Abstract
Microelectrode arrays provide the means to record electrophysiological activity critical to brain research. Despite its fundamental role, there are no means to customize electrode layouts to address specific experimental or clinical needs. Moreover, current electrodes demonstrate substantial limitations in coverage, fragility, and expense. Using a 3D nanoparticle printing approach that overcomes these limitations, we demonstrate the first in vivo recordings from electrodes that make use of the flexibility of the 3D printing process. The customizable and physically robust 3D multi-electrode devices feature high electrode densities (2600 channels/cm2 of footprint) with minimal gross tissue damage and excellent signal-to-noise ratio. This fabrication methodology also allows flexible reconfiguration consisting of different individual shank lengths and layouts, with low overall channel impedances. This is achieved, in part, via custom 3D printed multilayer circuit boards, a fabrication advancement itself that can support several biomedical device possibilities. This effective device design enables both targeted and large-scale recording of electrical signals throughout the brain.
3D nanoprinted electrodes enable targeted, experiment-specific recording of neural activity throughout the brain.
INTRODUCTION
Three-dimensional (3D) microelectrode arrays (MEAs) consisting of insulated, electrically conducting shanks are critical for a wide range of biological and biomedical applications (1, 2). They form the cornerstone of neuroscience and neuroengineering and serve as the basis for the transformational field of brain-computer interfaces. An MEA’s success is chiefly dependent on its sampling ability, a function of both the electrode density and ability to optimally target the regions of interest. While current fabrication methods have achieved substantial advances in recording density (3, 4) through developments in micro-electromechanical systems fabrication techniques (5–9), these silicon arrays are limited in their volumetric electrode densities, are difficult to customize, and are implemented with notoriously brittle silicon (Si) materials. Similarly, “Utah arrays,” which provide greater lateral sampling, are fabricated from metallized silicon using techniques such as dicing, etching, and photo or electron-beam lithography. Despite notable success of this approach over the past two decades, it is difficult to customize such probes, as their shank height variation is limited to uniformly varying lengths (i.e., on one plane). In addition, the pitch (distance between adjacent shanks) for such an array construction is limited by the kerf width of the dicing saw in the Si manufacturing process, limiting the recording density to a few hundred sites per square centimeter (5, 10), with a pitch of 400 μm, although efforts at enabling higher densities have also been successful (11–12). In another approach, multiple, high-density in-plane electrodes can be fabricated on a single silicon or printed circuit board (PCB) shank (13). By stacking multiple such shanks, a 2D or 3D device can be built with 400- to 700-μm pitch, but the distance between the stacked shanks is limited by assembly and bonding space requirements (14, 15). Recently, complementary metal-oxide semiconductor technology has enabled silicon-based Neuropixel probes, but the available probes are limited to what can be reached by a single linear shank or a few stacked shanks (16, 17).
The next generation of tools for electrophysiological recording must overcome the limiting factors above (18). In addition, advances in the rapidly expanding field of neuroscience would greatly benefit from tailored probes specific to the study or patient. We note that the fabrication methods for MEAs have followed the trends in the semiconductor industry, moving from microwires to lithography. Recently, 3D nanoparticle printing has emerged as a new method to fabricate electronic devices that either complements lithography or fills in the critical length-scale gaps left by current methods (19). Microelectronics fabrication by inkjet (20) and aerosol jet (AJ) (21) nanoparticle printing offers the freedom to use the target material in nanodispersion form. This process enables rapid changes to the device layouts and the use of a variety of device substrates, including flexible polymers. Flexible substrates can be used to create electrodes for uneven or moving surfaces, ideal for nerve-on-a-chip platforms (22) or interfacing with cardiac tissue. The AJ printing method can even print highly complex 3D metallic lattices and spirals without any support materials (23).
To this end, we developed a 3D nanoparticle printing system to open up a previously unexplored design space for 3D bioelectronic devices. The objective was to exploit the rapid customization offered by 3D printing to demonstrate a new class of MEAs having arbitrary variation in shank heights, diameters, and routing, and with a high density. We demonstrate that the multiscale, bottom-up printing method is able to assemble metal particles into 3D shanks with diameters of tens of micrometers and a length of several millimeters. Moreover, the printing method yields a notable cost and production time reduction, the latter on the order of a few hours, plus batch processes. Furthermore, we show that the high prototypability and reduction in the shank diameter leads to densities up to 2600 shanks/cm2 (i.e., shank-to-shank pitch of 200 μm) with arbitrary shank lengths, a major improvement over current methods (10). With these arrays, physiologists can optimize the MEA to target neural architectures spanning the brain, layer-specific cortical ensembles, or both, simultaneously.
RESULTS
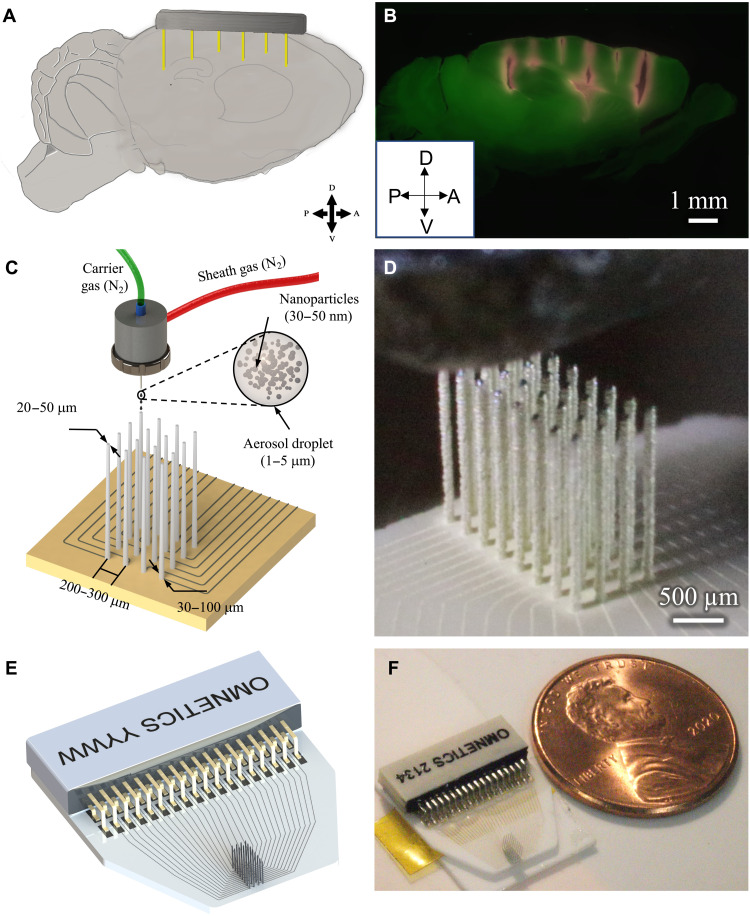
The drive to understand fundamental neural processes has created the need to monitor the spiking activity across select areas distributed throughout the brain [e.g., Fig. 1 (A and B)]. Even in more restricted studies, there is considerable benefit to study-specific electrodes; although, currently, the temporal and financial costs to prototyping electrodes all but preclude this approach. We demonstrate a novel electrode production technique, AJ conformal printing, that enables the rapid construction of fully customizable, acute-use “CMU array” microelectrodes as discussed below. In AJ printing, a 3D nanoparticle printing technique, a metal ink is atomized using ultrasonic energy to create an aerosol consisting of microdroplets. Each microdroplet carries metal particles from the ink. The aerosol is driven to a nozzle using an inert gas, while a sheath gas focuses the aerosol droplets onto the substrate at a resolution of about 10 μm (21). This 3D nanoparticle printing method can construct the protruding shanks (Fig. 1, C and D) required for the creation of MEAs that can record throughout a tissue volume and the 2D routing [also shown in Fig. 1 (C and D)] to carry the signal to connectors. The process of forming the three-dimensional structure involves stacking the metal nanoparticle–containing droplets on top of each other to form the high–aspect ratio shanks. Solvent evaporation with heating allows rapid solidification of the droplets once they approach the substrate. The solidified droplets then form the base for the subsequent droplets, leading to the formation of a 3D shank. The process is repeated at each shank location (see Materials and Methods for details of the manufacturing process and movie S1 for the shank printing process). Droplet dispense is controlled by a shutter that breaks and restarts the flow within 4 ms, enabling rapid printing of the array. The robust construction enables long, narrow shanks (aspect ratio at or above 50:1) that can then be targeted to the areas of interest (Fig. 1B). A schematic and an optical image of a 32-channel device used for recording in data presented later here are shown in Fig. 1 (E and F, respectively). The device consists of the shank array and the routing from individual shanks to the pads soldered to the Omnetics connector. Movies S2 and S3 show the printing process for the 2D routing and the 3D shanks, respectively, for the device shown in Fig. 1 (E and F).
Fig. 1. Example use-case and printing process.
(A) To understand circuit interactions, the activity of multiple brain areas must be recorded. The target brain areas will differ for any given study. In this conceptual schematic, the target areas to hit are (from left to right) mouse prefrontal cortex, motor cortex, caudal striatum, and hippocampal CA2 and CA3. (B) Slice of an actual mouse brain demonstrating the targeting of the brain areas mentioned in (A). The sagittal slice shows penetration of several 3D printed shanks (red) of a single probe into cortex, striatum, and hippocampus. Penetrations were parallel; appearance of shank trajectories are due to tissue dehydration in histological processing. (C) Schematic of AJ 3D printing of metal nanoparticles rapidly creates structurally robust, fully customizable neural probes, including the circuit board for routing of physiological signals. (D) Image during printing of a 32-channel probe. Also see movies S1, S2, and S3 for the printing process. (E) Schematic design of a 32-channel probe with routing and attached connector. (F) Probe printed on the basis of the design shown in (E) with a U.S. one cent coin for scale.
