Abstract
Valeriana plants are members of the Caprifoliaceae family, which include more than 200 species worldwide. We summarized previous reports on traditional clinical applications, bioactivities, and phytochemistry of Valeriana by searching electronic databases of Science Direct, Web of Science, PubMed, and some books. Some Valeriana species have been used as traditional medicines, demonstrating calming fright and tranquilizing mind, promoting Qi and blood, activating blood circulation and regulating menstruation, dispelling wind and eliminating dampness, regulating Qi-flowing to relieve pain, and promoting digestion and checking diarrhea, and treating diseases of the nervous, cardiovascular, and digestive systems, inflammation, gynecology, and others. Pharmacology studies revealed the effects of Valeriana, including sedative, hypnotic, antispasmodic, analgesic, antidepressant, anxiolytic, anticonvulsant, antiepileptic, neuroprotective, antibacterial, antiviral, cytotoxic, and antitumor effects as well as cardiovascular and cerebrovascular system improvements. More than 800 compounds have been isolated or identified from Valeriana, including iridoids, lignans, flavonoids, sesquiterpenoids, alkaloids, and essential oils. Constituents with neuroprotective, anti-inflammatory, cytotoxic, and sedative activities were also identified. However, at present, the developed drugs from Valeriana are far from sufficient. We further discussed the pharmacological effects, effective constituents, and mechanisms directly related to the traditional clinical applications of Valeriana, revealing that only several species and their essential oils were well developed to treat insomnia. To effectively promote the utilization of resources, more Valeriana species as well as their different medicinal parts should be the focus of future related studies. Clinical studies should be performed based on the traditional efficacies of Valeriana to facilitate their use in treating diseases of nervous, cardiovascular, and digestive systems, inflammation, and gynecology. Future studies should also focus on developing effective fractions or active compounds of Valeriana into new drugs to treat diseases associated with neurodegeneration, cardiovascular, and cerebrovascular, inflammation and tumors. Our review will promote the development and utilization of potential drugs in Valeriana and avoid wasting their medicinal resources.
Keywords: Valeriana plants, clinical application, phytochemistry, active constituent, pharmacological effect
1 Introduction
Valeriana L. is a group of perennial herbs, belonging to the family Caprifoliaceae, of which roots and rhizomes are used as medicines. Their roots and rhizomes give off special aroma and a slightly bitter taste (Zhu, 1984; Xi et al., 2002). At present, more than 200 species of Valeriana have been found all over the world (Du and Wu, 2006). Most wild or cultivated species are distributed in Germany, Holland, France, Belgium, Eastern Europe, India, Japan, Mexico, China and the United States (Ortiz et al., 1999; Raal et al., 2008; Sharma et al., 2012). However, only a few species have been widely reported in medicinal studies or clinical applications. For example, Valeriana officinalis L (V. officinalis) has been used in Europe and the United States for a long time to treat mild and moderate insomnia (Huang et al., 2004). There are 17 species and two varieties of Valeriana in China, most of which are distributed in regions ranging from northeast to southwest (Chinese Academy of Sciences, 2004). Among them, species of V. officinalis, Valeriana amurensis P. Smirn. ex Kom. (V. amurensis), Valeriana jatamansi Jones (V. jatamansi), Valeriana hardwickii Wall. (V. hardwickii), Valeriana alternifolia Bunge (V. alternifolia), and Valeriana fauriei Briq. (V. fauriei) have been used as the Chinese materia medica of Rhizoma et Radix Valerianae (RERV) (Ming and Guo, 1993; Castillo et al., 2000). These species were subjected to more studies due to their abundant resources (Huang et al., 2004; Zhang et al., 2006). In contrast, V. officinalis, Valeriana wallichii DC. (V. wallichii), and Valeriana edulis Nutt. (V. edulis) are the main medicinal species used in other countries or regions (Zuo et al., 2010; Huynh et al., 2013).
As resources of RERV, the roots and rhizomes of Valeriana have a long history in treating nervous system diseases in China (Tan et al., 2007). V. jatamansi was recorded in the Compendium of Materia Medica written by Li Shizhen in Ming Dynasty of China (Li, 1991). It is mainly used to treat neurasthenia, insomnia, hysteria, emotional disorders, palpitation, abdominal pain, lumbocrural pain, rheumatism pain, and dysmenorrhea. It is most commonly used for the treatment of insomnia (Duan et al., 2008; Zhou et al., 2008). The people of ancient Greece and Rome used the underground parts of Valeriana as a sedative approximately 1,000 years ago (Houghton, 1988). In recent years, an increasing number of pharmacological investigations on the effects of Valeriana have been reported, revealing the sedative, hypnotic (Valle-Mojica et al., 2011), antispasmodic, analgesic (Wei et al., 2016), antidepressant, anxiolytic (Andreatini et al., 2010), anticonvulsant, antiepileptic (Eadie, 2010), neuroprotective (Malva, 2004), antibacterial, antiviral (Rawat et al., 2017), cytotoxic, and antitumor effects (Yang et al., 2017) as well as cardiovascular and cerebrovascular system improvement (Xue et al., 2005; Chen et al., 2015). Chemical studies on Valeriana have identified constituents of iridoids (Wang F. F. et al., 2019), lignans (Zuo et al., 2018), flavonoids (Jugran et al., 2016), sesquiterpenes (Wang et al., 2009b), alkaloids (Torssell et al., 1967), essential oils (Rawat et al., 2017), and others. Although the efficacies, effective constituents and therapeutic mechanisms of Valeriana have been studied extensively and deeply, at present, only the sedative effect of Valeriana has been developed to treat insomnia clinically (Shinjyo et al., 2020). Obviously, the potential of Valeriana were not well utilized, resulting in a large waste of its resources. To promote the development and utilization of Valeriana, this review systematically summarizes the traditional clinical applications, bioactivities, and effective constituents of Valeriana as well as their relevance.
In this review, previous reports on Valeriana were searched by retrieving electronic databases of Science Direct, Web of Science, PubMed, Embase, China National Knowledge Infrastructure, Wanfang Database, China Biomedical Literature Database, VIP database, and Chinese Scientific Journals Database, as well as some books. Studies on Valeriana published between 1965 and 2022 were retrieved using search term “Valeriana.” The following terms were used in a combination for further search, which include clinical application, pharmacological effect, bioactivity, phytochemistry, active constituent, component, compound, natural product, traditional medicine, and traditional Chinese medicine. Relevant literatures were collected and irrelevant ones were removed, and then they were sorted out. The structures of compounds isolated from Valeriana were drawn with the software of ChemDraw 18.0 and shown in Figures 4–8. The detailed information on pharmacological studies of Valeriana is summarized in Table 1. The pharmacological effects and effective constituents related to traditional uses of Valeriana are summarized in Table 2. The detailed information on compounds and their activities is illustrated in Supplementary Tables S1–S5. The mechanisms for pharmacological effects of antidepressant, neuroprotective, and cardiovascular system improvement are elucidated in Figures 1–3, respectively. The quality of all the included studies was assessed in accordance with the best practice in research, overcoming common challenges in phytopharmacological research (Heinrich et al., 2020).
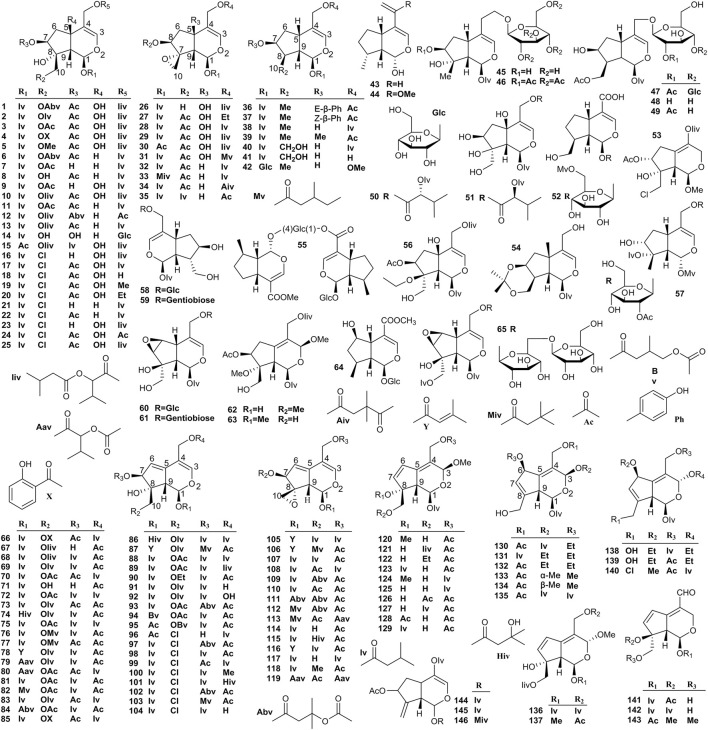
FIGURE 4.
Structures of monoethenoid (1–65) and diene (66–146) iridoids from the genus Valeriana.
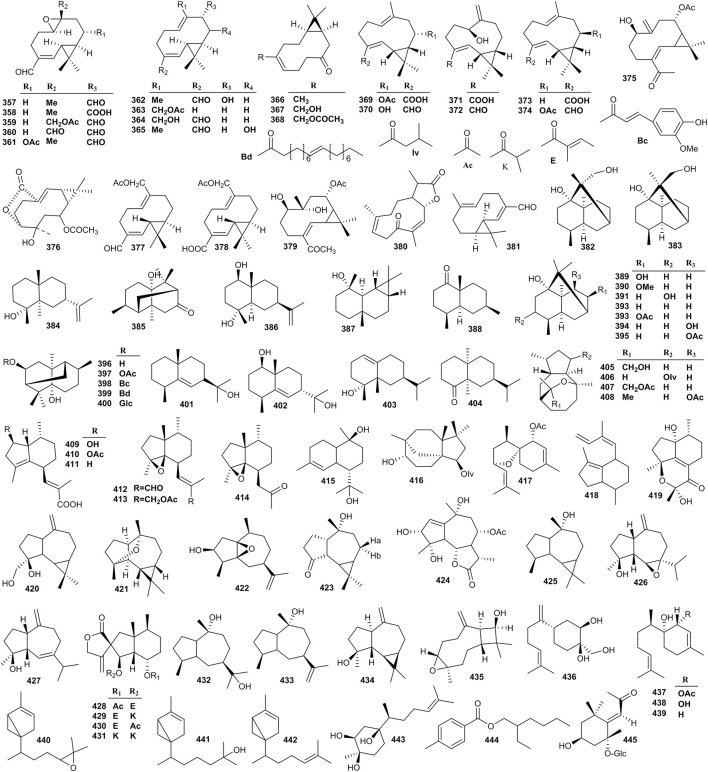
FIGURE 8.
Structures of sesquiterpenoids from the genus Valeriana.
TABLE 1.
Summary of pharmacology effects for Valeriana.
| Bioactivity | Source and extract | Model used | Positive drug and dose (administration) | Dose (administration) and duration | Minimum effective concentration | Molecular mechanism or outcome |
|---|---|---|---|---|---|---|
| Sedative and hypnotic | V. jatamansi roots and rhizomes, aqueous extract | In vivo normal mice | ― | 13.9, 27.8, and 55.6 g/kg (i.g.) | 13.9 g/kg | Activity time, the number of forelimb lifting ↓ |
| V. officinalis roots and rhizomes, aqueous extract | In vitro normal mice | Diazepam, 4 mg/kg (i.g.) | 1, 3, and 5 g/kg (i.g.), 1 week | 1 g/kg | IL-1β and TNF-α↑ | |
| V. amurensis roots and rhizomes, petroleum ether extract | In vivo pentobarbital sodium induced sleep in mice | Diazepam, 2.5 mg/kg (i.g.) | 7.5, 15, and 30 g/kg (i.g.), 2 weeks | 7.5 g/kg | GABA and 5-HT ↑ | |
| Antidepressant and anxiolytic | V. officinalis roots and rhizomes, aqueous extract | In vivo, CMS rats | Fluoxetine, 2.2 mg/kg (i.g.) | 100, 200, and 400 mg/kg (i.g.), 3 weeks | 100 mg/kg | 5-HT, cell in the hippocampus ↑ |
| V. jatamansi roots and rhizomes, total iridoids | In vivo, CUMS rats | Fluoxetine, 2.6 mg/kg (i.g.) | 5.7, 11.4, and 22.9 mg/kg (i.g.), 1 week | 5.7 mg/kg | 5-HT and NE ↑; SP and CRF ↓ | |
| V. wallichii roots and rhizomes, dichloromethane extract | In vivo, FST mice | Imipramine, 10 mg/kg (i.g.) | 10, 20, and 40 mg/kg (i.g.), 2 weeks | 20 mg/kg | NE and DA ↑ | |
| V. fauriei roots and rhizomes, ethanol extract | In vivo, CRS mice | ― | 100 and 200 mg/kg (i.g.), 2 weeks | 200 mg/kg | C-Fos, p-p38, COX-2, iNOS, Nrf2, and BDNF ↑ | |
| Anticonvulsant and antiepileptic | V. officinalis roots and rhizomes, aqueous extract | In vivo, PTZ mice | Diazepam, 5 mg/kg (i.g.) | 9 g/kg (i.g.), 2 weeks | ― | GABA, the threshold of PTZ seizure ↑ |
| V. jatamansi roots and rhizomes, aqueous extract | In vivo, TSZ mice | Diazepam, 5 mg/kg (i.p.) | 2.75, 5.5, and 11.0 g/kg (i.p.), 2 h | 11.0 g/kg | GABA ↑ | |
| V. officinalis roots and rhizomes, essential oil | In vivo, PTZ rats | Sodium valproate, 30 mg/kg (i.p.) | 30 mg/kg (i.p.), 2 weeks | 30 mg/kg | GABA ↑; Glu ↓ | |
| V. officinalis roots and rhizomes, total iridoids | In vivo, PTZ rats | ― | 0.5, 1, and 1.5 g/kg (i.g.), 4 weeks | 0.5 g/kg | GABA ↑; GAT-1 ↓ | |
| V. officinalis roots and rhizomes, aqueous extract | In vivo, TLC rats | ― | 200, 500, and 800 mg/kg (i.p.) | 500 mg/kg | Latency to the onset of bilateral forelimb clonuses↑; discharge duration, duration of stage five seizures ↓ | |
| Neuroprotective | V. officinalis roots and rhizomes, ethanol extract | In vivo, AD rats | ― | 25 and 100 mg/kg (i.g.), 3 weeks | 25 mg/kg | T-AOC, SOD, GSH-Px ↑; MDA ↓ |
| V. wallichii roots and rhizomes, 50% methanol extract | In vivo, PD mice | ― | 50, 100, and 200 mg/kg (i.g.), 3 weeks | 100 mg/kg | DA, TH+ cell count, antioxidant activity ↑; ROS, LPO, and GSH ↓ | |
| V. amurensis roots and rhizomes, the 50% ethanol eluted fraction from a 95% ethanol extract | In vitro, SDAD rats | Jiannao capsule 2.41 g/kg (i.g.) | 0.26 and 0.52 g/kg (i.g.), 1 week | 0.26 g/kg | Caspase-3, COX-2, microglia, and astrocytes, iNOS ↓ | |
| V. amurensis roots and rhizomes, 50% ethanol extract | In vivo, AD mice | Piracetam 0.5 g/kg; aricept 2 mg/kg (i.p.) | 0.2, 0.4, and 0.8 g/kg (i.g.), 2 weeks | 0.4 g/kg | ACh, ChAT, p-ERK, and Bcl-2 ↑; Bax ↓ | |
| Cardiovascular and cerebrovascular system improvements | V. officinalis roots and rhizomes, aqueous extract | In vitro, I/R rats | ― | 25, 50, and 100 mg/kg (i.p.), 2 h | 25 mg/kg | SOD, ATP-ase, GSHPx ↑; LDH, CK, MDA, and Ca2+ in cardiomyocytes ↓ |
| V. officinalis roots and rhizomes, aqueous extract | In vitro, I/R rabbits | ― | 100 mg/kg (i.p.), 2.5 h | ― | PGI2/TXA2 and coronary microcirculation ↑; platelet aggregation, TNF-α, and aseptic inflammation ↓ | |
| V. officinalis, roots and rhizomes, essential oil and ethyl acetate extracts | In vivo arrhythmic rats | Propranolol, 40 mg/kg (i.p.) | 50, 25, and 12.5 g/kg (i.g.), 3 h | 25 g/kg | Na+ influx of cardiomyocytes and cardiomyocyte β receptors ↓ | |
| V. officinalis roots and rhizomes, essential oil | In vivo acute cerebral ischemia, mice | Ligustrazine, 25 mg/kg (i.g.) | 200 and 300 mg/kg (i.g.), 1 h | 200 mg/kg | Cerebral blood flow and microcirculation ↑; platelet aggregation ↓ | |
| V. officinalis roots and rhizomes, aqueous extract | In vivo, SAH rabbits | Nimotop, 6 mg/kg (i.g.) | 500 mg/kg (i.g.), 5 days | ― | Free radicals, inflammation, platelet and leukocyte aggregation and adhesion, and immune response ↓ | |
| V. officinalis roots and rhizomes, essential oil | In vivo CaCl2 induced arrhythmic rats | Propranolol, 20 mg/kg (s.c.) | 400 and 500 mg/kg (s.c.), 30 min | 500 mg/kg | Ca2+ concentration ↓ |
TABLE 2.
