Abstract
The raging COVID-19 pandemic is in its third year of global impact. The SARS CoV 2 virus has a high rate of spread, protean manifestations, and a high morbidity and mortality in individuals with predisposing risk factors. The pathophysiologic mechanisms involve a heightened systemic inflammatory state, cardiometabolic derangements, and varying degrees of glucose intolerance. The latter can be evident as significant hyperglycemia leading to new-onset diabetes or worsening of preexisting disease. Unfortunately, the clinical course beyond the acute phase of the illness may persist in the form of a variety of symptoms that together form the so-called “Long COVID” or “Post-COVID Syndrome”. It is thought that a chronic, low-grade inflammatory and immunologic state persists during this phase, which may last for weeks or months. Although numerous insights have been gained into COVID-related hyperglycemia and diabetes, its prediction, course, and management remain to be fully elucidated.
Keywords: COVID-19, SARS CoV-2, Type 2 diabetes, Newly diagnosed diabetes, New-onset diabetes, Post-COVID syndrome, Long COVID, Syndemia
1. Introduction: clinical course of COVID-related syndromes
The COVID-19 pandemic has taken the world by storm. It started in late 2019, when a novel coronavirus termed severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) was etiologically linked to respiratory illness in the city of Wuhan, China. The infection has since spread globally and disrupted everyday human life significantly. Interestingly, there was previous evidence that viruses circulating in bats could spread to humans.1 Based on an international study of close to 4.5 million people in Europe regarding symptomatology, around one in ten patients who were positive for SARS-CoV-2 virus remain symptomatic beyond 3 weeks, while a smaller fraction displayed symptoms for months.2 The longitudinal impact of COVID-19 in patients using a 12-item Short Form Survey (SF-12) score (a Health-Related Quality of Life tool) identified predictors of developing Post COVID-19 Syndrome (PCS).3 With the acquisition of further data regarding the clinical course of COVID-19, it was seen to be a multiorgan disorder that sometimes led to the unfolding of complications while patients continue to suffer from persisting and cyclical symptoms.4 This spectrum of persisting symptoms can have variable presentation in people irrespective of the severity of their initial disease and can range from mild to chronic and debilitating. It has been named “Post-COVID-19 Syndrome” (PCS) or “Long COVID”. In fact, in terms of symptoms, more than 90 % took 35 weeks or longer to recover.5 This exacts a great toll on productivity, with more than one-third of the patients requiring a reduced work schedule compared to the prior, healthier state, and almost one-third still not be able to return to the workforce at 7 months of follow up due to continuing symptoms. Some researchers have used the term long COVID for symptoms lasting more than 4 weeks, whereas PCS refers to more than 12 weeks' duration.6
2. Symptoms of long- and post-COVID
The most common manifestations of PCS include extreme fatigue, tiredness, shortness of breath, brain fog, changes in taste and smell, and musculoskeletal and arthritic pains. In fact, surveys have identified literally hundreds of complaints. The picture may resemble chronic fatigue syndrome (CFS), which appears to be the most common complaint and restricts daily activities like cooking, showering, grocery shopping, or exercise.7
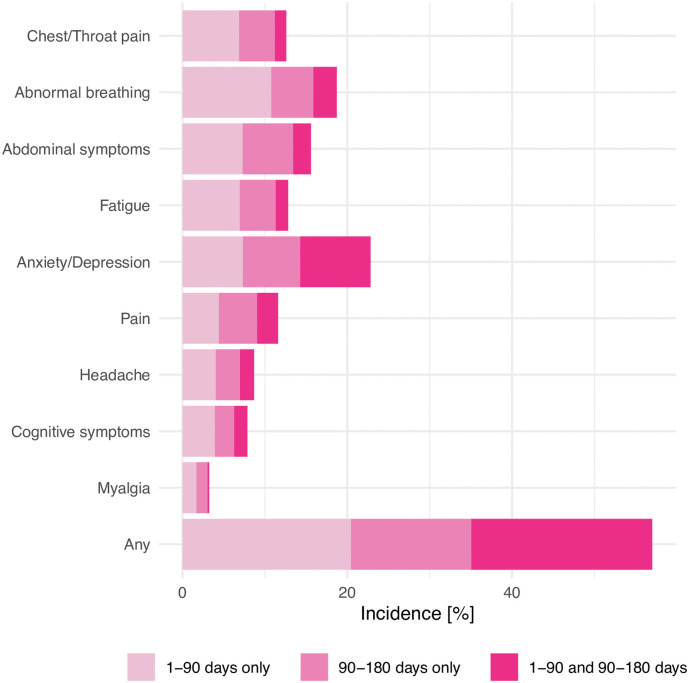