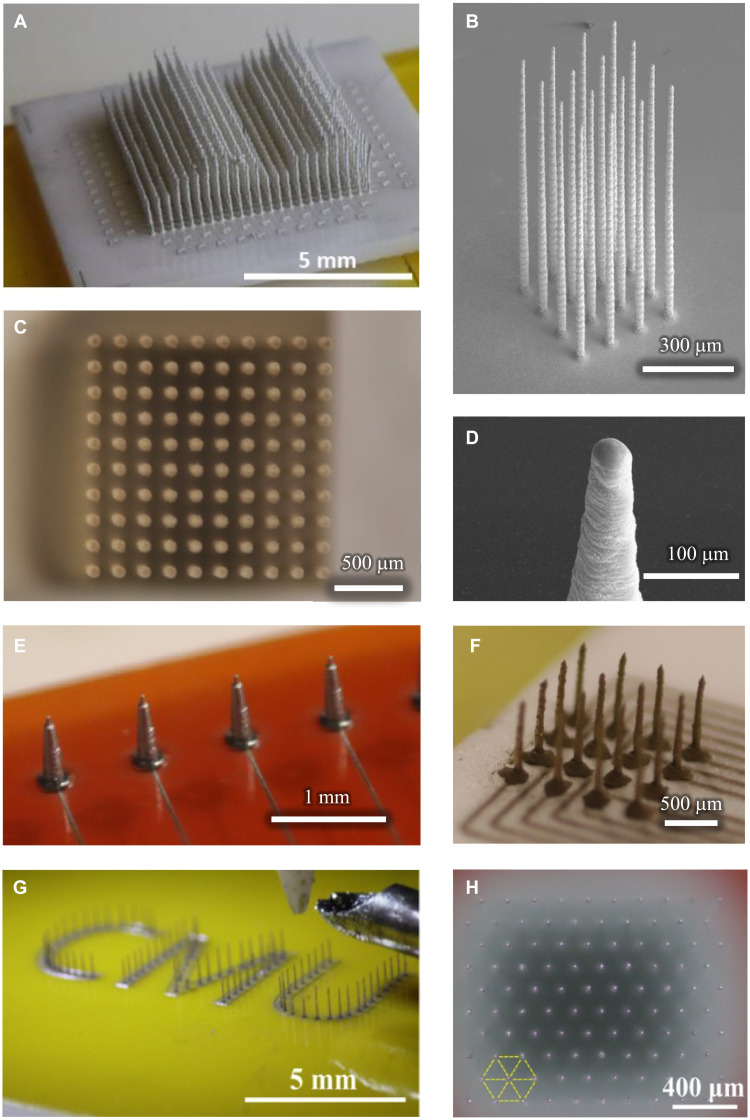
Because of the ease of customization afforded by computer-aided design (CAD), customized construction of individual shank height and location, along with electrical routing for each shank of the CMU array, requires minimal skill and effort. Specifics about the design space within which production and customization are possible are given in Table 1. In addition, different materials such as gold or silver can be printed to make the shanks. The shanks of varying heights within the same probe (Fig. 2A) allow for recording at different depths within tissue. This large 512-shank array at a density of 1600 shanks/cm2 (pitch of 250 μm) was built with variable shank heights of 1.0, 1.5, and 2.0 mm, demonstrating the potential for the production method (see movie S4 for a time-lapse video of the construction of this probe). Figure 2B demonstrates a series of printed shanks on an alumina substrate, each with a tip diameter of 10 μm, a base diameter of 30 μm, and an overall length of 1 mm. The average aspect ratio along the length of these shanks is 50:1. Despite the density and flexibility, fabrication processes create highly uniform shanks. Across all shanks here, the angle from vertical never exceeded 1°, evidenced well in the top-down view of a 10 × 10, 2600 shank/cm2 array in Fig. 2C. The AJ printing process also enables simple, CAD-driven control of the tip shape and profile (Fig. 2D), vital to successful insertion of high-density arrays. Because printing can be done on any substrate, a wide range of rigid and flexible substrates can be used for the construction of the probe. Figure 2E demonstrates an array printed on a flexible Kapton (polyimide) polymer substrate, enabling high-density, custom probes designed for potential use on curved or moving tissue, such as the heart. Also demonstrated here are conductive leads that can be printed onto the Kapton substrate to route electrical signals out to the recording device. Figure 2F shows shanks printed with gold. Printing enables unprecedented flexibility in the design and arrangement, as evidenced by shanks bearing “CMU,” the acronym of Carnegie Mellon University (Fig. 2G). To increase packing density by 15% over the traditional square array, the shanks can be arranged in a hexagonal pattern (Fig. 2H). Note that it is difficult if not impossible to create a hexagonal array pattern in traditional Utah arrays because of the limitations imposed by its manufacturing process.
Table 1. Manufacturing capability and current limitations for 3D printed CMU array presented here.
Process capabilities and limitations of the 3D nanoprinting process for neural probes developed in this work. PET, polyethylene terephthalate; PTFE, polytetrafluoroethylene.
| Parameter | Capabilities | Limitations |
| Pitch (density) | 200 μm min (2600 shanks/cm2) | Overspray, distortion of very narrow pillars. Overspray removal by PFIB can reduce the pitch down to 125 or 100 μm (i.e., shank densities of 6400–10,000/cm2); however, bed-of-nails effect may become a limiting factor as density increases. |
| Shank diameter | 20–150 μm; shank tip diameter can be reduced to 10 μm. |
Distortion during sintering. High-temperature sintering for longer shanks can help reduce the diameter further. |
| Shank length | 0.1–3 mm | Further process optimization will lead to straight shank longer than 3–5 mm in length. Higher susceptibility to buckling failure is expected for high shank length–to–diameter ratios. |
| Shank count | 512 demonstrated. More shank counts possible. |
Shank footprint and size of connectors relative to animal being studied, time in postprocessing |
| Substrate material | Polyimide, alumina, glass, and PCB | Sintering temperature, adhesion. Flash photonic sintering or spot laser sintering can enable substrates such as PET and PTFE. |
Fig. 2. CMU array fabrication using 3D nanoparticle printing.
(A) A 512-electrode shank array with variable shank heights demonstrating the customization possible by this method. Shank lengths are stepped to demonstrate regularity; individual shank lengths can be designated independently and to any value. A time-lapse video of the construction of this probe is shown in movie S2. (B) Printed platform with 1-mm-long shanks having an aspect ratio of 1:50. The substrate is alumina ceramic. It only takes under 1.5 min to create a 1-mm-long shank with this method. (C) A printed 10 × 10 array with a height of 2.4 mm and average width of 30 μm reveals straight and uniform shanks when viewed top-down. A similar geometry is used through the manuscript. (D) Close-up image of tapered shank tip, which facilitates their insertion into tissue. (E) Example of shanks directly printed on a flexible polyimide (Kapton) substrate. Printed wiring on the same substrate can enable connection to data acquisition hardware. (F) Example of shanks printed with gold nanoparticles, here demonstrating a wider base for increased robustness. Note that to demonstrate the diversity of geometries, the other panels in this figure are printed with less expensive silver ink. (G) Shank customization in any arrangement is possible: Here, shanks are printed to resemble the Carnegie Mellon University abbreviation “CMU.” (H) High-density, high–aspect ratio array printed in hexagonally packed pattern with 200-μm pitch (view from above). A 15% improvement in areal electrode density can be achieved by pattering the electrodes in hexagonal pattern rather than square packing.