Pharmacological effects and effective constituents related to traditional uses of Valeriana.
| Traditional efficacies | Traditional clinical applications | Pharmacological effects | Effective constituents |
|---|---|---|---|
| Calming fright and tranquilizing mind | Nervous system: insomnia; anxiety; hysteria; psychosis epilepsy; neurasthenia; and manic-depressive | Sedative and hypnotic | Iridoids; essential oil; and flavonoids |
| Antidepressant and anxiolytic | Iridoids; essential oil; and sesquiterpenoids | ||
| Anticonvulsant and antiepileptic | Iridoids and essential oil | ||
| Neuroprotective | Lignans; essential oil; sesquiterpenoids; and iridoids | ||
| Promoting Qi and blood and activating blood circulation regulating menstruation | Cardiovascular system: palpitation; coronary heart disease; pulmonary edema; and arrhythmias | Cardiovascular and cerebrovascular system improvements | Essential oil |
| Gynecology: anemia; menoxenia; Dysmenorrhea | Antispasmodic and analgesic | Iridoids and essential oil | |
| Dispelling wind and eliminating dampness and regulating Qi-flowing to relieve pain | Pain and inflammation: rheumatic arthralgia; hepatitis; abdominal distension and pain; traumatic injuries; gingivitis; pericoronitis and dental caries; toothache; soreness and weakness of waist and knees | Antispasmodic and analgesic | Iridoids and essential oil |
| Promoting digestion and checking diarrhea | Dyspepsia: indigestion; diarrhea and dysentery; and vomiting and diarrhea caused by heatstroke | Antibacterial and antiviral | Alkaloids and essential oil |
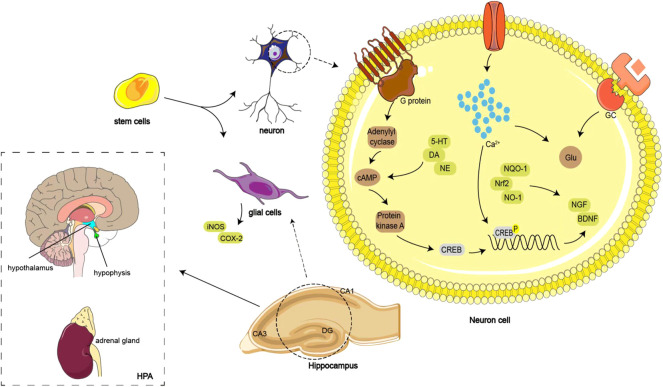
FIGURE 1.
Mechanisms of antidepressant effect of Valeriana: promoting the level of 5-HT, the proliferation of hippocampal neurons, and the expression of phosphorylated cAMP responsive element-binding protein. Inhibiting serum corticosterone and glutamate levels, reducing glucocorticoid elevation, and stabilizes the HPA.
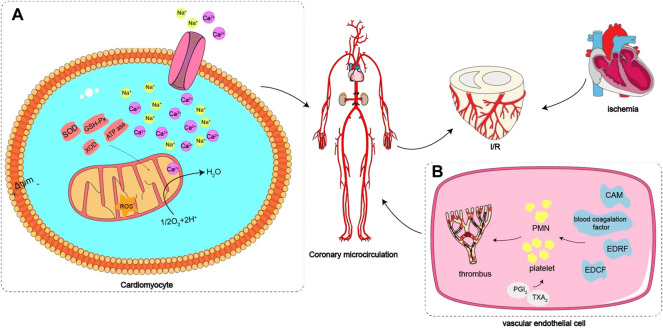
FIGURE 3.
Protective effect of Valeriana on myocardial ischemia–reperfusion injury: (A) SOD, GSHPx, and ATPase activities were increased in cardiomyocytes, and Ca2+ levels in cardiomyocytes were remarkably reduced. (B) Inhibiting a variety of CAMs, blood coagulation factor and EDRF, increasing EDCF and the value of PGI2/TXA2 and inhibiting platelet aggregation to improve coronary microcirculation.
2 Traditional clinical applications
From ancient China to present China, the roots and rhizomes from species of V. officinalis, V. amurensis, V. jatamansi, V. hardwickii, V. alternifolia, and V. fauriei were used as the traditional Chinese medicines (TCM) of RERV (Ming and Guo, 1993; Castillo et al., 2000). RERV was used to treat anxiety, palpitations, insomnia, manic-depressive psychosis, rheumatic arthralgia, abdominal distension and pain, and dysmenorrhea (Duan et al., 2008). In modern clinical practice, RERV is mainly used as a sedative, and occasionally for the treatment of arrhythmias and spasmolysis (Oshima et al., 1995; Pakseresht et al., 2011). For instance, ethanol or aqueous extracts of RERV were prepared into capsules, tablets, tinctures, liniments, and other preparations for treating neurasthenia and insomnia. The valtrate from RERV has also been used as a sedative (Zhou and Chen, 2011). RERV combined with the fruit of Humulus lupulus L. (Cannabaceae) can be made into tablets. The oral administration of two tablets can reduce the arousal caused by caffeine, whereas oral administration of six tablets can inhibit arousal completely (Zhou and Chen, 2011). As a medicine for insomnia, RERV is commonly used in the clinics in combination with Melissa officinalis L. (Lamiaceae), Schisandrae chinensis (Turcz.) Baill (Schisandraceae), Ziziphus jujuba var. spinosa (Bunge) H.H. Hu ex H.F. Chow (Rhamnaceae) semen, and other sedative and hypnotic TCMs (Huang et al., 2007). To treat arrhythmias, RERV was also combined with extracts of Adonis vernalis L (Ranunculaceae) and Crataegus L. (Rosaceae), camphor and sodium bromide to generate a complex phytomedicine (Alexander et al., 2014). In addition, RERV was made into chewing gum to treat anxiety, syrup for the treatment of insomnia in adults and children, or combined with decaffeinated coffee tea for sedation (Yuan, 1992).
V. jatamansi has a long history of medicinal use in Mongols, Tibetan, Miao, Uygur, and other ethnic minorities in China. In addition to treating insomnia, the water decoction of V. jatamansi can be used for treating urticaria, hepatitis, mosquito bites, and headaches caused by cold (Huang et al., 2006; Guan et al., 2021). Other studies have also revealed the therapeutic effects of V. jatamansi on treating peptic ulcers, anemia, abdominal distention, ephidrosis, irritability, and hyperactivity (Xu, 2006). The Dictionary of Traditional Chinese Medicine recorded the efficacies of V. jatamansi, which was used to treat abdominal distension and pain, vomiting and diarrhea, pulmonary edema, menoxenia, and tuberculosis cough (Miao et al., 2017). According to the Pharmacopoeia of the People’s Republic of China (2020 Edition), the roots and rhizomes of V. jatamansi were used to treat abdominal distension and pain, indigestion, diarrhea and dysentery, rheumatism arthralgia, soreness and weakness of waist and knees, and insomnia (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2020). In contrast, TCM Resources of China and Northeast Medicinal Plants and Local Medicines of Heilongjiang reported that V. amurensis was primarily used for treating nervous system diseases such as insomnia, neurasthenia, anxiety, hysteria, and epilepsy (Zhu, 1989; Zeng and Zeng, 1994; Yu et al., 1995). As early as ancient Greece and Rome, V. officinalis had been widely used as a mild sedative in Europe and was finally recorded in the European Pharmacopoeia in 1983. To date, V. officinalis and its extracts are widely used to treat mild and moderate insomnia in European and American countries (Zhao et al., 2011; Shinjyo et al., 2020). Preparations of V. officinalis have been accepted by the pharmacopoeias of more than 20 countries, including the Netherlands, Germany, and the United States (Wang R. J. et al., 2016). In 1985, the German Commission E monograph suggested preparing V. officinalis into tincture or infusion for relieving restlessness and nervous disturbance of sleep and defined its effects as calming and sleep-inducing (Bisset et al., 2004). As a mild sedative, V. officinalis was used in at least 25 sedative and hypnotic products in the United Kingdom and 400 other similar products in Germany (Houghton, 1999). Compared with plant raw materials and specific constituents of V. officinalis, extracts were more commonly used in these products. In addition, a double-blind experiment of 100 female students showed that V. officinalis effectively alleviated the pain of patients with dysmenorrhea (Mirabi et al., 2011). The essential oil of V. officinalis effectively treated coronary heart disease by significantly improving the symptoms of angina pectoris and myocardial ischemia in patients (Yang and Wang, 1994). In 2011, V. hardwickii was reported as a useful medicine, given its antiepileptic, sedative, diuretic, emmenagogue, spasmolytic, and antidiarrheal effects, but more other details were not found in Bashir et al. (2011).
3 Bioactivities
3.1 Sedative and hypnotic
Both the aqueous and ethanol extracts from Valeriana were effective in inducing sleep and sedative. These extracts alleviated anxiety without causing drowsiness (Bisset et al., 2004). Normal mice were administered different doses of V. jatamansi aqueous extract by intraperitoneal and gavage, separately, and activity changes at 30 and 60 min after administration were recorded by observing the activity time and the number of forelimb lifting in 2 min. As a result, the aqueous extract of V. jatamansi at doses of 2.78 (intraperitoneal) and 55.6 g/kg (gavage) significantly inhibited the autonomic activity of mice (Cao and Hong, 1994). Further studies revealed that the essential oil and iridoids fractions were responsible for the sedative effect of V. jatamansi (Wagner et al., 1980; Hendriks et al., 1981). V. officinalis aqueous extract inhibits the excitement of the cerebral cortex to reduce reflex excitability and smooth muscle spasm (Nam et al., 2013). The essential oil was determined as the effective fraction of V. officinalis that inhibits the autonomic activities of mice and strengthening the inhibitory effect of sodium pentobarbital and chloral hydrate on the central nervous system (CNS) (Xu et al., 1997). The mechanism of sedative and hypnotic effects of V. officinalis is potentially associated with increased expression of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) (Zhang J. P. et al., 2010). Other sedative and hypnotic components of V. officinalis include valerianone, flavonoids, and valeric acid. These compounds could act synergistically on γ-aminobutyric acid (GABA) receptors in the cerebral cortex, promote GABA release, and inhibit the combination of GABA with receptors to regulate the function of the CNS (Ortiz et al., 1999; Marder et al., 2003). The 70% ethanol extract of V. officinalis reduced the sleeping latency and increased the δ-wave activity of non-rapid eye movement, demonstrating that V. officinalis extract could not only induce sleep but also improve sleep quality (Shinomiya et al., 2005; Tokunaga et al., 2007). In terms of promoting sleep, the petroleum ether extract of V. amurensis roots and rhizomes showed a similar effect as V. officinalis but markedly increased the levels of the neurotransmitters GABA and 5-hydroxytryptamine (5-HT) in mouse brain tissue. These results indicate that the improving sleep effect of V. amurensis was due to regulation of the levels of neurotransmitters in the brain (Chen J. S. et al., 2013). The detailed information of sedative and hypnotic effects for Valeriana is summarized in Table 1.
3.2 Antispasmodic and analgesic
The essential oil of Valeriana not only showed effectiveness in calming and helping sleep but also exhibited significant antispasmodic and analgesic effects (Wang X. et al., 2019), such as the ethanol extract of V. wallichii. In the ileum test of guinea pigs, V. wallichii exhibited a more powerful antispasmodic effect than papaverine (Wagner and Jurcic, 1979). Similarly, methanol aqueous extract from V. hardwickii rhizome relaxed the spontaneous contractions on an isolated rabbit jejunum. The antispasmodic effect of V. hardwickii was verified to be related to its iridoids, which alleviated the contracture of smooth muscle cells (Gu et al., 2004; Gilani et al., 2005, 2007). In contrast, the antispasmodic effect of chloroform and aqueous extracts from V. jatamansi was verified by experiments of the rabbit jejunum, rabbit aorta, and guinea pig ileum, which might be attributed to the activation of K+-ATP channels (Chen et al., 2012). The analgesic effect of the V. jatamansi aqueous extract was evaluated with a hot plate, radiation hot stimulation, and double forearm experiments. As a result, the aqueous extract of V. jatamansi displayed a noticeable analgesic effect and demonstrated that strong polar constituents were the effective components (Mao et al., 2008). The peripheral analgesic effect of essential oil from V. jatamansi was demonstrated through acetic acid-induced writhing and tail flicking experiments in mice, and the effect was attributed to the essential oil inhibiting the prostaglandin synthesis (Sah et al., 2010a).
3.3 Antidepressant and anxiolytic
The aqueous extract of V. officinalis was capable of improving the behavioral activities of depressive model rats by promoting the level of 5-HT, the proliferation of hippocampal neurons, and the expression of phosphorylated cyclic adenosine monophosphate (cAMP) responsive element-binding protein (Tang et al., 2008a; Tang et al., 2008b; Zhou et al., 2010). The antidepressant effect of V. officinalis aqueous extract was also reflected in its improvement of the depressive behavior of ovalbumin-sensitized rats (Hosseini et al., 2014). Further study confirmed that the antidepressant effect of V. officinalis aqueous extract was due to promoting the proliferation of neural stem cells and reducing the production of caspase-3 positive neurons (Qin et al., 2009). Total iridoids of V. jatamansi effectively improved the sucrose preference, immobility time, and depression-like behaviors of the depressive mouse model induced by chronic unpredictable mild stress, as well as the levels of 5-HT, norepinephrine (NE), substance P, and corticotropin-releasing factor (CRF) expression in the hippocampus and colon. Therefore, the antidepressant effect of total iridoids from V. jatamansi was involved in the brain–gut axis. Further analysis of the metabolic markers in depressive mice revealed that the antidepressive effect of the total iridoids from V. jatamansi was related to the metabolic pathways of the tricarboxylic acid cycle, the synthesis of neurotransmitters, and amino acid metabolism (Wang L. W. et al., 2019; Li et al., 2020a, 2020b). A dichloromethane extract from roots and rhizomes of V. wallichii at 40 g/kg markedly decreased the immobility period of depressive mice, and the effect was involved in the extract increasing the NE and dopamine levels in the forebrain (Sah et al., 2011b). The same experiment was performed on the essential oil from the roots and rhizomes of V. wallichii. However, V. wallichii essential oil could not only increase the level of norepine in depressive mice but could also remarkably increas the level of serotonin in the forebrain, which was attributed to the inhibition of the NO signaling pathway (Sah et al., 2011c). Another study showed that the ethanol extract of V. fauriei roots could reduce the immobility time of chronic restraint stress-induced depressive mice by downregulating the level of corticosterone in serum. A more in-depth study found that the ethanol extract of V. fauriei improved the expression of C-Fos, p-p38, cyclooxygenase-2 (COX-2), and iNOS and microglial activation in the prefrontal cortex, hippocampus, and amygdala of depressive mice. In addition, the ethanol extract of V. fauriei strengthened the stimulation of nuclear factor erythroid 2-related factor 2 pathways and upregulated the expression of brain-derived neurotrophic factor (BDNF). Therefore, the antidepressant effect of V. fauriei was associated with anti-inflammatory and antioxidant activities (Choi et al., 2018). The mechanisms of the antidepressant effect of Valeriana are shown in Figure 1.
In the plus maze test, the 50% ethanol extract of V. officinalis roots improved the anxiety behavior of rats. Specifically, rats administered the 50% ethanol extract of V. officinalis roots, which spent more time on the open arms. Valerenic acid present in the 50% ethanol extract was the primary anxiolytic constituent (Murphy et al., 2010). Similarly, in the dark/light preference tank test, the 50% ethanol extract of V. officinalis roots increased the residence time of zebrafish on the white side, which was attributed to its interaction with metabotropic glutamate receptors of zebrafish (Valle-Mojica and Ortíz, 2012). The 95% ethanol extract of V. jatamansi markedly increased the percentages of anxiety model rats in open arm testing by reducing blood β-endorphin and corticosterone levels. Therefore, the anxiolytic effect of V. jatamansi was due to the regulation of hypothalamic pituitary adrenal axis dysfunction (Yan et al., 2010; Zhao et al., 2021). The detailed information of antidepressant and anxiolytic effects for Valeriana is summarized in Table 1.
