Abstract
In this article, we report a case wherein a brain tumor was suspected based on computed tomography and magnetic resonance imaging findings. We made an initial diagnosis of malignant brain tumor based on methionine-positron emission tomography (PET) findings, but the correct diagnosis was dural arteriovenous fistula (DAVF). The patient was a 45-year-old man with DAVF who developed headache. Methionine-PET imaging showed high methionine uptake in the lesion. Although the tumor was strongly suspected from the findings of methionine-PET, the diagnosis of DAVF could be made correctly only by interpreting digital subtraction angiography and computed tomographic angiography. The findings of methionine-PET, which is considered useful in the diagnosis and denial of brain tumors, made the diagnosis of DAVF more difficult. The increased uptake of methionine-PET in DAVF is an important finding because, to our knowledge, this study is the first to report such finding. The results of this study might be useful for differential diagnoses when the diagnosis is uncertain.
Keywords: methionine-PET, DSA, DAVF, transarterial embolization, brain tumor
Introduction
Dural arteriovenous fistula (DAVF) is considered an acquired disease, except in some pediatric cases, but the cause of its onset remains unknown. Symptoms associated with DAVF, such as headache, tinnitus, and cognitive impairment, are often nonspecific, and in some cases, hemorrhage and convulsions may occur. Digital subtraction angiography (DSA) is the gold standard for the definitive diagnosis of DAVF because it can evaluate venous perfusion and determine the indication for surgical treatment. However, due to its invasive examination and with the improvement in resolution since the installation of three Tesla magnetic resonance imaging (MRI), time-of-flight magnetic resonance angiography (TOF-MRA) has become a viable screening method.1,2) Some DAVFs show findings similar to those of malignant brain tumors or inflammatory diseases.3,4) In cases of DAVF with low shunt blood flow, the lesions are poorly visible and may be difficult to diagnose on MRI. In addition, venous congestion associated with shunt lesions can cause signal changes on MRI, and these changes may appear like tumors. Moreover, the symptoms are nonspecific, so it is difficult to differentiate between them.
When a brain tumor is suspected or other imaging findings are difficult to confirm as a brain tumor, fluorodeoxyglucose (FDG) or methionine-positron emission tomography (PET) scan can be performed to predict the malignancy and determine the spread of the tumor. In particular, methionine is more specifically taken up by tumors than FDG; thus, if a tumor is suspected, methionine is used.5-7) However, in Japan, methionine is not yet approved by the pharmaceutical affairs bureaucracy, and methionine-PET can only be performed at facilities with cyclotron equipment; therefore, the examinations can only be performed at a limited number of centers, such as university hospitals. Methionine-PET can be performed at our hospital, and simple computed tomography (CT), iodine-enhanced CT, simple MRI, gadolinium-enhanced MRI (Gd-MRI), FDG-PET, and methionine-PET are usually performed when a brain tumor is suspected.
Herein, we report a case that mimicked a brain tumor because of increased methionine uptake in the same areas highlighted on Gd-MRI, but the correct diagnosis was DAVF. It should be noted that the methionine-PET scan, which is supposed to be useful, led to a wrong diagnosis. Consent was obtained from all participants.
