Dear Editor,
We read with interest recent reports describing acute hepatic injury following vaccination against SARS-CoV-2 [1]. The clinical expression of the hepatic involvement ranges from mild hepatitis to acute liver failure requiring liver transplantation [2], and biochemical, serological and histological features are typical of autoimmune hepatitis (AIH). We herein describe suspected SARS-CoV-2 vaccine-related liver injury with autoimmune features occurring in three young patients, in whom other causes of acute hepatitis, such as hepatitis A, B, C, E, drug induced liver injury, alcohol, have been excluded after accurate clinical, biochemical, and virological assessment. Detailed liver biopsy findings for each case are presented in Table 1 .
Table 1.
Liver biopsy findings among three patients.
Data are presented according to Ishak grading and staging.
| Case I (11 portal spaces) | Case II (12 portal spaces) | Case III (12 portal spaces) | ||||
|---|---|---|---|---|---|---|
| Periportal or periseptal interface hepatitis | Severe (continuous around > 50% of portal areas). Moderate plasma cells infiltrate | 4 | Mild/moderate (focal, most portal areas). Scattered plasma cells and eosinophils | 2 | Moderate (continuous around <50% of tracts). Moderate plasma cells infiltrate | 3 |
| Confluent necrosis | Zone 3 necrosis in some areas | 2 | Absent | 0 | Absent | 0 |
| Focal (spotty) lytic necrosis, apoptosis and focal inflammation | Five to ten foci per 10X objective, perivenular necroinflammatory infiltrate | 3 | Two to four foci per 10X objective, perivenular necroinflammatory infiltrate | 2 | One focus per 10X objective | 1 |
| Portal inflammation | Marked, all portal areas | 4 | Mild, focal inflammation | 1 | Moderate, all portal areas | 3 |
| Fibrosis | No fibrosis | 0 | Fibrous expansion of some portal areas, without septa | 1 | Fibrous expansion of most portal areas, with short fibrous septa | 2 |
| Other features | Mild bile duct injury | Focal ductular hyperplasia, no rosettes | Mild biliary regression, focal rosettes | |||
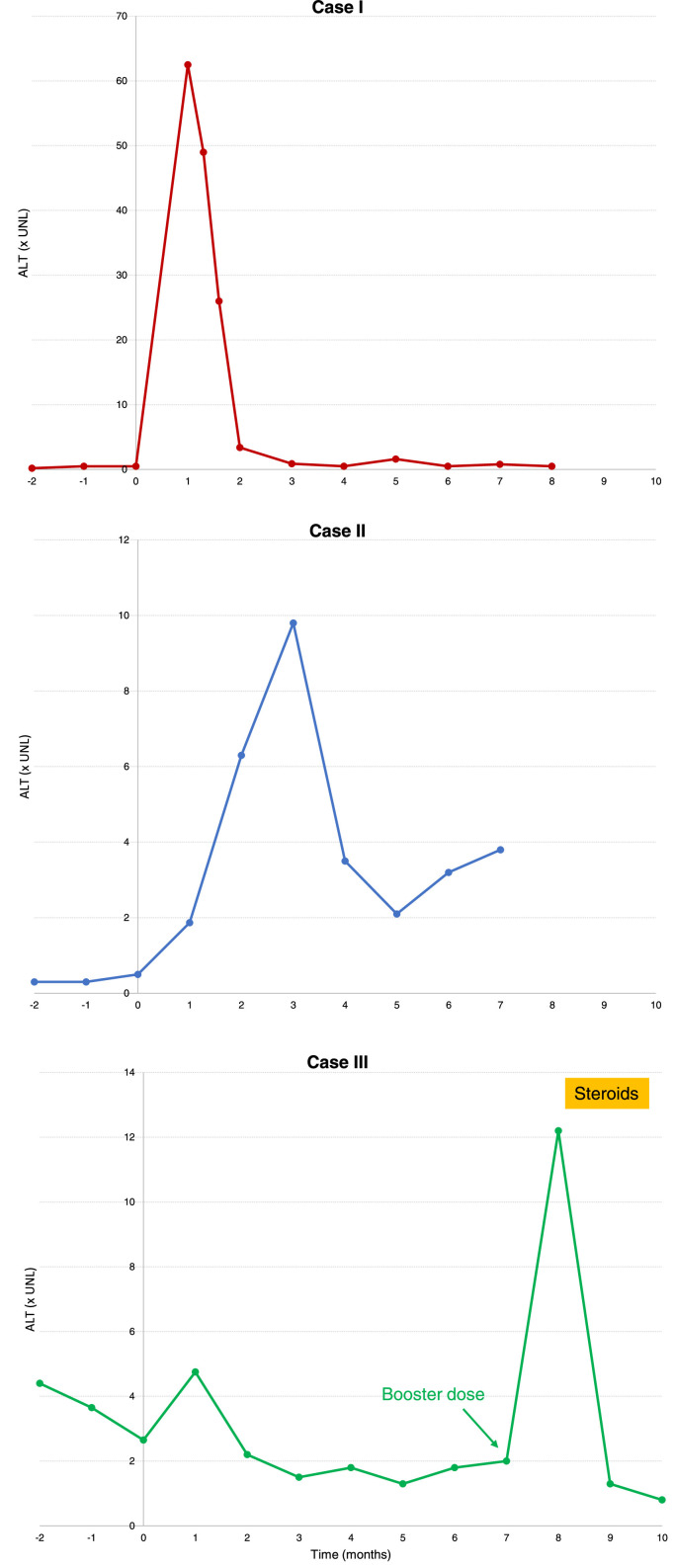
The first patient is a 30-years-old woman with Hashimoto thyroiditis and family history of autoimmune diseases (ADs) who received two doses of the Moderna vaccine in September and October 2021. In November 2021 she complained of asthenia, hyporexia, arthralgia and dark urine. Alanine transaminase (ALT) levels were 60-fold upper limit of normal (ULN), total bilirubin was 33.5 µmol/L, IgG