3.4 Anticonvulsant and antiepileptic
At present, it is generally believed that the anticonvulsant and antiepileptic effects of Valeriana are closely related to its regulation of GABA levels in the brain (Zhang et al., 2014). The aqueous extract of V. officinalis roots not only increased the sleeping time of pelltobarbitalum natricum-treated mice but also reduced the times of forelimb lifting, increased the seizure threshold, and prolonged the seizure latency of convulsions induced by pentylenetetrazole (PTZ). PTZ is a GABA receptor antagonist that interacts with GABA receptor to induce convulsions. Therefore, the anticonvulsant effect of V. officinalis is due to an increase in the levels of GABA neurotransmitters in convulsion mice (Wu et al., 2005; Nouri and Abad, 2011). Another similar study further confirmed that the aqueous extract of V. officinalis inhibited the absorption of [3H] GABA and promoted its release, which led to an increase in GABA concentration in synapses (Santos et al., 1994). The aqueous extract of V. jatamansi enhanced the sedative and hypnotic effects of pelltobarbitalum natricum on mice, which led to inhibition of spontaneous activity and writhing in mice. In addition to prolonging the latency period of seizures induced by picrotoxin, the aqueous extract of V. jatamansi resisted the convulsions induced by thiosemicarbazide (TSZ). TSZ is an inhibitor of GABA synthetase (glutamic acid decarboxylase), which leads to a decrease in GABA levels in the brain to induce convulsions. As a GABA receptor antagonist, picrotoxin interacts with the GABA receptor to induce convulsions. Therefore, the anticonvulsant effect of aqueous extract from V. jatamansi might be associated with the increase in GABA levels in the convulsion mouse model (Cao and Hong, 1994).
The essential oil from V. officinalis gradually alleviated the convulsive state of epileptic rats caused by PTZ. Moreover, the increased levels of GABA and decreased levels of glutamate in the hippocampus of epileptic rats indicated that the antiepileptic effect of the essential oil from V. officinalis was attributed to regulating the balance of excitatory and inhibitory amino acids in the brains of epileptic rats (Wu et al., 2008). Total iridoids of V. officinalis roots and rhizomes reduced the number of seizures of PTZ-induced epileptic rats, reduced the seizure time, and noticeably reduced the expression of the GABA uptake transporter GAT-1 in the hippocampus of epileptic rats. Therefore, the antiepileptic effect of total iridoids of V. officinalis might be related to enhancing the effect of GABA by inhibiting GAT-1 activity (Luo et al., 2004, 2005). The aqueous extract of V. officinalis roots reduced the discharge duration and duration of stage 5 seizures, prolonged latency to the onset of bilateral forelimb clonuses, and reduced seizure activity in an amygdala-kindled temporal lobe epilepsy rat model induced by 8-cyclopenthyl-1,3-dimethylxanthine. Moreover, the anticonvulsant effect of V. officinalis was decreased by 8-cyclopenthyl-1,3-dimethylxanthine, which is a selective adenosine A1 receptor antagonist. Therefore, the anticonvulsant effect of V. officinalis might be due to the activation of the adenosine system (Rezvani et al., 2010). The detailed information of anticonvulsant and antiepileptic effects for Valeriana is summarized in Table 1.
3.5 Neuroprotective
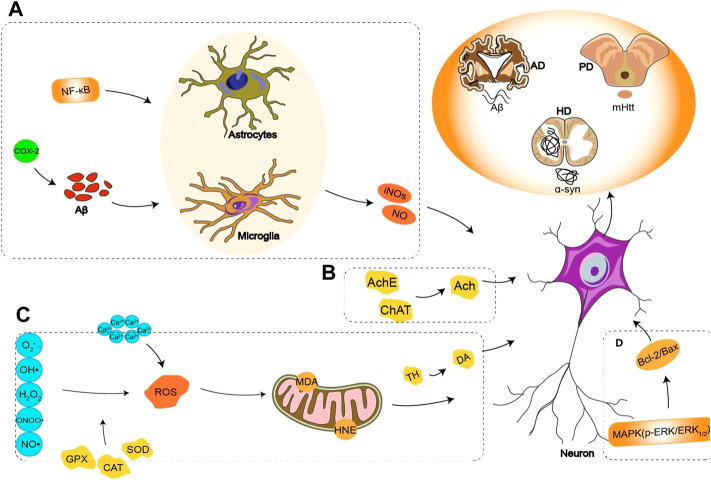
Valeriana can be used to treat nervous system diseases, such as PD, Alzheimer’s disease (AD), and Huntington’s disease, which are mostly related to brain neuron injuries or apoptosis. Therefore, some scholars have carried out studies on the neuroprotective effect of Valeriana in recent years. The ethanol extract of V. officinalis significantly improved the learning, memory, and autonomous activities of AD model rats. On the one hand, V. officinalis increased the level of catalase and total antioxidative capacity (T-AOC) in serum and reduced the activity of acetylcholinesterase. On the other hand, V. officinalis increased the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and reduced the level of lipid peroxide malondialdehyde (MDA). Therefore, the neuroprotective effect of V. officinalis should be involved in improving the function of the cholinergic nervous system and antioxidation (Yoo et al., 2015; Zhang and Zuo, 2018). The neuroprotective effect of the 70% ethanol extract from V. officinalis at 10 ng/ml–100 μg/ml was evaluated on Aβ25–35-induced hippocampal neuronal toxicity in rats, and the decrease in neuronal cell viability and number in the brain was prevented. The mechanisms involved inhibiting excess influx of Ca2+ following neuronal injury and partially inhibited ascorbate/iron-induced peroxidation (Malva et al., 2004). In addition, experiments on Drosophila melanogaster showed a protective effect of the aqueous extract from V. officinalis roots at 10 mg/ml on 500 mM rotenone-induced neuronal toxicity, and the restored expression of SOD and catalase mRNA confirmed that V. officinalis could represent a potential therapeutic strategy for neurodegenerative diseases, including PD (Sudati et al., 2013). Iridoids from V. jatamansi showed a significant protective effect against MPP+-induced SH-SY5Y cell death (Xu et al., 2012c). Using a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (PMTP) induced mouse PD model to evaluate the neuroprotective effect of V. wallichii, the results showed that the 50% methanol extract of V. wallichii upregulated the levels of dopamine and tyrosine hydroxylase, increased the number of midbrain tyrosine hydroxylase positive cells, enhanced antioxidant activity, reduced reactive oxygen species (ROS), lipid peroxidation (LPO), and inflammatory cytokines (Subhashree et al., 2015). The 50% ethanol eluted fraction (in macroporous adsorption resin column chromatography) from a 95% ethanol extract of V. amurensis roots and rhizomes could effectively inhibit the overexpression of β-APP- and Aβ1–40-positive cells in brain neurons of the AD rat model and prevent β-APP and Aβ1–40 aggregation in the brain. This fraction reduced the apoptosis or injury of neurons in the brain by inhibiting the activation of caspase-3, decreasing the activity of COX-2 and microglia and astrocytes and reducing the overexpression of inducible nitric oxide synthase (iNOS) and the inflammatory injury to cortical and hippocampal neurons to improve spatial exploration memory in AD rats (Zhang Z. L. et al., 2010; Janaína et al., 2018). The neuroprotective mechanism of V. amurensis was also examined in an AD mouse model established by intrahippocampal injection of Aβ1–42 in our previous study. The results showed that the 50% ethanol extract of V. amurensis roots and rhizomes remarkably improved cognitive function in AD mice by enhancing the activity of choline acetyltransferase and increasing the level of acetylcholine in the cerebral cortex and hippocampus of mice. The extract of V. amurensis also activated the p-ERK and Bcl-2 signaling pathways and inhibited the Bax pathway to protect brain neurons from Aβ1–42-induced apoptosis (Wang et al., 2013). According to our previous study, the lignans in V. amurensis might be responsible for the neuroprotective activity (Wang et al., 2021). Other experiments demonstrated that valeric acid in V. wallichii essential oil was similar to the structure of the neurotransmitter GABA, which could noticeably improve experimental dementia (Vishwakarma et al., 2016). In addition, V. officinalis tincture effectively reduced the volume of cerebral infarction, the density of C-Fos- and C-Jun-positive cells in the hippocampus and the degree of neuronal damage in the cerebral cortex in rats with experimental reversible middle cerebral artery occlusion. Therefore, the effect of V. officinalis on focal cerebral ischemia was associated with the inhibition of the expression of C-Fos and C-Jun in the hippocampus (Wang et al., 2004). The signaling pathways related to the neuroprotective effect of Valeriana are shown in Figure 2, and the detailed information of neuroprotective effects for Valeriana is summarized in Table 1.
FIGURE 2.
Signal pathways related to neuroprotective effect of Valeriana: (A) anti-inflammatory, inhibiting the activation of caspase-3, decreasing the activity of COX-2 and microglia and astrocytes, reducing the overexpression of iNOS and the inflammatory injury to cortical and hippocampal neurons. (B) Enhancing the activity of ChAT and increasing the level of Ach in the cerebral cortex and hippocampus. (C) Antioxidant, increasing the activities of SOD and GSH-Px and reducing the level of lipid peroxide MDA, upregulating the levels of DA and TH, increasing the number of midbrain tyrosine hydroxylase positive cells, enhancing antioxidant activity. (D) Anti-apoptotic, activating the p-ERK and Bcl-2 signaling pathways and inhibiting the Bax pathway to protect the brain neurons from Aβ1-42 induced apoptosis.
3.6 Cardiovascular and cerebrovascular system improvement
The effects of Valeriana on the cardiovascular and cerebrovascular systems include myocardial protection, antiarrhythmia, and anticerebral ischemia-reperfusion injury. V. officinalis aqueous extract noticeably alleviated myocardial spasm in an isolated cardiac ischemia–reperfusion rat model, which resulted in more regular, powerful, and smooth myocardium contraction and relaxation. Further study revealed that V. officinalis reduced the activity of lactic dehydrogenase, phosphochain kinase (CK), and the levels of MDA. In contrast, SOD, GSHPx, and adenosine triphosphatase activities were increased in cardiomyocytes, and Ca2+ levels in cardiomyocytes were remarkably reduced. Therefore, the protective effect of V. officinalis aqueous extract on myocardial ischemia-reperfusion injury was closely related to the decrease in Ca2+ concentration in cardiomyocytes and antilipid peroxidation (Yang et al., 2012). Another study showed that the protective effect of V. officinalis on myocardial ischemia–reperfusion injury might occur through inhibiting xanthine oxidase, reducing the production of free radicals, increasing the value of prostacyclin 2/thromboxane A2, inhibiting platelet aggregation, improving coronary microcirculation, decreasing TNF-α production, and reducing aseptic inflammation in the reperfusion area to alleviate myocardial ischemia–reperfusion injury (Yin et al., 2000). The ethanol extract of V. officinalis significantly slowed the heart rate and reduced the blood pressure and the partial pressure ratio of arteriovenous blood oxygen in anaesthetized cats, confirming the effects of reducing myocardial oxygen consumption and expanding coronary vessels (Zhang et al., 1982). The protective effect of Valeriana on myocardial ischemia–reperfusion injury is shown in Figure 3. The aqueous and n-butanol extracts of V. officinalis remarkably delayed the occurrence time of ventricular extrasystole and ventricular fibrillation (VF) induced by aconitine in rats and reduced the incidence of VF. In contrast, the essential oil and ethyl acetate extracts of V. officinalis significantly reduced the incidence of VF induced by chloroform in mice. The arrhythmia induced by aconitine in rats is caused by exciting the myocardium directly, opening the myocardial Na+ channel, and accelerating the Na+ influx of cardiomyocytes. Chloroform-induced VF in mice is related to the induction of adrenal medulla to secrete adrenaline to activate β receptors. Therefore, the antiarrhythmic effect of V. officinalis was associated with the inhibition of Na+ influx and blocking of cardiomyocyte β receptors (Wen et al., 2009). The essential oil from V. officinalis increased the uptake of technetium-99 m ethyl cysteinate dimer in mouse brain cells, brain radiation count and brain blood ratio to improve the microcirculation perfusion of mice brain tissue. V. officinalis essential oil also exhibited an antagonistic effect on acute cerebral ischemia caused by NE and significantly improved the blood supply insufficiency of brain cells. The mechanism might involve relieving arterial spasm, increasing cerebral blood flow, inhibiting platelet aggregation, and improving microcirculation (Li et al., 2004).
The essential oil of V. officinalis inhibited the contraction of rat vascular smooth muscle cells induced by angiotensin II, which confirmed the effect of dilating blood vessels to reduce blood pressure. The effect could not be blocked by the NOS inhibitor N′-nitro-L-arginine-methylesterhydrochloride, indicating that the inhibitory effect of the essential oil of V. officinalis on vascular smooth muscle cell contraction was not due to endogenous NO (Yang et al., 2002). The aqueous extract from V. officinalis accelerated the peak systolic velocity of the basilar artery and increased the diameter of the basilar artery after subarachnoid hemorrhage in rabbits, which dilated the spastic cerebral artery and reduced cerebral vasospasm effectively. The effect might be involved in reducing the damage to vascular endothelial cells by scavenging free radicals, inhibiting inflammation, preventing platelet, and leukocyte aggregation and adhesion, and inhibiting the immune response (Luo et al., 2001). The essential oil of V. officinalis shortened the duration of arrhythmia in rats caused by BaCl2, reduced its incidence, antagonized arrhythmia in mice induced by CaCl2, and reduced the number of mouse deaths, suggesting that V. officinalis plays an antiarrhythmic role as a Ca2+ antagonist (Chen and Yu, 1990). The detailed information of cardiovascular and cerebrovascular system improvements for Valeriana is summarized in Table 1.
3.7 Antibacterial and antiviral
The total alkaloids and essential oil of V. officinalis showed remarkable antibacterial effects and were particularly effective against Gram-positive bacteria. V. officinalis essential oil exhibited broad-spectrum antibacterial activity. The minimum inhibitory concentration (MIC) values ranged from 62.5 to 400 μg/ml, whereas the IC50 values ranged from 36.93 to 374.72 μg/ml. V. officinalis essential oil also showed inhibitory activity against fungi. For example, V. officinalis essential oil exhibited moderate inhibitory activity against the growth of Candida albicans and inhibited magnaporthe oryzae spore germination (Wang Y. F. et al., 2010). The essential oil of V. jatamansi whole plants exhibited potential antibacterial activity against Pseudomonas aeruginosa, Bacillus pumilus, Escherichia coli, Staphylococcus aureus, Candida albicans, and Staphylococcus epidermidis (Agnihotri et al., 2011). The 50% ethanol extract of V. jatamansi showed powerful antibacterial activity against the pathogens Micrococcus luteus, Escherichia coli, Escherichia coli mutans, Salmonella abony, Lactobacillus plantarum, and Staphylococcus epidermidis and exhibited antibacterial activity against multidrug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa. The antibacterial components of the ethanol extract were confirmed to be alkaloids (Babu et al., 2015). Both chloroform and n-hexane extracts of V. wallichii leaves showed noticeable antibacterial activity against Staphylococcus aureus and Bacillus subtilis, and the latter also exhibited antibacterial activity against Bacillus subtilis and Microsporum canis. Chloroform and aqueous extracts from V. wallichii leaves were efficient inhibitors of Microsporum canis and Aspergillus flavus (Khuda et L., 2012). In addition, the methanol extract of V. wallichii rhizomes inhibited HCV (Ganta et al., 2017). As a Rev-transport inhibitor, valtrate from V. fauriei showed a potential anti-HIV effect, which inhibited the P-24 production of HIV-1 virus but without any toxicity to host MT-4 cells (Murakami et al., 2002).
3.8 Cytotoxic and antitumor
Studies on the cytotoxic or antitumor activities of Valeriana have focused on their iridoid constituents. Valtrate, didrovaltrate, and baldrinal in V. wallichi exhibited powerful cytotoxicity to liver cancer cells. Among them, valtrate with the strongest cytotoxicity, and its activity was twice as toxic as didrovaltrate and eight times as toxic as baldrinal. Didrovaltrate had a more rapidly toxic effect on hepatoma cells. The dose-effect relationship study showed that the hepatoma cells died after 2 h of exposure to 66 μg/ml didrovaltrate, and all hepatoma cells died 5 h later (Bounthanh et al., 1981). Another study showed that didrovaltrate significantly inhibited the proliferation of Kreb’s II ascites cancer cells at a concentration of 100 mg/kg (Hude et al., 1986).