Case Report
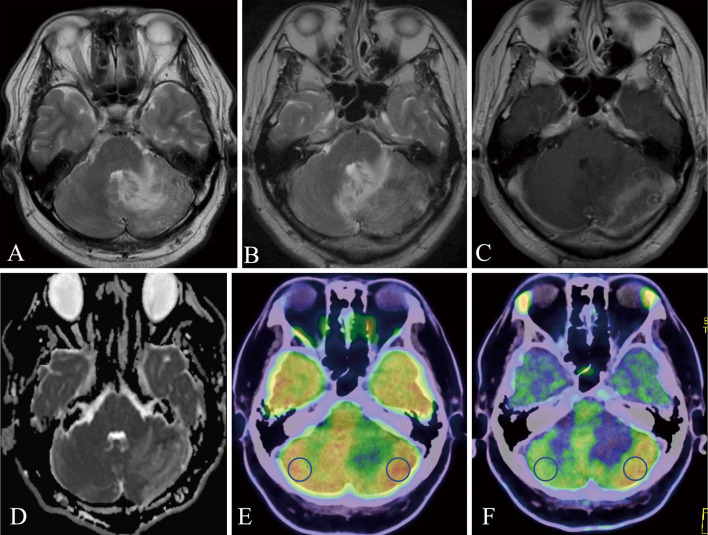
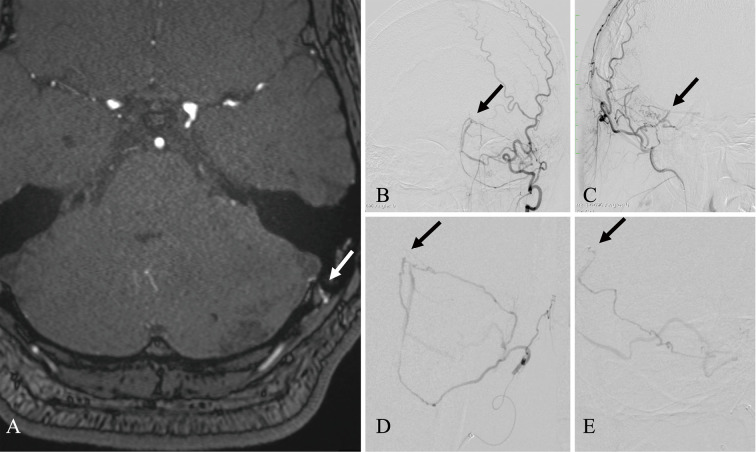
A 45-year-old man with diabetes mellitus controlled with insulin visited a primary doctor with headache and nausea. Although there was no worsening of symptoms, MRI T2-weighted image showed a high signal inside the left cerebellar hemisphere, which increased progressively over 1 month (Fig. 1A, B). With the diagnosis of a malignant brain tumor based on MRI T2-weighted image, Gd-enhanced MRI was performed. The outside of the hyperintensity region of MRI T2-weighted image was mottled and strongly enhanced, especially on the surface of the cerebellum (Fig. 1C). The apparent diffusion coefficient map image at this time showed an elevated signal, which suggested vasogenic cerebral edema (Fig. 1D). Based on these findings, a malignant brain tumor was initially suspected, but other diagnostic possibilities were also considered; thus, both FDG and methionine-PET were performed to confirm the diagnosis and estimate the extent of the tumor. At first, FDG-PET showed a slight increase in FDG uptake (tumor to normal [T:N] ratio: 1.22) in the region enhanced by Gd-MRI (Fig. 1E). Methionine-PET also showed increased methionine uptake (T:N ratio: 1.88) in the region enhanced by Gd-MRI (Fig. 1F). Therefore, we concluded that FDG and methionine-PET imaging also indicated the most suspected malignant brain tumor. Surgical submission of tissue was initially planned to confirm the diagnosis, but the discrepancy in FDG and methionine uptake was atypical; therefore, we reviewed the images again. A portion of the occipital artery ran close to the dura mater, and the possibility of an abnormal finding was considered. Since the TOF-MRA findings were faint and not convincing (Fig. 2A), DSA was performed. DSA revealed straight sinus DAVF (StS-DAVF), wherein feeders were dural branches of the left occipital artery. The shunt point was located just below the straight sinus on the tentorium cerebelli. These findings suggested Cognard type 3, nonsinus-type StS-DAVF (Fig. 2B-E).
Fig. 1.
Magnetic resonance and positron emission tomography images.
A: T2-weighted image. A high signal was observed in the left cerebellar hemisphere. B: One month after the first T2-weighted image. A high signal was expanded over time. C: Gd-enhanced T1-weighted image of the same period as B. Contrast effects are observed on the surface of the left cerebellar hemisphere. D: Apparent diffusion coefficient map showed a high signal, suggesting the finding of vasogenic cerebral edema. E: Fluorodeoxyglucose (FDG) positron emission tomography (PET) image. Mildly increased FDG uptake on the outside of the left cerebellar hemisphere, consistent with lesions imaged with gadolinium-enhanced magnetic resonance imaging (Gd-MRI). Region of interest was measured at the blue circles. F: Methionine-PET image. Increased methionine uptake on the outside of the left cerebellar hemisphere, consistent with lesions imaged with Gd-MRI. Region of interest (ROI) was measured at the blue circles.
Fig. 2.
Preoperative clinical images of magnetic resonance angiography and digital subtraction angiography.
A: An axial image of time-of-flight magnetic resonance angiography. The arrow indicates the mastoid branch, a feeder branching from the occipital artery and running over the dura mater. B and C: Frontal and lateral view of left-sided occipital angiography. D and E: Close-up image of super-selective angiography of the mastoid branch of the occipital artery. Black arrows indicate the shunt point.