Multilayer, multimaterial custom routing of signals
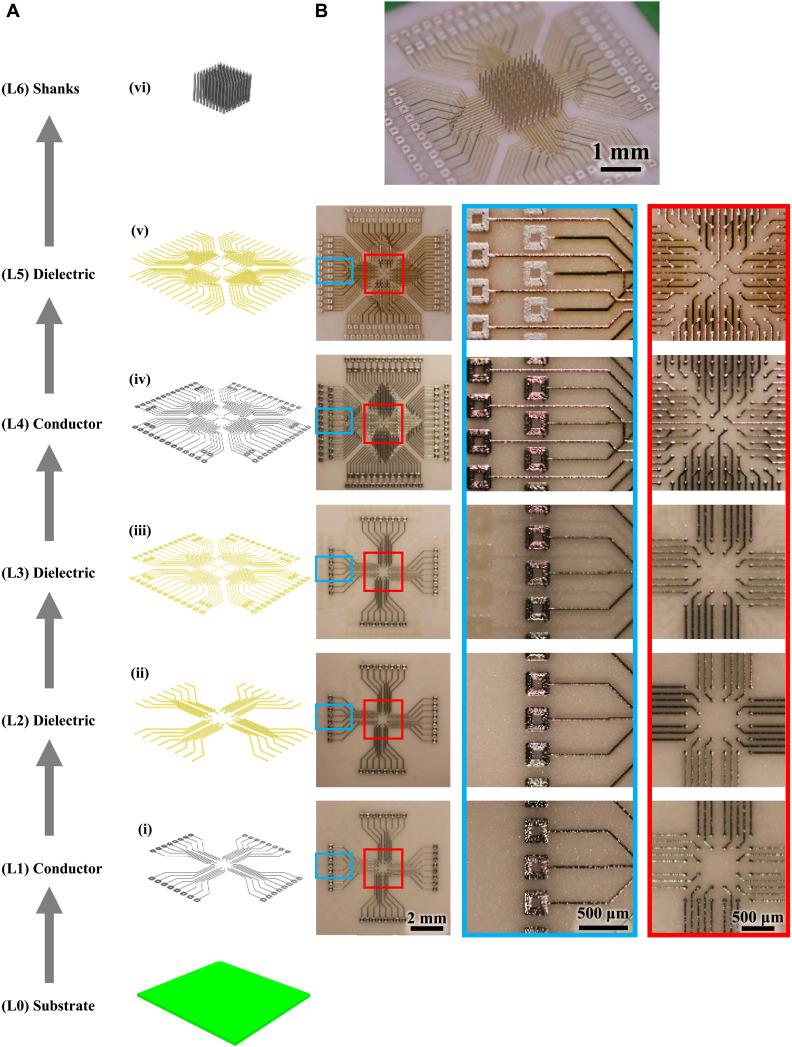
Although we are able to 3D print electrodes for acute, in vivo use, we required a creative, customizable back-end connectivity solution to fully realize the high-density applications. As the high-density shank arrangement leaves insufficient room for traditional electrical leads, routing out the electric signals required an innovative solution. Again, the flexibility that 3D printing affords provided a novel solution for high-density routing. We developed a multilayered, multimaterial printing method to route the electrical signal to the appropriate recording devices (Fig. 3). In the first step, a conductive layer of silver (layer L1 in Fig. 3A) was printed on an alumina substrate (L0) as shown in Fig. 3B (i) and sintered in an oven. The thickness of the silver as measured at five random locations was 4.14 ± 0.15 μm. A thin layer of liquid polyimide polymer (L2) was then printed, using the same AJ printing method, on top of the silver layer to form an insulating layer as shown in Fig. 3B (ii), leaving the ends of the leads exposed for future connections. The polyimide was heated to facilitate polymerization, completing the insulating layer L2. The thickness of the polyimide and silver together was 4.71 ± 0.15 μm. This process can be repeated as many times as desired, with a final polymer layer printed on the topmost metal layer [Fig. 3B (iii to v); note the underlying layers faintly visible under each layer]. The overall height of this multilayered circuit board (L1-L5) was 10.73 ± 0.29 μm. Note that the thickness of the printed layers of a current state-of-the-art two-layer PCB made by lithography and lamination is about 100 μm (24). Figure 3B (vi) shows shanks printed on the pads to create a 100-shank probe within an area of 2 mm by 2 mm [see fig. S1 for backscatter electron image of the probe in Fig. 3B (vi) to highlight 3D metal geometries]. Note that a linewidth of 20 μm and spacing of 100 μm are shown in the device for the planar 2D printing. In literature, a line/space of 10/30 μm via AJ printing was demonstrated by Cai et al. (25). The line/spacing obtained by the AJ printing are comparable to the state-of-the-art PCB/high-density interconnect technologies, which is about 10/10 μm, especially upon overspray removal (e.g., see next section) (24). The circuit thickness of the printed structures, however, represents an order of magnitude improvement over that by lithography-based processes used to make the PCBs (24). The primary advantage offered by the printing of multilayer board is the ability to change circuit/layout design on the fly that will enable customized study-specific wiring for probes. Furthermore, printing allows the flexibility in the materials to be used for the pads on which the 3D shanks are printed, an advantage not offered by lithography-based processes. We further exploit the flexibility offered by the nanoparticle printing process, and we have also explored the printing of the shanks directly onto commercial PCBs, as shown in fig. S2, enabling additional flexibility in the construction of the bioelectrodes. Note that the micropositioning accuracy for printing was within ±1 μm.
Fig. 3. Electrical routing of the high-density shanks.
Multilayer metal-polymer printing to fabricate a custom board to route signals from high-density MEA. (A) Schematic of the step-by-step process of printing the multilayer board shown via an exploded view and (B) optical images of the printed board. The layers are indicated as (i) to (vi) from bottom-up. (i) Printing of first metal layer on alumina ceramic with pads at both ends of each line. (ii) Polyimide printing to insulate the metal lines except at the pads. (iii) Middle polyimide layer to isolate the layer 4 from layer 1. (iv) Printing of the upper metal layer to create two conductive layers routing the signal out. (v) Polymer insulation printed on the upper metal layer except at the pads on the two ends. Note that the signal routing can be customized by simple changes to the printing program, allowing arbitrary locations and heights for the probes and pads. (vi) Printing of shanks on open pads via the AJ printing process to give a 100-shank probe over an area of 2 mm by 2 mm with printed multilayer wiring/routing.
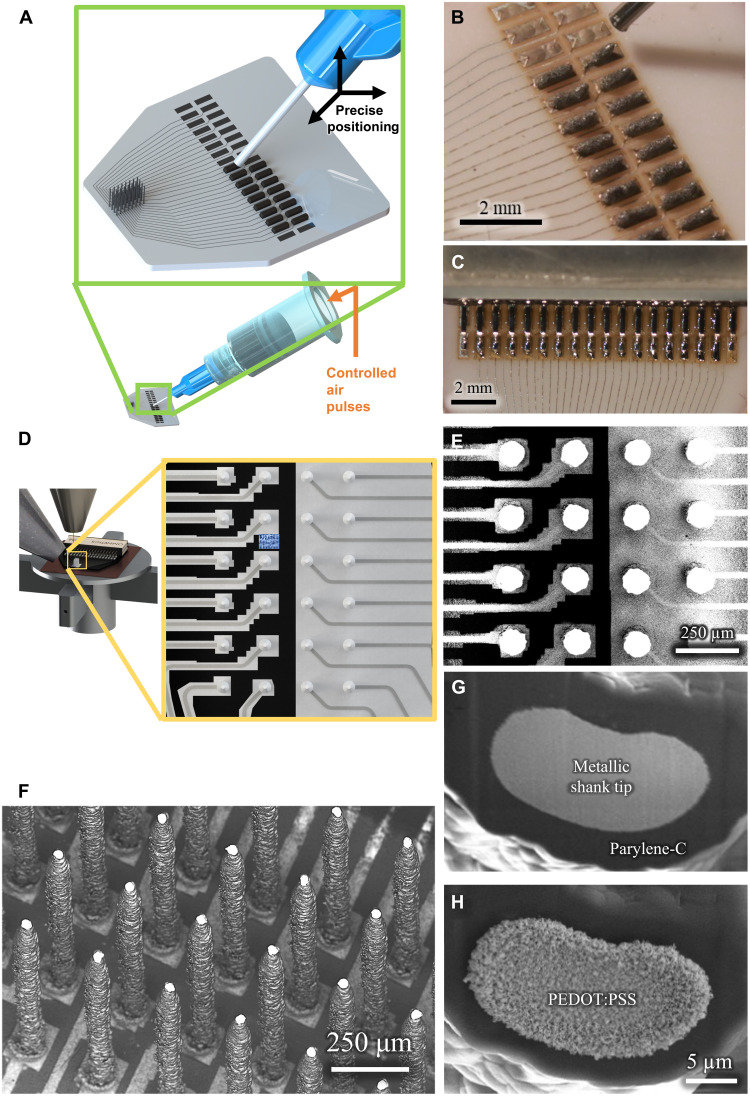
The next step is to route the signals from the printed and sintered pads to the leads of a connector (such as an Omnetics connector shown in Fig. 1F). Figure 4 (A and B) shows a schematic of the solder dispenser and the dispensed solder paste on the pads, respectively, for the 3D printed recording device shown in Fig. 1F. Figure 4C shows the melted solder via the thermal reflow process (see Materials and Method). Movie S5 shows the solder dispense and reflow processes for the recording device shown in Fig. 1F. These results establish that AJ printing offers enormous flexibility in routing the signals from the 3D printed neural probes with an easy interfacing for end users.
Fig. 4. Functionalization of the 3D printed array.
(A and B) A schematic and a picture of the solder deposition process to attach standard connectors to the 3D printed probe. (C) A picture of soldered joint after reflow. (D and E) A schematic and SEM of the removal of overspray using PFIB etching. The black color represents regions where the substrate is visible, i.e., the overspray has been removed or was never present. (F) Exposure of the tip using PFIB after parylene-C coating, thus enabling the probe to record neural voltages, through the exposed tip area only. (G and H) SEM before and after selective deposition of PEDOT:PSS at the tip using electroplating for reduced impedance.
Characterization of electrical and mechanical performance
Having achieved flexibility in shank diameter, placement, individual length, and routing, we next looked to functionalize the probe. Overspray, the satellite accumulation of nanoparticles outside the desired deposition area, can be removed readily by a CAD-controlled process involving rapid plasma focused ion beam (PFIB) machining. Figure 4 (D and E) shows a schematic and an actual image of the overspray removal using PFIB, respectively, for the 3D printed recording device shown in Fig. 1F (also see Materials and Methods). A video of a manual process demonstrating overspray removal is given in movie S6. The shanks are then coated with a 5-μm insulating layer of biocompatible parylene-C polymer in a vacuum chamber using a standard chemical vapor deposition process. The insulation was then removed selectively at the tip of the electrodes, also using PFIB, exposing a customizable area in the range of hundreds of square micrometers, yielding one site per shank (Fig. 4, F and G). We improved the interface impedance of the electrodes (see electrical characterization below) by coating the tips of the shanks with poly(3,4-ethylenedioxythiophene (PEDOT):polysodium styrene sulfonate (PSS), an electrically conductive polymer (Fig. 4H).