The spectrum of clinical presentation of COVID-19 varies from asymptomatic to severe illness. The recovery time from COVID-19 is highly variable and depends on the severity of disease, age and preexistence of comorbidities. Individuals with mild infection can have a recovery time as quick as a few days to within two weeks, while individuals with severe disease can have a longer recovery time of 2 to 3 months. If symptoms continue beyond 4 weeks since onset of infection, the term long COVID has been used, whereas persistence of symptoms for more than 12 weeks qualifies as PCS.5 Notably, ongoing symptoms and debility well after the acute phase is supposed to be over has been observed with other viral infections like SARS and MERS.8 However, unique to COVID-19, shortness of breath and fatigue may be seen up to 1 year after a symptomatic episode, coupled with reduced quality of life.9 Shah et al. identified fatigue and persisting shortness of breath as the most frequent symptoms of long COVID.10 The spectrum of symptomatology also includes cough, headache, myalgia, cognitive and mental disorders, chest and joint pains, smell and taste dysfunctions, insomnia, wheezing, rhinorrhea, sputum, and cardiac and gastrointestinal issues that may persist for six months after their onset.11 The frequency of prevalence of the common symptoms in long COVID has been summarized in Fig. 1 .
Fig. 1.
Long-COVID symptoms.
(From Taquet et al., PLOS Medicine 189: e1003773. https://doi.org/10.1371/journal.pmed.1003773. Open access. No copyright infringement intended).
3. Prevalence and underlying factors
Knowledge regarding the prevalence, pathology, predictors, or risk factors of developing long COVID-19 remains sparse. Based on data of the COVID-19 symptom study, the chances of developing long COVID are affected by age with rates being 1 to 2 % in patients in their twenties to about 5 % in people in their sixties.2., 3. The heterogeneity of long COVID with respect to symptom duration, frequency, initial disease severity and patient characteristics makes it highly unpredictable.
Several hypotheses have been suggested to understand the pathophysiology of post-COVID syndrome relating to hyperinflammatory states, oxidative stress, cytokine storm and DNA damage.12 In a follow-up study, 70 % of subjects had pulmonary radiological abnormalities at 12 weeks.13 Multiple studies have shown that abnormal lung functions as well as structural changes were detectable in some moderately sick COVID-19 patients for up to 6 months after the acute illness.14., 15., 16. In a German study that followed 100 COVID-19 patients, myocardial inflammation and cardiac abnormalities were found respectively in 60 % and 78 % of the subjects independent of their pre-existing disease severity.17 A literature review on long COVID suggests that the potential pathology may lie in persistent pulmonary, neurological, or cardiac tissue damage as well as virus-mediated inflammation and immune dysregulation.18
A constellation of features resembling chronic fatigue syndrome seems to be the hallmark in many patients who have persistent complaints. The term “myalgic encephalomyelitis” is used to describe a broad-spectrum condition with symptoms such as fatigue, post-exertional malaise, sleep disturbances, cognitive impairment, and non-provoked pain that persist for more than 6 months with substantial intensity and not completely explained by a medical condition. It is a heterogeneous, multifactorial etiology involving immune, virologic, psychological, musculoskeletal, and endocrine factors. Nutritional deficiencies could be a major challenge in some patients who have poor oral intake due to debility, lack of taste and smell, and medication side-effects, leading to negative protein balance and a lack of nutrients essential to proper musculoskeletal functioning. These factors are augmented in the elderly with previously existing chronic health issues. For example, a state of persistent low-grade neuroinflammation in the vulnerable population is a potential explanation for the chronic fatigue.19 Endocrine perturbations stemming from pituitary malfunction, hypothyroidism or hypothalamic-pituitary-adrenal axis disruption might be potential components that delay or prevent post-COVID recovery.