Endovascular treatment and postoperative course
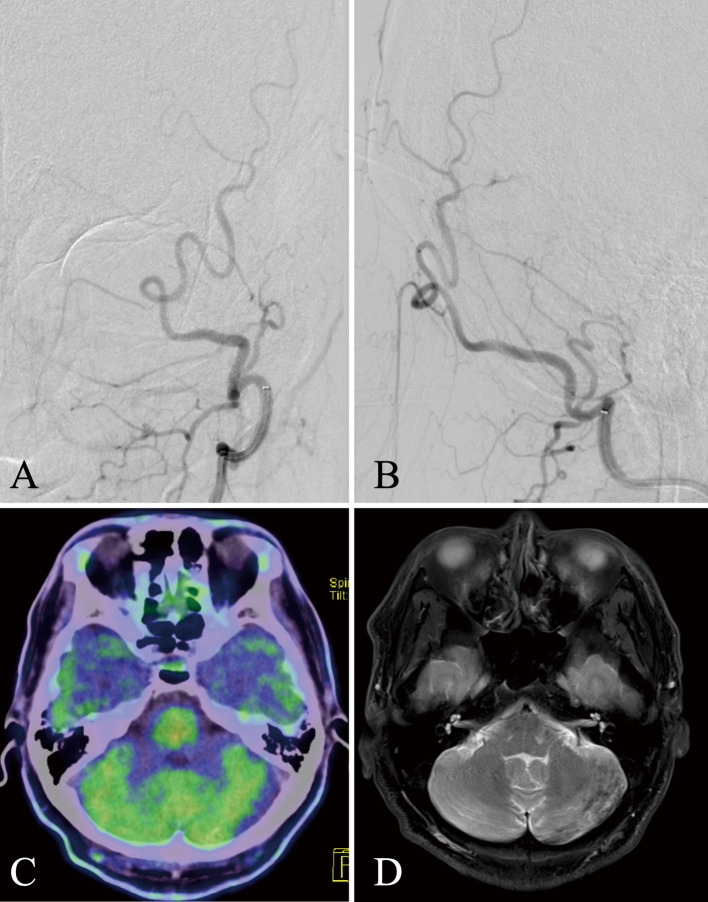
Transarterial embolization (TAE) was performed because the patient had a symptomatic nonsinus-type DAVF. A 7-Fr Roadmaster (Goodman, Aichi, Japan) was placed in the left external carotid artery and a 4-Fr Cerlian (Medikit, Tokyo, Japan) was placed in the left occipital artery for TAE using the liquid embolic substance Onyx 18 (Medtronic, Minneapolis, MN, USA). A micro guidewire Tenrou S1014 (Kaneka Medics, Osaka, Japan) was used to guide the Marathon (Medtronic, Minneapolis, MN, USA) as close to the shunt point as possible. Onyx was penetrated to the shunt point, resulting in complete occlusion (Fig. 3A, B). Headache and nausea disappeared after the operation, and it was confirmed that the hyperintensity area immediately disappeared during MRI T2-weighted image and methionine-PET 6 months after the operation (Fig. 3C, D).
Fig. 3.
Postoperative images of digital subtraction angiography, positron emission tomography, and T2-weighted image.
A and B: Frontal and lateral view of left-sided occipital angiography after Onyx transarterial embolization; the shunt flow disappeared completely. C: Six months after surgery, the high preoperative uptake found in methionine-positron emission tomography had disappeared. D: T2-weighted image. The abnormal hyperintense area in the left cerebellum had also been extensively improved.
Discussion
StS-DAVF is rare, accounting for 3%-5% of DAVF cases.8-10) Due to its rarity, there have been reports in the literature that DAVF is mistaken for other diseases, such as temporal arteritis,3) cerebral venous sinus thrombosis,4) and malignant glioma of the medulla oblongata.11-13) In this case, the findings were not well defined due to the fact that the shunt was faint. We considered a brain tumor as a differential diagnosis and therefore performed a methionine-PET scan. Methionine-PET showed findings consistent with a tumor diagnosis. This case showed that the diagnosis should not be solely based on the findings of methionine-PET and methionine uptake may also be elevated in cases of DAVF.