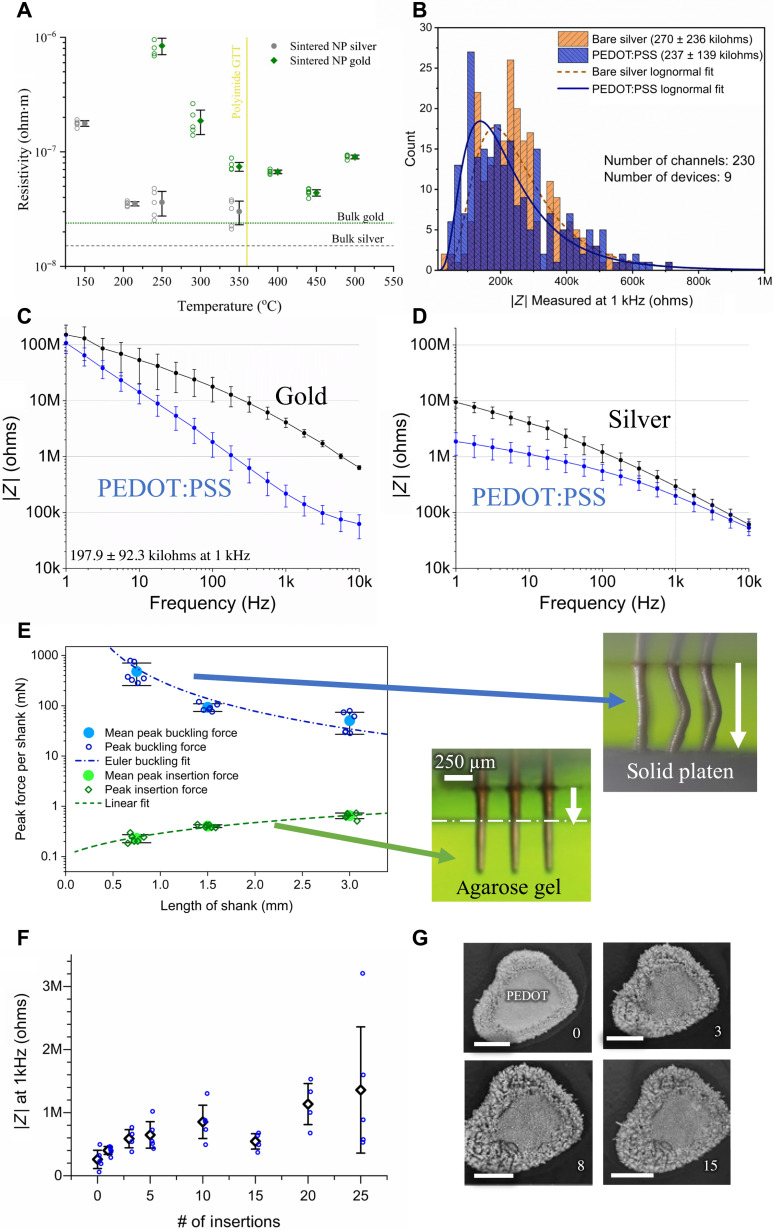
The resistivity of the printed lines was measured at different sintering conditions as shown in Fig. 5A. The resistivity for gold lines sintered at 300°C was 1.87 × 10−7 ohm·m and that for silver lines sintered at 250°C was 2.6 × 10−8 ohm·m, which are low and within a factor of two of the respective bulk properties. The resistivity can be reduced by sintering at temperatures higher than 300°C. This process, however, gives diminishing returns in terms of resistivity reduction (Fig. 5A) and limits the substrate options. Note that the resistance of the printed lines of a representative device (Fig. 1F) as a function of routing length measured by four-wire method is shown in fig. S3. As expected, the resistance scales linearly with the line length and is on the order of a few ohms.
Fig. 5. Electrical, electrochemical, and mechanical performance of the 3D printed probes.
(A) Electrical resistivity for printed gold and silver devices sintered at different temperatures. The resistivity is within one to two orders of magnitude of that for bulk metal for the sintering conditions used here (GTT, glass transition temperature). NP, nanoparticle. (B) Electrical impedance for 230 shanks across nine devices measured at 1-kHz frequency. All the 230 channels were electrically isolated after fabrication and had impedances below 1 megohm at 1-kHz frequency. (C and D) Impedance data for gold and silver electrodes, respectively, with (blue) and without (black) PEDOT:PSS-coated tips. The error bars represent the SDs for five different channels. In each case, PEDOT:PSS deposition reduced impedance to a range of about 100 to 250 kilohms at 1-kHz frequency. Impedance plots of individual gold and silver shanks with and without PEDOT:PSS coating are given in fig. S5 (A to E and F to J, respectively). (E) Measured peak force (per shank) to insert the probes of various lengths into agarose brain phantom and to buckle the probes when compressed under a solid platen. In each case, the insertion force is orders of magnitude smaller than the force in compression required for buckling. (F) Effect of repeated acute insertions of the silver shanks into phantom brain (agarose) on the impedance. The blue data points represent the average of the three repeat measurements taken after a given number of insertions. The average and SD of the impedance of the blue data points is also plotted. (G) Representative SEM images of a shank tip in (F) before insertion and after 3×, 8×, and 15× insertions. The PEDOT:PSS coating and parylene-C coatings are still intact after 15 insertions, consistent with the impedance measurements in (F). Scale bars, 10 μm.
Next, we determine the fabrication yield of our CMU array probes. The electrochemical impedance for 230 shanks across nine devices was measured at 1-kHz frequency. These data are plotted in Fig. 5B. All the 230 channels were electrically isolated after fabrication and had impedances below 1 megohm at 1-kHz frequency. Specifically, the average impedance reduced from 270 to 237 kilohms upon PEDOT:PSS coating on the tips. The impedance values in Fig. 5B are well within the requirements for high-fidelity recording in vivo. An analysis by Neto et al. (26) determines that the signal quality can suffer beyond an impedance of 1 megohm at 1-kHz frequency. The results in Fig. 5B shows that for a 95% confidence interval, the shank level yield is >98.8%. We note that the smallest pitch of 3D printed shanks demonstrated in this work is 200 μm. With PFIB removal, the overspray can be removed for shanks at a pitch down to 100 μm or less. However, at smaller pitches, the shank insertion into the brain is expected to be the limiting factor for the yield for this technology, an area that is yet to be explored.
We observed electrochemical impedance of PEDOT:PSS-tipped gold shanks (Fig. 5C) to be 197.9 ± 92.3 kilohms at 1-kHz frequency. Note that the data in Fig. 5C represent mean and SD of five channels. An example averaged impedance spectrum for a 16-channel silver device is also shown through the frequency range (Fig. 5D). This performance for both materials is well within the desired range for extracellular recording (14, 27). Low-cost silver probes, useful for physical prototyping, can be encapsulated by a thin gold layer via electroplating (see fig. S4). The individual impedance plots for the PEDOT:PSS-tipped gold and silver shanks are given in fig. S5 (A to E and F to J, respectively). Further details into the manufacturing processes are given below in Materials and Methods.
Electrode shanks must have sufficient strength and ductility to reliably penetrate the biological tissue of interest and to tolerate incidental outside forces during handling and insertion. Silicon probes exhibit high strength, but their brittleness can make the probes prone to breakage. Previously, we have demonstrated highly ductile metal pillars of a few tens of micrometers in diameter under a compressive load without substantial loss of strength (28). We evaluated the force required for the insertion of the shanks in agarose, a phantom brain, and the force required to buckle the shanks. For details of the experiment, please see Materials and Methods. Arrays of 3 × 3 shanks (pitch of 250 μm) with shank diameter of 80 μm were printed per sample at three different shank lengths of 0.75, 1.5, and 3 mm. A total of six samples per condition were evaluated for buckling tests and insertion tests. Thus, n = 162 shanks for insertion test and n = 162 shanks for the compression test. Figure 5E shows the peak agarose insertion force and peak force for buckling per shank for the three shank lengths. The peak insertion force scaled roughly linearly as a function of the shank length. This behavior is expected since the force during insertion is expected to be caused by the frictional force between the shank perimeter and the agarose. This peak force is of the order of 0.2 to 0.4 mN per shank (Fig. 5E). The array was then compressed using a solid platen (see Materials and Methods) until the shanks buckled (and broke), which gave the peak buckling force (Fig. 5E). Figure S6 shows force versus displacement measurements for representative insertion and buckling tests for the different shank lengths. The required buckling force was >2000× of that needed for insertion for the shortest pillars. As expected from the Euler’s buckling theory (29), the taller pillars (which were printed at higher temperatures; see Materials and Methods) buckled at a lower force per shank. However, even in the case of 3-mm-long shanks, the peak force per shank for buckling was about 80× that required for agarose insertion. We note, however, that the insertion force acts along the periphery of the shanks throughout the shank length, while the bucking force during compression acts at the shank tips. We do not expect buckling to be a failure mechanism once the shanks are inserted because of side supports, making this estimate highly conservative. In other words, the strength of our 3D printed shanks will enable their insertion into the brain for a length of at least several centimeters or more.
To address how repeat insertions during acute use affect impedance, we inserted PEDOT:PSS-tipped shanks in agarose phantom brain several times and measured its effect on shank impedances. The average impedances stayed in the range of 300 to 800 kilohms at a frequency of 1 kHz until 15 insertions (Fig. 5F); in addition, no shanks broke from the substrate, as confirmed by visual inspection between each insertion. The electrical performance also indicated intact PEDOT:PSS and parylene-C coating, a fact confirmed by scanning electron microscope (SEM) images (Fig. 5G).