V. jatamansi iridoid compounds didrovaltrate acetoxyhydrin, volvaltrate B, isovaleroxyhydroxy-dihydrovaltrate, 10-acetylpatrinoside, jatamanvaltrate Z1-Z3, valtrate, and acevaltrate exhibited moderate cytotoxicity against the metastatic prostate cancer (PC-3M), hepatoma (Bel7402), lung adenocarcinoma (A549), and colon cancer (HCT-8) cell lines with IC50 values ranging from 1.0 to 8.5 μM (Lin et al., 2014; Xie et al., 2019). Valejatanin A displayed powerful cytotoxicity against cancer cell lines, including human colon carcinoma HT29, human leukemia K562 and mouse melanoma B16 with IC50 values of 22.17, 15.26, and 3.53 μg/ml, respectively. Compounds 8-acetoxypatchouli alcohol and valerol A showed moderate activities against mice melanoma B16 cell lines with IC50 values of 31.43 and 30.78 μg/ml, respectively (Quan L. Q. et al., 2019). Another study showed that 8,9-dihydro-7-hydroxy-dolichodial and valeridoid F were cytotoxic to three types of human glioma stem cells and inhibited their growth effectively. The IC50 values of 8,9-dihydro-7-hydroxy-dolichodial and valeridoid F against GSC-3 were 35.84 and 42.45 μM, respectively. The respective IC50 values against GSC-12 were 36.67 and 41.40 μM. The respective IC50 values against GSC-18 were 30.19 and 47.55 μM (Quan L. Q. et al., 2020). Iridoids of valtrate, didrovaltrate, and baldrinal could induce apoptosis of MKN-45 gastric cancer cells, which might be related to increased expression of caspase-3 and caspase-9 in MKN-45 gastric cancer cells. Further studies showed that iridoids of valtrate, didrovaltrate, and baldrinal upregulated P53 protein expression and downregulated survivin protein expression in MKN-45 gastric cancer cells (Ye et al., 2004, 2007). The valepotriate in V. wallichii exhibited strong toxic effects on untransformed mouse early hematopoietic progenitor cells (CFU-GM and CFU-EOS) and human peripheral blood T-lymphocytes (Tortarolo et al., 1982). The inhibitory rate of valepotriate at concentrations of 100–150 mg/kg on transplanted tumor S180 in mice was 58%–68%. In addition, the survival time of Ehrlich ascites cancer mice was prolonged by 62%–66%, and the formation of erythrocyte rosettes in mice was also increased. Histopathological observation showed that valepotriate induced flake necrosis in the center of the tumor mass, which was surrounded by a large number of lymphocytes and macrophages. Therefore, valepotriate not only exhibited a significant antitumor effect but also enhanced the immune function of mice (Zhang S. Q. et al., 2010). In addition, the total flavonoids of V. jatamansi remarkably reduced the average tumor weight of liver cancer H22 mice, and the tumor inhibition rate was between 25% and 31%. These effects were associated with the inhibition of the JAK/STAT signaling pathway (Yan et al., 2011).
3.9 Others
In addition to the aforementioned pharmacological effects, other studies also reported the hepatoprotection of V. officinalis (Prasad et al., 2010), anti-inflammatory effects of V. wallichii flavonoids and tannins (Subhan et al., 2007), antioxidant effects of V. jatamansi flavonoids and tannins (Thusoo et al., 2014), inhibition of acute edema by V. jatamansi essential oil (Agnihotri et al., 2011), anticholinesterase effects of V. jatamansi iridoids and sesquiterpenoids (Dong et al., 2015b), and regulation of gastrointestinal disorder of V. jatamansi iridoids (Jugran, et al., 2019).
4 Phytochemistry
4.1 Iridoids
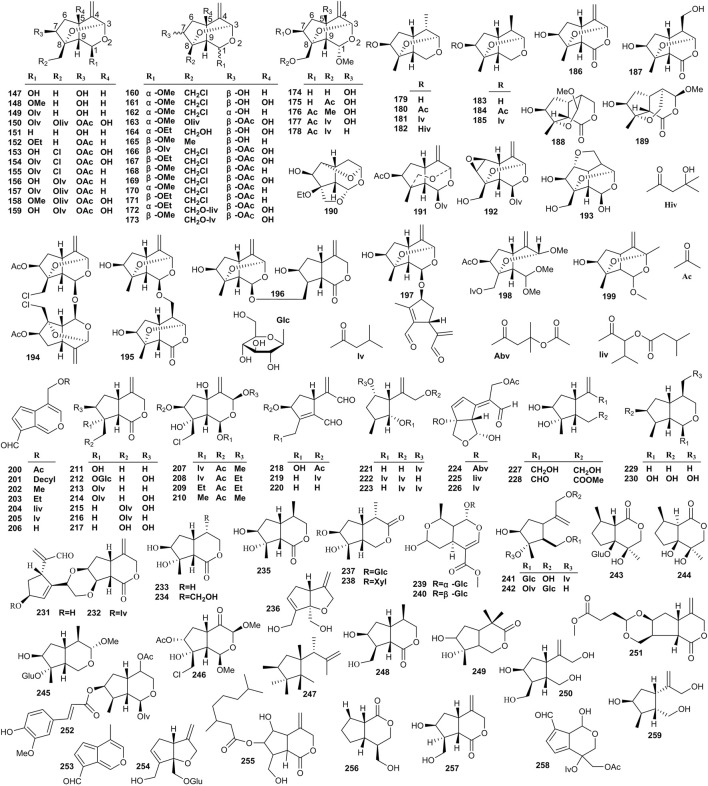
At present, 259 iridoids have been identified in Valeriana. Iridoids are acetal derivatives of iridoidium, which are mainly divided into monoethenoids, dienes, and oxygen bridges (Wang R. J. et al., 2016). The substitution of various acyl groups on the mother nuclear structure led to the structural diversity of iridoids, such as acetyl, isovaleryl, acetoxy isovaleryl, isovaleroxylisovaleroxyl, methyisovaleroxyl, and hydroxylisovaleroxyl (Liu, 2020). Generally, the structures of monoethenoid iridoids contain only one double bond located between C-3 and C-4 (Lin et al., 2009), that is, compounds 1–65 (Figure 4). The structures of diene iridoids contain two double bonds located between C-3 and C-4 and between C-5 and C-6, but some are located between C-4 and C-5 and between C-6 and C-7 (Xu et al., 2012a; Lin et al., 2014). The diene iridoids include compounds 66–146 (Figure 4).
The structures of oxygen-bridge iridoids contain an ether bond between C-3 and C-8 or between C-3 and C-10, and dioxygen bridge cage fragments exist in a minority of oxygen-bridge iridoids (Lin et al., 2010a). The oxygen-bridge iridoids include compounds 147–199 (Figure 5). In addition to the aforementioned three types of iridoids, other types of iridoids were also isolated from Valeriana. These compounds with lactone fragments formed a double bond out of the ring between C-4 and C-11. A free hydroxyl substitution typically occurs in these compounds, and relatively few acyl substituents are noted. Such compounds are formed when the A ring (five-membered ring) or B ring (six-membered ring) in the structure is split, and the substitution of hydroxyl, methoxy, ester and other groups occurs (Liu, 2020). Other iridoids include compounds 200–259 (Figure 5).
FIGURE 5.
Structures of oxygen-bridge (147–199) and other (200–259) iridoids from the genus Valeriana.
Iridoids are the main components with cytotoxic activities in Valeriana. Most compounds of iridoids were separated from the whole plants or roots and rhizomes of V. jatamansi, V. officinalis, V. amurensis, V. dioscoridis, and V. sorbifolia, such as jatamanvaltrate A-H (1–8), didrovaltrate acetoxyhydrin (11) (Wang et al., 2009a; Lin et al., 2009; Yu et al., 2010; Liu et al., 2021), chlorovaltrate E-K (16–22) (Lin et al., 2013), jatamanvaltrate L (26) and M (27), 5-hydroxydidrovaltrate (28), and didrovaltrate (32) (Thies, 1968b; Lin et al., 2009). Among them the diene iridoids showed more powerful cytotoxic activity on some tumor cell lines; for example, compounds, such as chlorovaltrate (98), rupesin B (99), valtrate (107), and acevaltrate (109) showed cytotoxic activity against metastatic prostate cancer, lung adenocarcinoma, hepatoma, and colon cancer cell lines (Lin et al., 2009; 2010b, 2013).
Thies et al. (1968a) isolated and identified valtrate (107, also known as valepotriate) from Valeriana for the first time and preliminarily demonstrated its sedative activity (Zhang et al., 2014). Valtrate mainly exists in the roots and rhizomes of V. jatamansi and the whole plants of V. officinalis. Other compounds with sedative activity were isolated from the aerial parts or roots and rhizomes of V. jatamansi and V. sorbifolia, including isovaltrate (108), baldrinal (200), and decyl baldrinal (201) (Thies, 1968b; Xu et al., 2007; Su, 2016). Some iridoids from Valeriana also exhibit neuroprotective activities. For example, jatamanvaltrate G (7) and H (8), valeriotriate B (9), and valeriandoid C (156) exhibited moderate neuroprotective effect against the neuronal SH-SY5Y cell model induced by 1-methyl-4-phenylpyridinium (MPP+) (Xu et al., 2011a; 2012b). Suspensolide F (14), jatadoid B (24), patrinoside (58), and kanokoside A (60) displayed significant neuroprotective effects against PC12 cell injury induced by Aβ25–35 (Wang et al., 2012a; Wan et al., 2016). Patrinoside-aglucone (41) and stenopterin B (54) showed promoting effects on NGF-induced neurite outgrowth in PC12 cells (Dong et al., 2015a). In addition, (4β,8β)-8-methoxy-3-methoxy-10-methylene-2,9-dioxatricyclo [4.3.1.0] decan-4-ol (165) (Quan L. Q. et al., 2019), isopatrinide (252), and vibutinal (253) showed neuroprotective effects against PC12 cell death induced by CoCl2 (Tan et al., 2016; Wang et al., 2017).
In addition, a study showed that jatadomin A (198), B (158), C (178), D (53), E (246), and jatamanvaltrate Q (89) exhibited anti-inflammatory activities by inhibiting nitric oxide (NO) release in murine microglial BV-2 cells induced by lipopolysaccharide (LPS) (Wang H. M. et al., 2020). Similarly, the anti-inflammatory activities of jatamanvaltrate B (2), E (5), and W (123), valeriotetrate C (15), 10-isovaleroxy-valtrathydrin (69), and patriscadoid I (124) were confirmed in RAW 264.7 cells stimulated with LPS (Liu et al., 2021). The details are shown in Supplementary Table S1 of the supplementary material.
4.2 Lignans
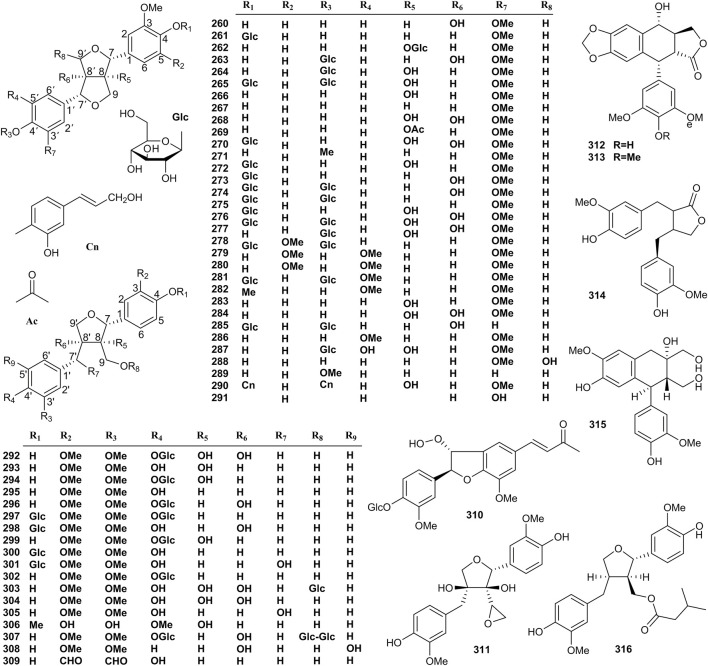
In recent years, more than 57 lignans have been isolated from Valeriana, which are divided into types of furofurans (260–291), tetrahydrofurans (292–309, 311, and 316), and others (310 and 312–315). The structures of lignans are shown in Figure 6. Lignans are the main components responsible for the neuroprotective effect of Valeriana. Specifically, furofurans lignans of pinoresinol-8-O-β-D-glucopyranoside (262), pinoresinol-4,4′-di-O-β-D-glucoside (265), 8-hydroxypinoresinol (266), pinoresinol (267), prinsepiol (268), prinsepiol-4-O-β-D-glucopyranoside (270), 8-hydroxypinoresinol-4,4′-di-O-β-D-glucopyranoside (274), 8,8′-di-hydroxyl-pinoresinol-4,4′-di-O-β-D-glucopyranoside (277), syringaresinol-4,4′-di-O-β-D-glucopyranoside (279), (+)-medioresinol-4,4′-di-O-β-D-glucopyranoside (282), tetrahydrofurans lignans of olivil-4′-O-β-D-glucopyranoside (296), lariciresinol-4,4′-di-O-β-D-glucopyranoside (297), olivil-4-O-β-D-glucopyranoside (298), 8-hydroxylariciresinol-4′-O-β-D-glucopyranoside (299), lariciresinol-4-O-β-D-glucopyranoside (300), neoarctin A (301), lariciresinol-4′-O-β-D-glucopyranoside (302), and massoniresinol-3a-O-β-D-glucopyranoside (303) exhibited significant protective effects against amyloid-beta (Aβ)-induced neurotoxicity in PC12 cells (Wang et al., 2012a; Wang et al., 2014 C. F.; Kırmızıbekmeza et al., 2018). Compounds 266 and 268 showed powerful antioxidant activity, and 266 also exhibited an appreciable vasodilation activity (Piccinelli et al., 2004). 4′-O-β-D-glucosyl-9-O-(6″-deoxysaccharosyl) olivil (307) was demonstrated to be a partial agonist of A1 adenosine receptors in rats and humans (Schumacher et al., 2002), and the inhibitory effect of (−)-matairesinol (314) on NO production in RAW 264.7 macrophages induced by LPS suggested that 314 exhibited obvious anti-inflammatory effect (Xie et al., 2019), respectively. By contrast, 4′-demethylpodophyllotoxin (312) and podophyllotoxin (313) showed significant cytotoxic activity, so it is not surprising that 313 and its derivatives are extensively applied as anticancer drugs (Glaser et al., 2015).
FIGURE 6.
Structures of lignans from the genus Valeriana.
The activities of other lignans, including 8′-hydroxypinoresinol (260), pinoresinol-4-O-β-D-glucopyranoside (261), 8′-hydroxypinoresinol-4′-O-β-D-glucopyranoside (263) (Schumacher et al., 2002; Wang et al., 2012b; Quan L. Q. et al., 2020), and (+)-1-acetoxypinoresinol (269) (Fan et al., 2020), have not been determined in a phytochemical study of Valeriana. The details are shown in Supplementary Table S2 of the supplementary material.
4.3 Flavonoids
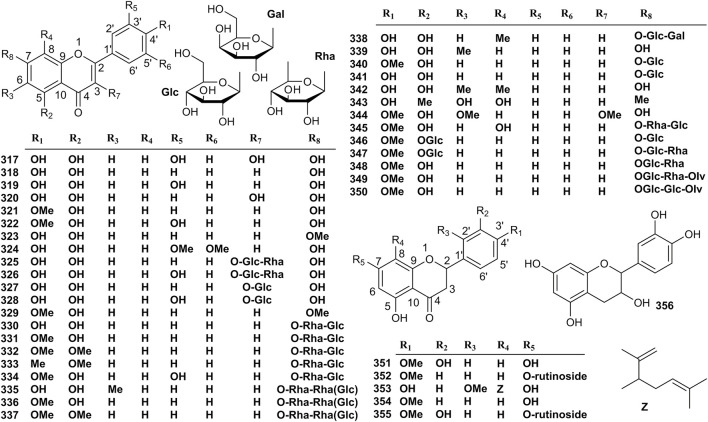
In recent years, 40 flavonoids have been isolated from Valeriana. The structures of 317–356 are shown in Figure 7. Compound 2S (-)-hesperidin (351) showed sedative and sleep enhancing activities, whereas 6-methylapigenin (339) exhibited anxiolytic and sleep-enhancing properties (Marder et al., 2003). In addition, some studies have proven that acacetin (321) and hesperidin (350) are the effective constituents with neuroprotective activity both in vitro and in vivo in Parkinson’s disease (PD) models (Kim et al., 2012; Tamilselvam et al., 2013; Antunes et al., 2014). The activities of other flavonoids, including quercetin (317), apigenin (318), luteolin (319), kaempferol (320), diosmetin (322), genkwanin (323), tricin (324) (Wang J. H. et al., 2010; Zhao et al., 2011; Chai et al., 2015), kaempferol-3-O-β-rutinoside (325), rutin (326), kaempferol-3-O-β-D-glucopyranoside (327), and quercetin-3-O-β-D-glucopyranoside (328) Tang et al. (2003) have not determined in phytochemical studies of Valeriana. The details are shown in Supplementary Table S3 of the supplementary material.
