Abstract
Background
Recent studies have investigated the importance of Galetin-3 in inflammation, fibrosis, cell proliferation, cardiac disease, diabetes, and tumor formation.
Aims
This study aims to investigate the role of the Galectin-3 level in the diagnosis of COVID-19 pneumonia and the value of the Galectin-3 level in predicting the clinical course of the patient.
Methods
This study employed a prospective, case-control study design and was conducted at Bakircay University Ciğli Training and Research Hospital. A total of 100 patients (40 had moderate and 60 had severe/critical COVID-19 disease according to World Health Organisation guidelines) and 50 non-symptomatic healthy volunteers participated in the study. Blood samples were taken from patients at the time of hospital admission, after which serum was isolated. Following the isolation of serum, Galectin-3 levels were evaluated using the enzyme-linked immunosorbent assay (ELISA) method.
Results
The serum Galectin-3 level was measured as 13.57 (10.9-16.4) ng/mL in the control group, 13.52 (10.69-16.6) ng/mL in the moderate disease group, and 11.65 (6.09-14.33) ng/mL in the severe/critical disease group. Serum Galectin-3 levels were significantly lower in the severe/critical disease group compared to the control and moderate disease groups (p=0.001 and p=0.019, respectively).
Using ROC analysis, a larger area under the curve (AUC) for the serum Galectin-3 levels of the control group (AUC=0.622, 95% CI =0.529-0.714; p=0.015) was calculated compared to the COVID-19 patient group for the diagnosis of COVID-19 disease. The Galectin-3 level was found to be 75% sensitive and 50% specific at a cut-off level of 11.3 ng/mL in predicting the need for ICU treatment.
Conclusion
Galectin-3 levels may be a beneficial biomarker in predicting the clinical severity of COVID-19 disease when used in conjunction with other known biomarkers, at the time of admission to the emergency department (ED).
Keywords: inflamation, clinical infectious disease, covid-19 pneumonia, covid-19, galectin-3
Introduction
The COVID-19 infection, which is transmitted from person to person through droplets and can lead to serious clinical conditions such as pneumonia, acute respiratory distress syndrome (ARDS), sepsis, and septic shock from the clinic of upper respiratory tract infection, continues to pose a significant problem worldwide [1,2].
In the follow-up of inflammatory processes caused by COVID-19, both clinical parameters and many laboratory parameters are evaluated. These parameters are used for estimating high-risk patients, determining the response to treatment, determining the intensive care unit (ICU) admission criteria, outcome prediction, and determining the discharge criteria [1].
In addition to known inflammatory markers such as C-reactive protein (CRP), procalcitonin, interleukins, neutrophil-to-lymphocyte ratio, serum amyloid protein, erythrocyte sedimentation rate, and ferritin, previous studies published during the pandemic have examined markers that may be associated with inflammation such as Annexin A1, S100B, and calprotectin for diagnosis and prognosis [2-5].
Galectin-3 is a chimera-type galectin of approximately 30 kDa and is expressed in the nucleus, cytoplasm, mitochondria, cell surface, and extracellular region in the cell. Galectin-3 and other galectin proteins are used in cell-cell communication and cell-matrix communication, and it plays a role in cell growth, cell differentiation, angiogenesis, and apoptosis. Recent studies have investigated its importance in inflammation, fibrosis, cell proliferation, cardiac disease, diabetes, and tumor formation [6-11]. A study conducted with mice found that stimulation of Galectin-3 activity was effective on direct bacteriostatic activity in severe pneumococcal infection and provided better clinical results [12].
Although a prospective study has been conducted to evaluate the role of Galectin-3 inhibitors in the treatment of COVID-19 previously, to the authors’ knowledge, there is no study examining the change of Galectin-3 protein level at the clinical level in COVID-19, and few studies have examined the usability of Galectin-3 level in the diagnosis of COVID-19 pneumonia [13,14]. This study aims to investigate the role of the Galectin-3 level in the diagnosis of COVID-19 pneumonia and the value of the Galectin-3 level in predicting the clinical course of the patient.