High-density probes successfully penetrate the brain
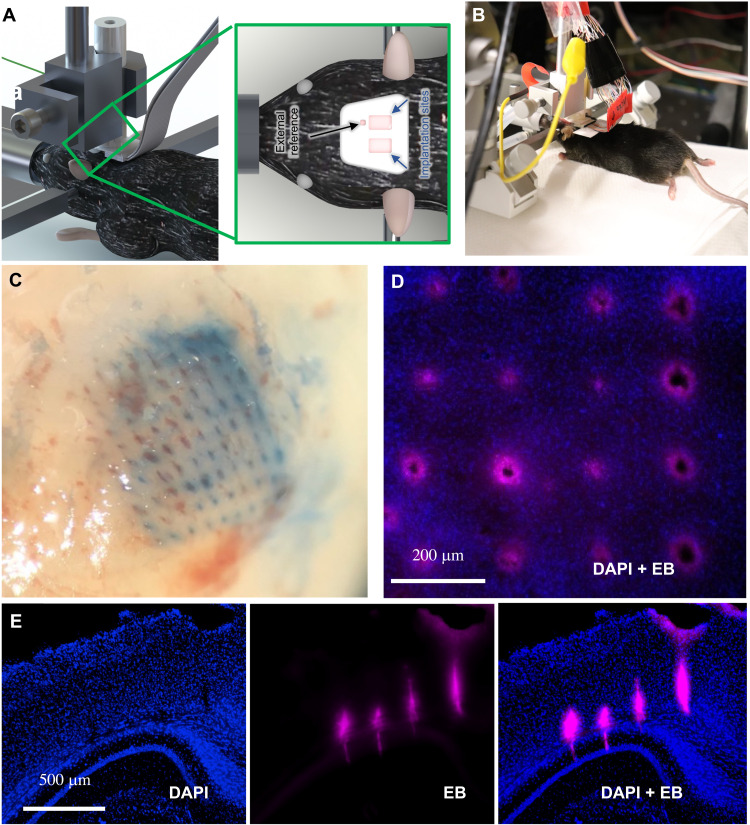
Having achieved more than sufficient structural and electrical properties, we tested probe performance in tissue. We inserted a 10 × 10 CMU array (2600 shank/cm2, pitch of 200 μm) of uniform-height shanks (with a diameter tapering from 50 μm at the base to 20 μm at the tip), which had been dipped in Evans blue (see Materials and Methods), into the cortex of an anesthetized mouse. Beyond providing the location of the electrode, Evans blue has often been used as a marker for bleeding because of its strong affinity for serum albumin. Higher spatial density of probes, even with densities half that shown here, often encounter a “bed of nails” effect in soft tissue, where the tissue is depressed rather than penetrated during insertion (30). However, owing to the small cross-sectional area and narrow tips, our array was capable of penetrating mouse brain with only a basic, benchtop manual manipulator at a rate of 0.1 to 0.2 mm/min (Fig. 6, A and B), thus precluding the need for accessory equipment such as vibration drives, pneumatic insertion hammers, or the need for staggered shank lengths to reduce the number of shanks penetrating at one time. Histological examination found no gross damage to the tissue beyond the presence of the probe (Fig. 6, C and D). Here, we define gross damage as tearing of tissue or missing tissue or cell bodies between shanks. This gross damage would indicate a bed of nails effect or other inability to cleanly penetrate the brain tissue, rather than an immunological response that would be more likely with a chronic implant. To further test the limits of penetration capability of our device, we fabricated and inserted another higher density 10 × 10 array (6400 shank/cm2, pitch of 125 μm) of uniform-height shanks (with a diameter tapering from 20 μm at the base to 10 μm at the tip) into a mouse brain. The insertion image and histological slices are shown in fig. S7 (A to C), which again show minimal gross damage from acute penetration, further confirming the effectiveness of our approach. Deeper penetrations made with longer shanks (length, 2.5 mm; diameter at tip, 15 μm; diameter at base, 75 μm; 25 shanks) also demonstrate the capability of the 3D printed shanks (Fig. 6E), with penetration in the mouse brain through area V2 to the hippocampus. In no case did we find gross tissue damage, tearing, or other damage as the acute probe slid through the brain tissue.
Fig. 6. Probe insertion into mouse brain.
(A and B) Schematic and photograph of form factor used during insertion for recording using a standard stereotaxic micromanipulator. (C) Evans blue dye left behind from the successful insertion of a dense (2600 shanks/cm2; pitch of 200 μm), 10 × 10 array of 20-μm tip diameter. (D) No gross tissue damage was observed following 30 min of insertion [blue, 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain; red, Evans blue (EB)]. (E) Successful insertion test in an anesthetized mouse without breaking the shanks or gross damage to the brain (coronal slice though area V2, hippocampus). Note that the lack of damage or tearing is caused by probe insertion in (C) to (E).
The 3D printed probe captures electrophysiological signals in vivo with low noise
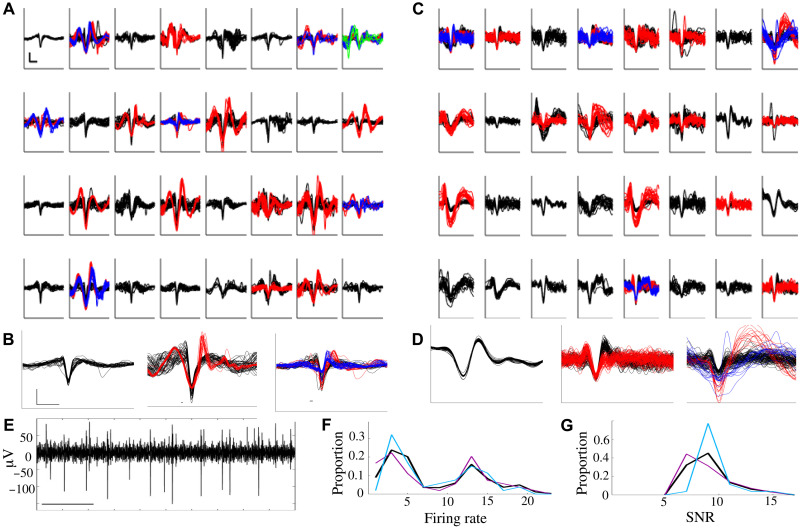
To test their in vivo performance, probes were inserted into sensorimotor cortex of anesthetized mice. For all in vivo benchmarking, we used square, silver arrays of uniform height analogous to that shown in Fig. 2C. This layout is similar to that of Utah arrays but at twice the density and at a size that can be readily used on mouse and rat. We were able to record and isolate action potential waveforms from individual cortical neurons (depth = 660 μm). The panels in Fig. 7A illustrate the neural activity observed from each channel within a single session (see Materials and Methods for more details). These represent the first ever in vivo neural recordings using a 3D printed electrode. On any given channel, we were able to identify individual action potential waveforms from typically one to three putative neurons; the means from arrays shown (Fig. 7, A to C) are 1.75 (SEM, 0.14) and 1.69 (SEM, 0.12) respectively, with a grand average across eight recordings of 1.71 (SEM, 0.14). In no case did we fail to find a putative unit with a signal-to-noise ratio (SNR) of at least 3. This yield is in line with other recently developed electrodes, which range from around one to three units per site (16, 31–32). For each of the two representative recording sessions shown (Fig. 7, A to C), recordings from three representative channels are shown in detail, each with either one, two, or three putative units on that channel (Fig. 7, B and D). A representative trace of neural activity from a single channel is also shown in Fig. 7E. Mean firing rates of isolated neurons were comparable to that typically observed (Fig. 7, A, C, and F), with a mean of 9.2 Hz (SEM, 0.8) and 7.1 Hz (SEM, 0.7), respectively, and a grand average of 9.7 Hz (SEM 1.0). The 3D printed electrodes demonstrated an SNR of 7.5 (SEM 0.6) and 9.5 (SEM 0.5) for the data shown in Fig. 7 (A and C, respectively). The grand average (Fig. 7G) was 8.6 (SEM, 0.6) with mean root mean square deviation (RMS) of 64.1 μV (SEM, 3.1). As an additional benchmark, recordings from mouse motor cortex were performed in a separate animal using a 32-channel silicon MEA from Cambridge NeuroTech, using the same data acquisition system and sorting analysis; this achieved similar performance with a mean firing rate of 10.1 and a mean SNR of 9.1.
Fig. 7. Recording of action potentials from mouse brain.
(A) Spike waveforms extracted from 32-channel electrode placed in mouse sensorimotor cortex. Scale bar, 1 ms × 100 μV. Individual isolated neurons are shown in different colors (black, 1; red, 2; blue, 3; green, 4). (B) Zoomed-in image of randomly selected waveforms from channels with one, two, or three putative neurons. Scale bar as in (A). (C and D) Waveforms as in (A) and (B), from the second use of a different electrode placed into sensorimotor cortex. Scale as in (A) and (B). (E) Representative trace of neural activity from a single channel. Scale bar, 100 ms. Distribution of (F) average firing rate and (G) SNR of neurons identified in (A) (magenta), (B) (cyan), and across all eight recording sessions (black).
DISCUSSION
The acute-use 3D printed CMU array demonstrated in this work overcomes several limitations in current in vivo recording devices from a variety of biological tissue and opens up several previously unexplored horizons for nanofabrication of biomedical solutions. First, the ability to print and route shanks at arbitrary locations allows the 3D printed array to target specific regions of interest across distant areas of the brain, thus enabling precision science and reducing damage due to unwanted shanks inherent in one-size-fits-all probes. Increased study of the cascade of areas within the 3D distribution of circuits in the brain appears to be the future of neuroscience (16, 33–35). The metal-polymer printing developed in this paper allows rapid prototyping and custom routing of signals from shanks via changes to the printing programs. This combination of creating 3D objects (shanks) along with layered 2D planar wires (routing) using a variety of metals will enable a rapid on-demand fabrication of study-specific probes or patient-specific neural interfaces. Second, the high shank density that we achieved (up to 2600 sites/cm2) represents a substantial improvement over current microelectrode techniques. The use of flexible substrates provides an additional degree of customization not offered by the current methods. While extensive focus has been placed on increasing electrode channel counts, these channels are only helpful if they are able to be placed in the correct locations. The 3D printed MEAs constitute a new avenue of targeted, optimized research that promises to reveal information processing strategies used by neural ensembles across brain areas (4).