Diagnostics of DAVF
DSA is the gold standard for the diagnosis of DAVF. Nevertheless, MRA is an easy and convenient modality for screening DAVF because DSA is an invasive examination.14) However, in rare cases such as the present case, or when the shunt is faint, it may be difficult to make a diagnosis due to poor imaging. In fact, Yu-Ching et al. reported that only 63.4% of cases diagnosed as DAVF by DSA could be diagnosed by TOF-MRA.15)
Methionine-PET
Tumor cells are more active in protein synthesis than normal cells, taking up more methionine.16) Thus, methionine-PET imaging is helpful for tumor examination. Some studies have reported the use of methionine-PET for brain tumor and radiation necrosis differentiation.17) In Japan, this test is not covered by insurance and is only available at university hospitals, but it is sometimes performed to confirm the characteristics and diagnosis of brain tumors. At our hospital, methionine-PET is performed in cases of suspected brain tumors. To our knowledge, there are no reports of methionine-PET in DAVF. Several studies have reported increased methionine uptake in other inflammatory diseases of the central nervous system.6,18) Ishiguro et al. reported that increased protein synthesis of intermediate filaments and elevated methionine uptake can be observed during gliotic reactions in cavernous malformations. Vasogenic cerebral edema and microangiogenesis of vascular endothelial cells may be caused by the disruption of the blood-brain barrier and the resulting gliotic reaction.16) Moreover, Nakajima et al. documented that focal blood-brain barrier disruption contributed to increased methionine uptake in cases of cavernous angioma.19) Furthermore, Ito et al. reported that gliotic reaction is associated with methionine uptake, which is falsely positive in intracranial vascular disease.20) Our patient had DAVF with regurgitation into cortical veins, which is expected to cause vasogenic edema (Fig. 1D) and focal disruption of the blood-brain barrier due to venous congestion, and methionine uptake may be increased by the exact mechanism, as in the previous report.
Discrepancy between FDG and methionine-PET images
In the present case, there was no clear concordance between the FDG-PET and methionine-PET findings, which led to further investigation. The T:N ratio is important for more accurate diagnoses based on PET findings.
The T:N ratio is defined as the ratio of the standardized uptake value max of the brain lesion to the contralateral normal gray matter in the same axial plane using a 10-mm circular region of interest. The T:N ratio in our case was 1.22 and 1.88 for FDG and methionine, respectively, both of which showed increased uptakes compared with normal tissue. However, according to previous studies, the T:N ratio of brain tumors generally ranges from 1.38 to 3.1, so the FDG uptake in our case was not significant.21,22) Moreover, Büsing et al. reported that FDG uptake in the normal brain is reduced in patients with high blood glucose levels, insulin users, and patients with obesity, suggesting that the T:N ratio is more enhanced because tumor areas are not affected.23) Because of this, the FDG uptake was not considered significant in this case, in which the T:N ratio on FDG-PET was only 1.22. The discrepancy in uptake between FDG and methionine was atypical in brain tumor, and additional tests were performed to reach a diagnosis.6,18) Therefore, if a brain tumor is suspected, it might be essential to obtain both FDG and methionine-PET scans and measure the T:N ratio of each, if possible, to distinguish it from other diseases.
Conclusion
We reported a rare StS-DAVF that mimicked a brain tumor on MRI and PET scans. PET scans are rarely performed on patients with DAVF. The present case is the first report of increased methionine uptake in DAVF. Methionine uptake may be increased in DAVF, and we should be careful to distinguish it from tumors.
Conflicts of Interest Disclosure
The authors declare no conflicts of interest.