was 1642 mg/dL and antinuclear antibodies (ANAs) tested positive at 1:640 titer with homogeneous pattern, while anti-smooth muscle antibody (ASMA), anti-SLA/LP, anti-LKM and anti-LC1 tested negative. A liver biopsy showed periportal lympho-plasmocytic infiltrate with interface hepatitis, without significant fibrosis (Table 1). The patient received high-dose N-acetylcysteine, while she refused steroid treatment. ALT levels decreased to normal in 8 weeks; one month later they were still moderately elevated (up to 3-fold), then normalized again spontaneously. The second patient is a 26-years-old male with vitiligo and pollen allergy, who has been taking maltodextrin and amino-acid supplements for several years. He performed routine blood tests on a regular basis and had his liver function consistently normal. He received two doses of the Moderna vaccine in June and July 2021, and in August he discovered altered ALT levels 1.9-fold ULN, progressively increasing up to a 10-fold peak in November, with IgG 1749 mg/dL, total bilirubin 15.4 µmol/L. ANAs were positive at 1:640 titer with homogeneous pattern, while ASMA, anti-SLA/LP and anti-LKM were negative. Liver biopsy showed mild portal lymphoplasmacytic infiltrate with mild-moderate interface hepatitis, without significant fibrosis (Table 1). HLA was A1, B8, DR3. Immunosuppressive treatment was declined, and transaminase levels remained altered (around 3-fold) in the following months. The third patient is a 21-years-old girl with no significant medical history. She came to our attention in November 2021 for persistent 2-fold ALT increase since March 2021 found on routine blood test, before the first vaccinal cycle. In November ALT were 2-fold ULN, ASMA were positive at 1:320 titer, ANAs at 1:80 titer with homogeneous pattern, IgG were 1502 mg/dL, and liver biopsy showed periportal lympho-plasmocytic and lobular inflammatory infiltrate and focal piecemeal necrosis with moderate periportal fibrosis (Table 1). HLA was A1, B8, DR3. A diagnosis of genuine AIH was made, but therapy was declined due to patient hesitancy. In December, a few days after the booster dose (Moderna), she developed a hepatitis flare with ALT levels up to 12-fold ULN, so steroid therapy was initiated with rapid normalization of ALT. Retrospectively, she was noted to have a slight ALT increase (peak 4.7 x ULN) two weeks after the second dose of the Pfizer vaccine in May 2021, with subsequent return to a 2-fold ULN during following months.