Materials and methods
Study design and study population
The present study is a prospective case-control study, and the ethics approval was obtained from the Ethics Committee of Pamukkale University (Numbered: E-60116787-020-67194). The study was conducted at Bakircay University Cigli Training and Educational Hospital. All procedures carried out on patients were in compliance with the Helsinki Declaration.
The patient groups and the healthy control group were informed in detail about the study, and they were requested to complete the written consent forms before participating in the study. Study groups were determined according to the inclusion and exclusion criteria. Patients who were clinically confirmed as COVID-19 infection according to World Health Organization (WHO) guidelines using a positive reverse transcriptase polymerase chain reaction (RT-PCR) test were included in the study. Participants were grouped in the moderate COVID-19 disease group (N=40), the severe/critical COVID-19 disease group (N=60), and the healthy control group (N=50). according to WHO guidelines and those who gave their written consent were included in the study [15]. The healthy control group included healthy volunteers with no history or diagnosis of any acute or chronic disease and infection and no known drug use.
Inclusion criteria
Patient Groups
Patients whose diagnoses of COVID-19 infection were confirmed by positive RT-PCR in ED [15].
Control Group
Subjects with no history of a known disease, no infectious symptoms, no drug use, and who provided written consent were included in the study.
Exclusion criteria
Patients who were diagnosed with heart, kidney or liver failure, who had a history of acute pulmonary embolism, deep venous thrombosis or chronic inflammatory disease, and who were pregnant were excluded from the study.
Clinical evaluation
The subjects included in the present study were clinically evaluated using WHO diagnosis and treatment guidelines for COVID-19. The patient management algorithms were administered due to the updates of these guidelines. The patients were categorized as moderate disease and severe/critical disease groups according to WHO guidelines. The severe/critical disease group includes the patients who have severe pneumonia or ARDS signs (adolescent or adult with clinical signs of pneumonia [fever, cough, dyspnoea, fast breathing] plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air and oxygenation impairment findings) [15]. Radiological examples were given in Figures 1A, 1B.
Figure 1. Radiological examples of COVID-19 patients.
(A) Severe COVID-19 pneumonia. (B) Moderate disease.
To evaluate the clinical severity, CURB-65 scores of patients were calculated as indicated in the literature. The CURB-65 score is a scoring system used in the evaluation of pneumonia [16].
Healthy group (control group)
This group included subjects who gave their written consent to participate in the study, who had no infection history within the last two weeks, no history of any particular medication, no history or diagnosis of any disease, and who were admitted to the emergency department (ED) with complaints other than infectious issues.
Data collection
Demographic information and vital findings of the subjects, and their laboratory findings (hemogram, C-reactive protein (CRP), liver function tests (aspartate aminotransferase, alanine aminotransferase), creatinine, blood urea nitrogen (BUN), D-dimer, creatinine kinase-MB, highly sensitive troponin T (hsTnT), blood gas analysis parameters, and hospitalization location ICU or not were recorded in the data set.
Galectin-3 level measurement
Venous blood samples that were taken when the patients were admitted to ED were withdrawn into a dry test tube. Samples were centrifugated for 10 minutes at 4,000 rpm. Serum samples obtained from centrifugation were collected for laboratory analysis. Serum Galectin-3 levels were analysed using a commercially available Galectin-3 enzyme-linked immunosorbent assay (ELISA) Kit (Human Galectin-3 ELISA Kit, Elabscience, Catalog No: E-EL-H1470), per the manufacturer’s protocol.
Statistical analysis
Given that a similarly organized reference study did not exist, a power analysis was performed in line with the presumptions. The results revealed that at least 88 people (min. 44 for each cohort) were needed to achieve 95% power at a 90% confidence interval, assuming that the projected effect size would be high (f=0.7). The dataset was analysed by using the SPSS package programme.
A Kolmogorov-Smirnov test was conducted to calculate the distribution type of the continuous variables. The continuous variables were presented as mean ± standard deviation or median (IQR). Kruskal-Wallis or Mann-Whitney U tests were used for analysing independent and non-parametric variables. Receiver operating characteristic (ROC) curve analysis was used for the discriminant performance serum Galectin-3 levels. The significance level was defined as p < 0.05 for all analyses.
