. However, data from the general population suggest beneficial effects of PAP also in subjects with mild sleep apnea (AHI ≥ 5 to < 15 events per h) [21]. The use of these criteria for PAP therapy could help to expand the PAP-treated population in future prospective studies. We admit that absence of an extensive search for PAP adherence predictors belongs to the important limitations of the current study. A detailed search for PAP settings and therapy adherence data including reasons for PAP therapy interruption should be considered in future prospective studies focused on PAP adherence in stroke subjects. Additional important factors including psychological variables, smoking status, socioeconomic status, or social support should be also included in future studies [22]. Future studies should consider also the management of well-known factors associated with non-compliance to treatment with PAP including discomfort from the mask, aerophagia, eye irritation from the pressurized air, or psychosocial issues including non-compliant personality, claustrophobia, and alcohol or drug abuse [23]. Discomfort and negative attitude to PAP therapy were the most frequent issues among PAP non-adherent subjects in our study. An additional promising approach to enhance PAP adherence is the implementation of PAP adherence programs (like SCOUTS intensive CPAP adherence program) focused on device adjustments, mask interface changes, SDB education, and encouragement [24]. The type of PAP device used for home therapy can represent an additional limitation. CPAP with constant inspiratory positive airway pressure, the first-line therapy choice covered by health insurance in regional settings, was used for therapy of OSA in the current study. We suppose that adherence might be enhanced by the therapeutic use of more sophisticated devices including Auto-CPAP, as described by Colelli et al. [10].
There is one more possible reason for PAP non-adherence. Spontaneous improvement of obstructive sleep apnea in stroke patients was described previously [25]. In a current study, a higher frequency of moderate sleep apnea and a lower frequency of severe sleep apnea was observed in the non-adherent group than in the adherent group. Similarly, when comparing minimal oxygen saturation in pulse oximetry (performed within the acute phase of stroke) and polysomnography (performed within the early subacute phase of stroke), a nonsignificant decrease was observed in PAP adherent group. On the contrary, a nonsignificant increase was found in PAP non-adherent group. This finding could suggest improvement of sleep apnea in the non-adherent group. Future studies should consider reassessment of sleep apnea in PAP nonadherent post-stroke subjects.
Conclusions
Despite early, complex in-hospital sleep apnea diagnostic work-up, PAP titration, and education of the patients, less than half of the subjects remained adherent to PAP therapy at 6 months post-PAP initiation. Approaches applied to enhance PAP therapy adherence in the current study seem insufficient. Strategies to augment adherence to PAP therapy in stroke patients with sleep apnea are warranted. Spontaneous improvement of sleep apnea in post-stroke patients is another possible reason for PAP non-adherence.
Abbreviations
- AHI
Apnea–hypopnea index
- BMI
Body mass index
- CPAP
Continuous positive airway pressure
- ESS
Epworth sleepiness scale
- EPAP
Expiratory positive airway pressure
- IPAP
Inspiratory positive airway pressure
- mRS
Modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- SDB
Sleep-disordered breathing
- PAP
Positive airway pressure
- PSQI
Pittsburgh sleep quality index
Author contribution
All authors have seen and approved the manuscript.
Funding
This study was supported by the Framework Programme for Research and Technology Development, Project: Building of Centre of Excellency for Sudden Cerebral Vascular Events, Comenius University Faculty of Medicine in Bratislava (ITMS:26240120023), co-financed by the European Regional Development Fund.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the Faculty of Medicine, Comenius University, and the University Hospital in Bratislava (Old Town Hospital).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pavel Šiarnik, Email: palo.siarnik@gmail.com.
Matúš Jurík, Email: jurik25@uniba.sk.
Katarína Valovičová, Email: valovicova.katarina@gmail.com.
Katarína Klobučníková, Email: klobucnikova@gmail.com.
Branislav Kollár, Email: branislavkollarmd@gmail.com.
Michal Poddaný, Email: michal.poddany@gmail.com.
Marek Rovňák, Email: rovnak.marek@gmail.com.
Peter Turčáni, Email: peter.turcani@sm.unb.sk.
Marek Sýkora, Email: marek.sykora@med.sfu.ac.at.