AIH is a persistent, fluctuating inflammation of the liver observed in genetically predisposed subjects after exposure to an initiating, mostly unknown, factor [3]. Vaccines have been proposed as potential triggers [4,5]. The association of SARS-CoV-2 vaccination and AIH has been increasingly reported: a systematic review recently reported 32 patients in the period December 2019 – November 21 [1].
In Supplementary material we reviewed the available case reports of AIH like liver injury until June 2022. The search was performed using PubMed, entering as keywords "COVID vaccine", "SARS-CoV-2 vaccine", "Autoimmune hepatitis" and “Liver injury”, last access in June 2022. A total of 31 reports was identified, covering a total of 52 cases of AIH-like liver injury after COVID-19 vaccination. The median age is 61 years (IQR 41-71), with a prevalence of female sex (N=35/52, 67%). Most relevant comorbidities are autoimmune diseases (ADs) (N=11/52, 21%) and liver diseases (N=12/52, 23%). Most of the reports involve mRNA vaccines (N=43/52, 83%). Onset time is generally quite short (median 15 days from the last dose, IQR 7-26) and a high proportion of patients present an acute hepatitis with jaundice (N=43/52, 40%). Autoantibodies useful for the diagnosis of AIH were often found (N=35/45, 78%, of which ANAs 31/45, 69%, ASMA in 11/45,24%, and anti-SLA/LP and anti-LC1 in one patient each). Other autoantibodies reported are anti-ds-DNA (N=2/45, 4%) and AMA (N=4/45, 9%). Immunoglobulin G values were increased in 68% of reports (N=23/34). Histological examination was suggestive of AIH among 98% of cases (N=45/46). In most of the reports, treatment involved the use of steroids (N=40/52, 77%), in some cases associated with azathioprine (N=8/52, 15%). In few cases (N=2/52) it was reported the use of budesonide with different results, in one case leading to hepatitis relapse requiring oral steroids. The clinical course was favorable in almost all cases, even if in three reports (6%) the disease evolved to overt liver failure, of which two died and one underwent to liver transplantation. One patient died of suspected opportunistic central nervous system infection.
These data are widely consistent with a recent retrospective study (ahead of print), which collected data from 18 countries on 87 cases and described their features and outcomes [2]. All the available evidence agree on the rarity of the phenomenon and its generally favorable outcome and emphasize the need for increased awareness, to promote early recognition and to provide guidance for adequate management.
Among our patients, the first two had stringent temporal correlation between vaccine and hepatic injury and a previously healthy liver has been documented, so direct causal correlation should be suspected. Interestingly, the kinetics of biochemical hepatic damage was quite different (graphic presentation in Supplementary material): hyperacute and self-limiting for the first patient, less acute but persistently abnormal and fluctuating for the second one. For the third patient, altered liver function tests were present before vaccination, but only after the Moderna booster a striking increase of ALT was observed, which was rapidly controlled with steroid treatment. In the latter case, previously altered ALT levels and liver fibrosis indicated a long-standing process, pointing to pre-existing AIH. Of note, as well as this “genuine AIH”, even the first two patients had a preexistent AD, highlighting predisposition to AIH-like liver injury after vaccination. Efe et al. reported that 28% of the AIH-like-liver-injury patients had been diagnosed with other AD before liver injury onset, of which most common are autoimmune thyroiditis (14%), inflammatory bowel diseases (3%) and sarcoidosis (3%) [2]. The significant proportion of patients with other ADs developing AIH-like liver injury suggest genetic predisposition, such as impaired clearance of nucleic acids, TLR polymorphism, HLA haplotype [7]. Various authors propose that vaccination may not generate new ADs, but rather triggers long-lasting latent autoimmunity [6,7]. The strong inflammatory response induced by pattern recognition receptors could act like a relapse trigger of a latent AIH, as shown for other ADs reactivation after SARS-CoV-2 vaccination [8]: this mechanism has been postulated since the early presentation with detectable pathogenic autoantibodies [8]. Other proposed mechanisms are bystander activation and epitope spreading [6] as well as molecular mimicry [7]. Interestingly, some reports suggest the possibility of SARS-CoV-2 Spike protein expression within hepatocytes after vaccination [9] and the presence of an immune infiltrate characterized by activated cytotoxic CD8 T-cells with SARS-CoV-2 Spike-protein specificity [10].