FIGURE 7.
Structures of flavonoids from the genus Valeriana.
4.4 Sesquiterpenoids
Eighty-nine sesquiterpenoids (357–445) have been isolated from Valeriana. The structures of sesquiterpenoids are shown in Figure 8. Sesquiterpenoids can enhance the activity of nerve growth factor (NGF). For example, compounds madolin A (357), madolin B (358), volvalerenal A (359), B (360), F (363), G (364), kissoone B (367), kissoone C (368), heishuixiecaoline B (374), 1β-hydroxyl-8α-acetoxyl-11,11-dimethyl-4-formyl-bicyclogermacren-E-4(5),10(14)-diene (375), volvalerenic acid D (378), isobicyclogermacrenal (381), and 4β,8aβ-dimethyl-6β-isopropenyl-3,4,4aα, 5,6,7,8,8a-octahydronaphthalen-1(2H)-one (388) remarkably promoted the effect on the neurite outgrowth of PC12 cells induced by NGF (Guo et al., 2006; Chen H. W. et al., 2013; Dong et al., 2019). Volvalerenal C (361) and heishuixiecaoline A (370), C (372), and B (374) showed protective effects on PC12 cells against Aβ25–35 induced toxicity (Glaser et al., 2015). In addition, volvalerenal D (362), kissoone B (367), and C (368), maaliol (387), 8-acetoxypatchoulol (393), 15-hydroxyspathulenol (420), and caryophyllenol A (435) prolonged Drosophila melanogaster total sleeping time and showed a significant sedative effect (Wu et al., 2014; Dong et al., 2015a). The activities of other sesquiterpenoids had not been determined in the phytochemistry study of Valeriana, including isovolvalerenal D (365), kissoone A (366) (Guo et al., 2006; Wu et al., 2014), and volvalerenic acid A (369), C (371), B (373) (Liu et al., 2012). The details are shown in Supplementary Table S4 of the supplementary material.
4.5 Essential oil
Essential oils are the main active constituents of Valeriana. Essential oil levels generally range from 0.5% to 3%. In some species, these values are up to 6%–8%. In addition, 0.03% essential oil has been reported in individual species (Wang et al., 2009a). To date, more than 385 essential oil constituents have been analyzed and identified from Valeriana, including monoterpenes and sesquiterpenes (Lokar and Moneghini, 1989). Among them, borneol (446), bornyl acetate (447), and bornyl isovalerate (448) are the main constituents of monoterpenes. There are many types of sesquiterpenoids in essential oil of Valeriana, such as valeric acid (449) and valerenal (450) (Chen et al., 2000; Zhou and Huang, 2008a); however, lower sesquiterpenoid levels are noted. Other constituents in the essential oil of Valeriana include α-pinene (460), β-pinene (461), and phellandrene (463) (Taherpour et al., 2010). The essential oil compounds of Valeriana are shown in Supplementary Table S5 of the supplementary material.
Essential oil is the main component with sedative activity in Valeriana demonstrate significantly prolong sleeping time of mice induced by a hypnotic dose of pentobarbital sodium (Ding, 2012). Essential oil also showed potential antibacterial effects against Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus pumilus, Candida albicans, Escherichia coli, and Staphylococcus epidermidis (Agnihotri et al., 2011). Essential oil also exhibited significant anti-inflammatory and antioxidant effects (Pandian and Nagarajan, 2015; Dyayiya et al., 2016).
4.6 Alkaloids
The content of total alkaloids in Valeriana is relatively low. Alkaloids are believed as the constituents with antibacterial effect (Babu et al., 2015; Li and Li, 2019). At present, only 13 alkaloids have been isolated from the roots and rhizomes of Valeriana, including volvalerine A (Wang P. C. et al., 2016), norphoebine, nantenine, nordelporphine, oxoaporphine, phoebine (Inouye et al., 1974), crystalline (Wang, 2007), valerianine (Franck et al., 1970), actindine (Yin et al., 2006), naphtyridylmethylketone (Thies, 1968a), valerine A, valerine B, and valerine (Wang J. H. et al., 2010). In recent years, there have been relatively few studies on the alkaloids of Valeriana due to their low content; thus, these compounds are unlikely to contribute to the therapeutic effect of Valeriana (Thies, 1968a).
4.7 Others
Except for the constituents mentioned earlier, some other compounds were also isolated, namely, caffeic acid, p-coumaric acid, gallic acid (Jugran et al., 2019), isoferulic acid (Wang S. J. et al., 2014), decursitin A, decursitin B, decursidin, 3′(S)-acetoxy-4′(R)-angeloyloxy-3′,4′-dihydroxanthyletin, dibutyl phthalate, phenanthrene, hydroxybenzoic acid, chlorogenic acid, benzoic acid, oleic acid, (-)-bornyl caffeate, tannin (Wang et al., 2009a, Wang et al., 2014 S. J.; Jugran et al., 2019; Wang S. L. et al., 2020), zansiumloside A (Wan et al., 2016), linoleic acid, palmitic acid, nonadecyl alcohol, β-carotene (Wang et al., 2009a, Wang et al., 2014 S. J.; Jugran et al., 2019; Wang S. L. et al., 2020), epoxylathyrol 3,5-dibutyrate (Thies, 1968a and β-sitosterol (Wang S. L. et al., 2020).
5 Conclusion and perspectives
There are more than 200 species of Valeriana, and only the species of V. officinalis, V. jatamansi, and V. amurensis had been systematically studied compared with other species. Most studies on the medicinal parts of Valeriana have focused on their roots and rhizomes or whole plants, and few studies have focused on the potential medicinal parts of leaves, seeds and flowers. To effectively promote the utilization of Valeriana resources, other common species, such as V. hardwickii, V. alternifolia, and V. fauriei should also be the focus of related studies, and these studies should include their different medicinal parts. The traditional efficacies of roots and rhizomes from Valeriana were calming fright and tranquilizing mind, promoting Qi and blood, activating blood circulation and regulating menstruation, dispelling wind and eliminating dampness, regulating Qi-flowing to relieve pain, promoting digestion, and checking diarrhea. The corresponding clinical applications were to treat nervous system diseases of insomnia, anxiety, hysteria, epilepsy, neurasthenia and manic-depressive psychosis, cardiovascular system diseases of palpitation, arrhythmias, coronary heart disease and pulmonary edema, pain and inflammation of rheumatism, rheumatic arthralgia, hepatitis, abdominal distension and pain, gynecological diseases of anemia, menoxenia and dysmenorrhea, dyspepsia of diarrhea, indigestion, and diarrhea and dysentery. As the scientific evidence, the pharmacological effects and effective constituents related to traditional uses of Valeriana are summarized in Table 2. At present, except for treating insomnia, other traditional clinical applications of Valeriana have not been conducted in modern clinical practice. Therefore, more clinical studies should be performed based on the traditional efficacies or uses of Valeriana so that they can be utilized in the treatment of diseases of the nervous system, cardiovascular system, gynecology, dyspepsia, and digestive system. In pharmacological studies, experiments assessing the sedative, hypnotic, antispasmodic, analgesic, antidepressant, anxiolytic, anticonvulsant, antiepileptic, neuroprotective, antibacterial, antiviral, cytotoxic, and antitumor effects as well as cardiovascular and cerebrovascular system improvements, were performed on the extracts or components of Valeriana roots and rhizomes. The phytochemistry of some Valeriana has been deeply investigated and more than 800 compounds have been isolated or identified, including 259 iridoids, 57 lignans, 40 flavonoids, 89 sesquiterpenoids, 13 alkaloids, and 385 essential oils. In vitro activity screening experiments found that lignans exhibited anti-inflammatory and neuroprotective effects, iridoids demonstrated anti-inflammatory and cytotoxic effects, flavonoids exhibited sedative and anti-inflammatory effects, sesquiterpenoids displayed sedative and neuroprotective effects, and essential oils demonstrated sedative effects. However, in fact, most effective fractions and active compounds of Valeriana have not been investigated in-depth studies related to drugs development. Only essential oil with the sedative effect of has been developed to treat insomnia clinically. Therefore, future studies should focus on developing effective fractions or active compounds of Valeriana into new drugs to treat diseases associated with neurodegeneration, cardiovascular and cerebrovascular, inflammation, and tumors. In addition, a large amount of polysaccharides in the water decoction of Valeriana has never been studied. In fact, recent studies on plant polysaccharides showed their extensive pharmacological activities and their medicinal values are worthy of development and utilization.
Compared with other similar reports recently (Jugran, et al., 2019; Dhiman, et al., 2020; Orhan, I. E., 2021), we pointed out for the first time that Valeriana as a traditional Chinese medicine, its potential drugs is far from being effectively developed. In view of this problem, we systematically summarized the available medicinal species of Valeriana, revealing the relationship between their traditional applications, bioactivities, effective fractions, and active constituents, based on which the future study is clarified for their subsequent development and utilization. Overall, our review will promote the development and utilization of potential drugs in Valeriana and avoid wasting their medicinal resources.
Author contributions
Conceptualization, MY; writing—original draft preparation, JL and XL; revision, QW; figures and tables preparation, MZ and CW; and funding acquisition, CW. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 82073995) and Guangzhou Municipal Science and Technology Project (grant number 201804010249).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.973138/full#supplementary-material.
References
- Agnihotri S., Wakode S., Ali M. (2011). Chemical composition, antibacterial and topical anti-inflammatory activity of Valeriana jatamansi Jones essential oil. J. Essent. Oil Bear. Plants 14, 417–422. 10.1080/0972060X.2011.10643596 [DOI] [Google Scholar]
- Alexander N. S., Olga N. P., Valery G. M., Hildebert W., Rob V., Michael H. (2014). Medicinal plants of the Russian pharmacopoeia; their history and applications. J. Ethnopharmacol. 154, 481–536. 10.1016/j.jep.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Andreatini R., Sartori V. A., Seabra M. L. V., Leite J. R. (2010). Effect of valepotriates (valerian extract) in generalized anxiety disor-der: A randomized placebo-controlled pilot study. Phytother. Res. 16, 650–654. 10.1002/ptr.1027 [DOI] [PubMed] [Google Scholar]
- Antunes M. S., Goes A., Boeira S. P., Prigol M., Jesse C. R. (2014). Protective effect of hesperidin in a model of Parkinson's disease induced by 6-hydroxydopamine in aged mice. Nutrition 30, 1415–1422. 10.1016/j.nut.2014.03.024 [DOI] [PubMed] [Google Scholar]
- Babu P., Verma S. K., Mathur A. (2015). Screening of solvent extracts of Valeriana jatamansi for isolation of antibacterial compound. Int. J. Pharma Sci. Res. 6, 2641–2648. 10.13040/IJPSR.0975-8232.6(6).2641-48 [DOI] [Google Scholar]
- Bashir S., Memon R., Gilani A. H. (2011). Antispasmodic and antidiarrheal activities of Valeriana hardwickii Wall. rhizomes are putatively mediated through calcium channel blockade. Evid. Based. Complement. Altern. Med. 2011, 304960–304966. 10.1155/2011/304960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset N. G., Wichtl M., Evans D. H. (2004). Herbal drugs and phytopharmaceuticals: A handbook for practice on a scientific basis. 3rd ed. Boca Raton, FL: CRC Press, 56–58. [Google Scholar]
- Bounthanh C., Bergmann C., Beck J., Haag-Berrurier M., Anton R. (1981). Valepotriates, a new class of cytotoxic and antitumor agents. Planta Med. 41, 21–28. 10.1055/s-2007-971668 [DOI] [PubMed] [Google Scholar]
- Chai S. W., Zhai Y. S., Wang M. Y. (2015). Chemical constituents from whole plants of Valeriana hardwickii . China J. Chin. Mat. Med. 40, 4007–4011. 10.4268/cjcmm20152021 [DOI] [PubMed] [Google Scholar]
- Cao B., Hong Y. X. (1994). Studies on central inhibition action of Valeriana jatamansi Jones. China J. Chin. Mat. Med. 19, 40–42, 63. 10.4268/cjcmm20121431 [DOI] [PubMed] [Google Scholar]
- Castillo P., Márquez J., Rubluo A., Hernández G., Lara M. (2000). Plant regeneration from callus and suspension cultures of Valeriana edulis ssp. procera via simultaneous organogenesis and somatic embryogenesis. Plant Sci. 151, 115–119. 10.1016/S0168-9452(99)00203-4 [DOI] [PubMed] [Google Scholar]
- Chen C., Li S. J., Tang S. H., Wu H. W., Xu H. M., Yang H., et al. (2012). Advance of pharmacological studies on Valeriana jatamansi . Chin. J. Chin. Mat. Med. 37, 2174–2177. [PubMed] [Google Scholar]
- Chen H. W., Chen L., Li B., Yin H. L., Tian Y., Wang Q., et al. (2013a). Three new germacrane-type sesquiterpenes with NGF-potentiating activity from Valeriana officinalis var. latiofolia . Molecules 18, 14138–14147. 10.3390/molecules181114138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Wei B. J., He X. H., Liu Y., Jie W. (2015). Chemical components and cardiovascular activities of Valeriana spp. Evid. Based. Complement. Altern. Med. 2015, 947619–947711. 10.1155/2015/947619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. S., Wu J. K., Ling L., Zhang Y., Wang F. J., Du X. W. (2013b). Studies on improving sleep function and relative mechanism of mice by petroleum extract of Valeriana amurensis . Chin. J. Exp. Tradit. Med. Form. 19, 245–249. [Google Scholar]
- Chen L., Qin L. P., Zheng H. C. (2000). Chemical constituents, plant resources and pharmacological activities of Valerian. J. Pharm. Prac. 18, 277–279. 10.4268/cjcmm20160807 [DOI] [Google Scholar]
- Chen Z. F., Yu F. (1990). Study on the antiarrhythmic effect of essential oil from Cucumis sinensis L. Mod. J. Integr. Tradit. Chin. West. Med. 10, 614–615. [Google Scholar]
- Chinese Pharmacopoeia Committee of People's Repulic of China (2020). Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science Press. [Google Scholar]