References
- 1.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. doi: 10.1161/CIRCOUTCOMES.1111.964783. [DOI] [PubMed] [Google Scholar]
- 2.Seiler A, Camilo M, Korostovtseva L, Haynes AG, Brill AK, Horvath T, Egger M, Bassetti CL. Prevalence of sleep-disordered breathing after stroke and TIA: A meta-analysis. Neurology. 2019;92(7):e648–e654. doi: 10.1212/wnl.0000000000006904. [DOI] [PubMed] [Google Scholar]
- 3.Devaraj NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24(4):1581–1590. doi: 10.1007/s11325-020-02040-1. [DOI] [PubMed] [Google Scholar]
- 4.Hermann DM, Bassetti CL. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology. 2016;87(13):1407–1416. doi: 10.1212/WNL.0000000000003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax. 2004;59(5):367–371. doi: 10.1136/thx.2003.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Eng J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Shukla G, Afsar M, Poornima S, Pandey RM, Goyal V, Srivastava A, Vibha D, Behari M. Role of positive airway pressure therapy for obstructive sleep apnea in patients with stroke: a randomized controlled trial. J Clin Sleep Med. 2018;14(4):511–521. doi: 10.5664/jcsm.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernasconi C, Ott SR, Fanfulla F, Miano S, Horvath T, Seiler A, Cereda CW, Brill AK, Young P, Nobili L, Manconi M, Bassetti CLA. SAS CARE 2 - a randomized study of CPAP in patients with obstructive sleep disordered breathing following ischemic stroke or transient ischemic attack. Sleep Med: X. 2020;2:100027. doi: 10.1016/j.sleepx.2020.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labarca G, Dreyse J, Drake L, Jorquera J, Barbe F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: Systematic review and meta-analysis. Sleep Med Rev. 2020;52:101312. doi: 10.1016/j.smrv.2020.101312. [DOI] [PubMed] [Google Scholar]
- 10.Colelli DR, Kamra M, Rajendram P, Murray BJ, Boulos MI. Predictors of CPAP adherence following stroke and transient ischemic attack. Sleep Med. 2020;66:243–249. doi: 10.1016/j.sleep.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Šiarnik P, Jurík M, Veverka J, Klobučníková K, Kollár B, Turčáni P, Sýkora M. Pulse oximetric routine examination of sleep apnea in acute stroke (PRESS) Sleep Med. 2020;73:208–212. doi: 10.1016/j.sleep.2020.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 13.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. doi: 10.1161/01.str.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 14.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus C, Vaughn BV (2012) The AASM manual for the scoring of sleep and associated events. Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine, Darien
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42(4):1062–1067. doi: 10.1161/strokeaha.110.597468. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura D, Otaka Y, Kamigaichi R, Honaga K, Kondo K, Liu M. Prevalence, effect on functional outcome, and treatment of sleep-disordered breathing in patients with subacute stroke. J Sleep Sleep Med. 2019;15(6):891–897. doi: 10.5664/jcsm.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin HS, Prasad AS, Pan CJ, Rowley JA. Factors associated with noncompliance to treatment with positive airway pressure. Arch Otolaryngol Head Neck Surg. 2007;133(1):69–72. doi: 10.1001/archotol.133.1.69. [DOI] [PubMed] [Google Scholar]
- 21.Wimms AJ, Kelly JL, Turnbull CD, McMillan A, Craig SE, O'Reilly JF, Nickol AH, Hedley EL, Decker MD, Willes LA, Calverley PMA, Benjafield AV, Stradling JR, Morrell MJ. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(4):349–358. doi: 10.1016/s2213-2600(19)30402-3. [DOI] [PubMed] [Google Scholar]
- 22.Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung. 2019;197(2):115–121. doi: 10.1007/s00408-018-00193-1. [DOI] [PubMed] [Google Scholar]
- 23.Pépin JL, Leger P, Veale D, Langevin B, Robert D, Lévy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest. 1995;107(2):375–381. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 24.Khot S, Barnett H, Davis A, Siv J, Crane D, Kunze A, Li Lue D, Bunnell A, McCann B, Bombardier C, Longstreth WT, Jr, Watson N, Billings M. Intensive continuous positive airway pressure adherence program during stroke rehabilitation. Stroke. 2019;50(7):1895–1897. doi: 10.1161/strokeaha.119.024795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slonkova J, Bar M, Nilius P, Berankova D, Salounova D, Sonka K. Spontaneous improvement in both obstructive sleep apnea and cognitive impairment after stroke. Sleep Med. 2017;32:137–142. doi: 10.1016/j.sleep.2016.1011.1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