Results
Symptom duration time was statistically higher in the severe/critical disease group than in the moderate disease group (7.5±1.7 and 5.9±1.1 days, respectively) (p=0.02). The post-hoc power analysis showed that the effect size of the Galectin-3 concentrations for the differences between the two groups (patients and control) was moderate-high (f=0.63), and the power level observed for this effect size was 95%, and the reliability level was 95.2%.
Serum Galectin-3 levels were measured as 13.57 (10.9-16.4) ng/mL in the control group; 13.52 (10.69-16.6) ng/mL in the moderate disease group; and 11.65 (6.09-14.33) ng/mL in the severe/critical disease group. Serum Galectin-3 levels were significantly lower in the severe/critical disease group compared to the control and moderate disease groups (p=0.001 and p=0.019, respectively) (Table 1).
Table 1. Galectin-3 levels of the study groups.
*p-value derived from Kruskal-Wallis' test and refers to the comparison between whole the groups
**p-value is derived from the Mann-Whitney U test and refers to the comparison between control and moderate disease groups.
***p-value is derived from the Mann-Whitney U test and refers to the comparison between control and severe/critical disease groups.
****p-value is derived from the Mann-Whitney U test and refers to the comparison between moderate disease groups and severe/critical disease groups.
| Controls (N=50) | Moderate Disease Group (N=40) | Severe/Critical Disease Group (N=60) | P-value | |||||
| Mean±SD | Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | Median (IQR) | |||
| Galectin-3 | 15.78±10.8 | 13.57 (10.9-16.4) | 12.58±5.34 | 13.52 (10.69-16.6) | 9.95±5.15 | 11.65 (6.09-14.33) | *p= 0.003 | |
| **p= 0.548 | ||||||||
| ***p= 0.001 | ||||||||
| ****p= 0.019 | ||||||||
Subjects included in patient groups and healthy control groups were matched by means of age and gender (p=0.686 and p=0.823, respectively) (Table 2).
Table 2. Clinical data and comorbidity data of the groups.
p-values are derived from the Mann-Whitney U test.
*p-values are derived from chi-square test
**p-values are derived from one-way ANOVA test
SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
| Controls (N=50) | Moderate Disease Group (N=40) | Severe/Critical Disease Group (N=60) | P-value | ||
| Gender, N(%) | Male | 19 | 18 | 22 | *0.686 |
| Female | 31 | 22 | 38 | ||
| Comorbidities, N(%) | |||||
| Diabetes Mellitus | 16 (40%) | 26 (43.3%) | *0.766 | ||
| Hypertension | 13 (32.5%) | 25 (41.6%) | *0.405 | ||
| Coronary artery disease | 6 (14.2%) | 6 (18.7%) | *0.41 | ||
| Mean±SD or Median(IQR) | |||||
| Age (year) | 65.32±16.1 | 64.05±13.5 | 65.8±12.67 | **0.823 | |
| CURB-65 Score | 2 (1-2) | 3 (2-4) | 0.0001 | ||
| Body temperature (0C) | 36.75 (36.5-37.1) | 36.8 (36.3-37) | 0.802 | ||
| Heart Rate (beat/min) | 88 (78.25-95.75) | 103 (93-120) | 0.003 | ||
| Respiratory Rate | 24 (20-28) | 27 (20-38) | 0.281 | ||
| sPO2 | 94 (90-96.75) | 85 (78-92) | 0.0001 | ||
| SBP (mm/Hg) | 137 (116-156.75) | 130 (114-143) | 0.106 | ||
| DBP (mm/Hg) | 73.5 (65.25-84.5) | 76 (70-82) | 0.732 | ||
Significant findings and clinical data for the study groups are given in Table 2, and Table 3 presents the laboratory parameters of the subjects.