The novel fabrication techniques also yield logistical improvements. Probe production time constitutes a matter of hours (followed by standard fabrication processes run in batch form the following day) rather than weeks. We note that the printing speed can be improved substantially (up to an order of magnitude or more) as follows. First, AJ printers with four nozzles that can print shanks simultaneously are commercially available and would further reduce the already rapid fabrication time. In addition, the speed of the platen movement during printing in this study is about 2 to 5 mm/s, while the maximum platen speed enabled by commercial printers is up to 200 mm/s. We expect a substantial reduction in the cost of the probes using this method, which will reduce the barriers to entry for researchers and clinicians that are either running hypothesis-driven experiments or aiming to create devices. Such “democratization” of the microelectrode technology will immensely benefit researchers and their targeted questions.
We note that the AJ printing method requires that the printed material be made of nanoparticles, a requirement for a stable aerosol to be formed and achieve the fine features described in this work. Furthermore, the proposed fabrication technique depends only on aerosol droplet dynamics (Fig. 1C) and not on the type of nanoparticles inside the droplets (36, 37). This flexibility is illustrated in this work by the construction of probes using gold shanks [Fig. 2F; with electrical characteristics in Fig. 5 (A and C)]. It is thus possible to construct the proposed probes from different materials, although the emphasis in this work largely has been on using silver because of its cost-effectiveness for prototyping. The silver shanks can readily be plated with PEDOT:PSS (Fig. 4H) and gold (fig. S4). The latter can serve as a low-cost alternative to the all-gold 3D printed probes demonstrated in this work. We also note that although AJ printing is used in the current work, other droplet-based printing methods such as fine inkjet printing (38) can be potentially explored to construct the 3D shanks. Such an effort will be part of a future investigation.
The flexibility of production may provide further inroads across biomedical and material science. The printing of three-dimensional features provides a means to fabricate hollow metallic tubes. Such a construct can be used as microneedles for drug delivery and/or extraction of fluids, all while recording the bioelectric signals. The current method can also be combined with printing of 3D transparent polymers (21) to construct optic fiber–paired probes for optogenetic stimulation and recording of neural signals. Last, the microelectrodes can be used in nonbiological applications such as changing surface hydrophobicity through texturing, increasing energy storage in batteries through an increase in the surface area and specific sensor devices.
In summary, we have demonstrated a rapid 3D additive printing method that we developed to create CMU array, a new class of customizable, high-density MEA, and demonstrated its operation in penetrating into and recording from biological tissue. The technology increases recording sites per unit area by an order of magnitude and enables the on-demand, study-specific prototyping and manufacture of electrode configurations in a few hours. This technology paves the way to customizable large-scale probes (thousands of channels; over several square centimeter area) with easily modified probe layouts that can capture and potentially manipulate the dynamics of large, multi-area neural circuits with single-neuron and single-millisecond resolution.
MATERIALS AND METHODS
3D nanoparticle printing
To construct the 3D nanoprinted array, we used Ag and Au nanoparticle inks. The Ag nanoparticle ink was Prelect TPS 50 G2 (Clariant Group, Frankfurt, Germany). The Ag nanoparticle size in the ink was 30 to 50 nm; the ink viscosity was about 1.5 cP, and the Ag particle loading in the ink was about 40 ± 2 weight % (wt %). The solvents used for this ink were deionized (DI) water and ethylene glycol. Ethylene glycol acts as a humectant and dispersant to help in the formation of the shank/pillar structures required for this work. The Au nanoparticle ink used in this work was UTDAu40 ink (UT Dots Inc., Champaign, IL). The average Au particle size was 4 nm; the ink viscosity was 3 cP, and particle loading in the ink was 40 wt %. The Au nanoparticles were dispersed in an organic nonpolar solvent, which was aerosolized during AJ printing via ultrasound energy. Different substrates were used for this work. One of the substrates was an alumina slab with 96% purity (ALN-101005S1, MTI Corp, Richmond, CA) cut in the desired shape using water jet machining. The second substrate was silicon wafer. For alumina and Si substrates, the printing of silver nanoparticles and polyimide was used to construct the leads to route the signal from the shanks. The third substrate was a PCB board with pads with dimensions of 340 μm by 340 μm and a metallization of electroless nickel immersion gold.
An AJ 3D printer (Model AJ-300, Optomec Inc., Albuquerque, NM) was used to print the MEAs. The AJ system at Carnegie Mellon University consists of three atomizers (two ultrasonic atomizers and a pneumatic atomizer), a programmable XY motion stage, an ultraviolet light source, and a deposition head. For the construction of the MEAs, we used one ultrasonic atomizer to aerosolize the gold or silver inks. The aerosolized ink droplets were carried to a printhead by a carrier gas (N2). Inside the printhead, the droplets were focused toward the nozzle with the help of a sheath gas (also N2) to form a microjet. In case of printing of polymers, we used the pneumatic atomizer. The printing process was carried out by a continuous flow of droplets, which was diverted or resumed by movement of a shutter. The diameter of the nozzle used for metals was 150 μm, which is known to give rise to an aerosol stream having a diameter of ~10 to 15 μm (19). Before printing, the geometry of the part was drawn in AutoCAD using a program in the software AutoLISP (AutoCAD 2015, Autodesk Inc., San Rafael, CA) and converted to a “prg” file compatible with the printer software. The shanks were printed by droplet over droplet in a method as described in our prior work (23), where semicircular printing was done to create a layer with thickness of about 20 μm within a fraction of a second. The gas flow rate in the AJ machine during printing of metals was about 25 sccm (standard cubic centimeters per minute), while that for the sheath gas was about 50 sccm. The platen temperature was kept at 80°C for Ag shanks <1 mm in length. For Ag shanks >1 mm in length, an external platen was used to heat the shanks to 300°C during printing. The 512-array device (Fig. 2A) had shank lengths below and above 1 mm and was printed at 80°C. All the gold shanks were printed at 300°C. Polyimide printing was achieved using the pneumatic atomizer of the AJ printer using an atomizing flow of 760 sccm, an exhaust flow of 720 sccm, and a sheath flow of 100 sccm. A 300-μm nozzle and platen temperature of 60°C were used for the printing of polyimide. Movies S1 and S3 show the printing process for 3D shanks, while movie S2 shows the process for printing of 2D interconnects and pads.
Note that for initial prototyping, we have used silver nanoparticles, while also demonstrating 3D printed electrodes with gold nanoparticles [Fig. 2F; electrical characteristics in Fig. 5 (A and C) and fig. S5 (A to E)]. We are aware of the cytotoxicity of silver, but (i) the electrodes described here are for acute use, (ii) all silver is concealed behind PEDOT:PSS and/or parylene-C, and (iii) the ink used is yet another customizable feature of the array. In addition, we demonstrate that the parylene-C and PEDOT:PSS coatings remain intact up to 15 acute insertions (Fig. 5, F and G). Despite the potential for concern, vigorous, normal action potentials were able to be maintained throughout the duration of the recordings, lasting in the tens of minutes, suggesting that the coatings provided a sufficient barrier. No evidence of gross cell death was observed in the histological analyses.
Sintering conditions
Printed gold MEAs were sintered at 300°C for 4 hours at a ramp rate of 1°C/min. The silver MEAs printed on alumina substrates were sintered at 300°C for 6 hours at a ramp rate of 1°C/min (for shanks <1 mm long) and 0.3°C/min (for shanks >1 mm long). The MEAs printed on the Kapton substrate were sintered at 335°C for 2 hours (same ramp rate as that for samples on alumina substrate). The MEAs printed on the PCB substrate were sintered at 195°C for 2 hours (ramp rate of 1°C/min). The sintering temperatures and times were chosen from our previous work on the sintering of silver nanoparticles (19, 28). The resistivity of five printed gold and five printed silver structures at different sintering condition are shown in Fig. 5A. The resistivity is between one and two orders of magnitude of the bulk for the sintering conditions used in this work. The sintering was carried out in a programmable oven with controlled heating rates (Neytech Vulcan furnace, Model 3-550, Degussa-Ney Dental Inc., Bloomfield, CT).
Soldering
Leads from a 32-channel standard Omnetics connector were soldered to the printed device to interface with the amplifier and recording equipment. Soldering was completed using a Sn-Bi-Ag low-temperature solder paste with a melting point of 137°C (low-temperature lead-free solder paste T3, SRA, Walpole, MA). The paste was dispensed using a pneumatic dispense system (digital precision fluid dispenser, SRA #205, SRA, Walpole, MA). The syringe was controlled using a 3D manual manipulation stage (see movie S5 for the solder dispense process). Using the same stage, the connector was located appropriately and held in place while the device was briefly heated to the melting point of the solder on a hot plate, creating a robust electrical connection. Note that the reflow process leads to self-alignment of the Omnetics connector with the printed pads. Movie S5 also illustrates the solder reflow. The feet of the connector were then encased in methyl cyanoacrylate glue (Loctite 430, Henkel AG & Co. KGaA, Düsseldorf, Germany), creating a robust mechanical connection to protect the solder joint and prevent any damage during handling and/or insertion and recording into the mouse brain.
Overspray removal
Using the attached connector to ground the printed routing lines to the stage within the vacuum chamber, the overspray can be removed by PFIB etching (FEI dual beam plasma, FEI Company, Hillsboro, OR). An accelerating voltage of 30 kV and beam current of 0.47 μA were used. A video of the overspray removal is given in movie S6. This process is highly effective in preventing shorting between the electrodes (data on 230 shanks across nine devices fabricated using 3D printing, followed by PFIB removal shows that no two shanks were shorted in any of the CMU array devices).