- Choi J. H., Lee M. J., Chang Y., Lee S., Kim H. J., Lee S. W., et al. (2018). Valeriana fauriei exerts antidepressant-like effects through anti-inflammatory and antioxidant activities by inhibiting brain-derived neurotrophic factor associated with chronic restraint stress. Rejuvenation Res. 23, 245–255. 10.1089/rej.2018.2157 [DOI] [PubMed] [Google Scholar]
- Dhiman B., Sharma P., Shivani A., Pal P. K. (2020). Biology, chemical diversity, agronomy, conservation and industrial importance of valeriana jatamansi : A natural sedative. J. Appl. Res. Med. Aromat. Plants 16, 100243–100503. 10.1016/j.jarmap.2020.100243 [DOI] [Google Scholar]
- Ding F. (2012). Study on the sedative and hypnotic effective substances and quality standards of Valeriana officinalis L. China: Hubei University of Chinese Medicine. [dissertation]. [Wuhan (HB)]. [Google Scholar]
- Dong F. W., Li F., Ren J. J., Zhao C. M., Diao H. L., Li B. J., et al. (2019). Sesquiterpenoids from the roots and rhizomes of Valeriana amurensis and their effects on NGF-induced neurite outgrowth in pc12 cells. Nat. Prod. Res. 35, 757–762. 10.1080/14786419.2019.1603223 [DOI] [PubMed] [Google Scholar]
- Dong F. W., Liu Y., Wu Z. K., Gao W., Zi C. T., Dan Y., et al. (2015b). Iridoids and sesquiterpenoids from the roots of Valeriana jatamansi Jones. Fitoterapia 102, 27–34. 10.1016/j.fitote.2015.01.021 [DOI] [PubMed] [Google Scholar]
- Dong F. W., Wu Z. K., Yang L., Zi C. T., Yang D., Ma R. J., et al. (2015a). Iridoids and sesquiterpenoids of Valeriana stenoptera and their effects on NGF-induced neurite outgrowth in PC12 cells. Phytochemistry 118, 51–60. 10.1016/j.phytochem.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Du X. W., Wu J. K. (2006). Chemical and pharmacological progress of Valeriana genera. World Phytomedicine 21, 10–14. [Google Scholar]
- Duan X. Y., Fang Y., Zhou Y., Gong Z. F., Liu Y. W. (2008). Overview of comprehensive studies on Valeriana. Chin. Pharm. 11, 793. [Google Scholar]
- Dyayiya N. A., Oyemitan I. A., Matewu R., Oyedeji O. O., Oluwafemi S. O., Nkeh-Chungag B. N., et al. (2016). Chemical analysis and biological potential of Valeriana root as used by herbal practitioners in the eastern cape province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 13, 114–122. 10.4314/ajtcam.v13i1.16 28487901 [DOI] [Google Scholar]
- Eadie M. J. (2010). Could Valerian have been the first anticonvulsant? Epilepsia 45, 1338–1343. 10.1111/j.0013-9580.2004.27904.x [DOI] [PubMed] [Google Scholar]
- Editorial Committee of flora of China, Chinese Academy of Sciences (2004). Flora of China. Beijing: Science Press. [Google Scholar]
- Fan H., Li Y. Z., Liang X. F., Yan S. T., Song X. M., Zhang H., et al. (2020). Chemical constituents isolated from Valeriana officinalis L. Biochem. Syst. Ecol. 93, 104143. 10.1016/j.bse.2020.104143 [DOI] [Google Scholar]
- Franck B., Petersen U., Hüper F. (1970). Valerianie, a tertiary monoterpene alkaloid from valerian (1). Angew. Chem. Int. Ed. Engl. 9, 891. 10.1002/anie.197008911 [DOI] [PubMed] [Google Scholar]
- Ganta K. K., Mandal A., Debnath S., Hazra B., Chaubey B. (2017). Anti‐HCV activity from semi‐purified methanolic root extracts of Valeriana wallichii . Phytother. Res. 31, 433–440. 10.1002/ptr.5765 [DOI] [PubMed] [Google Scholar]
- Gilani A. H., Bashir S., Janbaz K. H., Shah A. J. (2005). Presence of cholinergic and calcium channel blocking activities explains the traditional use of Hibiscus rosasinensis in constipation and diarrhoea. J. Ethnopharmacol. 102, 289–294. 10.1016/j.jep.2005.07.023 [DOI] [PubMed] [Google Scholar]
- Gilani A. H., Bashir S., Khan A. U. (2007). Pharmacological basis for the use of Borago officinalis in gastrointestinal, respiratory and cardiovascular disorders. J. Ethnopharmacol. 114, 393–399. 10.1016/j.jep.2007.08.032 [DOI] [PubMed] [Google Scholar]
- Glaser J., Schultheis M., Moll H., Hazra B., Holzgrabe U. (2015). Antileishmanial and cytotoxic compounds from Valeriana wallichii and identification of a novel nepetolactone derivative. Molecules 20, 5740–5753. 10.3390/molecules20045740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J. Q., Wang Y., Franzblau S. G., Montenegro G., Yang D., Timmermann B. N. (2004). Antitubercular constituents of Valeriana laxiflora . Planta Med. 70, 509–514. 10.1055/s-2004-827149 [DOI] [PubMed] [Google Scholar]
- Guan Y. L., Yang G. L., Li Q. H., Gao Y. G. (2021). Key techniques of artificial cultivation of Naxi traditional medicinal plant Valeriana jatamansi . Agric. Technol. 44, 78–79. 10.14051/j.cnki.xdyy.2021.13.084 [DOI] [Google Scholar]
- Guo Y. Q., Xu J., Li Y. S., Yamakuni T., Ohizumi Y. (2006). Three-membered ring sesquiterpenoids with NGF-potentiating activity from the roots of Valeriana fauriei . Planta Med. 72, 373–375. 10.1055/s-2005-916210 [DOI] [PubMed] [Google Scholar]
- Heinrich M., Appendino G., Efferth T., Furst R., Izzo A. A., Kayser O., et al. (2020). Best practice in research - overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. 10.1016/j.jep.2019.112230 [DOI] [PubMed] [Google Scholar]
- Hendriks H., Geertsma H. J., Malingré T. M. (1981). The occurrence of valeranone and crypto-fauronol in the essential oil of Valeriana officinalis L. s.l. collected in the northern part of the nether lands. Pharm. Weekbl. 3, 1316–1320. 10.1007/BF02193381 [DOI] [Google Scholar]
- Hosseini M., Boskabady M. H., Neamati A., Chaman F. (2014). The effects of Valeriana officinalis L. hydro-alcoholic extract on depression like behavior in ovalbumin sensitized rats. J. Pharm. Bioallied Sci. 6, 97–103. 10.4103/0975-7406.129174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton P. J. (1988). The biological activity of Valerian and related plants. J. Ethnopharmacol. 22, 121–142. 10.1016/0378-8741(88)90123-7 [DOI] [PubMed] [Google Scholar]
- Houghton P. J. (1999). The scientific basis for the reputed activity of Valerian. J. Pharm. Pharmacol. 51, 505–512. 10.1211/0022357991772772 [DOI] [PubMed] [Google Scholar]
- Huang B. K., Zheng H. C., Qin L. P. (2007). The hyprotic and sedative actions of Valeriana and its mechanism. J. Pharm. Pract. 25, 134–142. 10.3969/j.issn.1006-9690.2006.01.003 [DOI] [Google Scholar]
- Huang B. K., Zheng H. C., Qin L. P., Zheng Q., Xin H. (2004). Investigation on resource of genus Valeriana in China. J. Chin. Med. Mat. 27, 632–634. 10.13863/j.issn1001-4454.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Huang B. K., Zheng H. C., Zhang Q. Y., Qin L. P. (2006). Investigation on distribution and ethnomedicinal application of Valeriana pseud officinalis and V. jatamansi in China. Chin. Wild Plant Res. 25, 12–15. [Google Scholar]
- Hude W., Scheutwinkel-Reich M., Braun R. (1986). Bacterial mutagenicity of the tranquilizing constituents of Valerianaceae roots. Mutat. Res. 169, 23–27. 10.1016/0165-1218(86)90013-3 [DOI] [PubMed] [Google Scholar]
- Huynh L., Pacher T., Tran H., Novak J. (2013). Comparative analysis of the essential oils of Valeriana hardwickii Wall. from vietnam and Valeriana officinalis L. from Austria. J. Essent. Oil Res. 25, 409–414. 10.1080/10412905.2013.828325 [DOI] [Google Scholar]
- Inouye H., Ueda S., Uesato S., Shingu T., Thies P. W. (1974). Die absolute konfiguration von valerosidatum and von didrovaltratum. Tetrahedron 30, 2317–2325. 10.1016/S0040-4020(01)97098-X [DOI] [Google Scholar]
- Janaína M. D., Pereira A. O., Zachow L. L., Gehm A. Z., Santos M. Z., Mostardeiro M. A., et al. (2018). Chemical constituents from Valeriana polystachya smith and evaluation of their effects on the acetylcholinesterase and prolyl oligopeptidase activities. Fitoterapia 131, 80–85. 10.1016/j.fitote.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Jugran A. K., Bahukhandi A., Dhyani P., Bhatt I. D., Rawal R. S., Nandi S. K. (2016). Impact of altitudes and habitats on valerenic acid, total phenolics, flavonoids, tannins, and antioxidant activity of Valeriana jatamansi . Appl. Biochem. Biotechnol. 179, 911–926. 10.1007/s12010-016-2039-2 [DOI] [PubMed] [Google Scholar]
- Jugran A. K., Rawat S., Bhatt S. R., Rawal R. (2019). Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 33, 482–503. 10.1002/ptr.6245 [DOI] [PubMed] [Google Scholar]
- Khuda F., Iqbal Z., Khan Z., Nasir F. (2012). Antibacterial and anti-inflammatory activities of leaf extract of Valeriana wallichii DC. Pak. J. Pharma. Sci. 25, 715–719. 10.1124/mol.112.080614 [DOI] [PubMed] [Google Scholar]
- Kim H. G., Ju M. S., Ha S. K., Lee H., Lee H., Kim S. Y., et al. (2012). Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo . Biol. Pharm. Bull. 35, 1287–1294. 10.1248/bpb.b12-00127 [DOI] [PubMed] [Google Scholar]
- Kırmızıbekmeza H., Kúszb N., Bérdic P., Zupkóc I., Hohmann J. (2018). New iridoids from the roots of V. dioscoridis Fitoterapia. 130, 73–78. 10.1016/j.fitote.2018.08.007 [DOI] [PubMed] [Google Scholar]
- Li S. Z. (1991). Compendium of materia medica (herb section). Beijing: People's Health Publishing House. [Google Scholar]
- Li W. J., Li M. Y. (2019). Research progress of valeriana officinalis. North Horti. (12) 12, 139–145. 10.11937/bfyy.20182933 [DOI] [Google Scholar]
- Li Y. B., Chen C., Mao S., Guo C., Zhao T. T., Wu L. L., et al. (2020a). Antidepression effect and mechanism of Valerianae jatamansi Rhizoma et Radix. Chin. J. Exp. Tradit. Med. Form. 26, 235–240. 10.13422/j.cnki.syfjx.20192306 [DOI] [Google Scholar]
- Li Y. B., Wu L. L., Chen C., Wang L. W., Guo C., Zhao Y. X., et al. (2020b). Serum metabolic profil-ing reveals the antidepressive effects of the total iridoids of Valeriana jatamansi Jones on chronic unpredictable mild stress mice. Front. Pharmacol. 11, 338. 10.3389/fphar.2020.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xue C. K., He X. B., Shen K., Jiang L., Zeng P. (2004). Using 99mTc-ECD as the tracer to evaluate the effects of the extract from Valeriana officinalis on rat cerebral perfusion. Radiol. Pract. 19, 133–134. 10.13609/j.cnki.1000-0313.2004.02.025 [DOI] [Google Scholar]
- Lin S., Chen T., Liu X. H., Shen Y. H., Wang H., Shan L., et al. (2010a). Iridoids and lignans from Valeriana jatamansi . J. Nat. Prod. 73, 632–638. 10.1021/np900795c [DOI] [PubMed] [Google Scholar]
- Lin S., Fu P., Chen T., Ye J., Su Y. Q., Yang X. W., et al. (2014). Minor valepotriates from Valeriana jatamansi and their cytotoxicity against metastatic prostate cancer cells. Planta Med. 81, 56–61. 10.1055/s-0034-1383369 [DOI] [PubMed] [Google Scholar]
- Lin S., Shen Y. H., Li H. L., Yang X. W., Chen T., Lu L. H., et al. (2009). Acylated iridoids with cytotoxicity from Valeriana jatamansi . J. Nat. Prod. 72, 650–655. 10.1021/np800716f [DOI] [PubMed] [Google Scholar]
- Lin S., Shen Y. H., Zhang Z. X., Li H. L., Zhang W. D., Liu R. H., et al. (2010b). Revision of the structures of 1, 5-dihydroxy-3, 8-epoxyvalechlorine, volvaltrate B, and valeriotetrate C from Valeriana jatamansi and V officinalis. J. Nat. Prod. 73, 1723–1726. 10.1021/np100426j [DOI] [PubMed] [Google Scholar]
- Lin S., Zhang Z. X., Chen T., Ye J., Dai W., Shan L., et al. (2013). Characterization of chlorinated valepotriates from Valeriana jatamansi . Phytochemistry 85, 185–193. 10.1016/j.phytochem.2012.08.015 [DOI] [PubMed] [Google Scholar]
- Liu H., Liu D., Jiang M. Y., Zhao X. D., Li R. T., Li H. M. (2021). Iridoids from Valeriana jatamansi with anti-inflammatory and anti-proliferative properties. Phytochemistry 184, 112681. 10.1016/j.phytochem.2021.112681 [DOI] [PubMed] [Google Scholar]
- Liu H. (2020). Studies on iridoids and their bioactivities from Valeriana jatamansi Jones. Kunming: Kunming University of Science and Technology. [dissertation]. [Kunming (YN)]. [Google Scholar]
- Liu X. G., Gao P. Y., Wang G. S., Song S. J., Li L. Z., Li X., et al. (2012). In vivo antidepressant activity of sesquiter-penes from the roots of Valeriana fauriei Briq. Fitoterapia 83, 599–603. 10.1016/j.fitote.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Lokar L. C., Moneghini M. (1989). Geographical variation in the monoterpenes of Valeriana officinalis leaf. Biochem. Syst. Ecol. 17, 563–567. 10.1016/0305-1978(89)90100-2 [DOI] [Google Scholar]
- Luo G. J., He G. H., Wang X. X., Wang H. (2004). Effects and mechanism of Valeriana officinalis var. latifolia on epileptic rats induced by pentylenetetrazol. Shanghai J. Tradit. Chin. Med. 38, 45–48. 10.16305/j.1007-1334.2004.12.020 [DOI] [Google Scholar]
- Luo G. J., Wang H., He G. H. (2005). The effects of Valeriana officinalis var. latifolia on both EEG and behaviors of epileptic rats in-ducded by pentylenetetrazol. J. Clin. Elect. Physiol. 14, 95–98. 10.3969/j.issn.1674-8972.2005.02.011 [DOI] [Google Scholar]
- Luo G. J., Xi G. M., Fan H. Y., Ye T. X., Yu S. J. (2001). The effects of the Valeriana officinalis Linn. var. Latifolia Miq on the caliber of vertebro-basilarartery and blood flow velocity after subarachnoid hemorrhage in rabbits. Chin. J. Tradit. Med. Sci. Technol. 8, 310–311. [Google Scholar]
- Malva J. O., Santos S., Macedo T. (2004). Neuroprotective properties of Valeriana officinalis extracts. Neurotox. Res. 6, 131–140. 10.1007/BF03033215 [DOI] [PubMed] [Google Scholar]
- Mao X. J., Li J. P., Wang J. (2008). Main pharmacodynamic study on valeriana wallichii DC. J. Yunnan Univ. Tradit. Chin. Med. 31, 34–37. 10.19288/j.cnki.issn.1000-2723.2008.03.009 [DOI] [Google Scholar]
- Marder M., Viola H., Wasowski C., Fernández S., Medina J. H., Paladini A. C. (2003). 6-methylapigenin and hesperidin: New valeriana flavonoids with activity on the CNS. Pharmacol. Biochem. Behav. 75, 537–545. 10.1016/S0091-3057(03)00121-7 [DOI] [PubMed] [Google Scholar]
- Miao M. S., Sun Y. X., Wang X. T. (2017). Dictionary of traditional Chinese medicine (vol II). Shanxi: Shanxi Technology Press. [Google Scholar]
- Ming D. S., Guo J. X. (1993). The determination of the total valepotriate content in Valeriana plants. J. Shanghai Med. 20, 210–212. [Google Scholar]
- Mirabi P., Dolatian M., Mojab F., Majd H. A. (2011). Effects of Valerian on the severity and systemic manifestations of dysmenorrhea. Int. J. Gynaecol. Obstet. 115, 285–288. 10.1016/j.ijgo.2011.06.022 [DOI] [PubMed] [Google Scholar]
- Murakami N., Ye Y., Kawanishi M., Aoki S., Kudo N., Yoshida M., et al. (2002). New rev-transport inhibitor with anti-HIV activity from Valerianae radix. Bioorg. Med. Chem. Lett. 12, 2807–2810. 10.1016/S0960-894X(02)00624-8 [DOI] [PubMed] [Google Scholar]