Table 3. Laboratory parameters of the patient groups.
p-values are derived from the Kruskal-Wallis test
*p-values are derived from Student’s t-Test
IQR: Interquartile range; WBC: White blood cell; NLR: Neutrophil leukocyte ratio; CRP: C-reactive protein; BUN: Blood urea nitrogen; AST: Aspartate transaminase; ALT: Alanine transaminase; hsTnT: Highly sensitive troponin T; CKMB: Creatinine kinase MB; pH: Power of hydrogen; pCO2: Partial carbon dioxide pressure; HCO3: Bicarbonate.
| Moderate Disease Group (N=40) | Severe/Critical Disease Group (N=60) | P-value | |
| Mean±SD or Median (IQR) | Mean±SD or Median (IQR) | ||
| WBC (K/μL) | 5.52 (4.48-6.85) | 9.47 (7.76-14.2) | 0.0001 |
| Hemoglobin (g/dL) | 13.21±1.52 | 13.55±1.69 | *p=0.036 |
| Platelete (K/μL) | 205.56±57.33 | 263±94.83 | *p=0.007 |
| NLR | 3.17 (2.1-4.38) | 8.27 (3.67-10.2) | 0.0001 |
| CRP (mg/L) | 31.37 (10.01-92.28) | 123 (77.8-196.7) | 0.0001 |
| BUN (mg/dL) | 33 (25-45) | 55 (34.7-77) | 0.004 |
| Creatinine (mg/dL) | 0.85 (0.79-1.06) | 1.23 (0.91-1.52) | 0.321 |
| AST (U/L) | 27 (21-33) | 30 (21.5-46) | 0.626 |
| ALT (U/L) | 20 (13-29) | 26 (20.5-44.5) | 0.005 |
| D-Dimer (ng/mL) | 530 (290-1120) | 1,820 (718-4,400) | 0.0001 |
| hsTnT (μg/L) | 11.78 (6.7-20.37) | 25.5 (8.25-64.67) | 0.001 |
| CKMB (ng/mL) | 1.39 (0.83-2.45) | 2.67 (1.64-7.4) | 0.001 |
| pH | 7.41 (7.37-7.44) | 7.39 (7.3-7.44) | 0.437 |
| pCO2 (mmHg) | 38.9 (35.8-44) | 42.2 (35.87-62) | 0.045 |
| Lactate (mmol/L) | 1.8 (1.2-2) | 2.3 (1.72-3.2) | 0.001 |
| HCO3 (mEq/L) | 23.9 (22.8-25.2) | 25.6 (21.1-29) | 0.159 |
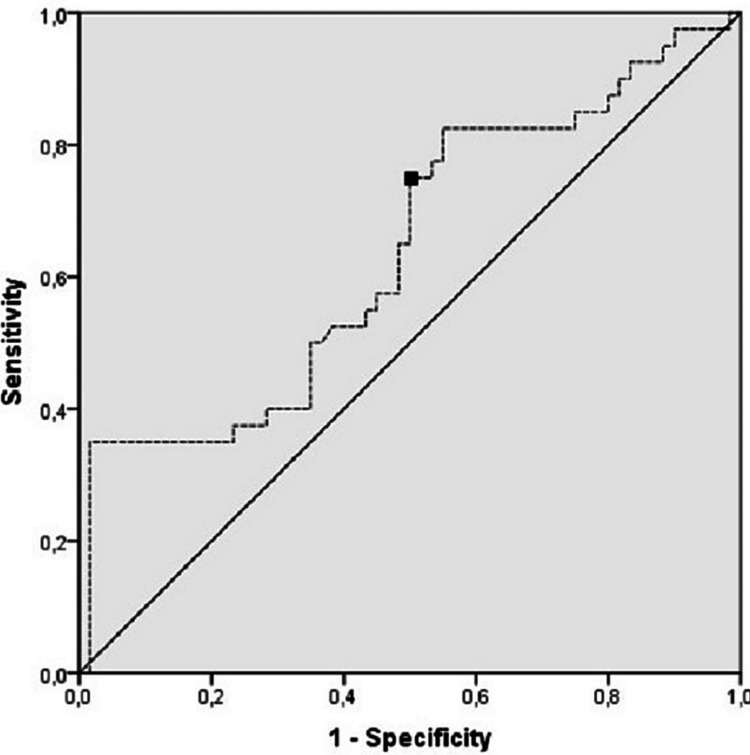
Using ROC analysis, a larger area under the curve (AUC) for the serum Galectin-3 level of the control group (AUC=0.622, 95% CI = 0.529-0.714; p=0.015) was calculated compared to the COVID-19 patient group for the diagnosis of COVID-19 disease. The Galectin-3 level was found to be 56.9% sensitive and 55.5% specific at a cut-off level of 13.23 ng/mL for the diagnosis of COVID-19 disease (Figure 2).