4. Hyperglycemia and new-onset diabetes
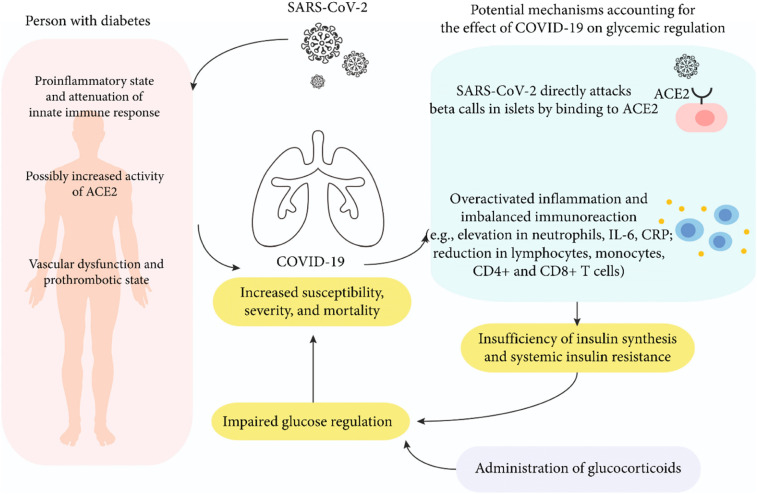
Evidence from the beginning of the COVID-19 pandemic has clearly established that patients with COVID-19 have a greater prevalence of hyperglycemia, new-diagnosed diabetes (NDD), and need for initiation or escalation of pharmacologic therapy.20., 21. Although most of the patients with NDD would be assumed to have new-onset diabetes (NOD), these two conditions were not necessarily equivalent and could not be referred to interchangeably, since an undiagnosed pre-existing diabetic state could not be ruled out. Post-COVID pathophysiologic changes that predispose to glucose intolerance are being actively investigated. Three prominent pathophysiologic mechanisms that have emerged relate to a heightened proinflammatory state, the role of angiotensin converting enzyme (ACE)-2 receptors, and pancreatic beta cell dysfunction.
The pathophysiologic pathways leading to increased inflammation and hyperglycemia are depicted in Fig. 2 .
Fig. 2.
Mechanisms leading to increased risk of hyperglycemia and diabetes in SARS CoV2 Infection.
(From Li et al., Int J Endocrinol. 2021 https://doi.org/10.1155/2021/7394378. Open access, No copyright infringement intended).
4.1. Proinflammatory factors
The International Study of Inflammation in COVID-19 (ISIC) is a multicenter observational study of 2044 patients hospitalized with COVID-19 to characterize the impact of DM on in-hospital outcomes and assess the contribution of inflammation and hyperglycemia to the risk attributed to DM. Based on this comprehensive database, Vasbinder and colleagues measured biomarkers of inflammation collected at hospital admission, and glucose levels and insulin data throughout hospitalization.22 Their findings suggested that the association between DM and outcomes in COVID-19 is largely mediated by a state of “hyperinflammation” reflected by elevated soluble urokinase plasminogen activator receptor (suPAR) levels as a significant predictor, followed by obesity, hyperglycemia, and age. The former is a useful biomarker whose levels reflect the degree of immune activation and predict morbidity and mortality across a spectrum of chronic conditions such as cardiovascular diseases and cancer. Interestingly, hyperglycemia and higher insulin requirements correlated only weakly with inflammatory biomarkers and seemed to be associated with outcomes independently of suPAR, suggesting that other mechanisms were at play.12., 18. Along these lines, in hospitalized patients with diabetes, a proinflammatory metabolic state resulting from the virus promotes severe insulin resistance, hyperglycemia, and a tendency toward rapidly evolving renal failure, hypotension, pressor and steroid use, and need for nutritional support.16., 22. Investigators have advocated timely implementation of glucose management protocols to address these complications while investigating the role of COVID-19-related inflammatory biomarkers.23
4.2. The role of ACE2 receptors
ACE2 receptors are present ubiquitously in the human body, including adipose tissue, liver, and small intestine. These tissues play an important role in insulin resistance and the pathophysiologic progression of diabetes. ACE-2 is a key regulator of the renin-angiotensin-aldosterone system (RAAS) and is expressed in the heart, kidneys, and lungs on cell surfaces through which SARS-CoV-2 enters the host cells and circulates in the plasma.24 SARS-CoV-2 causes disruption of the ACE/ACE2 balance and RAAS activation which contributes to insulin resistance and leads ultimately to COVID-19 symptomatology and progression, especially in patients with comorbidities such as hypertension, diabetes mellitus, and cardiovascular disease.25 With respect to the endocrine system, longitudinal and postmortem studies have found SARS-CoV RNA in the pituitary gland, parathyroid, pancreas and adrenal gland.26 However, the exact role that ACE2 receptors play in the facilitation or prevention of the coronavirus remains to be fully elucidated.27
4.3. Pancreatic damage
There is evidence that the SARS-CoV-2 virus directly attacks the endocrine pancreas and its beta cell assembly, leading to damage and subsequent impairment of insulin secretion.28 Although this can manifest in individuals without previously diagnosed diabetes, it is more severe and associated with poorer clinical outcomes in diabetic patients due to presence of comorbid conditions and baseline endothelial inflammation.29., 30. Additionally, there is emerging data that a scenario of insulinopenic hyperglycemia resembling type 1 diabetes (T1DM), possibly via immunologic pathways, might be associated with acute COVID-19 and its aftermath.31., 32.