Whether these cases represent genuine AIH triggered by vaccine or transient vaccine-induced liver injury is at present a matter of debate, which only a longer follow up could figure out: recurrent flares of ALT or persisting ALT and IgG alterations will suggest typical AIH and need for prolonged immunosuppression, whereas spontaneous and complete biochemical and histological resolution will indicate vaccine-induced liver injury. Cases of genuine AIH reactivation after vaccination emphasizes the need for close follow-up after vaccination, particularly when the immunosuppressive treatment has not been introduced yet. As of today, we are unable to prove the direct role of vaccination in the induction of hepatic damage, but we cannot disprove it either. Rigorous population-based studies and active pharmacovigilance are urgently needed to assess beyond reasonable doubt incidence and clinical significance of such observations. Until then, the final verdict should remain open.
Financial support
The authors received no financial support to produce this manuscript.
Authors contributions
MF co-wrote the original manuscript and edited the final submission; ML assisted with conceptualization and edited the final submission; LM provided patient care, co-wrote the original manuscript and edited the final submission (Fig. 1 ).
Fig. 1.
Declaration of Competing Interest
No conflict of interest to declare
References
- 1.Chow KW, Pham NV, Ibrahim BM, Hong K, Saab S. Autoimmune hepatitis-like syndrome following COVID-19 vaccination: a systematic review of the literature. Dig Dis Sci. 2022;29:1–7. doi: 10.1007/s10620-022-07504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efe C, Kulkarni AV, Beretta-Piccoli BT, Magro B, Stättermayer AF, Cengiz M, et al. Liver injury after SARS-CoV-2 vaccination: features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022:1–11. doi: 10.1002/hep.32572. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zachou K, Arvaniti P, Lyberopoulou A, Dalekos GN. Impact of genetic and environmental factors on autoimmune hepatitis. J Transl Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perumalswami P, Peng L, Odin JA. Vaccination as a triggering event for autoimmune hepatitis. Semin Liver Dis. 2009;29:331–334. doi: 10.1055/s-0029-1233537. [DOI] [PubMed] [Google Scholar]
- 5.Muratori P, Serio I, Lalanne C, Lenzi M. Development of autoimmune hepatitis after influenza vaccination; trigger or killer? Clin Res Hepatol Gastroenterol. 2019;43:e95–e96. doi: 10.1016/j.clinre.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Akinosoglou K, Tzivaki I, Marangos M. Covid-19 vaccine and autoimmunity: awakening the sleeping dragon. Clin Immunol. 2021;226 doi: 10.1016/j.clim.2021.108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to "potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases". Clin Immunol. 2021;224 doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camacho-Domínguez L, Rodríguez Y, Polo F, Restrepo Gutierrez JC, Zapata E, Rojas M, Anaya JM. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. J Transl Autoimmun. 2022;5 doi: 10.1016/j.jtauto.2022.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyein CM, Liew ZHS, Leow WQ, Yeong PSJ, Ho GH. Severe de novo liver injury after Moderna vaccination - not always autoimmune hepatitis. J Hepatol. 2022;77(2):556–558. doi: 10.1016/j.jhep.2022.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L, Bronsert P, Schwabenland M, Prinz M, Mogler C, Neumann-Haefelin C, Thimme R, Hofmann M, Bengsch B. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;21 doi: 10.1016/j.jhep.2022.03.040. S0168-8278(22)00234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]