Figure 2. ROC curve analysis of the clinical diagnosis of the patients.
AUC = 0.622; 95% CI = (0.529-0.714); Cut-off point = 13.23 ng/mL
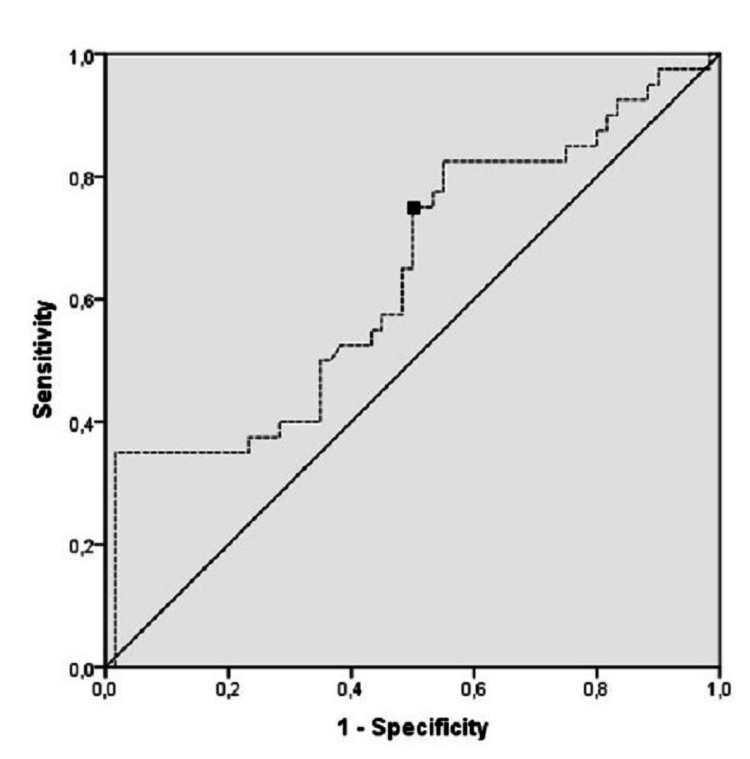
Furthermore, a larger AUC for the serum Galectin-3 levels of patients who have moderate disease (AUC = 0.701, 95% CI = 0.582-0.819; p=0.003) was calculated using ROC analysis. The Galectin-3 level was found to be 75% sensitive and 50% specific at a cut-off level of 11.3 ng/ml for predicting the severity of COVID-19 disease (Figure 3).
Figure 3. ROC curve analysis of the clinical severity of the patients.
AUC = 0.639; 95% CI = (0.525-0.752); cut-off point = 11.3 ng/mL
Discussion
This study examined the clinical value of serum Galectin-3 levels in COVID-19 pneumonia and found that serum Galectin-3 levels decreased especially in severe-critical disease patients. The Galectin-3 level was found to be 75% sensitive and 50% specific at a cut-off level of 11.3 ng/mL for predicting the severity of COVID-19 disease.
The Galectin-3 protein, which is known to have an effect on immune system cells and play a role in inflammation, fibrosis, apoptosis and host defense, has been studied in various viral infections in the literature [17]. For example, in their study, Alcendor et al. found that the Galectin-3 level decreased in Kaposi sarcoma-associated herpes virus infection [18]. In another study conducted by King et al., Galectin-3 levels were found to increase HSV infection [19]. In another study, Galectin-3 levels were found to increase chronic hepatitis [20]. All viruses do not have the same pathogenicity. We found different results when we compared this study but it may cause viral entry differences, viral proteins, etc.