5. The impact of antihyperglycemic drugs
Pursuant to the above-mentioned pathophysiologic mechanisms of COVID-related hyperglycemia, the purported anti-inflammatory effect of diabetic medications such as metformin, pioglitazone, sodium-glucose co-transporter-2 inhibitors (SGLT2-I), and the incretin-based therapies should be kept in mind.28 Some reports suggest that the dipeptidyl peptidase 4 (DPP4) enzyme receptor might also act as a binding target, possibly in conjunction with ACE2 receptors.33 Preliminary data, however, do not suggest a notable beneficial effect of DPP4 inhibitors in SARS-CoV-2 infection. Due to their unique side-effects profile, SGLT2-I might cause adverse effects in patients with COVID-19 as well. In fact, several major considerations point to insulin therapy as the most appropriate approach in the management of COVID-19 patients with hyperglycemia,28., 34. namely potency, flexibility, anti-inflammatory benefit, lack of interaction with other drugs, and effectiveness against diabetic ketoacidosis. The latter may be particularly relevant in situations where COVID-19 infection is a precipitant or aggravator of T1DM. Several inpatient insulin regimens have been developed for patients with COVID-19 and diabetes.35
6. Recent spotlight on persistent diabetes after COVID-19 infection
It is important to highlight two notable studies that examined the phenomenon of long-term hyperglycemia emerging after the diagnosis of diabetes coincident with infection with SARS CoV-2 infection, with somewhat divergent results. In a report from the Massachusetts General Hospital in Boston, Cromer and colleagues 1902 individuals admitted with COVID-19.36 Of these, 594 (31.2 %) had diabetes, 77 (13.0 %) of which had NDD. Compared to pre-existing DM, NDD was more common in younger patients and was associated with lower glycemic parameters and insulin requirements, longer length of stay, higher inflammatory markers, and higher likelihood of intensive care unit admission (but not death). Of the 64 patients with NDD who survived, 36 (56.3 %) continued to have DM and 26 (40.6 %) regressed to normoglycemia or pre-diabetes. The authors concluded that these patterns suggested stress hyperglycemia as a major physiologic mechanism, and approximately half of such individuals experience regression of DM.
In another large cohort study published recently, Xie and Al-Aly reported on the post-acute risk and burden of incident diabetes in people who survived the first 30 days of SARS-CoV-2 illness.37 The study was based on a review of national databases from the US Department of Veterans Affairs, consisting of a cohort of 181,280 participants who had a positive COVID-19 test between March 1, 2020, and Sept 30, 2021, and survived the first 30 days of COVID-19. They were compared with two groups, a contemporary control group (n = 4,118,441), and a historical control group (n = 4,286,911) that enrolled participants between March 1, 2018, and Sept 30, 2019, both without evidence of SARS-CoV-2 infection. All 3 groups were ascertained to be free of diabetes before entry and were followed for a median of 352 days. Two measures of risk were reported: hazard ratio (HR) and burden per 1000 people at 12 months. As depicted in 1, in the post-acute phase of the disease, compared with the contemporary control group, people with COVID-19 exhibited an increased risk (HR 1·40, 95 % CI 1·36–1·44) and excess burden (13·46, 95 % CI 12·11–14·84, per 1000 people at 12 months) of incident diabetes; and an increased risk (1·85, 1·78–1·92) and excess burden (12·35, 11·36–13·38) of incident antihyperglycemic use. Additionally, analyses to estimate the risk of a composite endpoint of incident diabetes or pharmaceutical agent use yielded a HR of 1·46 (95 % CI 1·43–1·50) and an excess burden of 18·03 (95 % CI 16·59–19·51) per 1000 people at 12 months. Risks and burdens of post-acute increased in a graded fashion according to the severity of the acute phase of COVID-19, whether patients were non-hospitalized, hospitalized, or admitted to intensive care (Table 1).