Parylene-C coating and tip exposure
The microelectrodes were insulated using 5-μm-thick parylene-C coating (SCS Labcoter-2; dimer mass, 8 g; furnace set point, 690°C; chamber gauge set point, 135°C; vaporizer set point, 175°C; and vacuum set point, 35 mtorr). Parylene-C was chosen as the insulator material because of its superior biocompatibility, high resistivity, impermeability to biological species, and conformal deposition process. Deposited thin-film thickness was verified by including a calibration Si wafer alongside the 3D printed electrode array in the deposition chamber using reflectometry (Nanospec 210XP, Nanometrics, Ottawa, ON, Canada). The Parylene-C deposition is a batch process with the ability to coat about a hundred probes or more in one run that lasts for about 5 hours. The metal at the tips of the shanks was exposed by removing parylene-C using a plasma Xe+ focused ion beam or PFIB machine (FEI dual-beam plasma, FEI Company, Hillsboro, OR). Using PFIB, the parylene-C coating removal from a shank tip takes about 1 to 2 min. The tip removal process can be automated via a CAD program.
PEDOT:PSS electrodeposition
PEDOT:PSS electrodeposition was carried out using a recipe optimized at CMU based on a prior report (39). The electroplating solution was prepared by combining and mixing thoroughly 2 ml of DI water and 41.24 mg of PSS in a test tube. 3,4-Ethylenedioxythiophene (2.2 μl) was added using a micropipette. This solution was mixed thoroughly via shaking. The solution was sealed and refrigerated for 24 hours before use. Before electroplating, the solution was brought to room temperature and mixed again before electrodeposition. An electrochemical workstation (VersaSTAT 3 Potentiostat Galvanostat, Princeton Applied Research, Oak Ridge, TN) as a current-controlled power supply was used for the electrodeposition. All chemicals were purchased from Sigma-Aldrich. Note that all the probes used in this study were used for recording within 4 weeks after fabrication.
Gold electroplating
Gold electroplating of silver shanks (fig. S4) was carried out using EarthCoat cyanide-free gold plating solution (24K) heated to 60°C on a hot plate with stirrer (Model MS300HS, Medline Scientific, Oxon, UK). The solution (filled to an adequate depth for submersion in a 1-liter thick glass beaker) was agitated to mix well before probe immersion, but no agitation was used during plating to prevent damage to the device. The probe was cleaned before plating by submerging it in DI water (three separate beakers used in sequence to reduce contamination). The probe was plated using Spa Plating (Bath, UK) multifunctional or MF Rectifier at a current of 0.1 A (for all pillars and routings in parallel) for 5 min, using a stainless-steel electrode. After plating, the rinsing process was repeated (using three beakers with fresh DI water) to remove any remaining plating solution. Energy-dispersive x-ray spectroscopy analysis was done to verify that the solution was plating gold without contamination.
Compression test for the shanks and insertion of shanks into agarose phantom brain
Shanks were printed onto an alumina substrate and tested under compression. The tests were carried out in a custom testing apparatus at a compression rate of 0.5 mm/min. For shanks with 750-μm height, a 5-lb load cell (Futek LCM100-FSH03829, FUTEK Advanced Sensor Technology Inc., Irvine, CA) was used, while for shanks with 1.5 and 3 mm in height, a 100-g load cell (Futek LSB200-FSH03870 FUTEK Advanced Sensor Technology Inc., Irvine, CA) was used. The load cells were independently calibrated before the experiment. A camera (Canon EOS Ti7 Rebel, Canon Corp., Tokyo, Japan) with a magnifying lens (model Tube TS-160, Infiniti Photo-Optical Company, Boulder, CO) was used to record the compression behavior. The shanks were compressed by a solid steel platen while measuring the force as a function of the displacement as monitored by the camera.
To prepare the agarose, we used a recipe described in literature (40–41). Agarose powder was mixed with DI water in a beaker to get a 0.6% concentration (w/v). The beaker was then kept in an oil bath at ~95°C for about 15 min. Subsequently, the beaker was cooled to about 35°C before pouring the mixture into a petri dish for curing overnight. The shank arrays were inserted using the same compression testing apparatus with the 100-g load cell at a speed of 0.2 mm/min to match with that for insertion of the CMU array using a manual stereotax (see the “Electrode insertion tests” section below). During shank insertion into agarose, the force was measured as a function of the insertion depth. The compression apparatus was calibrated in millinewton range before insertion tests.
Electrode insertion tests
Insertion testing was performed on the brains of adult C57BL/6J mice. Anesthesia was administered via 1.5 to 2% inhaled isoflurane and was continued throughout the implantation. Craniotomies were performed to access the brain, and the dura was resected. Probes were dipped in Evans blue dye and then lowered through the craniotomy using a manual stereotax manipulator at a rate of 100 to 200 μm/min. The probe was left in place for 5 min before being removed. Following several minutes following the removal of the probe, the mouse was euthanized with an overdose of isoflurane and decapitation. All procedures were approved by the Carnegie Mellon University Institutional Animal Care and Use Committee.
Electrophysiology
In a different set of experiments, neural signals were recorded from mouse sensorimotor cortex. Three 32-channel arrays were used in three mice, and one 16-channel array was used in the fourth mouse (one recording per hemisphere and one array per animal; a total of eight recording sessions across the mice and hemispheres). The pitch for the 32-channel arrays was 250 μm, while that for the 16-channel array was 200 μm. For acute recordings, anesthesia was induced via 1.5-2% inhaled isoflurane and maintained with urethane (1 mg/kg) and sedated with chlorprothixene (2 mg/kg) injections. Following craniotomies the isoflurane was reduced to 0.5-0.75% prior to recording. A reference wire shorted to ground was made isopotential to the brain by placing it in the saline within the craniotomy. Recorded voltages containing neural signals were routed out through the custom routing board described to an Intan-based amplifier and data acquisition system (Whisper System, Neural Circuits LLC) running SpikeGLX (github.com/billkarsh/SpikeGLX). Spikes from each channel were sorted offline using a custom-built spike sorter that applied a high-pass filter to sort out spike frequency bands (300 to 6000 Hz). To extract spikes, an amplitude discrimination threshold was set at 3 SDs below the mean of the recording segments. For each waveform exceeding the threshold in magnitude, a 5-ms putative waveform aligned on the absolute minimum of the waveform was then stored. Further manual sorting of the extracted spikes into distinct waveforms was then performed. The SNR for the waveforms over a session was calculated as the peak-to-peak amplitude of the average waveform divided by the calculated voltage RMS. Values of greater than 1.5 were considered to be quality units that were easily discriminable from the underlying noise floor.
Histology
For reconstruction of probe placement, the array was dipped in DiI (Fig. 1B) or Evans blue (Fig. 6) before implantation. Electrode tracks were imaged in the 40-μm slices, approximately orthogonal or parallel to the axis of entry. Brain slices were placed on slides for imaging electrode tracks using a mounting medium that included a 4′,6-diamidino-2-phenylindole (DAPI) stain, which labels the nuclei of individual cells.
Acknowledgments
We thank N. Anderson, B. He, and J. Beuth for helpful discussions as we prepared the study.
Funding: R.P.P. and E.A.Y. acknowledge support from the NIH (grants #1R21EY029441 and #RF1NS110483) and the DSF Foundation. M.C. acknowledges the support from the NIH (grant #1RF1NS113303) and the DSF Foundation. J.W.R. acknowledges the support of the Carnegie Mellon University Richard King Mellon Foundation Presidential Fellowship in the Life Sciences and the Axel Berny Presidential Graduate Fellowship. S.M.R. acknowledges the support of the Center for Machine Learning and Health (CMLH) Fellowship at CMU. We acknowledge use of the materials characterization facility at Carnegie Mellon University supported by grant MCF-677785 for SEM and PFIB.
Author contributions: R.P.P. and E.A.Y. conceived and designed the experiments. R.P.P., E.A.Y., S.M.R., M.S.S., M.A.N., H.L.G., C.H., B.Y., and S.J. designed the CMU array device used in this work. S.M.R., M.S.S., C.H., S.J., B.Y., and R.B. designed and carried out the CMU array shank and routing microfabrication using the AJ 3D nanoprinting process for silver and gold. S.M.R., M.S.S., C.H., S.J., and B.Y. designed and carried out clean-room microfabrication and packaging processes such as parylene-C coating, overspray removal, tip exposure using FIB, PEDOT:PSS coating, soldering, and impedance measurements. J.W.R., Z.A., and M.C. contributed to the cleanroom-based microfabrication process, troubleshooting device design, benchtop testing plans, including impedance measurement, PEDOT:PSS electrodeposition, and electronic packaging. S.M.R. developed gold electroplating process. S.M.R. and S.J. carried out the resistivity measurements. S.J., S.M.R., C.H., B.Y., and M.S.S. carried out the compression tests, acute repeated insertion tests, and microscopy of the probes. M.A.N. and H.L.G. carried out the biology side of the experiments including craniotomy, insertion, histology, and recording. All authors analyzed the data and discussed the results. E.A.Y. and R.P.P. cowrote the paper.