There are very few studies examining Galectin-3 levels in pneumonia cases. Chen et al.’s study revealed that Galectin-3 triggered avian H5N1 influenza-induced pulmonary inflammation by activating the NLRP3 inflammasome. They also suggested that Galectin-3 could be a therapeutic target [21].
In Baykan et al.’s study, patients with a diagnosis of COVID-19 infection and typical and atypical pneumonia findings on thorax CT were evaluated and the usability of Galectin-3 level as a diagnostic tool in COVID-19 pneumonia was calculated. The results showed that Galectin-3 level was higher in patients with typical pneumonia findings on thorax CT [14]. In addition, the Galectin-3 level was found to be higher in the patient group than in the control group. However, our study found the Galectin-3 level to be higher in the control group than in the patient group, and the Galectin-3 level was found to be lower, especially in severe-critical patients. We employed a different study design compared to Baykan et al.’s study in that the patients were evaluated by grouping them according to clinical seriousness. For this reason, we think that our study can better reveal the relationship between clinical severity and Galectin-3 level. The lower level in severe-critical patients may be due to a reason such as the release of cytokines during the inflammation process and the consequent consumption of Galectin-3 protein. In this regard, future research can look at the sequential Galectin-3 levels during the clinical follow-up of the patients. In addition, our study sheds light on the clinical status of the Galectin-3 protein level, which was suggested to be a treatment target in Chen et al.’s study.
Regarding the use of Galectin-3 level as a prognostic and diagnostic marker, Baykan et al. found Galectin-3 level to be important in diagnosing typical pneumonia and the AUC value to be 0.89 (95% CI = 0.83-94), and no association was made with clinical seriousness of the patients. However, in our study, we found lower AUC values in diagnosing COVID-19 disease and predicting severity. Although AUC=0.622 (95% CI =0.529-0.714; p=0.015) for the control group and AUC = 0.701 (95% CI = 0.582-0.819; p=0.003) for the moderate disease group were not higher than those in our study, that Galectin-3 level was found to be 75% sensitive and 50% specific at a value of 11.3 ng/mL indicates that it can be a diagnostic marker in the prognosis of the disease.
This study has some limitations. Firstly, we did not measure Galectin-3 levels continuously, and we have no data about the continuous changing of the Galectin-3 levels. Secondly, we did not follow up with the patients about mortality.
Conclusions
Many markers have been studied both in the diagnosis of COVID-19 and in predicting prognosis, and Galectin-3 can be used more appropriately for clinical classification of patients rather than diagnosis. Especially low Galectin-3 levels detected in severe-critical disease groups may shed light on the timing of Galectin-3 protein-targeted therapies. Galectin-3 levels may be a beneficial biomarker in predicting the clinical severity of COVID-19 disease when used in conjunction with other known biomarkers, at the time of admission to the ED.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Ethics Committee of Pamukkale University issued approval E-60116787-020-67194
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Biomarkers in COVID-19: an up-to-date review. Samprathi M, Jayashree M. Front Pediatr. 2020;8:607647. doi: 10.3389/fped.2020.607647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serum calprotectin as a novel biomarker for severity of COVID-19 disease. Kaya T, Yaylacı S, Nalbant A, et al. Ir J Med Sci. 2022;191:59–64. doi: 10.1007/s11845-021-02565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, Deng G. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Seyit M, Avci E, Nar R, et al. Am J Emerg Med. 2021;40:110–114. doi: 10.1016/j.ajem.2020.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association between S100B levels and COVID-19 pneumonia: a case control study. Mete E, Sabirli R, Goren T, Turkcuer I, Kurt Ö, Koseler A. In Vivo. 2021;35:2923–2928. doi: 10.21873/invivo.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Characterization of the advanced glycation end-product receptor complex in human vascular endothelial cells. Stitt AW, He C, Vlassara H. Biochem Biophys Res Commun. 1999;256:549–556. doi: 10.1006/bbrc.1999.0291. [DOI] [PubMed] [Google Scholar]