- Murphy K., Kubin Z. J., Shepherd J. N., Ettinger R. H. (2010). Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine 17, 674–678. 10.1016/j.phymed.2009.10.020 [DOI] [PubMed] [Google Scholar]
- Nam S. M., Choi J. H., Yoo D. Y., Kim W., Jung H. Y., Kim J. W., et al. (2013). Valeriana officinalis extract and its main component, valerenic acid, ameliorate d-galactose-induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation. Exp. Gerontol. 48, 1369–1377. 10.1016/j.exger.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Nouri M. H. K., Abad A. N. A. (2011). Gabaergic system role in aqueous extract of Valeriana officinalis L. root on PTZ-induced clonic seizure threshold in mice. Afr. J. Pharm. Pharmacol. 5, 1212–1217. 10.5897/ajpp11.241 [DOI] [Google Scholar]
- Orhan I. E. (2021). A review focused on molecular mechanisms of anxiolytic effect of valerina officinalis L. in connection with its phytochemistry through in vitro/in vivo studies. Curr. Pharm. Des. 27, 3084–3090. 10.2174/1381612827666210119105254 [DOI] [PubMed] [Google Scholar]
- Ortiz J. G., Nieves-Natal J., Chavez P. (1999). Effects of Valeriana officinalis extracts on [3H] flunitrazepam binding, synaptosomal [3H] GABA uptake, and hippocampal [3H] GABA release. Neurochem. Res. 24, 1373–1378. 10.1023/a:1022576405534 [DOI] [PubMed] [Google Scholar]
- Oshima Y., Matsuoka S., Ohizumi Y. (1995). Antidepressant principles of Valeriana fauriei roots. Chem. Pharm. Bull. 43, 169–170. 10.1248/cpb.43.169 [DOI] [PubMed] [Google Scholar]
- Pakseresht S., Boostani H., Sayyah M. (2011). Extract of valerian root (Valeriana officinalis L.) vs. placebo in treatment of obsessive-compulsive disorder: A randomized double-blind study. J. Complement. Integr. Med. 8, 32. 10.2202/1553-3840.1465 [DOI] [PubMed] [Google Scholar]
- Pandian D. S., Nagarajan N. S. (2015). Comparison of chemical composition and antioxidant potential of hydrodistilled oil and supercritical fluid CO2 extract of Valeriana wallichii DC. J. Nat. Prod. Res. 1, 25–30. [Google Scholar]
- Piccinelli A. L., Arana S., Caceres A., Bianca R. D. D., Rastrell I. L., Rastrelli L. (2004). New lignans from the roots of Valeriana prionophylla with antioxidative and vasorelaxant activities. J. Nat. Prod. 67, 1135–1140. 10.1021/np049879c [DOI] [PubMed] [Google Scholar]
- Prasad R., Naime M., Routray I., Mahmood A., Ali S. (2010). Valeriana jatamansi partially reverses liver cirrhosis and tissue hyperproliferative response in rat. Methods Finds Exp. Clin. Pharmacol. 32, 713–719. 10.1358/mf.2010.32.10.1522224 [DOI] [PubMed] [Google Scholar]
- Qin Y. J., Lin Y. K., Zhou C. C., Li Y., Zhong Z. Q., Zeng Y. S. (2009). Effects of Valerian on serum cortisol level and caspase-3 positive cells of hippocampus in depressive rats induced by chronic mild stress. Anat. Res. 31, 88–93. [Google Scholar]
- Quan L. Q., Hegazy A. M., Zhang Z. J., Zhao X. D., Li R. T. (2020b). Iridoids and bis-iridoids from Valeriana jatamansi and their cytotoxicity against human glioma stem cells. Phytochemistry 175, 112372. 10.1016/j.phytochem.2020.112372 [DOI] [PubMed] [Google Scholar]
- Quan L. Q., Liu H., Li R. T., Li H. M. (2019b). Three new 3, 8-epoxy iridoids from the roots and rhizomes of Valeriana jatamansi . Phytochem. Lett. 34, 35–38. 10.1016/j.phytol.2019.09.007 [DOI] [Google Scholar]
- Quan L. Q., Su L. H., Qi S. G., Xue Y., Yang T., Liu D., et al. (2019a). Bioactive 3, 8-epoxy iridoids from Valeriana jatamansi . Chem. Biodivers. 16, e1800474. 10.1002/cbdv.201800474 [DOI] [PubMed] [Google Scholar]
- Quan L. Q., Zhao Q., Li R. T., Li H. M. (2020a). The isolation of two new compounds from Valeriana jatamansi . Nat. Prod. Res. 1, 3280–3285. 10.1080/14786419.2020.1853728 [DOI] [PubMed] [Google Scholar]
- Raal A., Arak E., Orav A., Kailas T., Müürisepp M. (2008). Variation in the composition of the essential oil of commercial Valeriana officinalis L. roots from different countries. J. Essent. Oil Res. 20, 524–529. 10.1080/10412905.2008.9700079 [DOI] [Google Scholar]
- Rawat S., Jugran A. K., Bhatt I. D., Rawal R. S., Dhar U. (2017). Essential oil composition and antioxidant activity in Valeriana jatamansi jones: Influence of seasons and growing sources. J. Essent. Oil Res. 29, 101–107. 10.1080/10412905.2016.1189856 [DOI] [Google Scholar]
- Rezvani M. E., Roohbakhsh A., Allahtavakoli M., Shamsizadeh A. (2010). Anticonvulsant effect of aqueous extract of Valeriana officinalis in amygdala-kindled rats: Possible involvement of adenosine. J. Ethnopharmacol. 127, 313–318. 10.1016/j.jep.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Sah S. P., Mathela C. S., Chopra K. (2011b). Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: Behavioral and biochemical evidence. J. Ethnopharmacol. 135, 197–200. 10.1016/j.jep.2011.02.018 [DOI] [PubMed] [Google Scholar]
- Sah S. P., Mathela C. S., Chopra K. (2010a). Elucidation of possible mechanism of analgesic action of Valeriana wallichii DC chemotype (patchouli alcohol) in experimental animal models. Indian J. Exp. Biol. 48, 289–293. [PubMed] [Google Scholar]
- Sah S. P., Mathela C. S., Chopra K. (2011c). Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valeriana wallichii patchouli alcohol chemotype. Phytomedicine 18, 1269–1275. 10.1016/j.phymed.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Santos M. S., Ferreira F., Faro C., Pires E., Macedo T., Cunha A. P., et al. (1994). The amount of GABA present in aqueous extracts of Valerian is sufficient to account for [3H] GABA release in synaptosomes. Planta Med. 60, 475–476. 10.1055/s-2006-959538 [DOI] [PubMed] [Google Scholar]
- Schumacher B., Scholle S., Hölzl J., Khudeir N., Hess S., Muller C. E. (2002). Lignans isolated from valerian: Identification and characterization of a new olivil derivative with partial agonistic activity at a1 adenosine receptors. J. Nat. Prod. 65, 1479–1485. 10.1021/np010464q [DOI] [PubMed] [Google Scholar]
- Sharma P., Kirar V., Meena D. K., Suryakumar G., Misra K. (2012). Adaptogenic activity of Valeriana wallichii using cold, hypoxia and restraint multiple stress animal model. Biomed. Aging Pathology 2, 198–205. 10.1016/j.biomag.2012.10.001 [DOI] [Google Scholar]
- Shinjyo N., Waddell G., Green J. (2020). Valerian root in treating sleep problems and associated disorders-a systematic review and meta-analysis. J. Evid. Based. Integr. Med. 25, 2515690X20967323–31. 10.1177/2515690X20967323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya K., Inoue T., Utsu Y., Tokunaga S., Masuoka T., Ohmori A., et al. (2005). Effects of kava-kava extract on the sleep-wake cycle in sleep-disturbed rats. Psychopharmacology 59, 564–569. 10.1007/s00213-005-2196-4 [DOI] [PubMed] [Google Scholar]
- Su L. H. (2016). Studies on the chemical constituents and bioactivities of two medical plants. Nanjing: Nanjing University of Science and Technology. [dissertation]. [Nanjing (JS)]. [Google Scholar]
- Subhan F., Karim N., Ibrar M. (2007). Anti-inflammatory activity of methanolic and aqueous extracts of Valeriana wallichii Dc rhizome. Pak. J. Plant Sci. 13, 103–108. [Google Scholar]
- Subhashree S., Kumaravel M., Syam P. K. J., Divya S., Larance R., Kavimani S., et al. (2015). Neuroprotective effect of Valeriana wallichii rhizome extract against the neurotoxin MPTP in C57BL/6 mice. Neurotoxicology 51, 172–183. 10.1016/j.neuro.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Sudati J. H., Vieira F. A., Pavin S. S., Dias G. R. M., Seeger R. L., Golombieski R., et al. (2013). Valeriana officinalis attenuates the rotenone-induced toxicity in drosophila melanogaster. Neurotoxicology 37, 118–126. 10.1016/j.neuro.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Taherpour A. A., Maroofi H., Bajelani O., Larijani K. (2010). Chemical composition of the essential oil of Valeriana alliariifolia adams of Iran. Nat. Prod. Res. 24, 973–978. 10.1080/14786410902900010 [DOI] [PubMed] [Google Scholar]
- Tamilselvam K., Braidy N., Manivasagam T., Essa M. M., Prasad N. R., Karthikeyan S., et al. (2013). Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxid. Med. Cell. Longev. 2013, 102741. 10.1155/2013/102741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G. J., He J. Y., Li S. P., Zhou Y. (2007). The therapeutic effect of compound Valerian mixture on nervous system diseases. Proceedings of the 7th Biennial Meeting and the 5th Congress of the Chinese Society for Neuroscience. 311. [Google Scholar]
- Tan Y. Z., Yong Y., Dong Y. H., Wang R. J., Li H. X., Zhang H., et al. (2016). A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem. Lett. 17, 177–180. 10.1016/j.phytol.2016.07.020 [DOI] [Google Scholar]
- Tang J. Y., Zeng Y. S., Chen Q. G., Qin Y. J., Chen S. J., Zhong Z. Q. (2008a). Effect of Valerian on weight and behavior of depressive rats induced by Chronic Mild Stress. J. Sun Yat-sen Univ. Med. Sci. 29, 541–545. 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2008.0150 [DOI] [Google Scholar]
- Tang J. Y., Zeng Y. S., Chen Q. G., Qin Y. J., Chen S. J., Zhong Z. Q. (2008b). Effects of Valerian on the level of 5-hydroxytryptamine, cell proliferation and neurons in cerebral hippocampus of rats with depression induced by chronic mild stress. J. Chin. Integr. Med. 6, 283–288. 10.3736/jcim20080313 [DOI] [PubMed] [Google Scholar]
- Tang Y. P., Xin L., Yu B. (2003). Two new flavone glycosides from Valeriana jatamansi. J. Asian Nat. Prod. Res. 5, 257–261. 10.1080/1028602031000105867 [DOI] [PubMed] [Google Scholar]
- Thies P. W. (1968a). Constitution of valepotriates. Report on activeagents of valerian. Tetrahedron 24, 313–347. 10.1016/0040-4020(68)89032-5 [DOI] [Google Scholar]
- Thies P. W. (1968b). Die konstitution der valepotriate: Mitteilung über die wirkstoffe des baldrians. Tetrahedron 24, 313–347. 10.1016/0040-4020(68)89032-5 [DOI] [Google Scholar]
- Thusoo S., Gupta S., Sudan R., Kour J., Bhagat M., Hussain R., et al. (2014). Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. Biomed. Res. Int. 2014, 614187. 10.1155/2014/614187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga S., Takeda Y., Niimoto T., Nishida N., Kubo T., Ohno T., et al. (2007). Effect of valerian extract preparation (BIM) on the sleep-wake cycle in rats. Biol. Pharm. Bull. 30, 363–366. 10.1248/bpb.30.363 [DOI] [PubMed] [Google Scholar]
- Torssell K., Wahlberg K., Alnås Å., Hemmingsson S., Dumanović J. (1967). Isolation, structure and synthesis of alkaloids from Valeriana officinalis L. Acta Chem. Scand. 21, 53–62. 10.3891/acta.chem.scand.21-0053 [DOI] [PubMed] [Google Scholar]
- Tortarolo M., Braun R., Hübner G. E., Maurer H. R. (1982). In vitro effects of epoxide-bearing alepotriates on mouse early hemato-poietic progenitor cells and human T-lymphocytes. Arch. Toxicol. 51, 37–42. 10.1007/BF00279319 [DOI] [Google Scholar]
- Valle-Mojica L. M. D., Cordero-Hernández J. M., González-Medina G., Ramos-Vélez I., Ortíz J. G., Torres-Hernandez B. A., et al. (2011). Aqueous and ethanolic Valeriana officinalis extracts change the binding of ligands to glutamate receptors. Evid. Based Complement. Altern. Med. 2011, 1–7. 10.1155/2011/891819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle-Mojica L. M. D., Ortíz G. J. (2012). Anxiolytic properties of Valeriana officinalis in the zebrafish: A possible role for metabotropic glutamate receptors. Planta Med. 78, 1719–1724. 10.1055/s-0032-1315240 [DOI] [PubMed] [Google Scholar]
- Vishwakarma S., Goyal R., Gupta V., Dhar K. L. (2016). Gabaergic effect of valeric acid from Valeriana wallichii in amelioration of ICV STZ induced dementia in rats. Rev. Bras. Farmacogn. 26, 484–489. 10.1016/j.bjp.2016.02.008 [DOI] [Google Scholar]
- Wagner H., Jurcic K. (1979). On the spasmolytic activity of Valeriana extracts (author's transl). Planta Med. 37, 84–86. 10.1055/s-0028-1097303 [DOI] [PubMed] [Google Scholar]
- Wagner H., Jurcic K., Schaette R. (1980). Comparative studies on the sedative action of Valeriana extracts, valepotriates and their degradation products (author's transl). Planta Med. 39, 358–365. 10.1055/s-2008-1074930 [DOI] [PubMed] [Google Scholar]
- Wan Y. Y., Wang C. F., Wang Q. H., Yang X., Wang Z. B., Kuang H. X. (2016). Study on active constituents against Alzheimer's disease from Valeriana amurensis . China J. Chin. Mat. Med. 41, 1649–1653. 10.4268/cjcmm20160914 [DOI] [PubMed] [Google Scholar]
- Wang C. F., Wang Q. H., Xiao Y., Wu L. H., Wan Y. Y., Yang B. Y., et al. (2016a). Study on neuroprotective active constituents from root and rhizome of Valeriana amurensis . Chin. Tradit. Herb. Drugs 47, 1850–1855. 10.7501/j.issn.0253-2670.2016.11.007 [DOI] [Google Scholar]
- Wang C. F., Xiao Y., Yang B. Y., Wang Z. B., Wu L. H., Su X. L., et al. (2014a). Isolation and screened neuroprotective active constituents from the roots and rhizomes of Valeriana amurensis . Fitoterapia 96, 48–55. 10.1016/j.fitote.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Wang F. F., Zhang Y. M., Zheng X. W., Dai Z., Liu B., Ma S. C. (2019a). Research progress of the structure and biological activities of iridoids compounds. Chin. Pharm. Sci. 33, 323–330. 10.16153/j.1002-7777.2019.03.014 [DOI] [Google Scholar]
- Wang H. M., Song Z. T., Xing H. H., Shi Z. Y., Wu P., Zhang J., et al. (2020a). Nitric oxide inhibitory iridoids as potential anti-inflammatory agents from Valeriana jatamansi. Bioorg. Chem. 101, 103974. 10.1016/j.bioorg.2020.103974 [DOI] [PubMed] [Google Scholar]
- Wang J. H., Zhao J. L., Liu H., Zhou L. G., Liu Z. L., Wang J. G., et al. (2010b). Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis . Molecules 15, 6411–6422. 10.3390/molecules15096411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. W., Sun Y., Zhao T. T., Li Y. B., Zhao X. Q., Zhang L., et al. (2019b). Antidepressant effects and mechanisms of the total iridoids of Valeriana jatamansi on the brain-gut axis. Planta Med. 86, 172–179. 10.1055/a-1068-9686 [DOI] [PubMed] [Google Scholar]