References
- 1).Noguchi K, Melhem ER, Kanazawa T, et al. : Intracranial dural arteriovenous fistulas: evaluation with combined 3D time-of-flight MR angiography and MR digital subtraction angiography. AJR Am J Roentgenol 182: 183-190, 2004 [DOI] [PubMed] [Google Scholar]
- 2).Lin YH, Wang YF, Liu HM, et al. : Diagnostic accuracy of CTA and MRI/MRA in the evaluation of the cortical venous reflux in the intracranial dural arteriovenous fistula DAVF. Neuroradiology 60: 7-15, 2018 [DOI] [PubMed] [Google Scholar]
- 3).Naserrudin NS, Mohammad R: Dural arteriovenous fistula mimicking temporal arteritis. Clin Neurol Neurosurg 176: 44-46, 2019 [DOI] [PubMed] [Google Scholar]
- 4).Li-li S, Wen-Xiong T: Dural arteriovenous fistula disguised as cerebral venous sinus thrombosis. J Zhejiang Univ Sci B 18: 733-736, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Vincent D, Anastasia P: Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol 18: 426-434, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Gulyas B, Halldin C: New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging 56: 173-190, 2012 [PubMed] [Google Scholar]
- 7).Andreas H, Anne T: 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 46: 1948-1958, 2005 [PubMed] [Google Scholar]
- 8).Kuwayama N, Kubo M, Hori E, et al. : Dural arteriovenous fistulas: complications of endovascular treatment. Surg Cereb Stroke 34: 91-95, 2006 [Google Scholar]
- 9).Hiramatsu M, Sugiu K: Epidemiology of dural arteriovenous fistula in Japan: analysis of Japanese Registry of Neuroendovascular Therapy. Neurol Med Chir (Tokyo) 54: 63-71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Willinsky RA, Goyal M, TerBrugge K, Montanera W: Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: correlations with presentation, location, and MR findings in 122 patients. AJNR Am J Neuroradiol 20: 1031-1036, 1999 [PMC free article] [PubMed] [Google Scholar]
- 11).Shigekawa S, Inoue A, Nakamura Y, Kohno D, Tagawa M, Kunieda T: A rare case of spinal dural arteriovenous fistula mimicking malignant glioma of the medulla oblongata: significance of cerebral angiography for accurate diagnosis of brain stem region. Surg Neurol Int 11: 287, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Bernard F, Lemée JM, Faguer R, Fournier HD: Lessons to be remembered from a dural arteriovenous fistula mimicking medulla and high cervical cord glioma. World Neurosurg 113: 312-315, 2018 [DOI] [PubMed] [Google Scholar]
- 13).Sasagawa A, Mikami T, Kimura Y, et al. : Stroke mimics and chameleons from the radiological viewpoint of glioma diagnosis. Neurol Med Chir (Tokyo) 61: 134-143, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Balasubramanian AP, Kannath SK: Utility of silent magnetic resonance angiography in the evaluation and characterisation of intracranial dural arteriovenous fistula. Clin Radiol 76: 712.e1-712, 2021 [DOI] [PubMed] [Google Scholar]
- 15).Cheng YC, Chen HC, Wu CH, et al. : Magnetic resonance angiography in the diagnosis of cerebral arteriovenous malformation and dural arteriovenous fistulas: comparison of time-resolved magnetic resonance angiography and three dimensional time-of-flight magnetic resonance angiography. Iran J Radiol 13: e19814, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Ishiguro T, Nitta M, Komori T, Maruyama T, Muragaki Y, Kawamata T: Transient focal magnetic resonance imaging abnormalities after status epilepticus showed 11C-methionine uptake with positron emission tomography in a patient with cerebral cavernous malformation. World Neurosurg 114: 43-46, 2018 [DOI] [PubMed] [Google Scholar]
- 17).Hotta M, Minamimoto R, Miwa K: 11C-methionine-PET for differentiating recurrent brain tumor from radiation necrosis: radiomics approach with random forest classifier. Sci Rep 9: 15666, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Katsanos AH, Alexiou GA, Fotopoulos AD, et al. : Performance of 18F-FDG, 11C-methionine, and 18F-FET PET for glioma grading: a meta-analysis. Clin Nucl Med 44: 864-869, 2019 [DOI] [PubMed] [Google Scholar]
- 19).Nakajima R, Kimura K, Abe K, Sakai S: 11C-methionine PET/CT findings in benign brain disease. Jpn J Radiol 34: 279-288, 2017 [DOI] [PubMed] [Google Scholar]
- 20).Ito K, Matsuda H, Kubota K: Imaging spectrum and pitfalls of 11C-methionine positron emission tomography in a series of patients with intracranial lesions. Korean J Radiol 17: 424-434, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Hatakeyama J, Ono T, Takahashi M, Oda M, Shimizu H: differentiating between primary central nervous system lymphoma and glioblastoma: the diagnostic value of combining 18F-fluorodeoxyglucose positron emission tomography with arterial spin labeling. Neurol Med Chir (Tokyo) 61: 367-375, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Albano D, Bosio G, Bertoli M, Giubbini R, Bertagna F: 18F-FDG PET/CT in primary brain lymphoma. J Neurooncol 136: 577-583, 2018 [DOI] [PubMed] [Google Scholar]
- 23).Büsing KA, Schönberg SO, Brade J, Wasser K: Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol 40: 206-213, 2013 [DOI] [PubMed] [Google Scholar]