Competing interests: R.P.P., E.A.Y., and M.S.S. are inventors on a U.S. patent application no. 16/966,657 that covers the area of 3D printed neural probes. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S7
Other Supplementary Material for this manuscript includes the following:
Movies S1 to S6
REFERENCES AND NOTES
- 1.Spira M. E., Hai A., Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 8, 83–94 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Viventi J., Kim D.-H., Vigeland L., Frechette E. S., Blanco J. A., Kim Y.-S., Avrin A. E., Tiruvadi V. R., Hwang S.-W., Vanleer A. C., Wulsin D. F., Davis K., Gelber C. E., Palmer L., van der Spiegel J., Wu J., Xiao J., Huang Y., Contreras D., Rogers J. A., Litt B., Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14, 1599–1605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berényi A., Somogyvári Z., Nagy A. J., Roux L., Long J. D., Fujisawa S., Stark E., Leonardo A., Harris T. D., Buzsáki G., Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. 111, 1132–1149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zandvakili A., Kohn A., Coordinated neuronal activity enhances corticocortical communication. Neuron 87, 827–839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell P. K., Jones K. E., Huber R. J., Horch K. W., Normann R. A., A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 38, 758–768 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Jones K. E., Campbell P. K., Normann R. A., A glass/silicon composite intracortical electrode array. Ann. Biomed. Eng. 20, 423–437 (1992). [DOI] [PubMed] [Google Scholar]
- 7.Bhandari R., Negi S., Solzbacher F., Wafer-scale fabrication of penetrating neural microelectrode arrays. Biomed. Microdevices 12, 797–807 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Goncalves S. B., Peixoto A. C., Silva A. F., Correia J. H., Fabrication and mechanical characterization of long and different penetrating length neural microelectrode arrays. J. Micromech. Microeng. 25, 055014 (2015). [Google Scholar]
- 9.Normann R. A., Fernandez E., Clinical applications of penetrating neural interfaces and Utah electrode array technologies. J. Neural Eng. 13, 061003 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Maynard E. M., Nordhausen C. T., Normann R. A., The Utah intracortical electrode array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 102, 228–239 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Wark H. A. C., Mathews K. S., Normann R. A., Fernandez E., Behavioral and cellular consequences of high-electrode count Utah arrays chronically implanted in rat sciatic nerve. J. Neural Eng. 11, 046027 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Wark H. A. C., Sharma R., Mathews K. S., Fernandez E., Yoo J., Christensen B., Tresco P., Rieth L., Solzbacher F., Normann R. A., Tathireddy P., A new high-density (25 electrodes/mm2) penetrating microelectrode array for recording and stimulating sub-millimeter neuroanatomical structures. J. Neural Eng. 10, 045003 (2013). [DOI] [PubMed] [Google Scholar]
- 13.P. Van Zant, Microchip Fabrication: A Practical Guide to Semiconductor Processing (McGraw-Hill Professional, 2004), vol. 31. [Google Scholar]
- 14.Herwik S., Kisban S., Aarts A. A. A., Seidl K., Girardeau G., Benchenane K., Zugaro M. B., Wiener S. I., Paul O., Neves H. P., Ruther P., Fabrication technology for silicon-based microprobe arrays used in acute and sub-chronic neural recording. J. Micromech. Microeng. 19, 074008 (2009). [Google Scholar]
- 15.Barz F., Livi A., Lanzilotto M., Maranesi M., Bonini L., Paul O., Ruther P., Versatile, modular 3D microelectrode arrays for neuronal ensemble recordings: From design to fabrication, assembly, and functional validation in non-human primates. J. Neural Eng. 14, 036010 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Jun J. J., Steinmetz N. A., Siegle J. H., Denman D. J., Bauza M., Barbarits B., Lee A. K., Anastassiou C. A., Andrei A., Aydın Ç., Barbic M., Blanche T. J., Bonin V., Couto J., Dutta B., Gratiy S. L., Gutnisky D. A., Häusser M., Karsh B., Ledochowitsch P., Lopez C. M., Mitelut C., Musa S., Okun M., Pachitariu M., Putzeys J., Rich P. D., Rossant C., Sun W. L., Svoboda K., Carandini M., Harris K. D., Koch C., O’Keefe J., Harris T. D., Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinmetz N. A., Aydin C., Lebedeva A., Okun M., Pachitariu M., Bauza M., Beau M., Bhagat J., Böhm C., Broux M., Chen S., Colonell J., Gardner R. J., Karsh B., Kloosterman F., Kostadinov D., Mora-Lopez C., O’Callaghan J., Park J., Putzeys J., Sauerbrei B., van Daal R. J. J., Vollan A. Z., Wang S., Welkenhuysen M., Ye Z., Dudman J. T., Dutta B., Hantman A. W., Harris K. D., Lee A. K., Moser E. I., O’Keefe J., Renart A., Svoboda K., Häusser M., Haesler S., Carandini M., Harris T. D., Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledesma H. A., Li X., Carvalho-de-Souza J. L., Wei W., Bezanilla F., Tian B., An atlas of nano-enabled neural interfaces. Nat. Nanotechnol. 14, 645–657 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman M. T., McCloy J., Ramana C. V., Panat R., Structure, electrical characteristics, and high-temperature stability of aerosol jet printed silver nanoparticle films. J. Appl. Phys. 120, 075305 (2016). [Google Scholar]
- 20.Singh M., Haverinen H. M., Dhagat P., Jabbour G. E., Inkjet printing—Process and its applications. Adv. Mater. 22, 673–685 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Rahman T., Renaud L., Heo D., Renn M., Panat R., Aerosol based direct-write micro-additive fabrication method for sub-mm 3D metal-dielectric structures. J. Micromech. Microeng. 25, 107002 (2015). [Google Scholar]
- 22.Gribi S., du Bois de Dunilac S., Ghezzi D., Lacour S. P., A microfabricated nerve-on-a-chip platform for rapid assessment of neural conduction in explanted peripheral nerve fibers. Nat. Commun. 9, 4403 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleh M. S., Hu C., Panat R., Three-dimensional microarchitected materials and devices using nanoparticle assembly by pointwise spatial printing. Sci. Adv. 3, e1601986 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.W. Chen, B. Sammakia, I. Qin, J. Wilcox, E. Cotts, A. Teng, S. Rangarajan, M. Gerber, K. Erickson, V. Sukharev, L. Shan, B. Chan, K.-O. Lee, P. Ho, Single Chip and Multi Chip Integration, in Heterogeneous Integration Roadmap, P. Wesling, Ed. (IEEE Electronics Packaging Society, 2021), chap. 8. [Google Scholar]
- 25.Cai F., Chang Y. H., Wang K., Zhang C., Wang B., Papapolymerou J., Low-loss 3-D multilayer transmission lines and interconnects fabricated by additive manufacturing technologies. IEEE Trans. Microw. Theory Techn. 64, 3208–3216 (2016). [Google Scholar]
- 26.Neto J. P., Baião P., Lopes G., Frazão J., Nogueira J., Fortunato E., Barquinha P., Kampff A. R., Does impedance matter when recording spikes with polytrodes? Front. Neurosci. 12, 715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise K. D., Anderson D. J., Hetke J. F., Kipke D. R., Najafi K., Wireless implantable microsystems: High-density electronic interfaces to the nervous system. Proc. IEEE 92, 76–97 (2004). [Google Scholar]
- 28.Saleh M. S., HamidVishkasougheh M., Zbib H., Panat R., Polycrystalline micropillars by a novel 3-D printing method and their behavior under compressive loads. Scr. Mater. 149, 144–149 (2018). [Google Scholar]
- 29.S. P. Timoshenko, J. N. Goodier, Theory of Elasticity, (McGraw-Hill Book Company, 1970), vol. 37, pp. 888. [Google Scholar]
- 30.Okamura A. M., Simone C., O’Leary M. D., Force modeling for needle insertion into soft tissue. IEEE. Trans. Biomed. Eng. 51, 1707–1716 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Ulyanova A. V., Cottone C., Adam C. D., Gagnon K. G., Cullen D. K., Holtzman T., Jamieson B. G., Koch P. F., Chen H. I., Johnson V. E., Wolf J. A., Multichannel silicon probes for awake hippocampal recordings in large animals. Front. Neurosci. 13, 397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black B. J., Kanneganti A., Joshi-Imre A., Rihani R., Chakraborty B., Abbott J., Pancrazio J. J., Cogan S. F., Chronic recording and electrochemical performance of Utah microelectrode arrays implanted in rat motor cortex. J. Neurophysiol. 120, 2083–2090 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Shimaoka D., Steinmetz N. A., Harris K. D., Carandini M., The impact of bilateral ongoing activity on evoked responses in mouse cortex. eLife 8, e43533 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yttri E. A., Dudman J. T., Opponent and bidirectional control of movement velocity in the basal ganglia. Nature 533, 402–406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y., You S. S., Zhang A., Lee J.-H., Huang J., Lieber C. M., Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotechnol. 14, 783–790 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Hong K., Kim S. H., Mahajan A., Frisbie C. D., Aerosol jet printed p-and n-type electrolyte-gated transistors with a variety of electrode materials: Exploring practical routes to printed electronics. ACS Appl. Mater. Interfaces 6, 18704–18711 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Ouyang J., Cormier D., Williams S. A., Borkholder D. A., Photonic sintering of aerosol jet printed lead zirconate titanate (PZT) thick films. J. Am. Ceram. Soc. 99, 2569–2577 (2016). [Google Scholar]
- 38.Calvert P., Inkjet printing for materials and devices. Chem. Mater. 13, 3299–3305 (2001). [Google Scholar]
- 39.Cui X. T., Zhou D. D., Poly (3, 4-ethylenedioxythiophene) for chronic neural stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 15, 502–508 (2007). [DOI] [PubMed] [Google Scholar]
- 40.T. Proulx, Dynamic Behavior of Materials, Volume 1: Proceedings of the 2010 Annual Conference on Experimental and Applied Mechanics (Springer Science & Business Media, 2011), vol. 1. [Google Scholar]
- 41.Apollo N. V., Jiang J., Cheung W., Baquier S., Lai A., Mirebedini A., Foroughi J., Wallace G. G., Shivdasani M. N., Prawer S., Chen S., Williams R., Cook M. J., Nayagam D. A. X., Garrett D. J., Development and characterization of a sucrose microneedle neural electrode delivery system. Adv. Biosyst. 2, 1700187 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S7
Movies S1 to S6