- Wang P. C., Hu J. M., Ran X. H., Chen Z. Q., Jiang H. Z., Liu Y. Q., et al. (2009a). Iridoids and sesquiterpenoids from the roots of Valeriana officinalis . J. Nat. Prod. 72, 1682–1685. 10.1021/np9003382 [DOI] [PubMed] [Google Scholar]
- Wang P. C., Hu J. M., Ran X. H., Chen Z. Q., Jiang H. Z., Liu Y. Q., et al. (2009b). Sesquiterpenoids and lignans from the roots of Valeriana officinalis . L. Chem. Biodivers. 72, 1682–1685. 10.1021/np9003382 [DOI] [PubMed] [Google Scholar]
- Wang P. C., Ran X. H., Luo H. R., Ma Q. Y., Zhou J., Hu J. M., et al. (2016b). Volvalerine A, an unprecedented N-containing sesquiterpenoid dimer derivative from Valeriana officinalis var. latifolia . Fitoterapia 109, 174–178. 10.1016/j.fitote.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Wang Q. H., Wang C. F., Shu Z. P., Chan K., Huang S. M., Li Y., et al. (2013). Valeriana amurensis improves Amyloid-beta 1-42 induced cognitive deficit by enhancing cerebral cholinergic function and protecting the brain neurons from apoptosis in mice. J. Ethnopharmacol. 153, 318–325. 10.1016/j.jep.2013.11.017 [DOI] [PubMed] [Google Scholar]
- Wang Q. H., Wang C. F., Zuo Y. M., Wang Z. B., Yang B. Y., Kuang H. X. (2012a). Compounds from the roots and rhizomes of Valeriana amurensis protect against neurotoxicity in PC12 cells. Molecules 17, 15013–15021. 10.3390/molecules171215013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. H., Zuo Y. M., Zhang Z. L., Wang C. F., Kuang H. X. (2012b). Study on chemical constituents of effective parts of Valeriana amurensis Smir.ex Kom in the treatment of Alzheimer's disease. Inf. Tradit. Chin. Med. 29, 16–19. [Google Scholar]
- Wang R. J., Chen H. M., Fan Y., Deng Y., Ao H., Xie X. F., et al. (2017). Iridoids from the roots of Valeriana jatamansi jones. Phytochemistry 141, 156–161. 10.1016/j.phytochem.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Wang R. J., Huang Q., Yong Y., Li H. X., Zhang S. J., Chen B. H., et al. (2016c). Studies on chemical constituents of Valeriana plants and their biological activities. China J. Chin. Mat. Med. 41, 1405–1414. 10.4268/cjcmm20160807 [DOI] [PubMed] [Google Scholar]
- Wang R. J., Shi S. J., Tan Y. Z., Yao L. C., Zhu L. X. (2021). Chemical constituents from Valeriana jatamansi . Biochem. Syst. Ecol. 94, 104177. 10.1016/j.bse.2020.104177 [DOI] [Google Scholar]
- Wang R., Xiao D., Bian Y. H., Zhang X. Y., Li B. J., Ding L. S., et al. (2008). Minor iridoids from the roots of Valeriana wallichii . J. Nat. Prod. 71, 1254–1257. 10.1021/np070598p [DOI] [PubMed] [Google Scholar]
- Wang S. J., Qiu X. Q., Zhu J. Y., Ma X. Q., Lin B., Zheng C. J., et al. (2014b). Two new iridoids from the root and rhizome of Valeriana jatamansi jones. Helv. Chim. Acta 97, 722–726. 10.1002/hlca.201300287 [DOI] [Google Scholar]
- Wang S. L., Zeng Y. R., Li Y. A., He L., Hu Z. X., Huang L. J., et al. (2020b). Chemical constituents from Valeriana officinalis L. var. latifolia Miq. and their chemotaxonomic significance. Biochem. Syst. Ecol. 90, 104041. 10.1016/j.bse.2020.104041 [DOI] [Google Scholar]
- Wang X., Zhang J. H., Yuan Y., Liu X., Wang S. F. (2019c). Advances in research on chemical constituents and pharmacological effects of Valeriana officinalis L. Guizhou J. Anim. Husb. Vet. Med. 43, 6–9. [Google Scholar]
- Wang Y. (2007). Callus tissue induction and rapid propagation system of Valeriana amurensis . Jiamusi: Jiamusi University. [dissertation]. [Jiamusi (HL)]. [Google Scholar]
- Wang Y. F., Jin L. Q., Yu S. H., Shi Q. W., Gu Y. C., Kiyota H. (2010a). Chemical constituents of plants from the genus Valeriana . Mini. Rev. Org. Chem. 7, 161–172. 10.2174/157019310791065537 [DOI] [Google Scholar]
- Wang Y. F., Yan J., Luo G. J., Sun S. G., He G. H. (2004). Effects of Valeriana officinalis var latifolia Miq. in the treatment of cerebral infarction in rats. Chin. J. Rehabil. 19, 137–138. [Google Scholar]
- Wei G. M., Li D. W., Wang S. M., Yu E. R., Luo L. S., Wang J. (2016). Research progress of valerian. Agric. Technol. Servi. 33, 18–19. [Google Scholar]
- Wen L., Zhou Y., Zhou W., Duan X. Y., Fang Y. (2009). Effect of the extracts of Valeriana officinalis L. on cardiac arrhythmias. J. Chin. Pharm. Sci. 29, 191–194. [Google Scholar]
- Wu B., Fu Y. M., Huang A. H., Ma Y. J. (2008). Changes of GABA and Glu content in hippocampus of PTZ-Induced epileptic rats treated with volatile oil of Valeriana . Chin. Arch. Tradit. Chin. Med. 26, 2476–2477. 10.13193/j.archtcm.2008.11.173.wub.053 [DOI] [Google Scholar]
- Wu B., Ma Y. J., Fu Y. M. (2005). Studies on the Pharmacological effects of Valeriana officinalis L on the sedation and anticonvulsion. Chin. J. Mod. Appl. Pharm. 22, 587–588. [Google Scholar]
- Wu J. K., Wang G. S., Du X. W., Song N., Zou Z. M., Chen J. S., et al. (2014). A caryophyllane-type sesquiterpene, caryophyllenol a from Valeriana amurensis . Fitoterapia 96, 18–24. 10.1016/j.fitote.2014.03.025 [DOI] [PubMed] [Google Scholar]
- Xi J. K., Qu H. H., Luan X. H. (2002). Survey of studies on phytochemistry and pharmacology of Valeriana L. Chin. Pharm. J. 37, 729–733. [Google Scholar]
- Xie Y. G., Zhao X. C., Hassan S. S. U., Zhen X. Y., Muhammad I., Yan S. K., et al. (2019). One new sesquiterpene and one new iridoid derivative from Valeriana amurensis . Phytochem. Lett. 32, 6–9. 10.1016/j.phytol.2019.04.020 [DOI] [Google Scholar]
- Xu H., Yuan H. N., Pan L. H., Guo X. L. (1997). The Pharmacological effects of volatile oil from Valeriana on central nervous system. Chin. J. Pharm. Anal. 17, 399–401. 10.16155/j.0254-1793.1997.06.013 [DOI] [Google Scholar]
- Xu J., Guo P., Fang L. Z., Li Y. S., Guo Y. Q. (2012a). Iridoids from the roots of Valeriana jatamansi . J. Asian Nat. Prod. Res. 14, 1–6. 10.1080/10286020.2011.618804 [DOI] [PubMed] [Google Scholar]
- Xu J., Guo P., Guo Y. Q., Fang L. Z., Li Y. S., Su Z. P., et al. (2011a). Iridoids from the roots of Valeriana jatamansi and their biological activities. Nat. Prod. Res. 26, 1996–2001. 10.1080/14786419.2011.636747 [DOI] [PubMed] [Google Scholar]
- Xu J., Guo Y. Q., Xie C. F., Jin D. Q., Gao J., Gui L. P. (2012b). Isolation and neuroprotective activities of acylated iridoids from Valeriana jatamansi . Chem. Biodivers. 9, 1382–1388. 10.1002/cbdv.201100238 [DOI] [PubMed] [Google Scholar]
- Xu J., Li Y. S., Guo Y. Q., Guo P., Yammakuni T., Ohizumi Y. (2012c). Isolation, structural elucidation, and neuroprotective effects of iridoids from Valeriana jatamansi . Biosci. Biotechnol. Biochem. 76, 1401–1403. 10.1271/bbb.120097 [DOI] [PubMed] [Google Scholar]
- Xu J., Zhao P., Guo Y. Q., Xie C. F., Jin D. Q., Ma Y. G., et al. (2011b). Iridoids from the roots of Valeriana jatamansi and their neuroprotective effects. Fitoterapia 82, 1133–1136. 10.1016/j.fitote.2011.07.013 [DOI] [PubMed] [Google Scholar]
- Xu S. Y. (2006). Miao ethnic medicines: Application, research, and development. World sci. Technol. Moder. Tradit. Chin. Med. Mat. Med. 8, 73–78. [Google Scholar]
- Xu Y. M., Mclaughlin S. P., Gunatilaka A. (2007). Sorbifolivaltrates A-D, diene valepotriates from Valeriana sorbifolia L. J. Nat. Prod. 70, 2045–2048. 10.1021/np0704553 [DOI] [PubMed] [Google Scholar]
- Xue C. K., He X. B., Qu W., Li Y., Zeng L., Peng R. X. (2005). Improvement of Valerian-ligusticum extract on cerebral microcirculatory disturbance. Chin. J. Clin. Oncol. 9, 171–174. [Google Scholar]
- Yan Z. Y., Zhang T. E., Xiao T., Pan L. Z., Qin J. Z., Zhang Z. P., et al. (2010). Anti-anxiety effect of Valeriana jatamansi jones extract via regulation of the hypothalamus-pituitary-adrenal axis. Neural. Regen. Res. 5, 1071–1075. 10.3969/j.issn.1673-5374.2010.14.006 [DOI] [Google Scholar]
- Yan Z. Y., Zuo C. R., Chen C., Li S. H., Chen C. Y. (2011). Anti-hepatoma effects and influence on JAK/STAT signaling pathway of total flavonoids from Valeriana jatamansi Jones. Chin. J. Pharmacol. Toxicol. 25, 78. [Google Scholar]
- Yang B., Zhu R., Tian S., Wang Y., Lou S., Zhao H. (2017). Jatamanvaltrate P induces cell cycle arrest, apoptosis and autophagy in human breast cancer cells in vitro and in vivo . Biomed. Pharmacother. 89, 1027–1036. 10.1016/j.biopha.2017.02.065 [DOI] [PubMed] [Google Scholar]
- Yang G. Y., Qing X. U., Wang J. F. (2002). Valeriana officinalis var. latifolia Miq regulates vascular smooth muscle cell contraction and growth. J. Yunyang Med. Coll. 21, 324–326. [Google Scholar]
- Yang G. Y., Wang W. (1994). Clinical studies on treatment of coronary heart disease with Valeriana officinalis var latifolia . Chin. J. Integr. Tradit. West Med. 14, 540–542. [PubMed] [Google Scholar]
- Yang S. H., Chen F., Ma H. M., Hongmei M. A. (2012). Protection of Valeriana officinalis L extract preconditioning on ischemia-reperfusion injury in rat hearts in vitro . Med. J. Wuhan. Univ. 33, 639–643. 10.14188/j.1671-8852.2012.05.003 [DOI] [Google Scholar]
- Ye J. M., Hu P. J., Yi C. Q., Xue C. K., Hu C. Y., Chen F. M., et al. (2007). Valepotriate-induced apoptosis of gastric cancer cell line MKN-45. World Chin. J. Digestol. 15, 22–28. 10.11569/wcjd.v15.i1.22 [DOI] [Google Scholar]
- Ye J. M., Yi C. Q., Xue C. K., Hu C. Y., Chen F. M., Qian W. (2004). Relationship between signal molecule expression of valepotriate-induced apoptosis of gastric cancer cell. Chin. J. Dig. 24, 619. [Google Scholar]
- Yin H., Xue C. K., Ye J. M., Zhu X. Z., Li Y., Zeng L. (2000). An experimental study of Valerian extract combating myocardial ischemia reperfusion injury. Chin. J. Microcirc. 10, 12–14. [Google Scholar]
- Yin Y. G., Zhu Q. H., Wang C. Y. (2006). Chemical studies on Valeriana genera. Her. Med. 25, 230–231. [Google Scholar]
- Yoo D. Y., Jung H. Y., Nam S. M., Kim J. W., Hwang I. K., Kwak Y. G., et al. (2015). Valeriana officinalis extracts ameliorate neuronal damage by suppressing lipid peroxidation in the gerbil hippocampus following transient cerebral ischemia. J. Med. Food 18, 642–647. 10.1089/jmf.2014.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. B., Zhou M. Q., Liu S. M. (1995). Heilongjiang local medicine. 1st ed. Harbin, China: Heilongjiang Science and Technology Press, 48–49. [Google Scholar]
- Yu L. L., Han C. R., Huang R., Lv Y. P., Gui S. H., Chen Y. G. (2010). New iridoid triesters from Valeriana jatamansi . Helv. Chim. Acta 88, 1059–1062. 10.1002/hlca.200590077 [DOI] [Google Scholar]
- Yuan S. Q. (1992). Research progress on chemistry and pharmacology of Valeriana . Int. J. Pharm. Sci. Res. 19, 346–352. [Google Scholar]
- Zeng M. Y., Zeng J. F. (1994). Chinese traditional medicine resources. Beijing s: Science Pres. [Google Scholar]
- Zhang B. H., Meng H. P., Wang T., Dai Y. C., Yuan S. H., Tao C., et al. (1982). Effects of Valeriana officinalis L. extract on cardiovascular system. Acta Pharm. Sin. B 17, 382–384. 10.16438/j.0513-4870.1982.05.011 [DOI] [PubMed] [Google Scholar]
- Zhang D., Zhou L. X., Lin N. M. (2014). Research progress on pharmacological action of Valeriana officinalis . Chin. J. Clin. Pharma. 23, 397–402. [Google Scholar]
- Zhang J. P., Han G. T., Zhang J. F., Gong Y. (2010a). Effect of Valeriana officials on IL-1β and TNF-α gene expression in mice. J. Tradit. Chin. Med. 12, 237–238. 10.13194/j.jlunivtcm.2010.11.239.zhangjp.079 [DOI] [Google Scholar]
- Zhang S. Q., Xue C. K., He X. B., Liu Z. G. (2010b). Experimental study of anti-tumor effect of Valerian iridoids. Mod. J. Integr. Tradit. Chin. West Med. 30, 1008–1010. [Google Scholar]
- Zhang Z. L., Zuo Y. M. (2018). Effect of extracts from Valeriana officinalis on spatial learning memory and antioxidant capacity in rat model of sleep disorder Alzheimer's disease. Chin. J. Gerontol. 38, 3976–3979. 10.4103/1673-5374.205108 [DOI] [Google Scholar]
- Zhang Z. L., Zuo Y. M., Wang Q. H., Xiao H. B., Kuang H. X. (2010c). Effects of Valeriana amurensis on the expressions of iNOS, COX-2 and IkappaCB-alpha in Alzheimer's disease model rat's brain. J. Chin. Med. Mat. 33, 581–583. 10.13863/j.issn1001-4454.2010.04.037 [DOI] [PubMed] [Google Scholar]
- Zhang Z. X., Dou D. Q., Liu K., Yao X. S. (2006). Studies on the chemical constituents of Valeriana fauriei Briq. J. Asian Nat. Prod. Res. 8, 397–400. 10.1080/10286020500172418 [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang Y. Y., Huang L. L., Zhao H., Li T. L. (2021). Research progress on active components and mechanism of traditional Chinese herbal medicine for antianxiety. Shanghai J. tradit. Chin. Med. 55, 96–100. 10.16305/j.1007-1334.2021.2011013 [DOI] [Google Scholar]
- Zhao Y. X., Ma Q. Y., Wang P. C., Li N., Huang S. Z. (2011). Research progress on constituents of Valeriana officinalis linn. And its pharmacological activities. J. Anhui Agric. Sci. 39, 7078–7080. 10.13989/j.cnki.0517-6611.2011.12.291 [DOI] [Google Scholar]
- Zhou C. C., Zeng Y. S., Qin Y. J., Ding Y., Li Y., Zhong Z. Q. (2010). Effects of Valerian on number of p-CREB positive neurons in cerebral hippocampus of rats with depression induced by chronic mild stress. Anat. Res. 32, 81–87. 10.1089/cbr.2009.0707 [DOI] [Google Scholar]
- Zhou H. Y., Chen S. L. (2011). Sedative and hypnotic effects of Valerian plants. Med. Pharm. Yunnan 2, 261–263. [Google Scholar]
- Zhou T., Huang B. K. (2008a). Advancement on the chemical constituents and bioactivities of essential oil of Valeriana officinlais L. Lishizhen Med. Mat. Med. Res. 19, 2663–2664. [Google Scholar]
- Zhou Y., Fang Y., Liu Y. W. (2008b). Research progress of valerian. Hubei J. Tradit. Chin. Med. 30, 61–64. [Google Scholar]
- Zhu Y. C. (1984). Medicinal plant resources of valeriana L. In northeast China. Nat. Resour. Res. 1, 35–40. [Google Scholar]
- Zhu Y. C. (1989). Northeast medicinal plants. Harbin: Heilongjiang Science and Technology Press. [Google Scholar]
- Zuo Y. M., Wang Y. F., Zhang Z. L., Yan H., Xu Y. L. (2018). Study on the chemical components of lignans of Valeriana officinalis . J. Chin. Med. Mat. 41, 1091–1094. 10.13863/j.issn1001-4454.2018.05.016 [DOI] [Google Scholar]
- Zuo Y. M., Zhang Z. L., Wang Q. H., Xie N., Kuang H. X. (2010). Effects of Valeriana amurensis on the expressions of beta-APP, Abeta (1-40) and caspase-3 in Alzheimer's disease model rat's brain. J. Chin. Med. Mat. 33, 233–236. 10.13863/j.issn1001-4454.2010.02.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.