Table 1.
Increased risk of new-onset diabetes and new antidiabetic agent use in the VA study.37
| Incident diabetes | Incident antihyperglycemic use | Both | |
|---|---|---|---|
| Hazard ratioa | 1.4 1·36–1·44 |
1.85 1.78–1.92 |
1.46 1.43–1.50 |
| Excess burdena | 13.46 12.11–14.84 |
12.35 11.36–13.38 |
18.03 16.59–19.51 |
With 95 % CI's.
7. Long-term and public health perspectives
The impact of the SARS CoV2 virus on the prevalence of diabetes has had profound epidemiologic and public health implications.38 It is a true bidirectional relationship, with diabetes contributing significantly to COVID-19 morbidity, and the latter having had a devastating effect on the diabetic population.39 It is sobering to review the statistics in this regard. Approximately 30–40 % of people with severe COVID-19–related morbidity (hospitalization requiring intensive care) and mortality have diabetes.40 Among hospitalized individuals with diabetes and COVID-19, anywhere between 21 and 43 % required intensive care, and the case fatality was 25 %.41 Sathish et al. reported a high prevalence of previously undiagnosed diabetes among persons hospitalized for COVID-19.42 Alarmingly, the risk of severe morbidity and mortality is 100–250 % higher among patients with COVID-19 with diabetes compared to those without, even after adjustment for sociodemographic and comorbid factors.40 The economic impact of this collision of diseases has been astronomical.43 Unfortunately, the epidemiologic burden of this new “COVID-diabetes pandemic” is disproportionately higher in the developing countries where most of the vulnerable population resides. It is imperative that the problem begs a multi-pronged strategy for optimal management, addressing both individual patient therapies and public health challenges. Finally, a sizeable percentage of the affected population is facing the prospect of chronic glucometabolic sequelae in the “long COVID” phase, with ongoing morbidity and an uncertain quality of life.44
Footnotes
The authors have no relevant conflicts of interest to disclose. This work is original and has not been submitted for publication elsewhere.
References
- 1.Menachery V.D., Yount B.L., Jr., Debbink K., Jr., et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. Erratum in: Nat Med. 2016;22(4):446. Erratum in: Nat Med. 2020;26(7):1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID Symptom Study How long does COVID-19 last? https://covid.joinzoe.com/post/covid-long-term Available from.
- 3.O'Kelly B., Vidal L., Avramovic G., et al. Assessing the impact of COVID-19 at 1 year using the SF-12 questionnaire: data from the anticipate longitudinal cohort study. Int J Infect Dis. 2022;S1201–9712:00152–00157. doi: 10.1016/j.ijid.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis A., et al. COVERSCAN Study Investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiyegbusi O.L., et al. TLC Study Group. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114:428–442. doi: 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batawi S., Tarazan N., Al-Raddadi R., et al. Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS) Health Qual Life Outcomes. 2019;17:101. doi: 10.1186/s12955-019-1165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han Q., Zheng B., Daines L., Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372 doi: 10.1136/bmj.n136. Epub. 01/24. [DOI] [PubMed] [Google Scholar]
- 11.Crook H., Raza S., Nowell J., Young M., Edison P. Long COVID-mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 12.Bektas A., Schurman S.H., Franceschi C., Ferrucci L. A public health perspective of aging: do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short- and long-term inflammaging? Immun Ageing. 2020;17:23. doi: 10.1186/s12979-020-00196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y.M., Shang Y.M., Song W.B., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiss R., Grodzki D.M., Horger W., Uder M., Nagel A.M., Bickelhaupt S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn Reson Imaging. 2021;76:49–51. doi: 10.1016/j.mri.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno-Pérez O., et al. COVID19-ALC research group. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah A.S., Wong A.W., Hague C.J., et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76:402–404. doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- 17.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. Erratum in: JAMA Cardiol. 2020;5(11):1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021:1–18. doi: 10.1080/23744235.2021.1924397. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller C., Lin J.C., Sheriff S., Maudsley A.A., Younger J.W. Evidence of widespread metabolite abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2020;14:562–572. doi: 10.1007/s11682-018-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metwally A.A., Mehta P., Johnson B.S., Nagarjuna A., Snyder M.P. COVID-19-induced new-onset diabetes: trends and technologies. Diabetes. 2021 Dec;70:2733–2744. doi: 10.2337/dbi21-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michalakis K., Ilias I. COVID-19 and hyperglycemia/diabetes. World J Diabetes. 2021;12:642–650. doi: 10.4239/wjd.v12.i5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasbinder A., et al. On behalf of the ISIC Study Group. Inflammation, hyperglycemia, and adverse outcomes in individuals with diabetes mellitus hospitalized for COVID-19. Diabetes Care. 2022;45:692–700. doi: 10.2337/dc21-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianchandani R., Esfandiari N.H., Ang L., et al. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes. 2020;69:2048–2053. doi: 10.2337/dbi20-0022. [DOI] [PubMed] [Google Scholar]
- 24.Scialo F., Daniele A., Amato F., et al. The major cell entry receptor for SARS-CoV-2. Lung. 2020;198:867–877. doi: 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyerstedt S., Casaro E.B., Rangel É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y., He L., Zhang Q., et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Huneidi W., Hamad M., Taneera J. Expression of SARS-CoV-2 receptor "ACE2" in human pancreatic β cells: to be or not to be! Islets. 2021;13:106–114. doi: 10.1080/19382014.2021.1954458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuraishy H.M., Al-Gareeb A.I., Alblihed M., Guerreiro S.G., Cruz-Martins N., Batiha G.E. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.644095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unnikrishnan R., Misra A. Diabetes and COVID19: a bidirectional relationship. Nutr Diabetes. 2021;11:21. doi: 10.1038/s41387-021-00163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao S., Yang Q., Pan R., Yu X., Chen Y. Interaction of severe acute respiratory syndrome coronavirus 2 and diabetes. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.731974. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boddu S.K., Aurangabadkar G., Kuchay M.S. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14:2211–2217. doi: 10.1016/j.dsx.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner G., Fraker C.A. Natural killer cells as key mediators in type I diabetes immunopathology. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.722979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valencia I., Peiró C., Lorenzo Ó., Sánchez-Ferrer C.F., Eckel J., Romacho T. DPP4 and ACE2 in diabetes and COVID-19: therapeutic targets for cardiovascular complications? Front Pharmacol. 2020;11:1161. doi: 10.3389/fphar.2020.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim S., Bae J.H., Kwon H.S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Diabetes Association Inpatient insulin protocols - COVID-19. https://professional.diabetes.org/content-page/inpatient-insulin-protocols-covid-19
- 36.Cromer S.J., Colling C., Schatoff D., et al. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: associated factors, short-term outcomes, and long-term glycemic phenotypes. J Diabetes Complications. 2022;36 doi: 10.1016/j.jdiacomp.2022.108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Y., Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riddle M.C., Bakris G., Blonde L., et al. A lesson from 2020: public health matters for both COVID-19 and diabetes. Diabetes Care. 2021;44:8–10. doi: 10.2337/dci20-0071. [DOI] [PubMed] [Google Scholar]
- 39.Gregg E.W., Sophiea M.K., Weldegiorgis M. Diabetes and COVID-19: population impact 18 months into the pandemic. Diabetes Care. 2021;44:1916–1923. doi: 10.2337/dci21-0001. [DOI] [PubMed] [Google Scholar]
- 40.Ko J.Y., Danielson M.L., Town M., et al. COVID-NET Surveillance Team. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2021;72:e695–e703. doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shang L., Shao M., Guo Q., et al. Diabetes mellitus is associated with severe infection and mortality in patients with COVID-19: a systematic review and meta-analysis. Arch Med Res. 2020;51:700–709. doi: 10.1016/j.arcmed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;23:870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bain S.C., Czernichow S., Bøgelund M., et al. Costs of COVID-19 pandemic associated with diabetes in Europe: a health care cost model. Curr Med Res Opin. 2021;37:27–36. doi: 10.1080/03007995.2020.1862775. [DOI] [PubMed] [Google Scholar]
- 44.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. Erratum in: Nat Med. 2021;27(6):1116. [DOI] [PMC free article] [PubMed] [Google Scholar]