- 7.Identification of galectin-3 as a factor in pre-mRNA splicing. Dagher SF, Wang JL, Patterson RJ. Proc Natl Acad Sci U S A. 1995;92:1213–1217. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galectin-3 level predicts response to ablation and outcomes in patients with persistent atrial fibrillation and systolic heart failure. Clementy N, Garcia B, André C, et al. PLoS One. 2018;13:0. doi: 10.1371/journal.pone.0201517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galectin-3 is associated with left ventricular reverse remodeling and outcome after percutaneous mitral valve repair. Zuern CS, Floss N, Mueller II, et al. Int J Cardiol. 2018;263:104–110. doi: 10.1016/j.ijcard.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Differential and predictive value of galectin-3 and soluble suppression of tumorigenicity-2 (sST2) in heart failure with preserved ejection fraction. Cui Y, Qi X, Huang A, Li J, Hou W, Liu K. Med Sci Monit. 2018;24:5139–5146. doi: 10.12659/MSM.908840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Tan KC, Cheung CL, Lee AC, Lam JK, Wong Y, Shiu SW. Diabetologia. 2018;61:1212–1219. doi: 10.1007/s00125-018-4552-z. [DOI] [PubMed] [Google Scholar]
- 12.Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Farnworth SL, Henderson NC, Mackinnon AC, et al. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A potential role for Galectin-3 inhibitors in the treatment of COVID-19. Caniglia JL, Guda MR, Asuthkar S, Tsung AJ, Velpula KK. PeerJ. 2020;8:0. doi: 10.7717/peerj.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galectin-3: can it be a diagnostic tool for pneumonia in covid-19 patients? Kartal Baykan E, Şebin E, Karaşahin Ö, et al. Turk J Med Sci. 2021;51:2256–2262. doi: 10.3906/sag-2102-202. [DOI] [PubMed] [Google Scholar]
- 15.Clinical management of COVID- 19: interim guidance. [ Oct; 2021 ];World Health Organization. (2020. https://www.bing.com/search?q=Clinical+management+of+COVID-+19%3A+interim+guidance&qs=n&form=QBRE&sp=-1&pq=clinical+management+of+covid-+19%3A+interim+guidance&sc=9-50&sk=&cvid=69BC194B0A69487BAE9E28AD0FB587DF&ghsh=0&ghacc=0&ghpl= 2020 22:2021. [Google Scholar]
- 16.Applicability of the CURB-65 pneumonia severity score for outpatient treatment of COVID-19. Nguyen Y, Corre F, Honsel V, Curac S, Zarrouk V, Fantin B, Galy A. J Infect. 2020;81:0–8. doi: 10.1016/j.jinf.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The role of galectins in virus infection - a systemic literature review. Wang WH, Lin CY, Chang MR, et al. J Microbiol Immunol Infect. 2020;53:925–935. doi: 10.1016/j.jmii.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Identifying dysregulated genes induced by Kaposi's sarcoma-associated herpesvirus (KSHV) Alcendor D, Knobel S. J Vis Exp. 2010;14:2078. doi: 10.3791/2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herpes simplex virus type 1 infection increases the carbohydrate binding activity and the secretion of cellular galectin-3. King RD, Lubinski JM, Friedman HM. Arch Virol. 2009;154:609–618. doi: 10.1007/s00705-009-0351-7. [DOI] [PubMed] [Google Scholar]
- 20.Serum galectin-3 levels in children with chronic hepatitis B infection and inactive hepatitis B carriers. Uluca Ü, Şen V, Ece A, et al. Med Sci Monit. 2015;21:1376–1380. doi: 10.12659/MSM.894035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galectin-3 enhances avian H5N1 influenza A virus-induced pulmonary inflammation by promoting NLRP3 inflammasome activation. Chen YJ, Wang SF, Weng IC, et al. Am J Pathol. 2018;188:1031–1042. doi: 10.1016/j.ajpath.2017.12.014. [DOI] [PubMed] [Google Scholar]